HTPFA-Coated AlB2 with Enhanced Combustion Performance as a High-Energy Fuel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
3. Results and Discussion
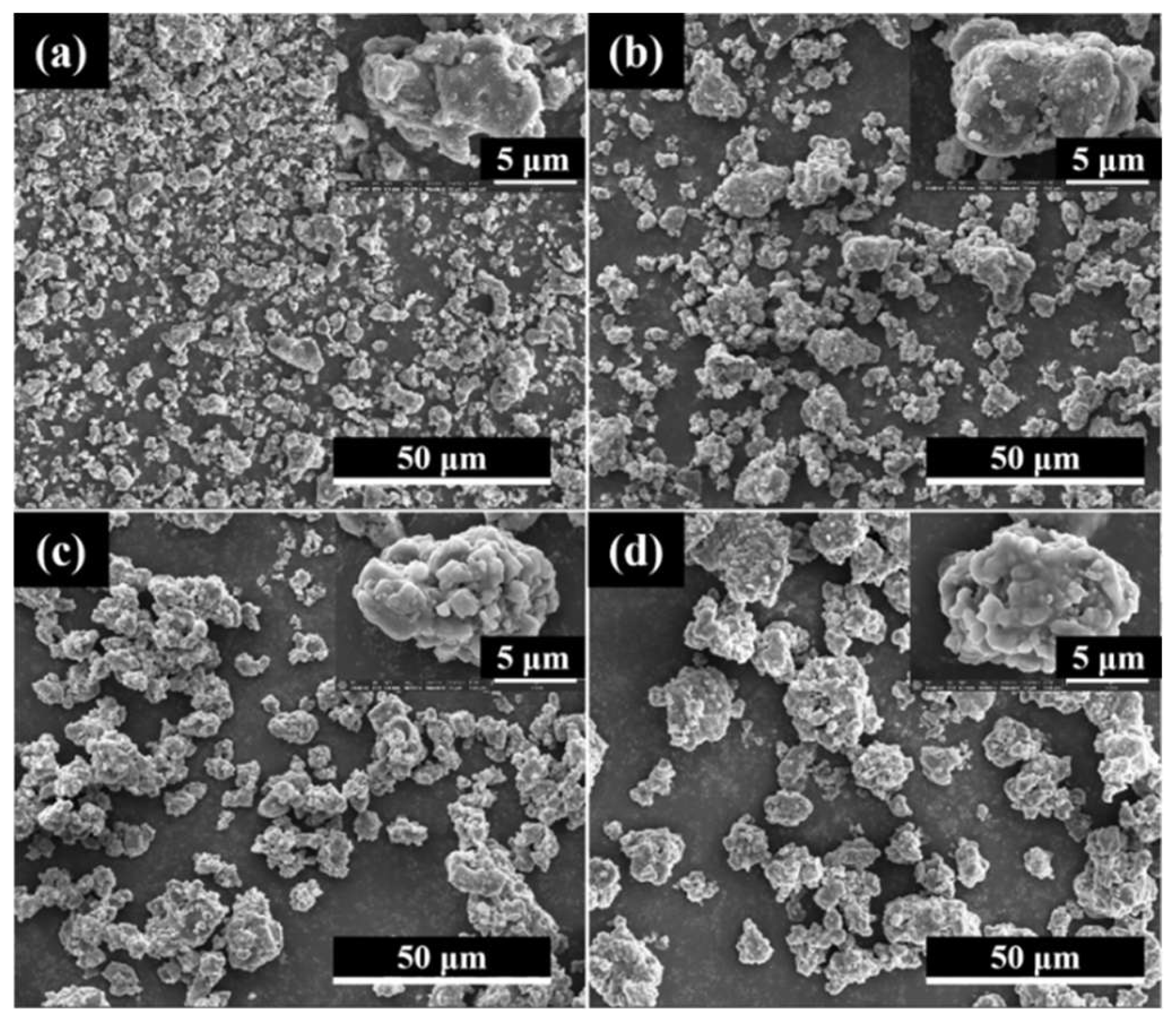
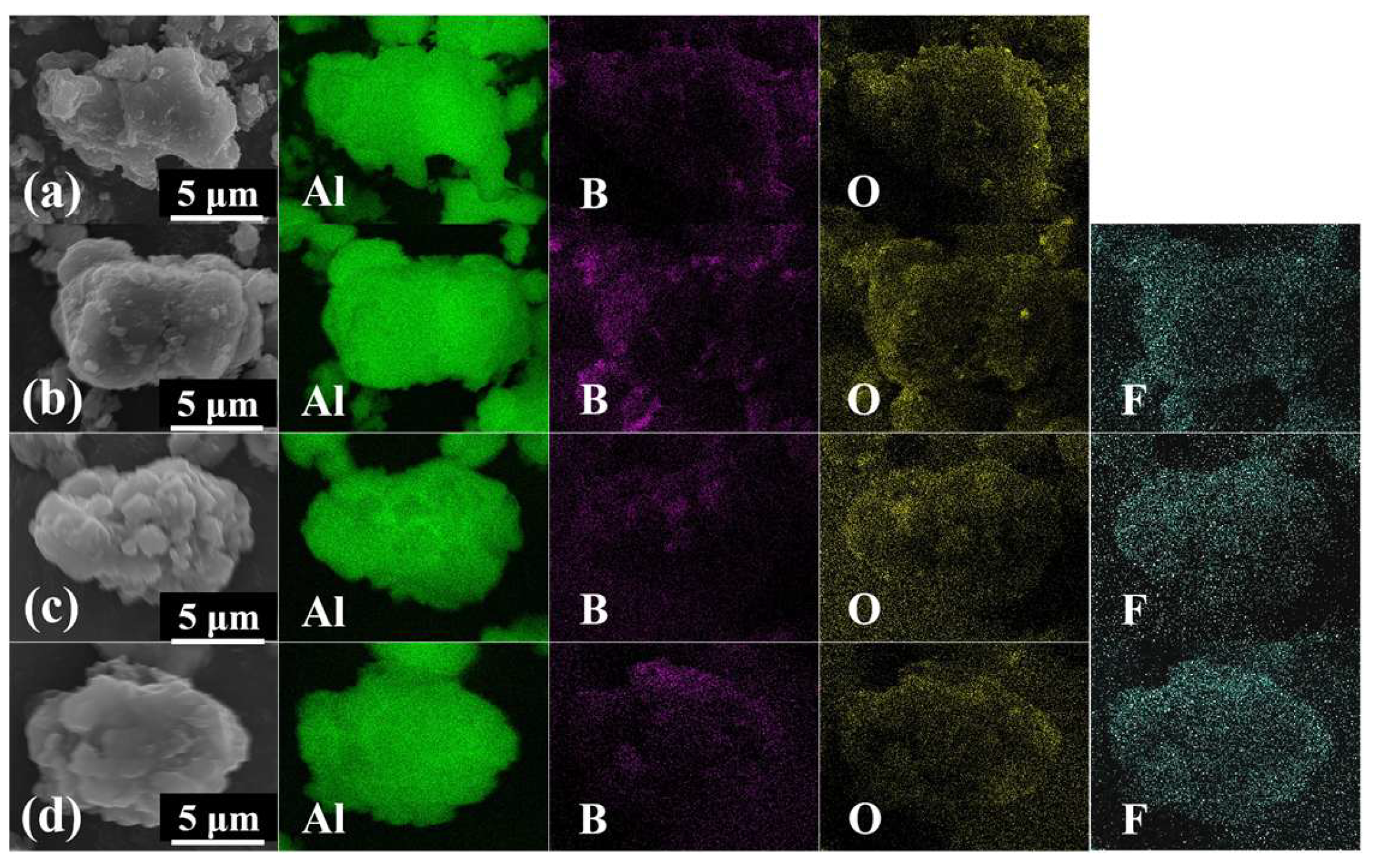
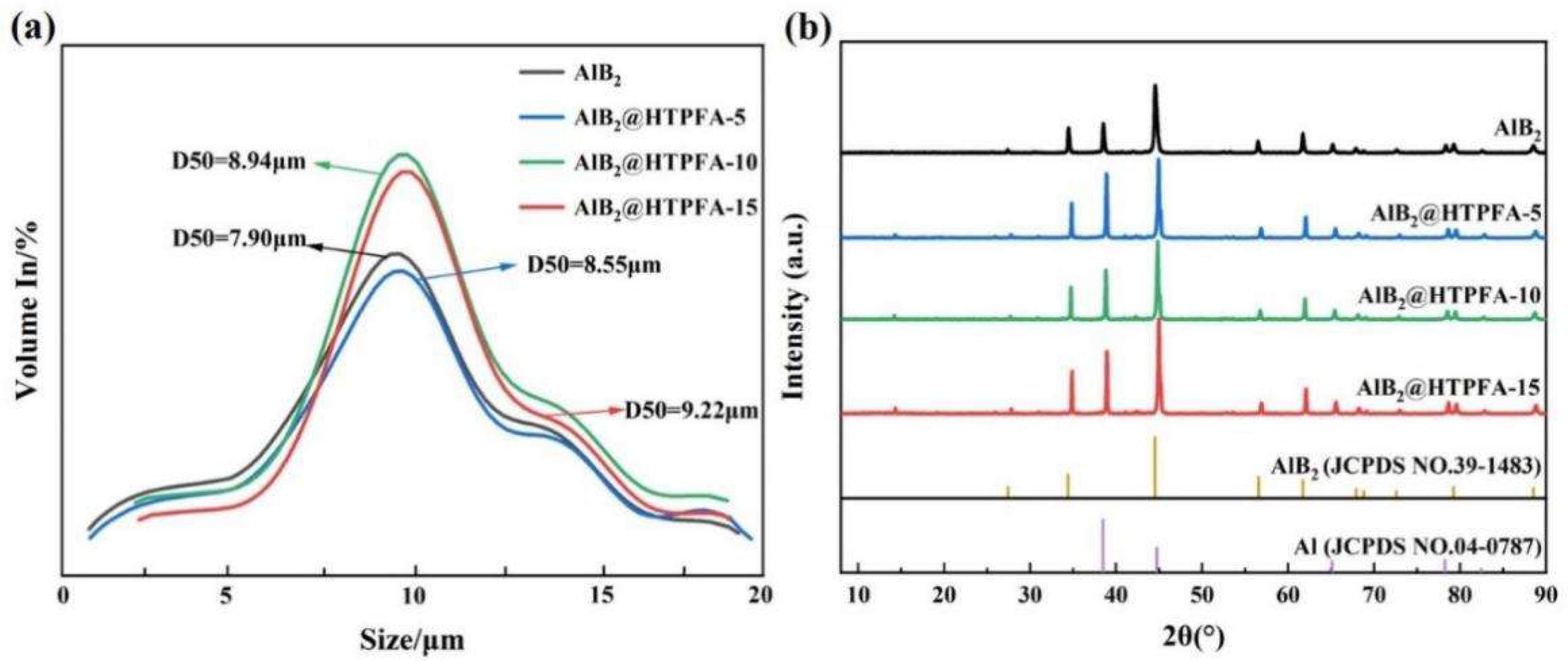
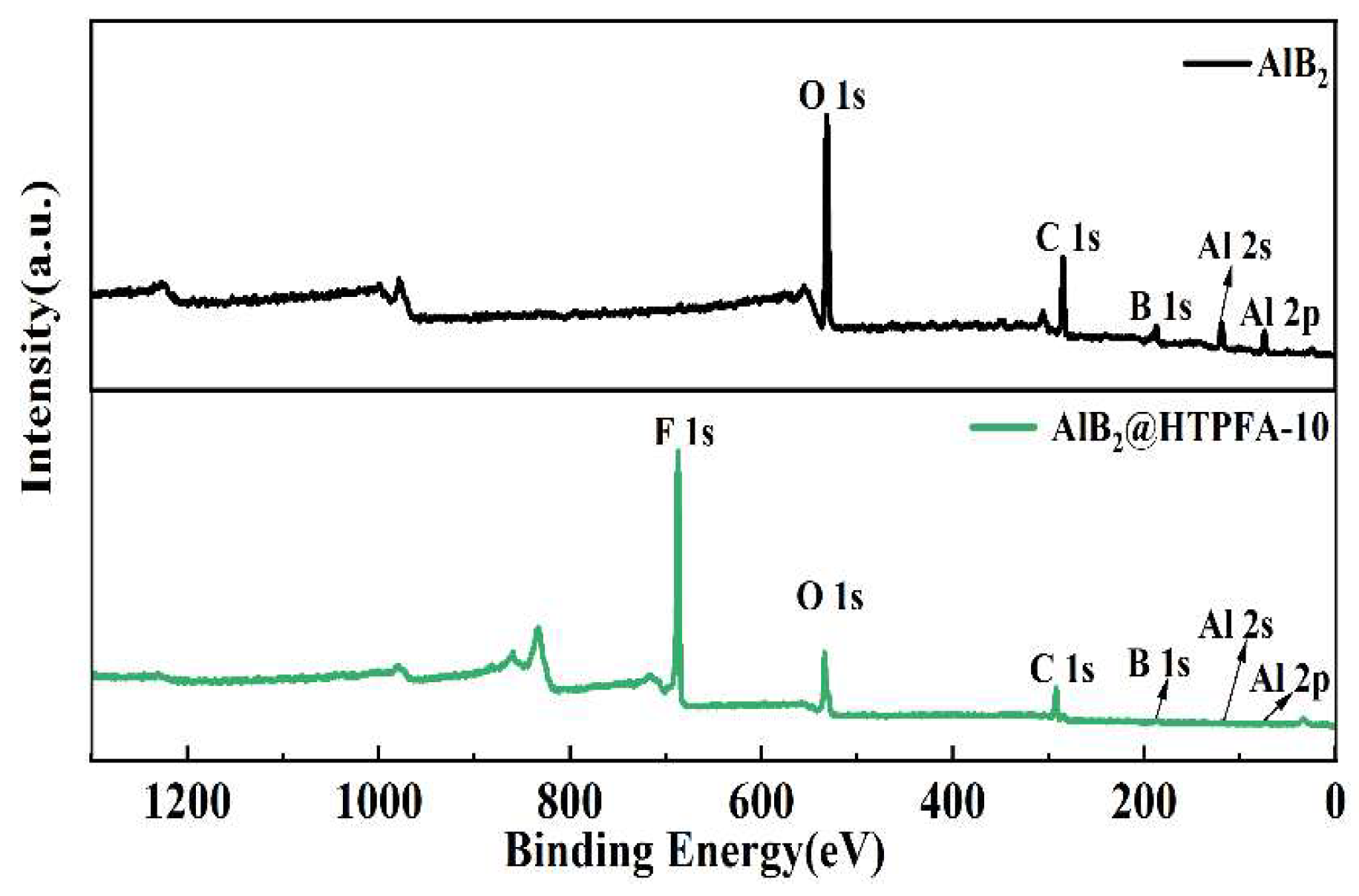
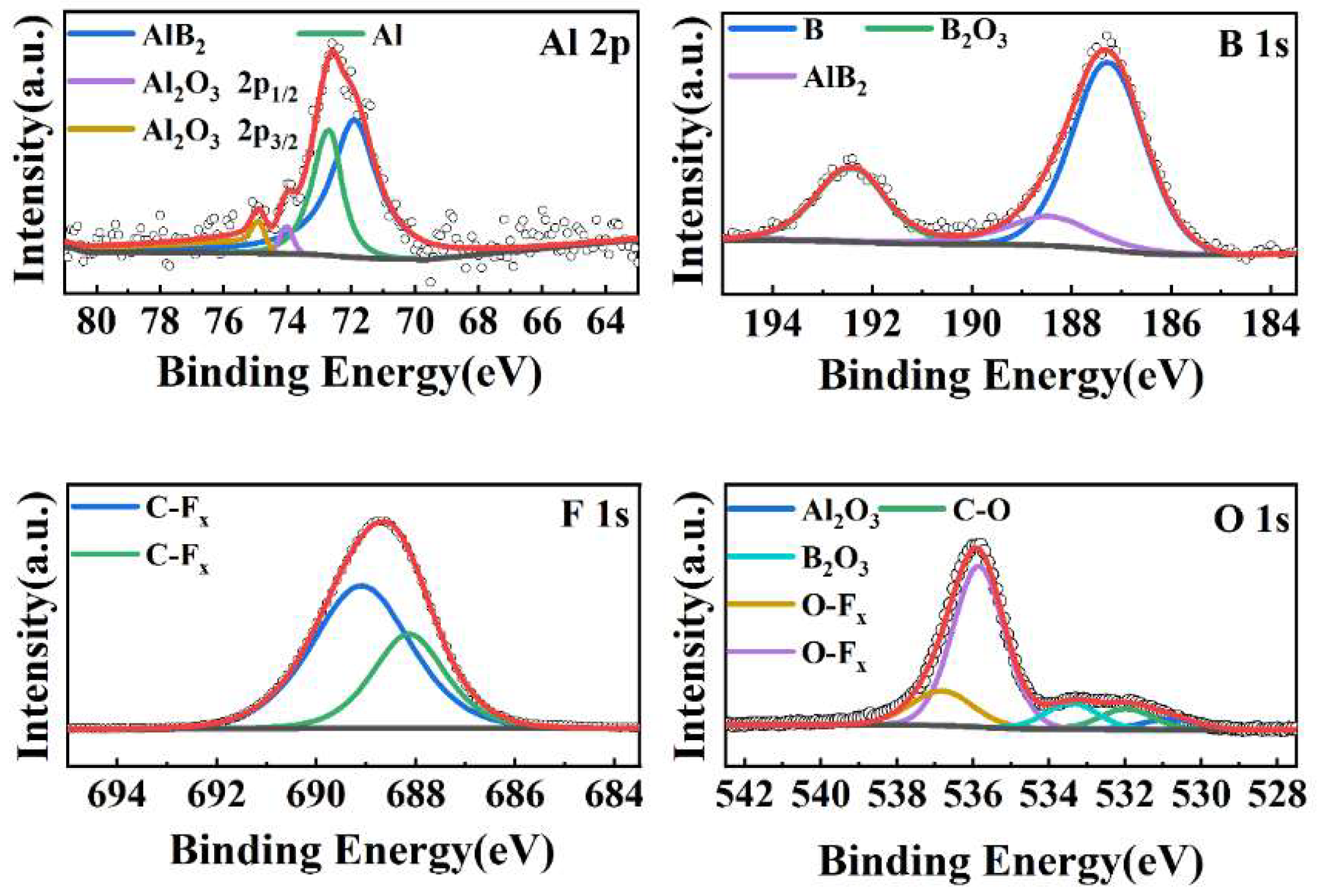
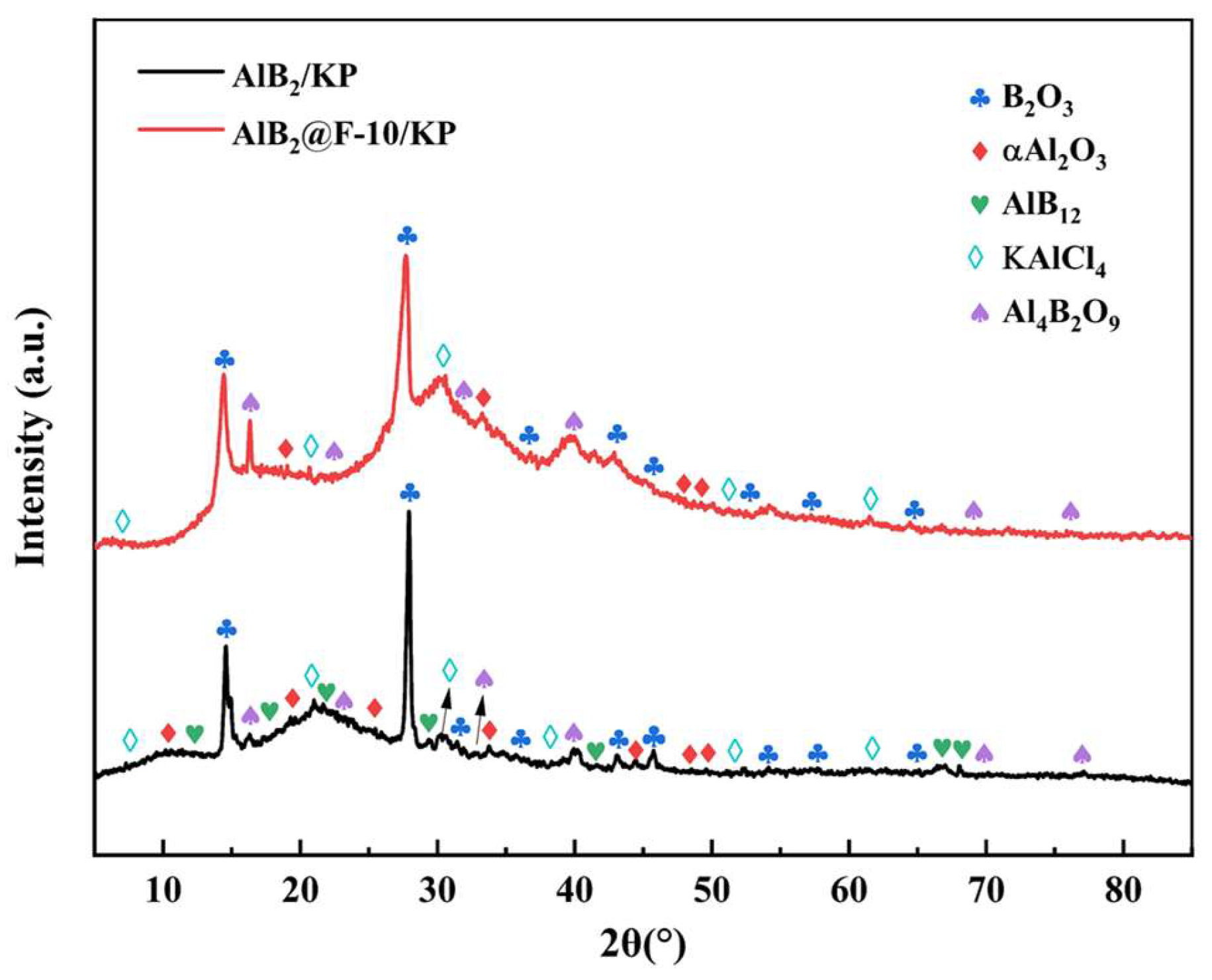
3.1. Characterization of Morphology and Components
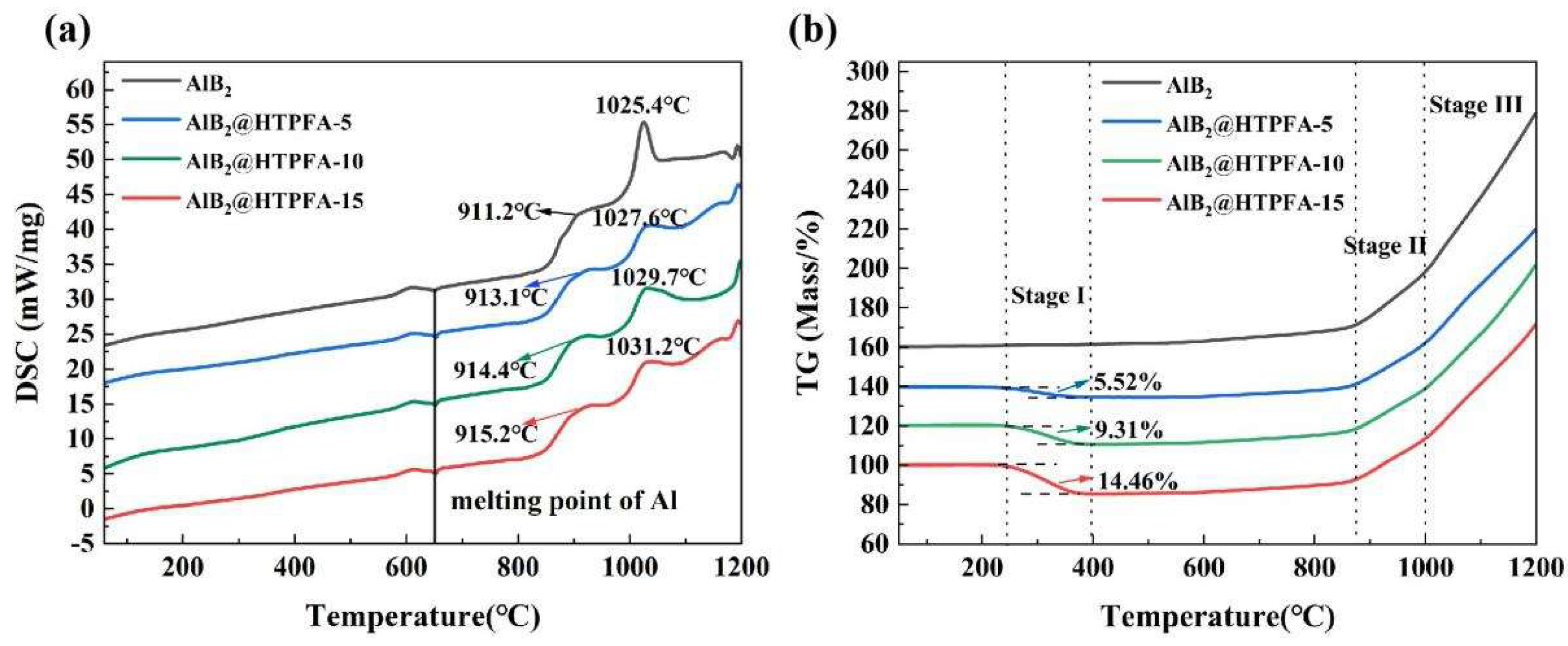
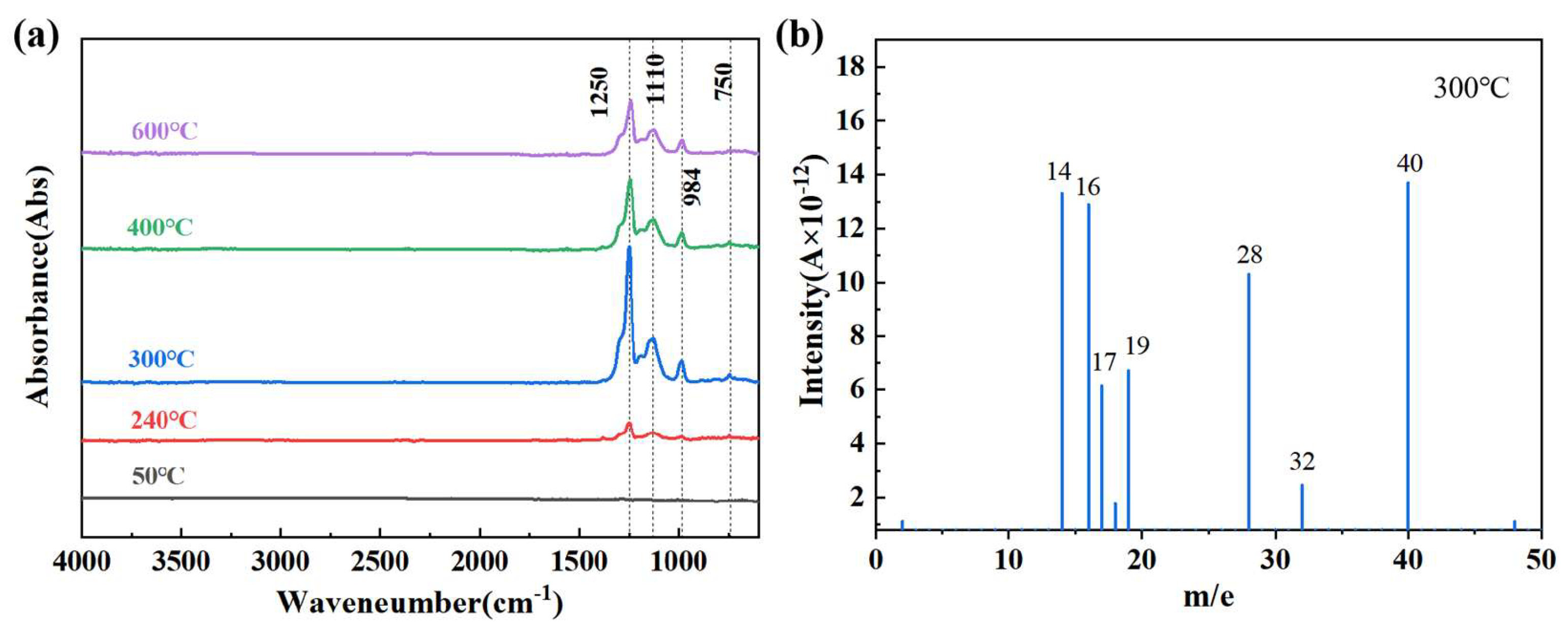
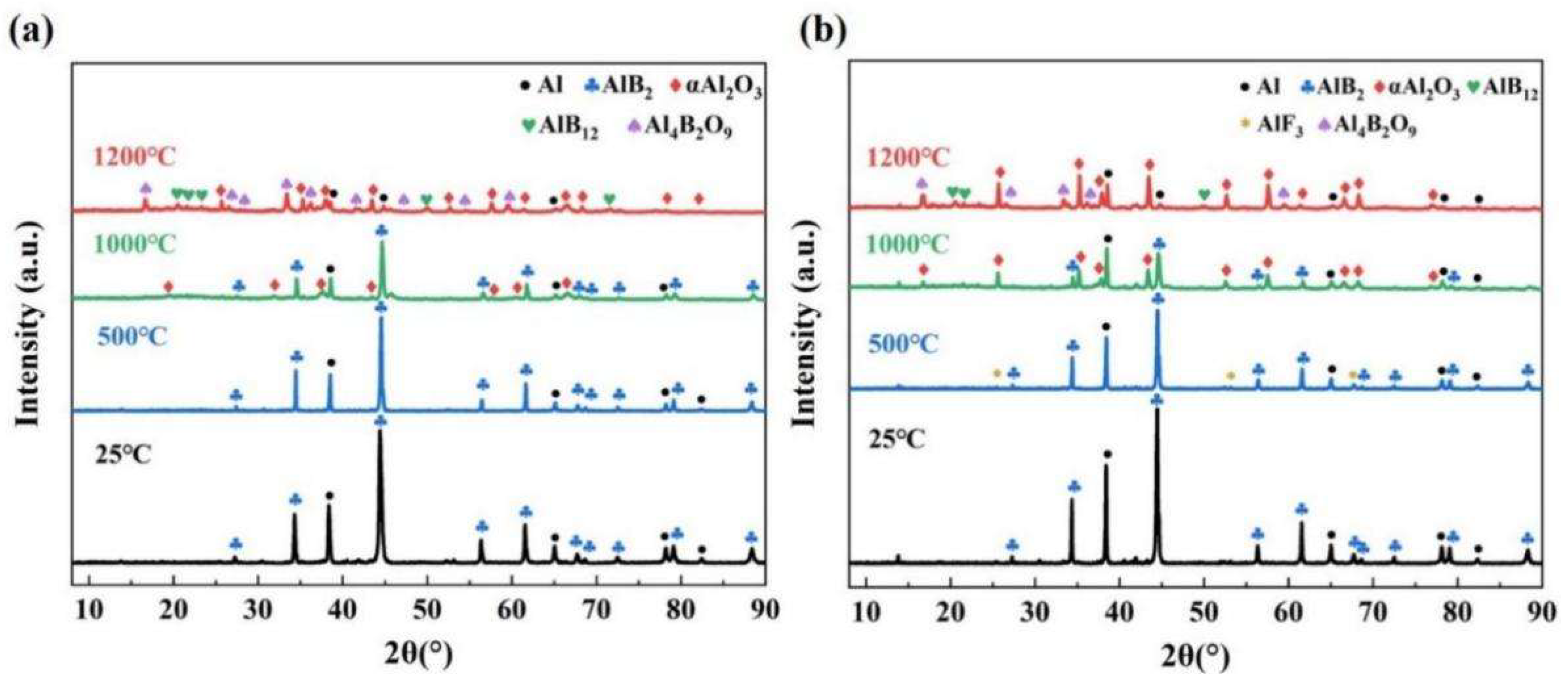
3.2. Oxidation Process
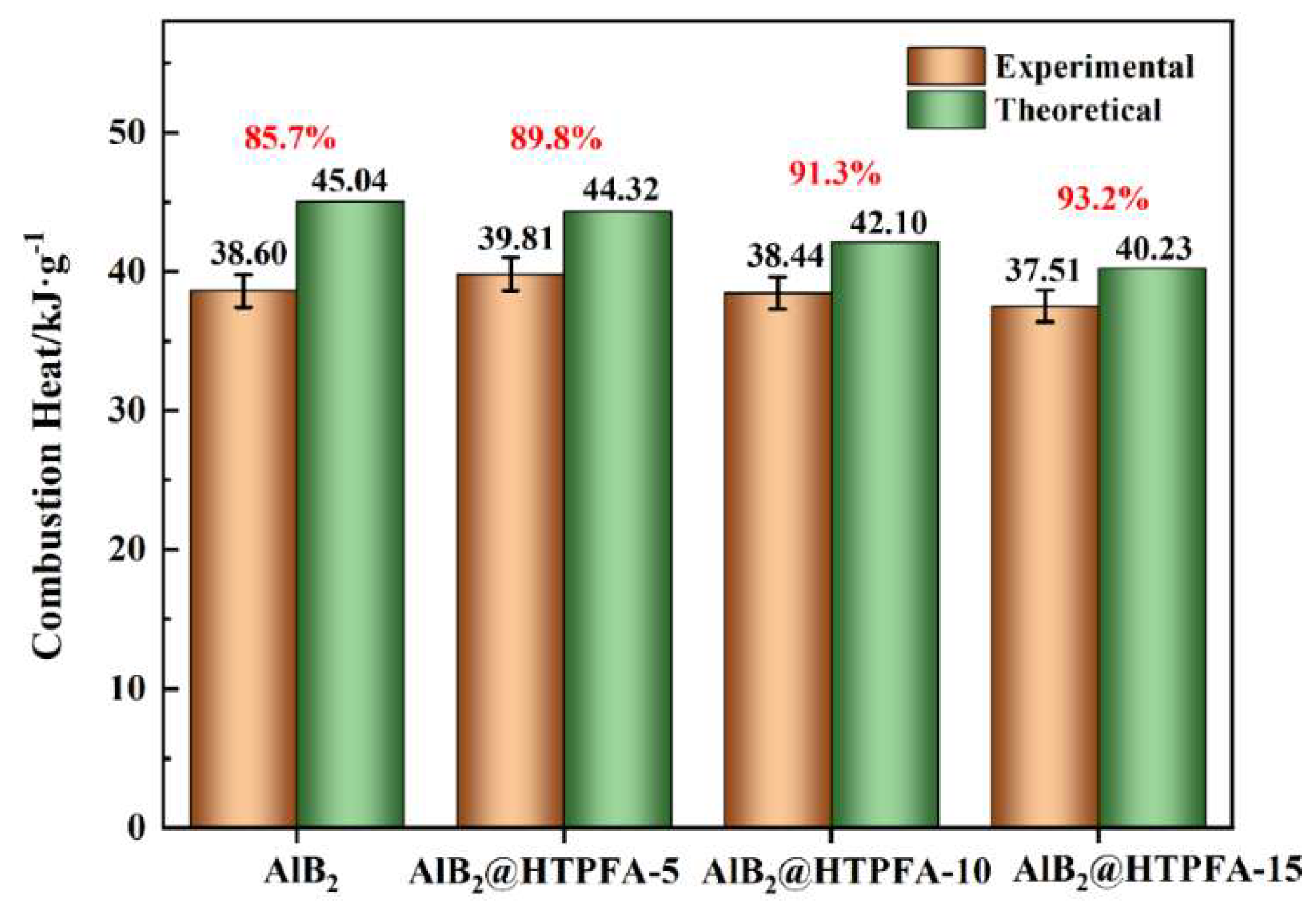
3.3. Calorimeter and Combustion Test
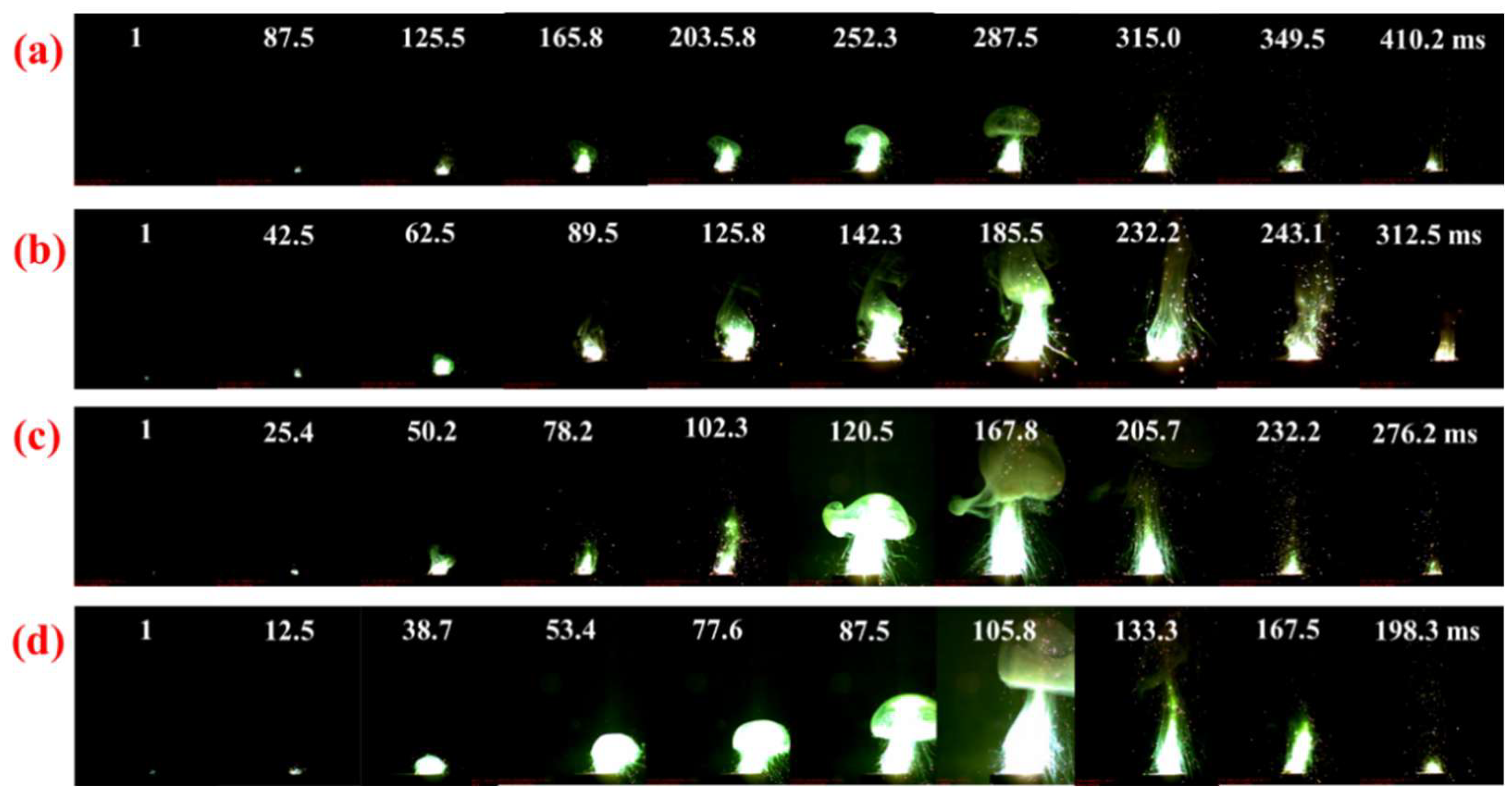
3.4. Laser Ignition Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padwal, M.; Castaneda, D.; Natan, B. Hypergolic combustion of boron-based propellants. Proc. Combust. Inst. 2021, 38, 6703–6711. [Google Scholar] [CrossRef]
- Korotkikh, A.G.; Arkhipov, V.A.; Slyusarsky, K.V.; Sorokin, I.V. Study of Ignition of High-Energy Materials with Boron and Aluminum and Titanium Diborides. Combust. Explos. Shock. Waves 2018, 54, 350–356. [Google Scholar] [CrossRef]
- Sivan, J.; Haas, Y.; Grinstein, D.; Kochav, S.; Yegudayev, G.; Kalontarov, L. Boron particle size effect on B/KNO3 ignition by a diode laser. Combust. Flame 2015, 162, 516–527. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, X.; Tang, G.; Guo, X.; Pang, A. Differences of thermal decomposition behaviors and combustion properties between CL-20-based propellants and HMX-based solid propellants. J. Therm. Anal. Calorim. 2020, 140, 2529–2540. [Google Scholar] [CrossRef]
- Chintersingh, K.L.; Nguyen, Q.; Schoenitz, M.; Dreizin, E.L. Combustion of boron particles in products of an air–acetylene flame. Combust. Flame 2016, 172, 194–205. [Google Scholar] [CrossRef]
- Deng, P.; Chen, P.; Fang, H.; Liu, R.; Guo, X. The combustion behavior of boron particles by using molecular perovskite energetic materials as high-energy oxidants. Combust. Flame 2022, 241, 112118. [Google Scholar] [CrossRef]
- Dayanand, S.; Boppana, S.B.; Hemanth, J.; Telagu, A. Microstructure and corrosion characteristics of in situ aluminum diboride metal matrix composites. J. Bio-Tribo-Corros. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Kenawy, S.H. Synthesis and Characterization of Aluminum Borate Ceramic Whiskers. Int. J. Appl. Ceram. Technol. 2011, 8, 783–792. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; He, Y.; Fu, Y.; Wang, J.; Li, A.; Guo, X.; Wang, M.; Guo, W.; Zhang, T. Determination of detonation characteristics by laser-induced plasma spectra and micro-explosion dynamics. Opt. Express 2022, 30, 4718–4736. [Google Scholar] [CrossRef]
- Yanovskii, L.S.; Varlamova, N.I.; Borodako, P.V.; Popov, I.M. On influence of energy-producing additives upon the service characteristics of aviation fuels. Russ. Aeronaut. 2013, 56, 314–318. [Google Scholar] [CrossRef]
- Weast, R.C.; Astle, M.J.; Beyer, W.H.; Company, C.R.; Selby, S.M.; Lide, D.R.; Frederikse, H.P.R.; Haynes, W.M.; Bruno, T.J. CRC handbook of chemistry and physics. Am. J. Med. Sci. 1982, 257, 423. [Google Scholar]
- Liu, T.K.; Luh, S.P.; Perng, H.C. Effect of Boron Particle Surface Coating on combustion of solid propellants for ducted rockets. Propellants Explos. Pyrotech. 2010, 16, 156–166. [Google Scholar] [CrossRef]
- Vellaisamy, U.; Biswas, S. Effect of metal additives on neutralization and characteristics of AP/HTPB solid propellants. Combust. Flame 2021, 234, 111627. [Google Scholar] [CrossRef]
- Pang, W.; Fan, X.; Zhang, W.; Xu, H.; Li, J.; Li, Y.; Shi, X.; Li, Y. Application of Amorphous Boron Granulated with Hydroxyl-Terminated Polybutadiene in Fuel-Rich Solid Propellant. Propellants Explos. Pyrotech. 2011, 36, 360–366. [Google Scholar] [CrossRef]
- Lyu, J.Y.; Chen, S.; He, W.; Zhang, X.; Tang, D.; Liu, P.; Yan, Q. Fabrication of High-Performance Graphene Oxide doped PVDF/CuO/Al Nanocomposites via Electrospinning. Chem. Eng. J. 2019, 368, 129–137. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, F.; Xu, H.; Pei, Q.; Jiang, H.; Yi, J.; Xuan, C.; Chen, S. Hydrogen-enhanced combustion of a composite propellant with ZrH2 as the fuel. Combust. Flame 2018, 187, 67–76. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Hussain, I.; Yang, G.; Zhang, K. Core–Shell Structured Nanoenergetic Materials: Preparation and Fundamental Properties. Adv. Mater. 2020, 32, 2001291. [Google Scholar] [CrossRef]
- He, W.; Li, Z.H.; Chen, S.; Yang, G.; Yang, Z.; Liu, P.; Yan, Q. Energetic Metastable n-Al@PVDF/EMOF Composite Nanofibers with Improved Combustion Performances. Chem. Eng. J. 2019, 41, 123146. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, X.; Wu, C.; Cheng, H.; Lu, Z.; Zhang, K. Si Wire Supported MnO2/Al/Fluorocarbon 3D Core/Shell Nanoenergetic Arrays with Long-Term Storage Stability. Chin. Ophthalmic Res. 2017, 7, 6678. [Google Scholar] [CrossRef]
- Deng, S.; Jiang, Y.; Huang, S.; Shi, X.; Zhao, J.; Zheng, X. Tuning the morphological, ignition and combustion properties of micron-Al/CuO thermites through different synthesis approaches. Combust. Flame 2018, 195, 303–310. [Google Scholar] [CrossRef]
- Li, X.; Huang, C.; Yang, H.; Li, Y.; Cheng, Y. Thermal reaction properties of aluminum/copper (II) oxide/poly(vinylidene fluoride) nanocomposite. J. Therm. Anal. Calorim. 2016, 124, 899–907. [Google Scholar]
- DeLisio, J.B.; Hu, X.; Wu, T.; Egan, G.C.; Young, G.; Zachariah, M.R. Probing the Reaction Mechanism of Aluminum/Poly(vinylidene fluoride) Composites. J. Phys. Chem. B 2016, 120, 5534–5542. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, P.; He, G.; Gozin, M.; Yan, Q. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, 1706293. [Google Scholar] [CrossRef]
- Wang, H.; Rehwoldt, M.; Kline, D.J.; Wu, T.; Wang, P.; Zachariah, M.R. Comparison study of the ignition and combustion characteristics of directly-written Al/PVDF, Al/Viton and Al/THV composites. Combust. Flame 2019, 201, 181–186. [Google Scholar] [CrossRef]
- Young, G.; Stoltzi, C.A.; Mayo, D.H.; Roberts, C.W.; Milby, C.L. Combustion Behavior of Solid Fuels Based on PTFE/Boron Mixtures. Combust. Sci. Technol. 2013, 185, 1261–1280. [Google Scholar] [CrossRef]
- Pang, W.Q. Boron-Based Fuel-Rich Propellant: Properties, Combustion and Technology Aspects; Taylor & Francis Group: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Keerthi, V.; Nie, H.; Pisharath, S.; Hng, H.H. Combustion characteristics of fluoropolymer coated boron powders. Combust. Sci. Technol. 2020, 194, 1183–1198. [Google Scholar] [CrossRef]
- Ulas, A.; Kuo, K.K.; Gotzmer, C. Ignition and combustion of boron particles in fluorine-containing environments. Combust. Flame 2001, 127, 1935–1957. [Google Scholar] [CrossRef]
- Young, G.; Roberts, C.W.; Stoltz, C.A. Ignition and combustion enhancement of boron with polytetrafluoroethylene. J. Propuls. Power 2015, 31, 386–392. [Google Scholar] [CrossRef]
- Hedman, T.D.; Demko, A.R.; Kalman, J. Enhanced ignition of milled boron-polytetrafluoroethylene mixtures. Combust. Flame 2018, 198, 112–119. [Google Scholar] [CrossRef]
- Jiang, Y.; Demko, A.R.; Baek, J.; Shi, X.; Vallez, L.; Ning, R.; Zheng, X. Facilitating laser ignition and combustion of boron with a mixture of graphene oxide and graphite fluoride. Appl. Energy Combust. Sci. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Q.Z.; Zhao, C.W. Liquid phase in-situ synthesis of LiF coated boron powder composite and performance study. Def. Technol. 2020, 16, 635–641. [Google Scholar] [CrossRef]
- Zhou, T.; Li, X.; Liu, C.; Shi, X.; Wu, B.; Duan, X.; Yang, G. Butterfly wing fans the fire: High efficient combustion of CWs/CL-20/AP nanocomposite for light ignited micro thruster using multi-channeled hierarchical porous structure from butterfly wing scales. Combust. Flame 2021, 231, 111505. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, H.; Yang, Y.; Li, Y.; Meng, Y.; Li, Y.; Song, D.; Chen, H.; Artiaga, R. Preparation of B/Nitrocellulose/Fe particles and their effect on the performance of an ammonium perchlorate propellant—ScienceDirect. Combust. Flame 2020, 211, 456–464. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, B.; Chen, J.; Sun, Y. Study on nano-boron particles modified by PVDF to enhance the combustion characteristics. Combust. Flame 2023, 248, 112556. [Google Scholar] [CrossRef]
- Tang, W.; Zeng, T.; Hu, J.; Li, J.; Yang, R. Investigation on the thermal decomposition of the elastomer containing fluoroolefin segment by DSC-TG-MS-FTIR. Polym. Adv. Technol. 2021, 32, 4880–4890. [Google Scholar] [CrossRef]
- Ge, Z.; Zhang, X.; Dai, J.; Li, W.; Luo, Y. Synthesis, characterization and properties of a novel fluorinated polyurethane. Eur. Polym. J. 2009, 45, 530–536. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Li, L.; Liu, Y.; Li, Y.; Dong, Q. Influences of fluorine on microphase separation in fluorinated polyurethanes. Polymer 2016, 98, 311–319. [Google Scholar] [CrossRef]
- Wen, J.; Sun, Z.; Xiang, J.; Fan, H.; Chen, Y.; Yan, J. Preparation and characteristics of waterborne polyurethane with various lengths of fluorinated side chains. Appl. Surf. Sci. 2019, 494, 610–618. [Google Scholar] [CrossRef]
- Cobranchi, D.P.; Botelho, M.; Buxton, L.W.; Buck, R.C.; Kaiser, M.A. Vapor pressure determinations of 8-2 fluorortelomer alcohol and 1-H perfluorooctane by capillary gas chromatography: Relative retention time versus headspace methods. J. Chromatogr. A 2006, 1108, 248–251. [Google Scholar] [CrossRef]
- Silva, G.M.C.; Morgado, P.; Haley, J.D.; Montoya, V.M.T.; McCabe, C.; Martins, L.F.G.; Filipe, E.J.M. Vapor pressure and liquid density of fluorinated alcohols: Experimental, simulation and GC-SAFT-VR predictions. Fluid Phase Equilibria 2016, 425, 297–304. [Google Scholar] [CrossRef]
- Ou, Y.; Sun, Y.; Jiao, Q. Properties related to linear and branched network structure of hydroxyl terminated polybutadiene. e-Polymers 2018, 18, 267–274. [Google Scholar] [CrossRef]
- Ma, M.; Kwon, Y. Preparation of energetic polyurethane binders with enhanced properties by nonmigratory reactive monocyclic plasticizers. Eur. Polym. J. 2019, 123, 109414. [Google Scholar] [CrossRef]
- Shimada, S. Thermosonimetry and thermomicroscopy of the decomposition of NaClO4 and KClO4. J. Therm. Anal. 1993, 40, 1063–1068. [Google Scholar] [CrossRef]
- Lee, J.-S. Thermal properties and firing characteristics of the Zr/KClO4/Viton A priming compositions. Thermochim. Acta 2002, 392, 147–152. [Google Scholar] [CrossRef]
- Tan, L.; Lu, X.; Liu, N.; Yan, Q. Further enhancing thermal stability of thermostable energetic derivatives of dibenzotetraazapentene by polydopamine/graphene oxide coating. Appl. Surf. Sci. 2021, 543, 148825. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.; Dreizin, E.L. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 140, 310–318. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, S.; Yu, Z.; Huang, R.; He, C.; Ye, Z.; Zhang, W.; Chen, X. Silver(I)-based molecular perovskite energetic compounds with exceptional thermal stability and energetic performance. Inorg. Chem. 2022, 61, 4143–4149. [Google Scholar] [CrossRef]
- Noor, F.; Zhang, H.; Korakianitis, T.; Wen, D. Oxidation and ignition of aluminum nanomaterials. Phys. Chem. Chem. Phys. PCCP 2013, 15, 20176–20188. [Google Scholar] [CrossRef]
- Zhu, S.; Cao, X.; Cao, X.; Feng, Y.; Lin, X.; Han, K.; Li, X.; Deng, P. Metal-doped (Fe, Nd, Ce, Zr, U) graphitic carbon nitride catalysts enhance thermal decomposition of ammonium perchlorate-based molecular perovskite. Mater. Des. 2021, 199, 109426. [Google Scholar] [CrossRef]
- Yan, T.; Liu, P.; Song, N.; Liu, J.; Ou, Y. Thermally active nano-aluminum particles with improved flowability prepared by adsorbing multi-component layer. Combust. Flame 2021, 234, 111680. [Google Scholar] [CrossRef]
- Hall, A.; Economy, J. The Al (L) + AlB12 AlB2 peritectic transformation and its role in the formation of high aspect ratio AlB2 flakes. J. Phase Equilibria 2000, 21, 63–69. [Google Scholar] [CrossRef]
- Velasco, F.; Guzman, S.; Moral, C.; Bautista, A. Oxidation of micro-sized aluminium particles: Hollow alumina spheres. Oxid. Met. 2013, 80, 403–422. [Google Scholar] [CrossRef]
- Blackburn, P.E.; Büchler, A.; Stauffer, J.L. Thermodynamics of Vaporization in the Aluminum Oxide—Boron Oxide System1a. J. Phys. Chem. 1966, 70, 2469–2474. [Google Scholar] [CrossRef]
- Nagai, T.; Ogasawara, Y.; Maeda, M. Thermodynamic measurement of (Al2O3 + B2O3) system by double Knudsen cell mass spectrometry. J. Chem. Thermodyn. 2009, 41, 1292–1296. [Google Scholar] [CrossRef]
- Whittaker, M.L.; Sohn, H.Y.; Cutler, R.A. Oxidation kinetics of aluminum diboride. J. Solid State Chem. 2013, 207, 163–169. [Google Scholar] [CrossRef]
- Crouse, C.A.; Pierce, C.J.; Spowart, J.E. Synthesis and reactivity of aluminized fluorinated acrylic (AlFA) nanocomposites. Combust. Flame 2012, 159, 3199–3207. [Google Scholar] [CrossRef]
- Yan, T.; Liu, P.; Song, N.; Ou, Y. Insight into combustion characteristics of micro- and nano-sized boron carbide. Combust. Flame 2023, 251, 112721. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhao, W.; Shen, C.; Ou, Y.; Jiao, Q. HTPFA-Coated AlB2 with Enhanced Combustion Performance as a High-Energy Fuel. Materials 2025, 18, 1452. https://doi.org/10.3390/ma18071452
Wang J, Zhao W, Shen C, Ou Y, Jiao Q. HTPFA-Coated AlB2 with Enhanced Combustion Performance as a High-Energy Fuel. Materials. 2025; 18(7):1452. https://doi.org/10.3390/ma18071452
Chicago/Turabian StyleWang, Jiangfeng, Wanjun Zhao, Chen Shen, Yapeng Ou, and Qingjie Jiao. 2025. "HTPFA-Coated AlB2 with Enhanced Combustion Performance as a High-Energy Fuel" Materials 18, no. 7: 1452. https://doi.org/10.3390/ma18071452
APA StyleWang, J., Zhao, W., Shen, C., Ou, Y., & Jiao, Q. (2025). HTPFA-Coated AlB2 with Enhanced Combustion Performance as a High-Energy Fuel. Materials, 18(7), 1452. https://doi.org/10.3390/ma18071452





