Meta-Analysis of Materials and Treatments Used in Contact Lenses: Implications for Lens Characteristics
Highlights
- Cellulose hydrogel lenses and nanogels enhance transparency and biocompatibility.
- pH-sensitive polymers enable sustained drug release for up to 10 days.
- Surface treatments reduce protein adsorption and improve hydration.
- Oxygen permeability remains consistent across advanced and conventional materials.
- Biodegradable materials show potential for reducing environmental impact.
Abstract
1. Introduction
- -
- Health monitoring: measuring intraocular pressure, glucose levels, or other health metrics;
- -
- Drug delivery: the sustained release of medications directly to the eye;
- -
- Augmented reality: providing visual overlays for enhanced navigation, training, or entertainment.
- -
- Wettability enhancements: coatings and plasma treatments improve water retention, reducing dryness and increasing comfort;
- -
- Anti-protein adsorption: specialized treatments minimize the buildup of proteins and lipids, maintaining lens clarity and reducing irritation risks;
- -
- Oxygen permeability optimization: advanced technologies ensure sufficient oxygen flow to the cornea, preventing hypoxia and promoting corneal health;
- -
- Therapeutic functionalities: coatings for sustained drug release convert lenses into therapeutic devices, delivering medications over extended periods;
- -
- Self-lubricating surfaces: innovative coatings reduce the friction between the lens and eyelid, enhancing comfort and minimizing irritation.
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Source
2.3. Search Methods for Identification of Studies
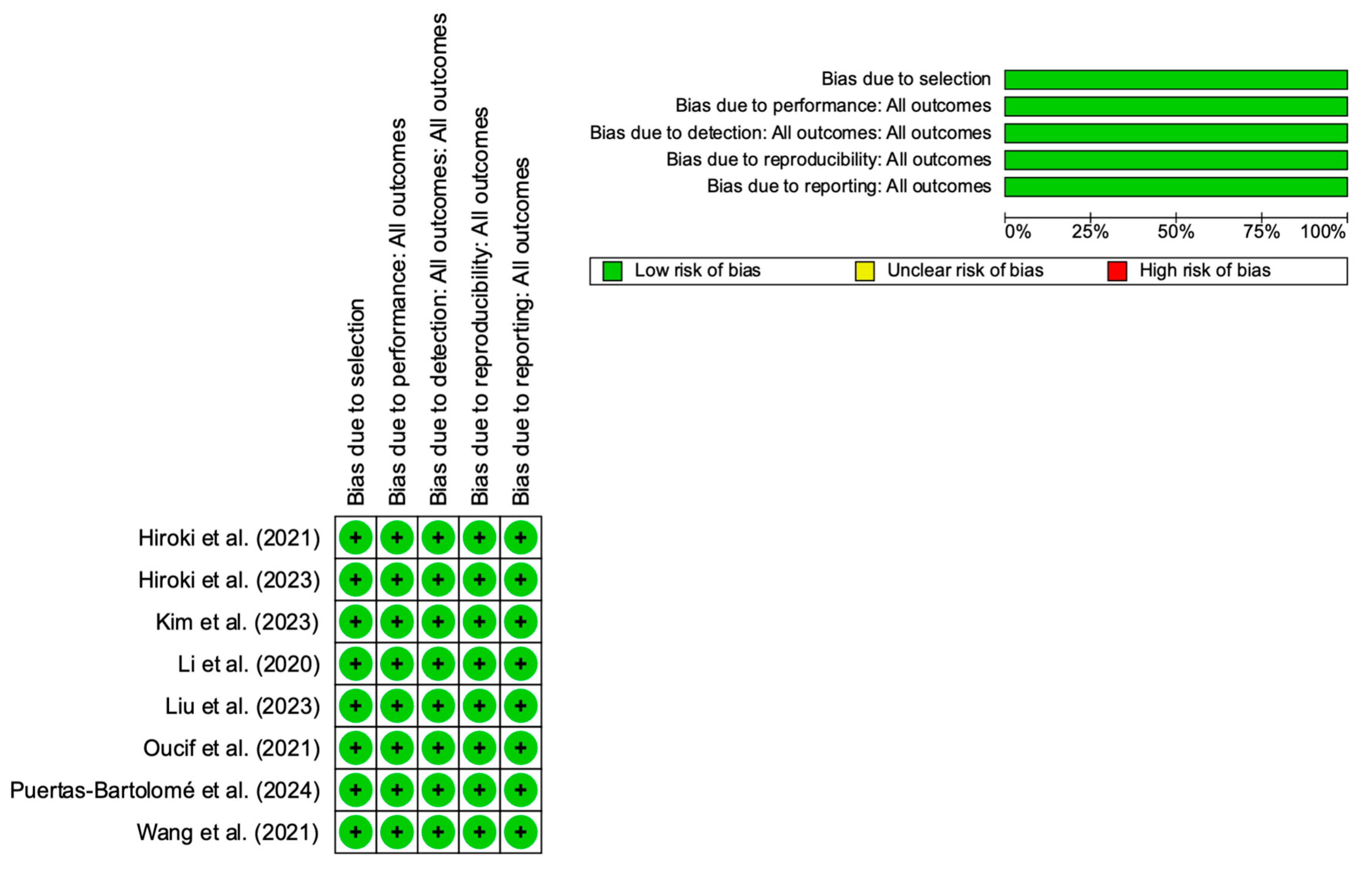
2.4. Assessment of Results
2.5. Publication Bias
2.6. Additional Analyses
3. Results
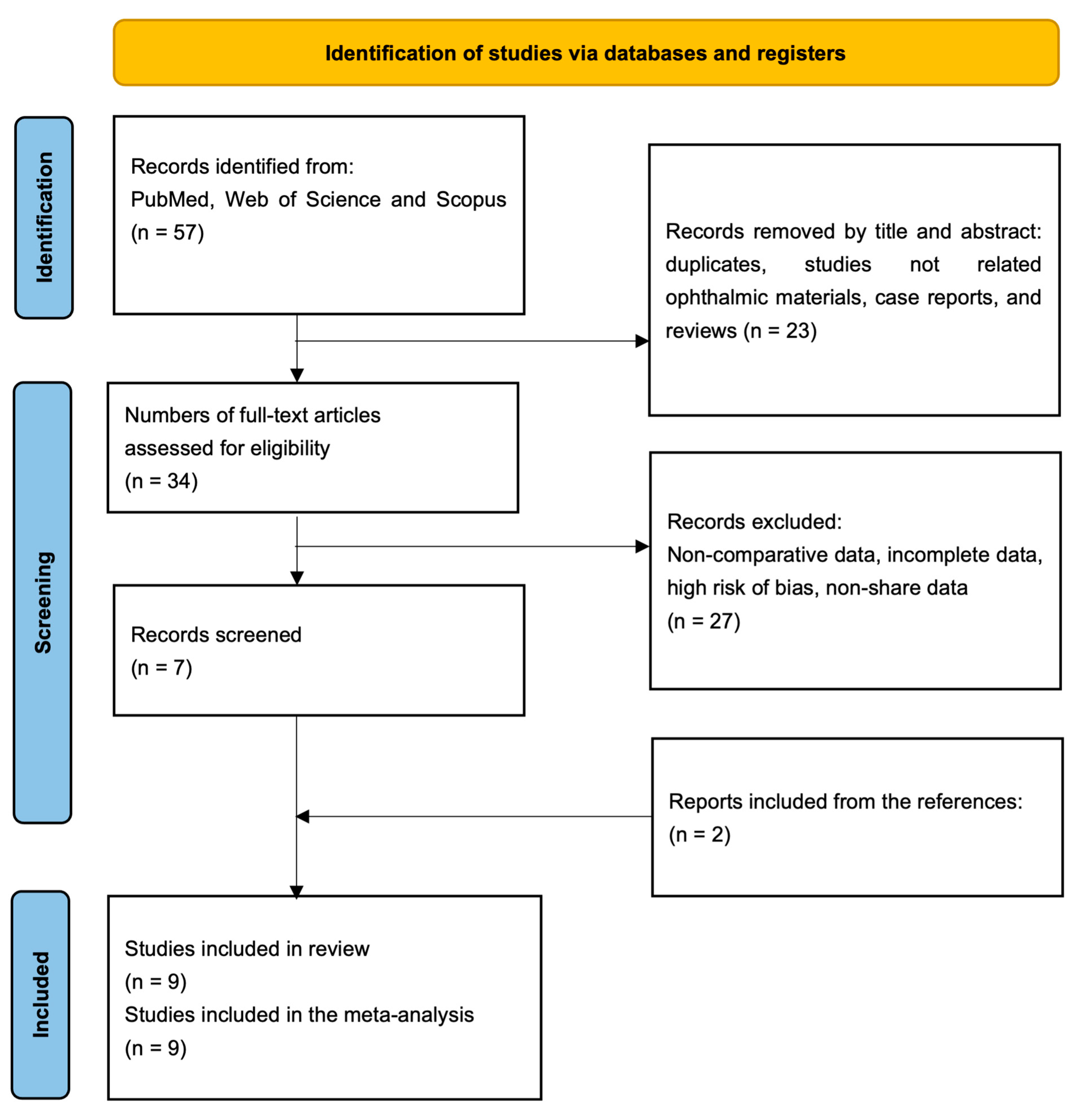
3.1. Study Selection
3.2. Study Characteristics
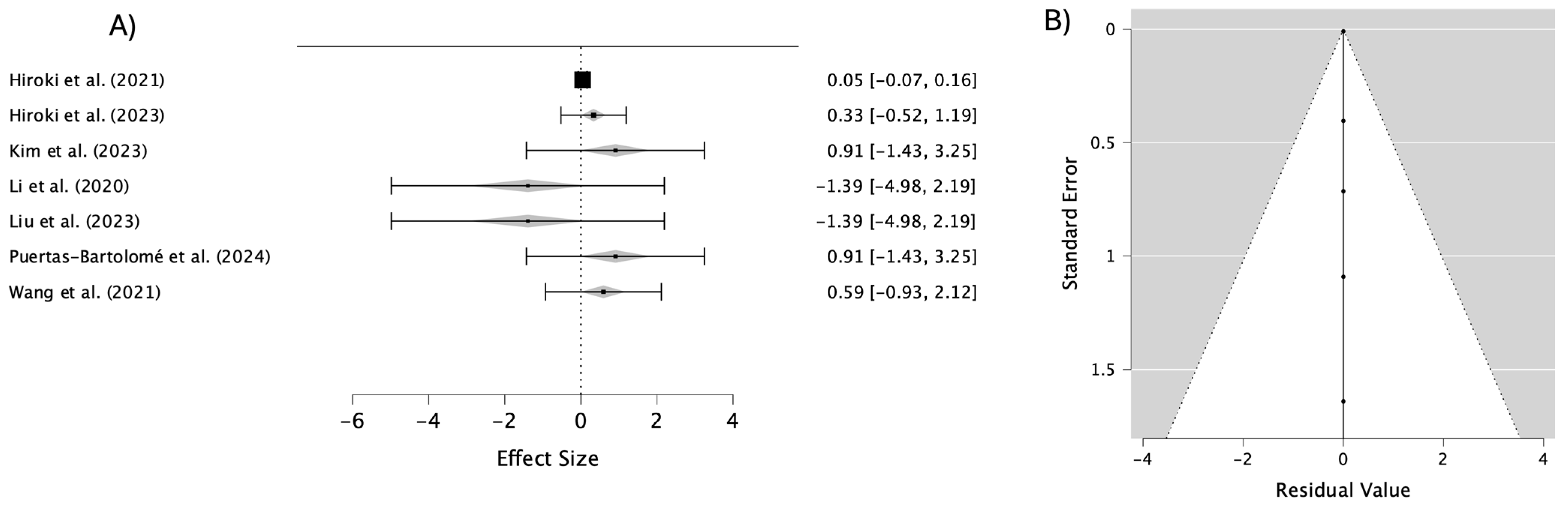
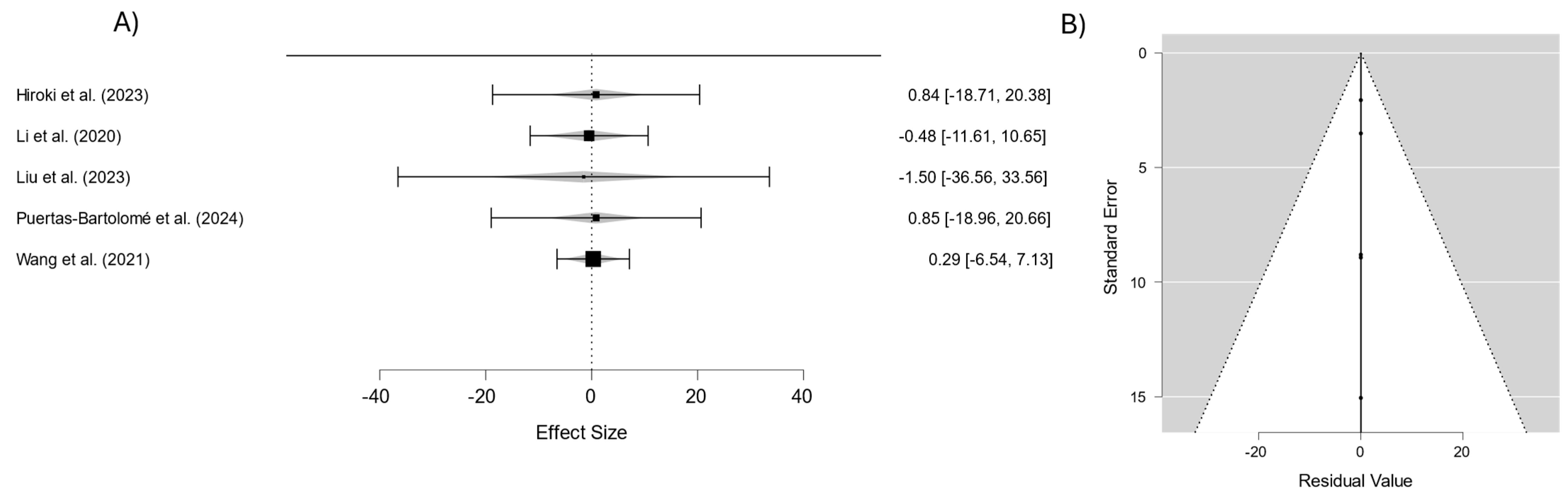
3.3. Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulamier, A.A.; Shaker, L.M.; Al-Amiery, A.A. Advancements in the Chemistry of Contact Lenses: Innovations and Applications. Results Chem. 2024, 12, 101872. [Google Scholar]
- Maldonado-Codina, C.; Carnt, N.; Wagner, H.; Stapleton, F. Reimagining Approaches to Solving Common Contact Lens Conundrums. Ophthalmic Physiol. Opt. 2024, 44, 679–685. [Google Scholar]
- Shaker, L.M.; Al-Amiery, A.; Wan Isahak, W.N.R. Revolutionizing Contact Lens Manufacturing: Exploring Cutting-Edge Techniques and Innovations for Enhanced Vision and Comfort. Int. J. Low-Carbon Technol. 2024, 19, 359–385. [Google Scholar] [CrossRef]
- Shaker, L.M.; Al-Amiery, A.A.; Al-Azzawi, W.K. A Clearer Vision: A Mini-Review on Contact Lenses. J. Opt. 2024, 53, 949–958. [Google Scholar] [CrossRef]
- Khan, H.; Kansal, K.; Subharti Medical College, D.O. Comparison of Vision Related Quality of Life between Wearing Contact Lenses and Spectacles: A Review. Int. J. Ophthalmol. Optom. 2023, 5, 29–32. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Ge, Y.; Qu, J.; Liedberg, B.; Zhang, Q.; Wang, Y. Smart Contact Lenses for Healthcare Monitoring and Therapy. ACS Nano 2024, 18, 6817–6844. [Google Scholar] [PubMed]
- Muhsin, Z.; Qahwaji, R.; Ghanchi, F.; Al-Taee, M. Review of Substitutive Assistive Tools and Technologies for People with Visual Impairments: Recent Advancements and Prospects. J. Multimodal User Interfaces 2023, 18, 135–156. [Google Scholar] [CrossRef]
- Sawarkar, S.; Pimple, P.; Sawant, A.; Nair, S. Current Insights into Targeting Strategies for the Effective Therapy of Diseases of the Posterior Eye Segment. Crit. Rev. Ther. Drug Carr. Syst. 2023, 41, 1–50. [Google Scholar] [CrossRef]
- Horizon. Contact Lenses Market Size, Share & Growth Report, 2030. Contact Lenses Market Size, Share & Trends Analysis Report By Material (Hybrid Lens, Silicone Hydrogel), By Design (Spherical Lens, Toric Lens), By Application (Corrective, Therapeutic), By Distribution Channel, By Usage, And Segment Forecasts, 2024–2030. Horizon Grand View Research. 2023. Available online: https://www.grandviewresearch.com/industry-analysis/contact-lenses-market/toc (accessed on 3 March 2025).
- Schifrin, L.G.; Rich, W.J. The Contact Lens Industry: Structure, Competition, and Public Policy (Health Technology Case Study 31); U.S. Congress, Office of Technology Assessment: Washington, DC, USA, 1984; OTA-HCS-31.
- Efron, N. Rigid Lens Materials; Elsevier: Amsterdam, The Netherlands, 2024; pp. 122–130. ISBN 9780702084270. [Google Scholar]
- Hajirasouliha, E.; Zandi, M.; Hashemi Tabatabaei, M.; Zarrinbakhsh, P. Ocular Contact Lenses: Smart Materials for Biomedical Applications. Polym. Bull. 2023, 81, 1–42. [Google Scholar] [CrossRef]
- Stapleton, F.; Tan, J. Impact of Contact Lens Material, Design, and Fitting on Discomfort. Eye Contact Lens Sci. Clin. Pract. 2017, 43, 32–39. [Google Scholar] [CrossRef]
- Dar, U.A.; Tantary, A.A.; Ali, A. Chapter 2-Polysaccharide-Based Hydrogels: History and Chronological Developments. In Polysaccharides-Based Hydrogels; Ahmed, S., Ali, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 21–42. ISBN 978-0-323-99341-8. [Google Scholar]
- Dhara, M. Polymer Hydrogels: Classification and Recent Advances. J. Macromol. Sci. Part A 2024, 61, 265–288. [Google Scholar]
- Janarthanan, S.D.; Samiyullah, K.; Madheswaran, G.; Ballae Ganeshrao, S.; Watt, K. Exploring the Impact of Optical Corrections on Visual Functions in Myopia Control–a Scoping Review. Int. Ophthalmol. 2024, 44, 47. [Google Scholar] [PubMed]
- Ramasubramanian, D.; Hernández-Verdejo, J.L.; López-Alonso, J.M. Contact Lens Fitting and Changes in the Tear Film Dynamics: Mathematical and Computational Models Review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024, 262, 2751–2764. [Google Scholar]
- Sarac, B.; Yücer, S.; Sahin, H.; Unal, M.; Ciftci, F. Wearable and Implantable Bioelectronic: Biosensing Contact Lens and Applications. Chem. Eng. J. 2024, 491, 152016. [Google Scholar] [CrossRef]
- Shaker, L.; Al-Amiery, A.; Isahak, W.; Al-Azzawi, W. Vinyl Polymers as Key Materials in Contact Lens Design: A Review of Progress and Future Directions. Starch-Stärke 2024, 76, 2300213. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Huang, C.C.; Lai, J.Y. Therapeutic Hydrogel Sheets Programmed with Multistage Drug Delivery for Effective Treatment of Corneal Abrasion. Chem. Eng. J. 2022, 429, 132409. [Google Scholar] [CrossRef]
- Ma, C.; Nikiforov, A.; Hegemann, D.; De Geyter, N.; Morent, R.; Ostrikov, K. Plasma-Controlled Surface Wettability: Recent Advances and Future Applications. Int. Mater. Rev. 2022, 68, 1–38. [Google Scholar] [CrossRef]
- Kim, E.; Saha, M.; Ehrmann, K. Mechanical Properties of Contact Lens Materials. Eye Contact Lens Sci. Clin. Pract. 2017, 44 (Suppl. S2), S148–S156. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef]
- Collins, C.; Dennehy, D.; Conboy, K.; Mikalef, P. Artificial Intelligence in Information Systems Research: A Systematic Literature Review and Research Agenda. Int. J. Inf. Manag. 2021, 60, 102383. [Google Scholar] [CrossRef]
- Ofori-Boateng, R.; Aceves-Martins, M.; Wiratunga, N.; Moreno-García, C. Towards the Automation of Systematic Reviews Using Natural Language Processing, Machine Learning, and Deep Learning: A Comprehensive Review. Artif. Intell. Rev. 2024, 57, 200. [Google Scholar] [CrossRef]
- Patil, D.; Rane, N.; Desai, P.; Rane, J. Machine Learning and Deep Learning: Methods, Techniques, Applications, Challenges, and Future Research Opportunities; Deep Science Publishing: Mumbai, India, 2024; pp. 28–81. ISBN 978-81-981367-4-9. [Google Scholar]
- Mobarak, M.H.; Mimona, M.A.; Islam, M.A.; Hossain, N.; Zohura, F.T.; Imtiaz, I.; Rimon, M.I.H. Scope of Machine Learning in Materials Research—A Review. Appl. Surf. Sci. Adv. 2023, 18, 100523. [Google Scholar] [CrossRef]
- Woelfle, T.; Hirt, J.; Janiaud, P.; Kappos, L.; Ioannidis, J.P.A.; Hemkens, L.G. Benchmarking Human–AI Collaboration for Common Evidence Appraisal Tools. J. Clin. Epidemiol. 2024, 175, 111533. [Google Scholar] [CrossRef]
- Kolaski, K.; Logan, L.R.; Ioannidis, J.P.A. Guidance to Best Tools and Practices for Systematic Reviews. Syst. Rev. 2023, 12, 1699–1731. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews That Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, A.; Taguchi, M. Development of Environmentally Friendly Cellulose Derivative-Based Hydrogels for Contact Lenses Using a Radiation Crosslinking Technique. Appl. Sci. 2021, 11, 9168. [Google Scholar] [CrossRef]
- Hiroki, A.; Kimura, A.; Taguchi, M. Development of Environmentally Friendly Soft Contact Lenses Made from Cellulose-Derived Hydrogel Materials. Radiat. Phys. Chem. 2023, 213, 111257. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, S.Y.; Kang, H.; Kim, D.I.; Yoo, H.J.; Han, S.M.; Lu, P.; Moon, G.D.; Hyun, D.C. Contact Lens with PH Sensitivity for On-Demand Drug Release in Wearing Situation. ACS Appl. Bio. Mater. 2023, 6, 5372–5384. [Google Scholar] [CrossRef]
- Li, R.; Guan, X.; Lin, X.; Guan, P.; Zhang, X.; Rao, Z.; Du, L.; Zhao, J.; Rong, J.; Zhao, J. Poly(2-Hydroxyethyl Methacrylate)/β-Cyclodextrin-Hyaluronan Contact Lens with Tear Protein Adsorption Resistance and Sustained Drug Delivery for Ophthalmic Diseases. Acta Biomater. 2020, 110, 105–118. [Google Scholar] [CrossRef]
- Liu, D.; Zang, Y.; Hu, Z.; Yu, C.; Han, Z.; Wang, M.; Xu, M.; Zhao, X.; Yue, W.; Nie, G. Synthesis of Silicone Hydrogel for Soft Contact Lens (SCLs) and Sustainable Release of Dexamethasone. React. Funct. Polym. 2023, 186, 105532. [Google Scholar] [CrossRef]
- Oucif, A.; Haddadine, N.; Zakia, D.; Bouslah, N.; Benaboura, A.; Beyaz, K.; Guedouar, B.; El-Shall, M.S. Poly (Hydroxyethyl Methacrylate-Co-Hydroxyethyl Acrylate) Soft Contact Lenses for Acetazolamide Release. Polym. Bull. 2022, 79, 1535–1554. [Google Scholar] [CrossRef]
- Puertas-Bartolomé, M.; Gutiérrez-Urrutia, I.; Teruel-Enrico, L.L.; Duong, C.N.; Desai, K.; Trujillo, S.; Wittmann, C.; del Campo, A. Self-Lubricating, Living Contact Lenses. Adv. Mater. 2024, 36, 2313848. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. [Google Scholar] [CrossRef] [PubMed]
- Wuchte, L.; DiPasquale, S.A.; Byrne, M.E. In Vivo Drug Delivery via Contact Lenses: The Current State of the Field from Origins to Present. J. Drug Deliv. Sci. Technol. 2021, 63, 102413. [Google Scholar] [CrossRef]
- Delgado-Pujol, E.J.; Martínez, G.; Casado-Jurado, D.; Vázquez, J.; León-Barberena, J.; Rodríguez-Lucena, D.; Torres, Y.; Alcudia, A.; Begines, B. Hydrogels and Nanogels: Pioneering the Future of Advanced Drug Delivery Systems. Pharmaceutics 2025, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Nayak, P. PH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar]
- Rolsky, C.; Kelkar, V.P.; Halden, R.U. Nationwide Mass Inventory and Degradation Assessment of Plastic Contact Lenses in US Wastewater. Environ. Sci Technol. 2020, 54, 12102–12108. [Google Scholar] [CrossRef]
- Shaker, L.; Mahmood, A.; Mohammad, Z.; Hussain, F.; Al-Amiery, A.; Abdalelah, S.; Fayad, M. Biodegradation of Polymeric Contact Lenses: A Comprehensive Review of Biological Activity. Results Surf. Interfaces 2024, 17, 100338. [Google Scholar] [CrossRef]
- Encarnação, T.; Nicolau, N.; Ramos, P.; Silvestre, E.; Mateus, A.; de Carvalho, T.A.; Gaspar, F.; Massano, A.; Biscaia, S.; Castro, R.A.E.; et al. Recycling Ophthalmic Lens Wastewater in a Circular Economy Context: A Case Study with Microalgae Integration. Materials 2024, 17, 75. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Martinez-Perez, C.; Barqueira, A.; Alvarez-Peregrina, C.; Sánchez-Tena, M.Á. Optical Material Recycling Practices: A Look at Portuguese Optical Centers. Sustainability 2024, 16, 5931. [Google Scholar] [CrossRef]
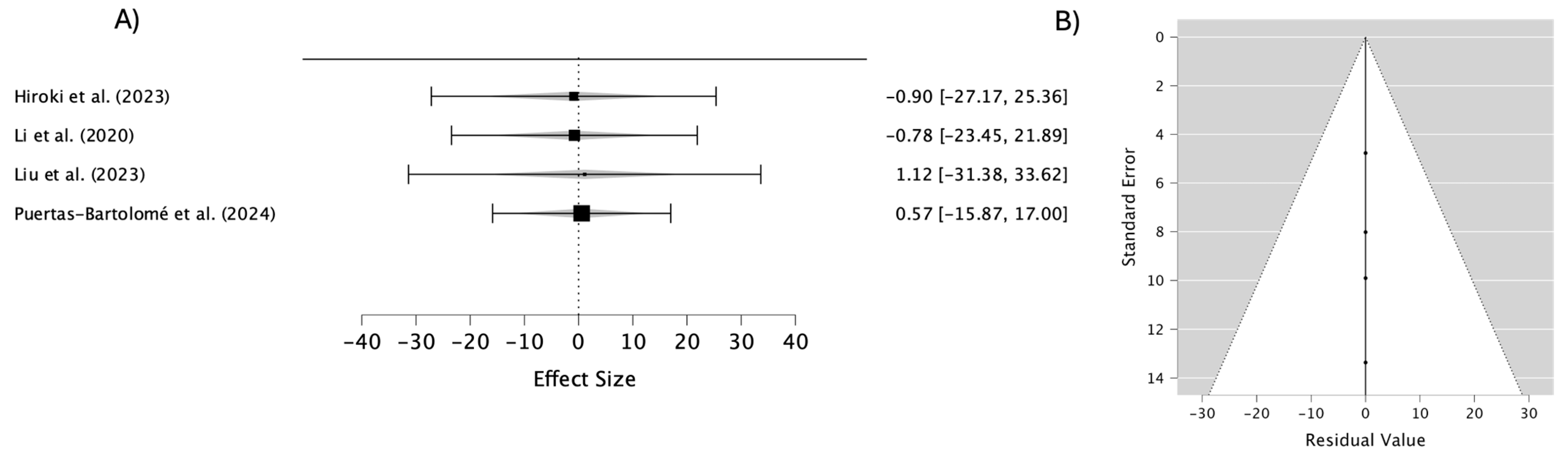
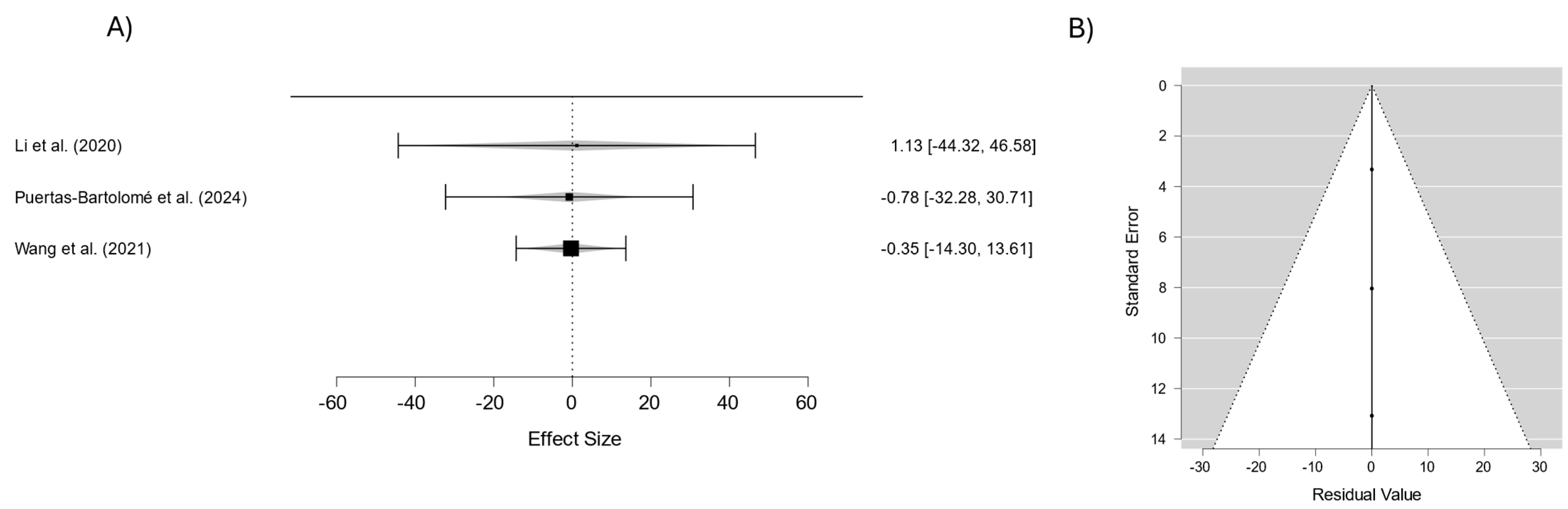

| Study | Year | Lens Material/Coating | Key Findings | Drug Release Characteristics | Mechanical Properties |
|---|---|---|---|---|---|
| Hiroki et al. (2021) [32] | 2021 | Cellulose derivative-based hydrogels | Improved mechanical properties and elasticity | Not applicable | Tensile strength 0.2 MPa |
| Hiroki et al. (2023) [33] | 2023 | Hydroxypropyl cellulose hydrogels | Biodegradable, high transparency, reduced protein deposition | Sustained release for >48 h | Tensile strength 0.2 MPa |
| Kim et al. (2023) [34] | 2023 | pH-sensitive polymer with silica | Controlled, temperature-sensitive drug release | pH-sensitive release for <35 °C | Elastic modulus maintained |
| Li et al. (2020) [35] | 2020 | pHEMA/β-CD-crHA hydrogels * | Improved wettability and sustained drug delivery | Prolonged release for 72 h | Elastic modulus 1.8 MPa |
| Liu et al. (2023) [36] | 2023 | Silicone hydrogels | High oxygen permeability and controlled drug release | Release improved by 200% | Oxygen permeability 65.8 |
| Oucif et al. (2021) [37] | 2021 | HEMA-co-HEA hydrogels ** | Adjustable swelling and extended drug release | Non-Fickian release behavior | Enhanced swelling properties |
| Puertas-Bartolomé et al. (2024) [38] | 2024 | Self-lubricating hydrogel lenses | Long-term lubrication and HA secretion | Sustained HA *** release over 3 weeks | Functional flexibility |
| Wang et al. (2021) [39] | 2021 | Nanogel-embedded lenses | Extended drug release and improved bioavailability | Sustained drug delivery for 10 days | Mechanical durability maintained |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.P.; Martinez-Perez, C. Meta-Analysis of Materials and Treatments Used in Contact Lenses: Implications for Lens Characteristics. Materials 2025, 18, 1445. https://doi.org/10.3390/ma18071445
Oliveira AP, Martinez-Perez C. Meta-Analysis of Materials and Treatments Used in Contact Lenses: Implications for Lens Characteristics. Materials. 2025; 18(7):1445. https://doi.org/10.3390/ma18071445
Chicago/Turabian StyleOliveira, Ana Paula, and Clara Martinez-Perez. 2025. "Meta-Analysis of Materials and Treatments Used in Contact Lenses: Implications for Lens Characteristics" Materials 18, no. 7: 1445. https://doi.org/10.3390/ma18071445
APA StyleOliveira, A. P., & Martinez-Perez, C. (2025). Meta-Analysis of Materials and Treatments Used in Contact Lenses: Implications for Lens Characteristics. Materials, 18(7), 1445. https://doi.org/10.3390/ma18071445