Advanced Technologies for Nitrogen Removal and Recovery from Municipal and Industrial Wastewater
Abstract
1. Introduction
1.1. Origin and Consequences of Nitrogen Emissions in Municipal and Industrial Wastewater
1.2. Methods of Nitrogen Input into the Environment
1.3. Environmental Impact and Toxicity of Various Nitrogen Forms
1.4. Objectives and Scope of Review
2. Conventional Nitrogen Removal Methods
2.1. The Nitrification Process: Mechanism and Environmental Conditions
2.1.1. Nitrification Microorganisms
2.1.2. Impact of Environmental Factors on Activity of Nitrifying Microorganisms
2.1.3. Kinetic Modeling of Nitrification and Its Impact on Reactor Sizing
- r = reaction rate.
- μmax = maximum specific growth rate of nitrifying bacteria.
- S = substrate concentration (ammonium or nitrite).
- Ks = half-saturation constant (substrate concentration at which growth rate is half of μmax).
2.1.4. Comammox
2.2. Denitrification: Key Stages and Environmental Conditions
2.3. Summary of Biological Nitrification and Denitrification Processes
3. Advanced Nitrogen Removal and Recovery Methods
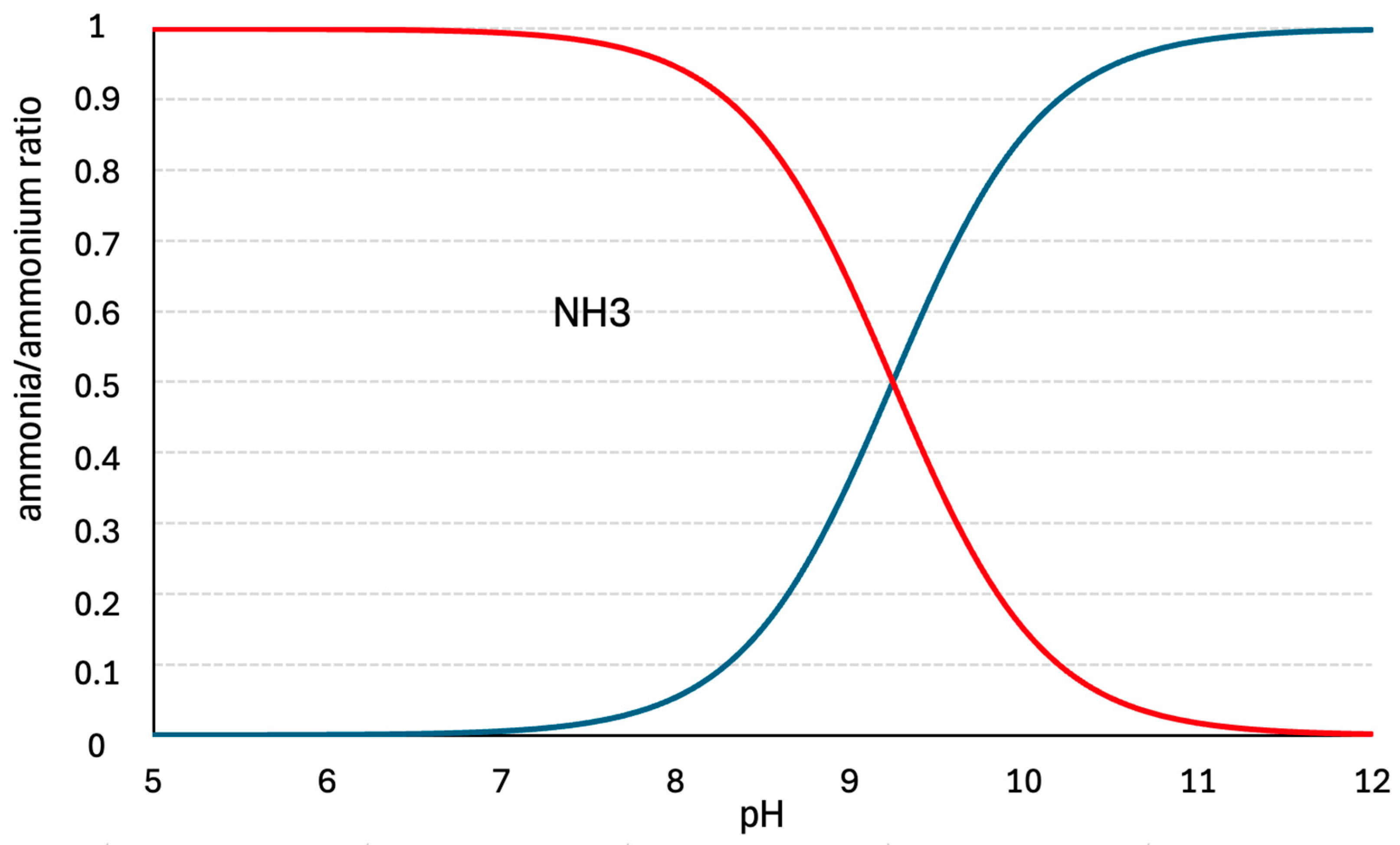
3.1. Ammonia Stripping
3.2. Novel Biological Processes Based on Shorcut in Nitrogen Removal
3.3. Membrane Technologies
3.4. Electrochemical Methods
3.5. Technology Readiness and Scalability of Nitrogen Removal and Recovery Methods
3.6. Fate of Nitrogen Within Biological Sludge Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lindström, K.; Mousavi, S.A. Rhizobium and Other N -fixing Symbioses. In Encyclopedia of Life Sciences; Wiley: Hoboken, NJ, USA, 2010; ISBN 978-0-470-01617-6. [Google Scholar]
- Galloway, J.N.; Schlesinger, W.H.; Levy, H.; Michaels, A.; Schnoor, J.L. Nitrogen Fixation: Anthropogenic Enhancement-environmental Response. Glob. Biogeochem. Cycles 1995, 9, 235–252. [Google Scholar] [CrossRef]
- Intergovernmental Panel On Climate Change (Ed.) Carbon and Other Biogeochemical Cycles. In Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2014; pp. 465–570. ISBN 978-1-107-05799-9. [Google Scholar]
- Winiwarter, W.; Erisman, J.W.; Galloway, J.N.; Klimont, Z.; Sutton, M.A. Estimating Environmentally Relevant Fixed Nitrogen Demand in the 21st Century. Clim. Change 2013, 120, 889–901. [Google Scholar] [CrossRef]
- Van Drecht, G.; Bouwman, A.F.; Knoop, J.M.; Beusen, A.H.W.; Meinardi, C.R. Global Modeling of the Fate of Nitrogen from Point and Nonpoint Sources in Soils, Groundwater, and Surface Water. Glob. Biogeochem. Cycles 2003, 17, 2003GB002060. [Google Scholar] [CrossRef]
- Czerwionka, K.; Makinia, J.; Pagilla, K.R.; Stensel, H.D. Characteristics and Fate of Organic Nitrogen in Municipal Biological Nutrient Removal Wastewater Treatment Plants. Water Res. 2012, 46, 2057–2066. [Google Scholar] [CrossRef]
- Litwack, G. Metabolism of Amino Acids. In Human Biochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–394. ISBN 978-0-12-383864-3. [Google Scholar]
- Ladd, J.N.; Jackson, R.B. Biochemistry of Ammonification. In Agronomy Monographs; Stevenson, F.J., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 2015; pp. 173–228. ISBN 978-0-89118-216-0. [Google Scholar]
- Wrong, O. Nitrogen Metabolism in the Gut. Am. J. Clin. Nutr. 1978, 31, 1587–1593. [Google Scholar] [CrossRef]
- Henze, M.; Van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008; ISBN 978-1-78040-186-7. [Google Scholar]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Li, J.-M.; Du, Y.-P.; Dong, Z.-Y.; Zhao, X.-L. Air Stripping of High Concentration Ammonia Wastewater in Fertilizer Plant. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 2908–2910. [Google Scholar]
- Visigalli, S.; Turolla, A.; Bellandi, G.; Bellucci, M.; Clagnan, E.; Brusetti, L.; Jia, M.; Di Cosmo, R.; Menin, G.; Bargna, M.; et al. Autotrophic Nitrogen Removal for Decentralized Treatment of Ammonia-Rich Industrial Textile Wastewater: Process Assessment, Stabilization and Modelling. Environ. Sci. Pollut. Res. 2021, 28, 46643–46654. [Google Scholar] [CrossRef]
- Aziz, A.; Basheer, F.; Sengar, A.; Irfanullah; Khan, S.U.; Farooqi, I.H. Biological Wastewater Treatment (Anaerobic-Aerobic) Technologies for Safe Discharge of Treated Slaughterhouse and Meat Processing Wastewater. Sci. Total Environ. 2019, 686, 681–708. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Noor, A.; Isah, A.S.; Lawal, I.M.; Birniwa, A.H.; Usman, A.K.; Abubakar, S. Kinetics of Pulp and Paper Wastewater Treatment by High Sludge Retention Time Activated Sludge Process. J. Hunan Univ. Nat. Sci. 2022, 49, 242–251. [Google Scholar] [CrossRef]
- Jones, E.R.; van Vliet, M.T.H.; Qadir, M.; Bierkens, M.F.P. Country-Level and Gridded Estimates of Wastewater Production, Collection, Treatment and Reuse. Earth Syst. Sci. Data 2021, 13, 237–254. [Google Scholar] [CrossRef]
- Food and Feed Information Portal Database|FIP. Available online: https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search (accessed on 10 December 2024).
- Guzik, M. Związki azotu w płytkich wodach podziemnych w rejonie Lublińca. Przegląd Geol. 2003, 51, 139–141. [Google Scholar]
- Aspelin, V.; Ekholm, J. Inhibition of Nitrification in Industrial Wastewater—Identification of Sources. Master’s Thesis, Lund University, Lund, Sweden, 2017. [Google Scholar]
- Levine, A.D.; Tchobanoglous, G.; Asano, T. Characterization of the Size Distribution of Contaminants in Wastewater: Treatment and Reuse Implications. Water Pollut. Control Fed. 1985, 57, 805–816. [Google Scholar]
- Guellil, A.; Boualam, M.; Quiquampoix, H.; Ginestet, P.; Audic, J.-M.; Block, J. Hydrolysis of Wastewater Colloidal Organic Matter by Extracellular Enzymes Extracted from Activated Sludge Flocs. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2001, 43, 33–40. [Google Scholar] [CrossRef]
- Hu, Z.; Chandran, K.; Smets, B.F.; Grasso, D. Evaluation of a Rapid Physical–Chemical Method for the Determination of Extant Soluble COD. Water Res. 2002, 36, 617–624. [Google Scholar] [CrossRef]
- Jørgensen, N. Organic Nitrogen; Encyclopedia of Inland Waters; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Huo, S.; Xi, B.; Yu, H.; Qin, Y.; Zan, F.; Zhang, J. Characteristics and Transformations of Dissolved Organic Nitrogen in Municipal Biological Nitrogen Removal Wastewater Treatment Plants. Environ. Res. Lett. 2013, 8, 044005. [Google Scholar] [CrossRef]
- Sattayatewa, C.; Pagilla, K.; Sharp, R.; Pitt, P. Fate of Organic Nitrogen in Four Biological Nutrient Removal Wastewater Treatment Plants. Water Environ. Res. 2010, 82, 2306–2315. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Ahmad, A.; Das, S.; Ghangrekar, M.M. Removal of Xenobiotics from Wastewater by Electrocoagulation: A Mini-Review. J. Indian Chem. Soc. 2020, 97, 493–500. [Google Scholar]
- Son, D.-J.; Kim, C.-S.; Lee, J.-H.; Yoon, J.-K.; Lee, S.-H.; Jeong, D.-H. Occurrence Assessment of Pharmaceuticals in Various Sewage Treatment Plants and Effluent-Receiving Streams in Korea. Water 2023, 15, 3897. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-Wide Monitoring Survey on Emerging Polar Organic Contaminants in Wastewater Treatment Plant Effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Anumol, T.; Vijayanandan, A.; Park, M.; Philip, L.; Snyder, S.A. Occurrence and Fate of Emerging Trace Organic Chemicals in Wastewater Plants in Chennai, India. Environ. Int. 2016, 92–93, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.L.; Katz, B.G.; Meyer, M.T. Occurrence and Potential Transport of Selected Pharmaceuticals and Other Organic Wastewater Compounds from Wastewater-Treatment Plant Influent and Effluent to Groundwater and Canal Systems in Miami-Dade County, Florida; U.S. Geological Survey: Reston, VA, USA, 2012; pp. 1–64.
- Plósz, B.G.; Leknes, H.; Liltved, H.; Thomas, K.V. Diurnal Variations in the Occurrence and the Fate of Hormones and Antibiotics in Activated Sludge Wastewater Treatment in Oslo, Norway. Sci. Total Environ. 2010, 408, 1915–1924. [Google Scholar] [CrossRef]
- König, M.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherová, K.; Novák, J.; Teodorović, I.; Schulze, T.; Seidensticker, S.; Kamal Hashmi, M.A.; et al. Impact of Untreated Wastewater on a Major European River Evaluated with a Combination of in Vitro Bioassays and Chemical Analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef]
- Xiny, Y. Identification and Transformation of Nitrogen Compounds in Coking Wastewater During O/H/O Biological Treatment Process. Available online: https://www.semanticscholar.org/paper/Identification-and-transformation-of-nitrogen-in-O-Xiny/ff36f509647495c08a1f000f6f8d148235fbf40c (accessed on 13 December 2024).
- Mondal, M.; Mukherjee, R.; Sinha, A.; Sarkar, S.; De, S. Removal of Cyanide from Steel Plant Effluent Using Coke Breeze, a Waste Product of Steel Industry. J. Water Process Eng. 2019, 28, 135–143. [Google Scholar] [CrossRef]
- Manso Cobos, I.; Ibáñez García, M.I.; de la Peña Moreno, F.; Sáez Melero, L.P.; Luque-Almagro, V.M.; Castillo Rodríguez, F.; Roldán Ruiz, M.D.; Prieto Jiménez, M.A.; Moreno Vivián, C. Pseudomonas Pseudoalcaligenes CECT5344, a Cyanide-Degrading Bacterium with by-Product (Polyhydroxyalkanoates) Formation Capacity. Microb. Cell Factories 2015, 14, 77. [Google Scholar] [CrossRef]
- Schmidt, T.C.; Steinbach, K.; von Löw, E.; Stork, G. Highly Polar Metabolites of Nitroaromatic Compounds in Ammunition Wastewater. Chemosphere 1998, 37, 1079–1090. [Google Scholar] [CrossRef]
- Spanggord, R.J.; Gibson, B.W.; Keck, R.G.; Thomas, D.W.; Barkley, J.J. Effluent Analysis of Wastewater Generated in the Manufacture of 2,4,6-Trinitrotoluene. 1. Characterization Study. Environ. Sci. Technol. 1982, 16, 229–232. [Google Scholar] [CrossRef]
- Schwarz, G.; Lingens, F. Bacterial Degradation of N-Heterocyclic Compounds. In Biochemistry of Microbial Degradation; Ratledge, C., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 459–486. ISBN 978-94-011-1687-9. [Google Scholar]
- Ghosh, P.; Mukherji, S. Degradation of Carbazole, Fluorene, Dibenzothiophene and Their Mixture by P. Aeruginosa RS1 in Petroleum Refinery Wastewater. J. Water Process Eng. 2020, 37, 101454. [Google Scholar] [CrossRef]
- Peng, C.; Chai, L.; Tang, C.; Min, X.; Song, Y.; Duan, C.; Yu, C. Study on the Mechanism of Copper–Ammonia Complex Decomposition in Struvite Formation Process and Enhanced Ammonia and Copper Removal. J. Environ. Sci. 2017, 51, 222–233. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, L.; Jiao, G.; Wen, Y.; Liao, Q.; Zhou, H.; Tang, S. Enhanced Removal of Metal-Cyanide Complexes from Wastewater by Fe-Impregnated Biochar: Adsorption Performance and Removal Mechanism. Chemosfere 2023, 331, 138719. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, M.; Peñuelas, J.; Liu, X.; Paerl, H.W.; Elser, J.J.; Sardans, J.; Couture, R.-M.; Larssen, T.; Hu, H.; et al. Improvement in Municipal Wastewater Treatment Alters Lake Nitrogen to Phosphorus Ratios in Populated Regions. Proc. Natl. Acad. Sci. USA 2020, 117, 11566–11572. [Google Scholar] [CrossRef] [PubMed]
- Powley, H.R.; Dürr, H.H.; Lima, A.T.; Krom, M.D.; Cappellen, P.V. Direct Discharges of Domestic Wastewater Are a Major Source of Phosphorus and Nitrogen to the Mediterranean Sea. Environ. Sci. Technol. 2016, 50, 8722–8730. [Google Scholar] [CrossRef] [PubMed]
- Holeton, C.; Chambers, P.A.; Grace, L. Wastewater Release and Its Impacts on Canadian Waters. Can. J. Fish. Aquat. Sci. 2011, 68, 1836–1859. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; van Clemput, O. Closing the Global N2O Budget: Nitrous Oxide Emissions Through the Agricultural Nitrogen Cycle. Nutr. Cycl. Agroecosyst. 1998, 52, 225–248. [Google Scholar] [CrossRef]
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing Ammonia Emissions from Livestock Production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef]
- Tantawy, M.F.; Abou Elnaga, S.A.; Abou Hussien, E.A.; Abou Alfotoh, M.S. Effect of Nitrogenous Fertilizers Factory (Talkha Fertilizers Factory) on Nitrate Status in the Agricultural Environment. J. Soil Sci. Agric. Eng. 2014, 5, 687–704. [Google Scholar] [CrossRef]
- O’Connell, M. Identification of Anthropogenic Impact on Nitrogen Cycling Using Stable Isotopes and Distributed Hydrologic Modeling; University of Virginia: Charlottesville, VA, USA, 2016. [Google Scholar]
- Liu, H.; Jin, Q.; Shi, R.; Lv, C.; Luo, J.; He, Y.; Yang, W.; Xu, X.; Qian, S.; Li, W. Effect of vegetation distribution driven by hydrological fluctuation on sedimental stoichiometry regulating N2O emissions in freshwater wetland. Biogeosci. Discuss. 2021, 2021, 1–23. [Google Scholar]
- Pang, L.; Sun, Y.; Yue, Y.; Liu, C.; An, C.; Yang, T.; Lu, X.; Xu, Q.; Mei, J.; Liu, M.; et al. Stability of Aquatic Nitrogen Cycle Under Dramatic Changes of Water and Sediment Inflows to the Three Gorges Reservoir. GeoHealth 2022, 6, e2022GH000607. [Google Scholar] [CrossRef]
- Kanter, D.R.; Winiwarter, W.; Bodirsky, B.L.; Bouwman, L.; Boyer, E.; Buckle, S.; Compton, J.E.; Dalgaard, T.; de Vries, W.; Leclère, D.; et al. A Framework for Nitrogen Futures in the Shared Socioeconomic Pathways. Glob. Environ. Change 2020, 61, 102029. [Google Scholar] [CrossRef]
- Eddy, F.B. Ammonia in Estuaries and Effects on Fish. J. Fish Biol. 2005, 67, 1495–1513. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic Effects on Bioaccumulation, Hematological Parameters, Oxidative Stress, Immune Responses and Tissue Structure in Fish Exposed to Ammonia Nitrogen: A Review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Resch, C.M.; Gibson, J. Ammonia Uptake and Retention in Some Cyanobacteria. Arch. Microbiol. 1984, 138, 287–292. [Google Scholar] [CrossRef]
- Dai, G.-Z.; Shang, J.-L.; Qiu, B.-S. Ammonia May Play an Important Role in the Succession of Cyanobacterial Blooms and the Distribution of Common Algal Species in Shallow Freshwater Lakes. Glob. Change Biol. 2012, 18, 1571–1581. [Google Scholar] [CrossRef]
- van der Eerden, L.J.M. Toxicity of Ammonia to Plants. Agric. Environ. 1982, 7, 223–235. [Google Scholar] [CrossRef]
- Pearson, J.; Stewart, G.R. The Deposition of Atmospheric Ammonia and Its Effects on Plants. New Phytol. 1993, 125, 283–305. [Google Scholar] [CrossRef]
- Tilak, K.S.; Veeraiah, K.; Raju, J.M.P. Effects of Ammonia, Nitrite and Nitrate on Hemoglobin Content and Oxygen Consumption of Freshwater Fish, Cyprinus Carpio (Linnaeus). J. Environ. Biol. 2007, 28, 45–47. [Google Scholar]
- Hamlin, H.J. Nitrate Toxicity in Siberian Sturgeon (Acipenser baeri). Aquaculture 2006, 253, 688–693. [Google Scholar] [CrossRef]
- McGurk, M.D.; Landry, F.; Tang, A.; Hanks, C.C. Acute and Chronic Toxicity of Nitrate to Early Life Stages of Lake Trout (Salvelinus Namaycush) and Lake Whitefish (Coregonus Clupeaformis). Environ. Toxicol. Chem. 2006, 25, 2187–2196. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate Toxicity to Aquatic Animals: A Review with New Data for Freshwater Invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Barker, T.; Hatton, K.O.; Connor, L.; Moss, B. Effects of Nitrate Load on Submerged Plant Biomass and Species Richness: Results of a Mesocosm Experiment. Fundam. Appl. Limnol. 2008, 173, 89–100. [Google Scholar] [CrossRef]
- Ho, Y.B.; Wong, W. Growth and Macronutrient Removal of Water Hyacinth in a Small Secondary Sewage Treatment Plant. Resour. Conserv. Recycl. 1994, 11, 161–178. [Google Scholar] [CrossRef]
- Aoi, T.; Hayashi, T. Nutrient Removal by Water Lettuce (Pisitia Stratiotes). Water Sci. Technol. 1996, 34, 407–412. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Jing, S.-R.; Wang, T.-W.; Lee, D.-Y. Effects of Macrophytes and External Carbon Sources on Nitrate Removal from Groundwater in Constructed Wetlands. Environ. Pollut. 2002, 119, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Sooknah, R.D.; Wilkie, A.C. Nutrient Removal by Floating Aquatic Macrophytes Cultured in Anaerobically Digested Flushed Dairy Manure Wastewater. Ecol. Eng. 2004, 22, 27–42. [Google Scholar] [CrossRef]
- Araki, R.; Mori, M.; Mori, M.; Hasegawa, H. Genetic Differences in Nitrate Uptake in Two Clones of the Common Reed, Phragmites Australis. Breed. Sci. 2005, 55, 297–302. [Google Scholar] [CrossRef]
- Ayyasamy, P.M.; Rajakumar, S.; Sathishkumar, M.; Swaminathan, K.; Shanthi, K.; Lakshmanaperumalsamy, P.; Lee, S. Nitrate Removal from Synthetic Medium and Groundwater with Aquatic Macrophytes. Desalination 2009, 242, 286–296. [Google Scholar] [CrossRef]
- Wang, R.; Bai, N.; Xu, S.; Zhuang, G.; Bai, Z.; Zhao, Z.; Zhuang, X. The Adaptability of a Wetland Plant Species Myriophyllum Aquaticum to Different Nitrogen Forms and Nitrogen Removal Efficiency in Constructed Wetlands. Environ. Sci. Pollut. Res. 2018, 25, 7785–7795. [Google Scholar] [CrossRef]
- Stuart, M.E.; Gooddy, D.C.; Bloomfield, J.P.; Williams, A.T. A Review of the Impact of Climate Change on Future Nitrate Concentrations in Groundwater of the UK. Sci. Total Environ. 2011, 409, 2859–2873. [Google Scholar] [CrossRef]
- Kent, R.; Landon, M.K. Trends in Concentrations of Nitrate and Total Dissolved Solids in Public Supply Wells of the Bunker Hill, Lytle, Rialto, and Colton Groundwater Subbasins, San Bernardino County, California: Influence of Legacy Land Use. Sci. Total Environ. 2013, 452–453, 125–136. [Google Scholar] [CrossRef]
- Sheikhy Narany, T.; Aris, A.Z.; Sefie, A.; Keesstra, S. Detecting and Predicting the Impact of Land Use Changes on Groundwater Quality, a Case Study in Northern Kelantan, Malaysia. Sci. Total Environ. 2017, 599–600, 844–853. [Google Scholar] [CrossRef]
- Fan, A.M. Nitrate and Nitrite in Drinking Water: A Toxicological Review. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Burlington, MA, USA, 2011; pp. 137–145. ISBN 978-0-444-52272-6. [Google Scholar]
- Sataieva, T.; Zadnipryany, I.; Zukow, W. Sodium Nitrate Affects Myocardium in Pregnant Rats and Their Pups. Ecol. Quest. 2018, 29, 55–62. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr.; Morris, D.P. Toxicity of Nitrite to Fish: A Review. Trans. Am. Fish. Soc. 1986, 115, 183–195. [Google Scholar] [CrossRef]
- Kocour Kroupová, H.; Valentová, O.; Svobodová, Z.; Šauer, P.; Máchová, J. Toxic Effects of Nitrite on Freshwater Organisms: A Review. Rev. Aquac. 2018, 10, 525–542. [Google Scholar] [CrossRef]
- Huey, D.W.; Beitinger, T.L. Hematological Responses of larvalRana Catesbiana to Sublethal Nitrite Exposures. Bull. Environ. Contam. Toxicol. 1980, 25, 574–577. [Google Scholar] [CrossRef]
- Jensen, F.B. Sublethal Physiological Changes in Freshwater Crayfish, Astacus Astacus, Exposed to Nitrite: Haemolymph and Muscle Tissue Electrolyte Status, and Haemolymph Acid-Base Balance and Gas Transport. Aquat. Toxicol. 1990, 18, 51–60. [Google Scholar] [CrossRef]
- Jensen, F.B. Uptake, Elimination and Effects of Nitrite and Nitrate in Freshwater Crayfish (Astacus Astacus). Aquat. Toxicol. 1996, 34, 95–104. [Google Scholar] [CrossRef]
- Harris, R.R.; Coley, S. The Effects of Nitrite on Chloride Regulation in the Crayfish Pacifastacus Leniusculus Dana. J. Comp. Physiol. B 1991, 161, 199–206. [Google Scholar] [CrossRef]
- Cheng Jiann-Chu, W.; Liu, C.; Chen, J. Effect of Nitrite on Interaction between the Giant Freshwater Prawn Macrobrachium Rosenbergii and Its Pathogen Lactococcus Garvieae. Dis. Aquat. Org. 2002, 50, 189–197. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Toxic Effects of Ammonia, Nitrite, and Nitrate to Decapod Crustaceans: A Review on Factors Influencing Their Toxicity, Physiological Consequences, and Coping Mechanisms. Rev. Fish. Sci. 2013, 21, 1–21. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Cong, W.; Cai, Z.; Ouyang, F. Utilization of Nitrite as a Nitrogen Source by Botryococcus Braunii. Biotechnol. Lett. 2004, 26, 239–243. [Google Scholar] [CrossRef]
- Massara, T.M.; Malamis, S.; Guisasola, A.; Baeza, J.A.; Noutsopoulos, C.; Katsou, E. A Review on Nitrous Oxide (N2O) Emissions during Biological Nutrient Removal from Municipal Wastewater and Sludge Reject Water. Sci. Total Environ. 2017, 596–597, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Winogradsky, S. Contributions à la Morphologie des Organismes de la Nitrification; Arkhiv Biologischeski Nauk: St. Petersbourg, Russia, 1892. [Google Scholar]
- Vilajeliu-Pons, A.; Koch, C.; Balaguer, M.D.; Colprim, J.; Harnisch, F.; Puig, S. Microbial Electricity Driven Anoxic Ammonium Removal. Water Res. 2018, 130, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sievers, M.; Vorlop, K.D.; Hahne, J.; Schlieker, M.; Schäfer, S. Pre-Nitrification by Encapsulated Nitrifiers—A Possibility for Self-Sufficient Energy Operation of Domestic WWTPs. Water Sci. Technol. 2003, 47, 173–180. [Google Scholar] [CrossRef]
- Yao, G.-J.; Ren, J.-Q.; Zhou, F.; Liu, Y.-D.; Li, W. Micro-Nano Aeration Is a Promising Alternative for Achieving High-Rate Partial Nitrification. Sci. Total Environ. 2021, 795, 148899. [Google Scholar] [CrossRef]
- Almén, E. Impact of Plant Elicitors on Ammonia Oxidizing Root and Rhizosphere Communities in Winter Wheat. Master’s Thesis, Swedish University of Agricultural Sciences, SLU, Uppsala, Sweden, 2024. [Google Scholar]
- Yin, Z.; Bi, X.; Xu, C. Ammonia-Oxidizing Archaea (AOA) Play with Ammonia-Oxidizing Bacteria (AOB) in Nitrogen Removal from Wastewater. Archaea 2018, 2018, 8429145. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Berube, P.M.; Urakawa, H.; de la Torre, J.R.; Stahl, D.A. Ammonia Oxidation Kinetics Determine Niche Separation of Nitrifying Archaea and Bacteria. Nature 2009, 461, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L. Adaptations to Energy Stress Dictate the Ecology and Evolution of the Archaea. Nat. Rev. Microbiol. 2007, 5, 316–323. [Google Scholar] [CrossRef]
- Sharma, P.; Kanta Pandey, K.; Lepcha, A.; Sharma, S.; Maurya, N.; Kumar Sharma, S.; Pradhan, R.; Kumar, R. Elucidating the Potential of Nitrifying Bacteria in Mitigating Nitrogen Pollution and Its Industrial Application. Microsphere 2023, 2, 246–259. [Google Scholar] [CrossRef]
- Martens-Habbena, W.; Stahl, D.A. Nitrogen Metabolism and Kinetics of Ammonia-Oxidizing Archaea. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 496, pp. 465–487. ISBN 978-0-12-386489-5. [Google Scholar]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lücker, S.; Vejmelkova, D.; Kostrikina, N.A.; Kleerebezem, R.; Rijpstra, W.I.C.; Damsté, J.S.S.; Le Paslier, D.; Muyzer, G.; Wagner, M.; et al. Nitrification Expanded: Discovery, Physiology and Genomics of a Nitrite-Oxidizing Bacterium from the Phylum Chloroflexi. ISME J. 2012, 6, 2245–2256. [Google Scholar] [CrossRef]
- Lebedeva, E.V.; Alawi, M.; Maixner, F.; Jozsa, P.-G.; Daims, H.; Spieck, E. Physiological and Phylogenetic Characterization of a Novel Lithoautotrophic Nitrite-Oxidizing Bacterium, “Candidatus Nitrospira Bockiana”. Int. J. Syst. Evol. Microbiol. 2008, 58, 242–250. [Google Scholar] [CrossRef]
- Nowka, B.; Off, S.; Daims, H.; Spieck, E. Improved Isolation Strategies Allowed the Phenotypic Differentiation of Two Nitrospira Strains from Widespread Phylogenetic Lineages. FEMS Microbiol. Ecol. 2015, 91, fiu031. [Google Scholar] [CrossRef]
- Ushiki, N.; Fujitani, H.; Aoi, Y.; Tsuneda, S. Isolation of Nitrospira Belonging to Sublineage II from a Wastewater Treatment Plant. Microb. Environ. 2013, 28, 346–353. [Google Scholar] [CrossRef]
- Gao, J.; Fan, X.; Wu, G.; Li, T.; Pan, K. Changes of Abundance and Diversity of Ammonia-Oxidizing Archaea (AOA) and Bacteria (AOB) in Three Nitrifying Bioreactors with Different Ammonia Concentrations. Desalin. Water Treat. 2016, 57, 21463–21475. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, T. Ammonia-Oxidizing Bacteria Dominates over Ammonia-Oxidizing Archaea in a Saline Nitrification Reactor under Low DO and High Nitrogen Loading. Biotechnol. Bioeng. 2011, 108, 2544–2552. [Google Scholar] [CrossRef] [PubMed]
- Yapsakli, K.; Aliyazicioglu, C.; Mertoglu, B. Identification and Quantitative Evaluation of Nitrogen-Converting Organisms in a Full-Scale Leachate Treatment Plant. J. Environ. Manag. 2011, 92, 714–723. [Google Scholar] [CrossRef]
- Shourjeh, M.S.; Kowal, P.; Drewnowski, J.; Szeląg, B.; Szaja, A.; Łagód, G. Mutual Interaction between Temperature and DO Set Point on AOB and NOB Activity during Shortcut Nitrification in a Sequencing Batch Reactor in Terms of Energy Consumption Optimization. Energies 2020, 13, 5808. [Google Scholar] [CrossRef]
- Stenstrom, M.K.; Poduska, R.A. The Effect of Dissolved Oxygen Concentration on Nitrification. Water Res. 1980, 14, 643–649. [Google Scholar] [CrossRef]
- Guo, X.; Kim, J.H.; Behera, S.K.; Park, H.S. Influence of Dissolved Oxygen Concentration and Aeration Time on Nitrite Accumulation in Partial Nitrification Process. Int. J. Environ. Sci. Technol. 2008, 5, 527–534. [Google Scholar] [CrossRef]
- Kim, T.; Hite, M.; Rogacki, L.; Sealock, A.W.; Sprouse, G.; Novak, P.J.; LaPara, T.M. Dissolved Oxygen Concentrations Affect the Function but Not the Relative Abundance of Nitrifying Bacterial Populations in Full-Scale Municipal Wastewater Treatment Bioreactors during Cold Weather. Sci. Total Environ. 2021, 781, 146719. [Google Scholar] [CrossRef]
- Park, H.-D.; Noguera, D. Nitrospira Community Composition in Nitrifying Reactors Operated with Two Different Dissolved Oxygen Levels. J. Microbiol. Biotechnol. 2008, 18, 1470–1474. [Google Scholar] [PubMed]
- Kalvelage, T.; Lavik, G.; Lam, P.; Contreras, S.; Arteaga, L.; Löscher, C.R.; Oschlies, A.; Paulmier, A.; Stramma, L.; Kuypers, M.M.M. Nitrogen Cycling Driven by Organic Matter Export in the South Pacific Oxygen Minimum Zone. Nat. Geosci. 2013, 6, 228–234. [Google Scholar] [CrossRef]
- Casciotti, K.L.; Buchwald, C. Insights on the Marine Microbial Nitrogen Cycle from Isotopic Approaches to Nitrification. Front. Microbiol. 2012, 3, 33877. [Google Scholar] [CrossRef]
- Lam, P.; Kuypers, M.M.M. Microbial Nitrogen Cycling Processes in Oxygen Minimum Zones. Annu. Rev. Mar. Sci. 2011, 3, 317–345. [Google Scholar] [CrossRef]
- Khangembam, C.D. Effect of Temperature on Nitrifying Microbes, Emphasizing on Ammonia Oxidizing Archaea and Bacteria. J. Biol. Sci. Med. 2016, 2, 7–14. [Google Scholar]
- Taylor, A.E.; Giguere, A.T.; Zoebelein, C.M.; Myrold, D.D.; Bottomley, P.J. Modeling of Soil Nitrification Responses to Temperature Reveals Thermodynamic Differences Between Ammonia-Oxidizing Activity of Archaea and Bacteria. ISME J. 2017, 11, 896–908. [Google Scholar] [CrossRef]
- Nishizawa, M.; Sakai, S.; Konno, U.; Nakahara, N.; Takaki, Y.; Saito, Y.; Imachi, H.; Tasumi, E.; Makabe, A.; Koba, K.; et al. Nitrogen and Oxygen Isotope Effects of Ammonia Oxidation by Thermophilic Thaumarchaeota from a Geothermal Water Stream. Appl. Environ. Microbiol. 2016, 82, 4492–4504. [Google Scholar] [CrossRef]
- Lee, S.; Cho, K.; Lim, J.; Kim, W.; Hwang, S. Acclimation and Activity of Ammonia-Oxidizing Bacteria with Respect to Variations in Zinc Concentration, Temperature, and Microbial Population. Bioresour. Technol. 2011, 102, 4196–4203. [Google Scholar] [CrossRef]
- Pan, K.-L.; Gao, J.-F.; Fan, X.-Y.; Li, D.-C.; Dai, H.-H. The More Important Role of Archaea than Bacteria in Nitrification of Wastewater Treatment Plants in Cold Season despite Their Numerical Relationships. Water Res. 2018, 145, 552–561. [Google Scholar] [CrossRef]
- Fan, X.-Y.; Gao, J.-F.; Pan, K.-L.; Li, D.-C.; Dai, H.-H.; Li, X. Temporal Heterogeneity and Temperature Response of Active Ammonia-Oxidizing Microorganisms in Winter in Full-Scale Wastewater Treatment Plants. Chem. Eng. J. 2019, 360, 1542–1552. [Google Scholar] [CrossRef]
- Alawi, M.; Off, S.; Kaya, M.; Spieck, E. Temperature Influences the Population Structure of Nitrite-Oxidizing Bacteria in Activated Sludge. Environ. Microbiol. Rep. 2009, 1, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.; Lipski, A.; Sanders, T.; Eva-Maria-Pfeiffer; Spieck, E. Cultivation of a Novel Cold-Adapted Nitrite Oxidizing Betaproteobacterium from the Siberian Arctic. ISME J. 2007, 1, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Hüpeden, J.; Wegen, S.; Off, S.; Lücker, S.; Bedarf, Y.; Daims, H.; Kühn, C.; Spieck, E. Relative Abundance of Nitrotoga Spp. in a Biofilter of a Cold-Freshwater Aquaculture Plant Appears To Be Stimulated by Slightly Acidic pH. Appl. Environ. Microbiol. 2016, 82, 1838–1845. [Google Scholar] [CrossRef]
- Fernandez-Fontaina, E.; Carballa, M.; Omil, F.; Lema, J.M. Modelling Cometabolic Biotransformation of Organic Micropollutants in Nitrifying Reactors. Water Res. 2014, 65, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Le, T.T.H.; Fettig, J.; Meon, G. Kinetics and Simulation of Nitrification at Various pH Values of a Polluted River in the Tropics. Ecohydrol. Hydrobiol. 2019, 19, 54–65. [Google Scholar] [CrossRef]
- Kowal, P.; Mehrani, M.-J.; Sobotka, D.; Ciesielski, S.; Mąkinia, J. Rearrangements of the Nitrifiers Population in an Activated Sludge System Under Decreasing Solids Retention Times. Environ. Res. 2022, 214, 113753. [Google Scholar] [CrossRef]
- Fan, F.; Li, M.; Dou, J.; Zhang, J.; Li, D.; Meng, F.; Dong, Y. Functional Characteristics and Mechanisms of Microbial Community Succession and Assembly in a Long-Term Moving Bed Biofilm Reactor Treating Real Municipal Wastewater. Environ. Res. 2025, 267, 120602. [Google Scholar] [CrossRef]
- Liu, M.; Chen, M.; Qi, R.; Yu, D.; Yang, M.; Zheng, J.; Wei, Y.; Du, H. Model-Based Solution for Upgrading Nitrogen Removal for a Full-Scale Municipal Wastewater Treatment Plant with CASS Process. Processes 2021, 9, 527. [Google Scholar] [CrossRef]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete Nitrification by Nitrospira Bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- Van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op Den Camp, H.J.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete Nitrification by a Single Microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, F.; Jiang, H.; Ren, S.; Wang, W.; Peng, Y. Nitrite Accumulation in Comammox-Dominated Nitrification-Denitrification Reactors: Effects of DO Concentration and Hydroxylamine Addition. J. Hazard. Mater. 2020, 384, 121375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, B.; Wei, J.; Ye, Y.; Xu, J.; Chen, L.; Lu, C. Temperature Influenced the Comammox Community Composition in Drinking Water and Wastewater Treatment Plants. Microb. Ecol. 2021, 82, 870–884. [Google Scholar] [CrossRef]
- Burghate, S.P.; Ingole, N.W. Biological Denitrification—A Review. J. Environ. Sci. Comput. Sci. Eng. Technol. 2014, 3, 9–27. [Google Scholar]
- Fajardo, C.; Mora, M.; Fernández, I.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Cross Effect of Temperature, pH and Free Ammonia on Autotrophic Denitrification Process with Sulphide as Electron Donor. Chemosphere 2014, 97, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, Y.; Sun, J.; Wang, X.; Yuan, J. Improvement in Denitrification Efficiency at Low Temperature with Addition of Redox Mediators. Desalin. Water Treat. 2017, 81, 80–86. [Google Scholar] [CrossRef]
- Poh, L.S.; Jiang, X.; Zhang, Z.; Liu, Y.; Ng, W.J.; Zhou, Y. N2O Accumulation from Denitrification under Different Temperatures. Appl. Microbiol. Biotechnol. 2015, 99, 9215–9226. [Google Scholar] [CrossRef]
- Chen, C.; Ali, A.; Su, J.; Wang, Y.; Huang, T.; Gao, J. Pseudomonas Stutzeri GF2 Augmented the Denitrification of Low Carbon to Nitrogen Ratio: Possibility for Sewage Wastewater Treatment. Bioresour. Technol. 2021, 333, 125169. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Li, J.; Li, B. The Denitrifying Nitrogen Removal Experiment with Low Carbon to Nitrogen Ratio and Organic Loading. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, Nanjing, China, 24–26 June 2011; pp. 8635–8638. [Google Scholar]
- Wyffels, S.; Van Hulle, S.W.H.; Boeckx, P.; Volcke, E.I.P.; Cleemput, O.V.; Vanrolleghem, P.A.; Verstraete, W. Modeling and Simulation of Oxygen-Limited Partial Nitritation in a Membrane-Assisted Bioreactor (MBR). Biotechnol. Bioeng. 2004, 86, 531–542. [Google Scholar] [CrossRef]
- Mulder, A.; van de Graaf, A.A.; Robertson, L.A.; Kuenen, J.G. Anaerobic Ammonium Oxidation Discovered in a Denitrifying Fluidized Bed Reactor. FEMS Microbiol. Ecol. 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Palakodeti, A.; Azman, S.; Rossi, B.; Dewil, R.; Appels, L. A Critical Review of Ammonia Recovery from Anaerobic Digestate of Organic Wastes via Stripping. Renew. Sustain. Energy Rev. 2021, 143, 110903. [Google Scholar] [CrossRef]
- Undalkar, R. Ammonia Removal from Wastewater of Different Sources and Recent Development in Ammonia Stripping. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 673–677. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, D.; Hua, X.; Xu, Y.; Guo, Z.; Liang, D. Ammonia Nitrogen Removal and Recovery from Acetylene Purification Wastewater by Air Stripping. Water Sci. Technol. 2017, 75, 2538–2545. [Google Scholar] [CrossRef]
- Dos Santos, H.A.P.; De Castilhos Júnior, A.B.; Nadaleti, W.C.; Lourenço, V.A. Ammonia Recovery from Air Stripping Process Applied to Landfill Leachate Treatment. Environ. Sci. Pollut. Res. 2020, 27, 45108–45120. [Google Scholar] [CrossRef]
- Barros, A.J.M.; Leite, V.D.; Prasad, S.; Lopes, W.S.; De Sousa, J.T. Study on ammonia stripping process of leachate from the packed towers. J. Urban Environ. Eng. 2013, 7, 215–222. [Google Scholar] [CrossRef]
- Liao, P.H.; Chen, A.; Lo, K.V. Removal of Nitrogen from Swine Manure Wastewaters by Ammonia Stripping. Bioresour. Technol. 1995, 54, 17–20. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Ammonia Stripping for Enhanced Biomethanization of Piggery Wastewater. J. Hazard. Mater. 2012, 199–200, 36–42. [Google Scholar] [CrossRef]
- Finzi, A.; Ferrari, O.; Riva, E.; Provolo, G. Nitrogen Recovery from Intensive Livestock Farms Using a Simplified Ammonia Stripping Process. Front. Sustain. Food Syst. 2024, 8, 1406962. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Xiang, S.; Huang, Z.; Ruan, R.; Liu, Y. Nutrient Removal from Digested Swine Wastewater by Combining Ammonia Stripping with Struvite Precipitation. Environ. Sci. Pollut. Res. 2019, 26, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Başakçilardan-Kabakci, S.; İpekoğlu, A.N.; Talinli, I. Recovery of Ammonia from Human Urine by Stripping and Absorption. Environ. Eng. Sci. 2007, 24, 615–624. [Google Scholar] [CrossRef]
- Liu, B.; Giannis, A.; Zhang, J.; Chang, V.W.-C.; Wang, J.-Y. Air Stripping Process for Ammonia Recovery from Source-Separated Urine: Modeling and Optimization: Air Stripping Process for Ammonia Recovery from Source-Separated Urine. J. Chem. Technol. Biotechnol. 2015, 90, 2208–2217. [Google Scholar] [CrossRef]
- Aung, S.L.; Choi, J.; Cha, H.; Woo, G.; Song, K.G. Ammonia-Selective Recovery from Anaerobic Digestate Using Electrochemical Ammonia Stripping Combined with Electrodialysis. Chem. Eng. J. 2024, 479, 147949. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Trinh, M.V.; Chen, Y.-H.; Lu, Y.-J.; Wang, L.-P.; Cheng, S.; Li, Z.; Santikunaporn, M.; Asavatesanupap, C. Steam Stripping for Recovery of Ammonia from Wastewater Using a High-Gravity Rotating Packed Bed. Environments 2024, 11, 206. [Google Scholar] [CrossRef]
- Hwang, Y.L.; Keller, G.E.I.; Olson, J.D. Steam Stripping for Removal of Organic Pollutants from Water. 1. Stripping Effectiveness and Stripper Design. Ind. Eng. Chem. Res. 1992, 31, 1753–1759. [Google Scholar] [CrossRef]
- Toth, A.J.; Mizsey, P. Comparison of Air and Steam Stripping: Removal of Organic Halogen Compounds from Process Wastewaters. Int. J. Environ. Sci. Technol. 2015, 12, 1321–1330. [Google Scholar] [CrossRef]
- Proceedings of the 43rd Industrial Waste Conference May 1988, Purdue University. Available online: https://www.routledge.com/Proceedings-of-the-43rd-Industrial-Waste-Conference-May-1988-Purdue-University/Bell/p/book/9781315896915 (accessed on 19 January 2025).
- Sakaji, R.H.; Persoff, P.; Daughton, C.G. Steam Stripping of Oil Shale Wastewaters LBL/SEEHRL Steam Stripper: Design, Operation, and Maintenance Manual; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1984. [Google Scholar]
- Zeng, L.; Mangan, C.; Li, X. Ammonia Recovery from Anaerobically Digested Cattle Manure by Steam Stripping. Water Sci. Technol. 2006, 54, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Tettenborn, F.; Behrendt, J.; Otterpohl, R. Resource Recovery and Removal of Pharmaceutical Residues Treatment of Separate Collected Urine; Institute of Wastewater Management and Water Protection Hamburg University of Technology: Hamburg, Germany, 2007. [Google Scholar]
- Leverenz, H.; Adams, R.; Hazard, J.; Tchobanoglous, G. Continuous Thermal Stripping Process for Ammonium Removal from Digestate and Centrate. Sustainability 2021, 13, 2185. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Xu, Z.; Yuan, S.; Cao, M.; Liu, H.; Lu, X. Removal of Ammonia Nitrogen in Wastewater by Microwave Radiation: A Pilot-Scale Study. J. Hazard. Mater. 2009, 168, 862–867. [Google Scholar] [CrossRef]
- Ata, O.N.; Kanca, A.; Demir, Z.; Yigit, V. Optimization of Ammonia Removal from Aqueous Solution by Microwave-Assisted Air Stripping. Water Air Soil. Pollut. 2017, 228, 448. [Google Scholar] [CrossRef]
- Yin, S.; Chen, K.; Srinivasakannan, C.; Guo, S.; Li, S.; Peng, J.; Zhang, L. Enhancing Recovery of Ammonia from Rare Earth Wastewater by Air Stripping Combination of Microwave Heating and High Gravity Technology. Chem. Eng. J. 2018, 337, 515–521. [Google Scholar] [CrossRef]
- Song, Y.-C.; Woo, J.-H.; Oh, G.-G.; Kim, D.-H.; Lee, C.-Y.; Kim, H.-W. External Electric Field Promotes Ammonia Stripping from Wastewater. Water Res. 2021, 203, 117518. [Google Scholar] [CrossRef]
- Melgaço, L.A.O.; Meers, E.; Mota, C.R. Ammonia Recovery from Food Waste Digestate Using Solar Heat-Assisted Stripping-Absorption. Waste Manag. 2020, 113, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Strous, M.; Jetten, M.S.M. Anaerobic Oxidation of Methane and Ammonium. Annu. Rev. Microbiol. 2004, 58, 99–117. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Cirpus, I.; Kartal, B.; van Niftrik, L.; van de Pas-Schoonen, K.T.; Sliekers, O.; Haaijer, S.; van der Star, W.; Schmid, M.; van de Vossenberg, J.; et al. 1994–2004: 10 Years of Research on the Anaerobic Oxidation of Ammonium. Biochem. Soc. Trans. 2005, 33, 119–123. [Google Scholar] [CrossRef]
- Kartal, B.; Koleva, M.; Arsov, R.; Van Der Star, W.; Jetten, M.S.M.; Strous, M. Adaptation of a Freshwater Anammox Population to High Salinity Wastewater. J. Biotechnol. 2006, 126, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Kartal, B.; Van Niftrik, L.; Rattray, J.; Van De Vossenberg, J.L.C.M.; Schmid, M.C.; Sinninghe Damsté, J.; Jetten, M.S.M.; Strous, M. Candidatus ‘Brocadia Fulgida’: An Autofluorescent Anaerobic Ammonium Oxidizing Bacterium: Autofluorescent Anammox Bacteria. FEMS Microbiol. Ecol. 2008, 63, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, D.; Zhai, J.; Makinia, J. Generalized Temperature Dependence Model for Anammox Process Kinetics. Sci. Total Environ. 2021, 775, 145760. [Google Scholar] [CrossRef]
- De Cocker, P.; Bessiere, Y.; Hernandez-Raquet, G.; Dubos, S.; Mozo, I.; Gaval, G.; Caligaris, M.; Barillon, B.; Vlaeminck, S.E.; Sperandio, M. Enrichment and Adaptation Yield High Anammox Conversion Rates under Low Temperatures. Bioresour. Technol. 2018, 250, 505–512. [Google Scholar] [CrossRef]
- Van Dongen, U.; Jetten, M.S.M.; Van Loosdrecht, M.C.M. The SHARON®-Anammox® Process for Treatment of Ammonium Rich Wastewater. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef]
- Volcke, E.I.P.; Van Hulle, S.W.H.; Donckels, B.M.R.; Van Loosdrecht, M.C.M.; Vanrolleghem, P.A. Coupling the SHARON Process with Anammox: Model-Based Scenario Analysis with Focus on Operating Costs. Water Sci. Technol. 2005, 52, 107–115. [Google Scholar] [CrossRef]
- Wett, B. Development and Implementation of a Robust Deammonification Process. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2007, 56, 81–88. [Google Scholar] [CrossRef]
- Ganigué, R.; Gabarró, J.; Sànchez-Melsió, A.; Ruscalleda, M.; López, H.; Vila, X.; Colprim, J.; Balaguer, M.D. Long-Term Operation of a Partial Nitritation Pilot Plant Treating Leachate with Extremely High Ammonium Concentration Prior to an Anammox Process. Bioresour. Technol. 2009, 100, 5624–5632. [Google Scholar] [CrossRef] [PubMed]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; Van Loosdrecht, M.C.M. Full-Scale Partial Nitritation/Anammox Experiences—An Application Survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Leal, C.D.; Pereira, A.D.; Nunes, F.T.; Ferreira, L.O.; Coelho, A.C.C.; Bicalho, S.K.; Mac Conell, E.F.A.; Ribeiro, T.B.; De Lemos Chernicharo, C.A.; De Araújo, J.C. Anammox for Nitrogen Removal from Anaerobically Pre-Treated Municipal Wastewater: Effect of COD/N Ratios on Process Performance and Bacterial Community Structure. Bioresour. Technol. 2016, 211, 257–266. [Google Scholar] [CrossRef]
- Kartal, B.; Kuenen, J.G.; Van Loosdrecht, M.C.M. Sewage Treatment with Anammox. Science 2010, 328, 702–703. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M. Mainstream Deammonification. Water Intell. Online 2016, 15, 9781780407852. [Google Scholar] [CrossRef]
- Malaeb, L.; Ayoub, G.M. Reverse Osmosis Technology for Water Treatment: State of the Art Review. Desalination 2011, 267, 1–8. [Google Scholar] [CrossRef]
- Chan, Q.; Entezarian, M.; Zhou, J.; Osterloh, R.; Huang, Q.; Ellefson, M.; Mader, B.; Liu, Y.; Swierczek, M. Gold Nanoparticle Mixture Retention Test with Single Particle Detection: A Fast and Sensitive Probe for Functional Pore Sizes of Ultrafiltration Membranes. J. Membr. Sci. 2020, 599, 117822. [Google Scholar] [CrossRef]
- Teow, Y.H.; Sum, J.Y.; Ho, K.C.; Mohammad, A.W. 3—Principles of Nanofiltration Membrane Processes. In Osmosis Engineering; Hilal, N., Ismail, A.F., Khayet, M., Johnson, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–95. ISBN 978-0-12-821016-1. [Google Scholar]
- Luo, J.; Ding, L.; Qi, B.; Jaffrin, M.Y.; Wan, Y. A Two-Stage Ultrafiltration and Nanofiltration Process for Recycling Dairy Wastewater. Bioresour. Technol. 2011, 102, 7437–7442. [Google Scholar] [CrossRef]
- Choosing Between Ultrafiltration and Nanofiltration for Water Treatment|Hydramem 2024. Available online: https://hydramem.com/choosing-between-ultrafiltration-and-nanofiltration-for-water-treatment/ (accessed on 30 January 2025).
- Ong, Y.K.; Li, F.Y.; Sun, S.-P.; Zhao, B.-W.; Liang, C.-Z.; Chung, T.-S. Nanofiltration Hollow Fiber Membranes for Textile Wastewater Treatment: Lab-Scale and Pilot-Scale Studies. Chem. Eng. Sci. 2014, 114, 51–57. [Google Scholar] [CrossRef]
- Schiopu, A.-M.; Piuleac, G.C.; Cojocaru, C.; Apostol, I.; Mămăligă, I.; Gavrilescu, M. Reducing Environmental Risk of Landfills: Leachate Treatment by Reverse Osmosis. Environ. Eng. Manag. J. 2012, 11, 2319–2331. [Google Scholar] [CrossRef]
- Fatima, S.; Jehangir, A.; Bhat, G.A.; Scholar, M.P. Treatment of Landfill Leachate Using Reverse Osmosis and Its Potential for Reuse. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 15825–15832. [Google Scholar]
- Qiang, X.; Fei-Fei, Z.; Zhang, Y. Ultrafiltration and Nanofiltration for Advanced Treatment of Casing Wastewater. Food Mach. 2012, 1, 59–61. Available online: https://www.semanticscholar.org/paper/Ultrafiltration-and-nanofiltration-for-advanced-of-Zhang/c00520327c3885ab0fa0af4d17a50201b7f51988#citing-papers (accessed on 30 January 2025).
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pía, A.; Alcaina-Miranda, M.I.; Dhahbi, M. Reactive Dyes Rejection and Textile Effluent Treatment Study Using Ultrafiltration and Nanofiltration Processes. Desalination 2012, 297, 87–96. [Google Scholar] [CrossRef]
- Suksaroj, C.; Héran, M.; Allègre, C.; Persin, F. Treatment of Textile Plant Effluent by Nanofiltration and/or Reverse Osmosis for Water Reuse. Desalination 2005, 178, 333–341. [Google Scholar] [CrossRef]
- Singh, R. Analysis of Energy Usage at Membrane Water Treatment Plants. Desalin. Water Treat. 2011, 29, 63–72. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Koutsou, C.P.; Kostoglou, M.; Sioutopoulos, D.C. Analysis of Specific Energy Consumption in Reverse Osmosis Desalination Processes. Desalination 2018, 431, 15–21. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-85445-7. [Google Scholar]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A Review of Pressure-Driven Membrane Processes in Wastewater Treatment and Drinking Water Production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Tran, T.; Bolto, B.; Gray, S.; Hoang, M.; Ostarcevic, E. An Autopsy Study of a Fouled Reverse Osmosis Membrane Element Used in a Brackish Water Treatment Plant. Water Res. 2007, 41, 3915–3923. [Google Scholar] [CrossRef]
- Speth, T.F.; Gusses, A.M.; Scott Summers, R. Evaluation of Nanofiltration Pretreatments for Flux Loss Control. Desalination 2000, 130, 31–44. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Yun, T.I.; Coffey, B.M.; Suffet, I.H. “Mel” Effects of Aluminum Sulfate and Ferric Chloride Coagulant Residuals on Polyamide Membrane Performance. Desalination 2002, 150, 15–30. [Google Scholar] [CrossRef]
- Ivnitsky, H.; Katz, I.; Minz, D.; Shimoni, E.; Chen, Y.; Tarchitzky, J.; Semiat, R.; Dosoretz, C.G. Characterization of Membrane Biofouling in Nanofiltration Processes of Wastewater Treatment. Desalination 2005, 185, 255–268. [Google Scholar] [CrossRef]
- Abeynayaka, A.; Visvanathan, C. Performance Comparison of Mesophilic and Thermophilic Aerobic Sidestream Membrane Bioreactors Treating High Strength Wastewater. Bioresour. Technol. 2011, 102, 5345–5352. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Manenti, S.; Todeschini, S.; Bertanza, G.; Pedrazzani, R. Treatment of Aqueous Wastes by Means of Thermophilic Aerobic Membrane Reactor (TAMR) and Nanofiltration (NF): Process Auditing of a Full-Scale Plant. Environ. Monit. Assess. 2019, 191, 708. [Google Scholar] [CrossRef] [PubMed]
- Hai, F.I.; Yamamoto, K. Membrane Biological Reactors. In Treatise on Water Science; Elsevier: Amsterdam, The Netherlands, 2011; pp. 571–613. ISBN 978-0-444-53199-5. [Google Scholar]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of Total Ammonia Nitrogen (TAN), Nitrate and Total Organic Carbon (TOC) from Aquaculture Wastewater Using Electrochemical Technology: A Review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Dortsiou, M.; Kyriacou, G. Electrochemical Reduction of Nitrate on Bismuth Cathodes. J. Electroanal. Chem. 2009, 630, 69–74. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Zhang, Z.; Lei, X.; Chen, R.; Yang, Y.; Sugiura, N. Simultaneous Reduction of Nitrate and Oxidation of By-Products Using Electrochemical Method. J. Hazard. Mater. 2009, 171, 724–730. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Reduction of Nitrate on Copper Single Crystals in Acidic and Alkaline Solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Jiang, X.; Ying, D.; Ye, D.; Zhang, R.; Guo, Q.; Wang, Y.; Jia, J. Electrochemical Study of Enhanced Nitrate Removal in Wastewater Treatment Using Biofilm Electrode. Bioresour. Technol. 2018, 252, 134–142. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Yin, L.; Ji, Y.; Niu, J.; Yu, Y. Electrochemical Removal of Nitrate in Industrial Wastewater. Front. Environ. Sci. Eng. 2018, 12, 9. [Google Scholar] [CrossRef]
- Georgeaud, V.; Diamand, A.; Borrut, D.; Grange, D.; Coste, M. Electrochemical Treatment of Wastewater Polluted by Nitrate: Selective Reduction to N2 on Boron-Doped Diamond Cathode. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2011, 63, 206–212. [Google Scholar] [CrossRef]
- Bunce, N.J.; Bejan, D. Mechanism of Electrochemical Oxidation of Ammonia. Electrochim. Acta 2011, 56, 8085–8093. [Google Scholar] [CrossRef]
- Oswin, H.G.; Salomon, M. The anodic oxidation of ammonia at platinum black electrodes in aqueous koh electrolyte. Can. J. Chem. 1963, 41, 1686–1694. [Google Scholar] [CrossRef]
- Gerischer, H.; Mauerer, A. Untersuchungen Zur Anodischen Oxidation von Ammoniak an Platin-Elektroden. J. Electroanal. Chem. Interfacial Electrochem. 1970, 25, 421–433. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Wang, J.; Chen, J. Process Optimization of Electrochemical Oxidation of Ammonia to Nitrogen for Actual Dyeing Wastewater Treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ni, J.; Lai, P. Advanced Treatment of Biologically Pretreated Coking Wastewater by Electrochemical Oxidation Using Boron-Doped Diamond Electrodes. Water Res. 2009, 43, 4347–4355. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Gong, B.; Feng, C. Electrochemical Oxidation of Ammonia-Containing Wastewater Using Ti/RuO2-Pt Electrode. Water Sci. Eng. 2009, 2, 103–109. [Google Scholar] [CrossRef]
- Li, Y.F.; Duan, Y.H. Treatment of Wastewater Containing High Concentration Ammonia-Nitrogen by Electrochemical Oxidation Process. Adv. Mater. Res. 2012, 393–395, 1587–1590. [Google Scholar] [CrossRef]
- Muster, T.H.; Jermakka, J. Electrochemically-Assisted Ammonia Recovery from Wastewater Using a Floating Electrode. Water Sci. Technol. 2017, 75, 1804–1811. [Google Scholar] [CrossRef]
- Zangeneh, A.; Sabzalipour, S.; Takdatsan, A.; Yengejeh, R.J.; Khafaie, M.A. Ammonia Removal Form Municipal Wastewater by Air Stripping Process: An Experimental Study. S. Afr. J. Chem. Eng. 2021, 36, 134–141. [Google Scholar] [CrossRef]
- Löschau, M. Effects of Combustion Temperature on Air Emissions and Support Fuel Consumption in Full Scale Fluidized Bed Sludge Incineration: With Particular Focus on Nitrogen Oxides and Total Organic Carbon. Waste Manag. Res. 2018, 36, 342–350. [Google Scholar] [CrossRef]
- Nuagah, M.B.; Boakye, P.; Oduro-Kwarteng, S.; Sokama-Neuyam, Y.A. Valorization of Faecal and Sewage Sludge via Pyrolysis for Application as Crop Organic Fertilizer. J. Anal. Appl. Pyrolysis 2020, 151, 104903. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, Y.; Zhao, H.; Peng, H. Nitrogen Removal during Anaerobic Digestion of Wasted Activated Sludge under Supplementing Fe(III) Compounds. Chem. Eng. J. 2018, 332, 711–716. [Google Scholar] [CrossRef]


| Nitrogen Species | Typical Concentration Range (mg/L) | Remarks |
|---|---|---|
| Total Nitrogen (TN) | 20–85 | Sum of all nitrogen species, including organic and inorganic forms. |
| Organic Nitrogen | 8–35 | Includes proteins, amino acids, urea, and humic substances. |
| Ammonium (NH4+) | 12–50 | Predominant form of nitrogen in wastewater due to urea hydrolysis. |
| Nitrite (NO2−) | <0.1–0.5 | Intermediate oxidation product, usually present in very low concentrations. |
| Nitrate (NO3−) | <0.1–1.0 | Minimal in raw wastewater, but increases after nitrification in treatment processes. |
| Dissolved Organic Nitrogen (DON) | 1–10 | Comprises low-molecular-weight organic nitrogen compounds. |
| Factor | Influence on Nitrification | Influence on Denitrification |
|---|---|---|
| Dissolved Oxygen (DO) | Requires aerobic conditions (high DO). Inhibited by low DO. | Requires anoxic/anaerobic conditions (low or no DO). Inhibited by high DO. |
| pH | Optimal range: 7.0–8.5. Inhibited by very low or high pH. | Optimal range: 6.5–8.0. Can occur at a wider range but is generally slowed outside this range. |
| Temperature | Increases with temperature up to an optimal range (25–35 °C). Decreases at very low or high temperatures. | Increases with temperature up to an optimal range (30–35 °C.) Decreases at very low or high temperatures. |
| Carbon Source | Requires an inorganic carbon source (CO2, bicarbonates). | Requires an organic carbon source (e.g., BOD, COD). |
| Ammonium (NH4+) Concentration | Substrate availability. High concentrations may be inhibitory. | Generally, not directly relevant, but nitrate availability is crucial. |
| Nitrate (NO3−) Concentration | Product of nitrification. | Substrate availability. High concentrations may be inhibitory. |
| Inhibitory Substances | Heavy metals, sulfides, certain organic compounds. | Heavy metals, sulfides, certain organic compounds, high nitrite concentrations. |
| Hydraulic Retention Time (HRT) | Sufficient HRT is necessary for bacterial growth and reaction completion. | Sufficient HRT is necessary for bacterial growth and reaction completion. |
| Alkalinity | Consumed during nitrification. | Produced during denitrification. |
| Wastewater Type | Ammonia Concentr. [mg/L] | pH Adjuster | Reactor Type | Operational Conditions | NH3 Capture | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| pH | Q [m3/L·h] | T [°C] | |||||||
| Industrial WW 1 | ~2000 | Lime | Packed tower | 11.0 | 420 | 25 | 99% | NR 2 | [139] |
| Acetylene Purification WW | 125 | NaOH | Stripping column | 12.0 | 0.5 | 60 | 91% | H2SO4 0.2 mol/L | [140] |
| Landfill Leachate | 1158 | Lime, NaOH, | Raschig ring-packed column | 12.0 | 0.9 | 25 | 98% | H3PO4 0.24 mol/L | [141] |
| 2738 | NaOH, Ca(OH)2 | Packed tower | 8.0–11 | 0.18–0.63 | 25–30 | 91–95.5% | H2SO4 | [142] | |
| Swine WW | ~2000 | Lime | Stripping tower | 11.2 | 0.065 | 20 | 90% | NR | [143] |
| 4950 | NaOH | Laboratory reactor | 10.0 | 0.4 | 37 | 88.2% | 50% H2SO4 | [144] | |
| Mixed Livestock WW | 2.970 | NaOH | Pilot stripping reactor | 9.0–10.7 | NR | 30–40 | 82% | H2SO4 | [145] |
| Anaerobic Digestate | 7170 | NaOH, Ca(OH)2 | Stripping column | 10.0 | 0.2–0.5 | 40–70 | 91–96% | H2SO4 | [138] |
| 297.6 | MgO | Stripping column | 10.0 | 0.48 | 40 | 94.34% | H3PO4 | [146] | |
| Human Urine | 1963.5 | NaOH | Laboratory reactor | 11–13.5 | 0.09–0.27 | 16 | 98.7% | H2SO4 0.5 mol/L | [147] |
| ~4000 | NaOH | Stripping column | 9.3–11 | 0.12–0.84 | 25–60 | 97% | H2SO4 1 mol/L | [148] | |
| Wastewater Type | Ammonia Concentr. [mg/L] | Reactor Design | Operational Conditions | Ref. | |||
|---|---|---|---|---|---|---|---|
| G/L | T [°C] | Other | |||||
| Metallurgical Industry Wastewater | ~5200 | Small-scale pilot countercurrent steam stripping reactor | ~2.55 lb/gal | ~101 | pH: 11.5 | up to 99.9% | [153] |
| Process Water with Nitrobenzene contamination | Not specified | Oldershaw glass column, 10–20 sieve trays, φ = 1 inch | ~0.065 kg/kg | ~100 | NR 1 | up to 99.8% | [151] |
| Shale Oil Wastewater | 1065–24,689 | Packed bed column with countercurrent flow | ~1.0 kg/kg | ~93.3 | NR | 66.5–99% | [154] |
| Cattle Manure Digestate | 910–45,000 | Ceramic-filled steam stripping tower | ~63 kg/m3 ~11,000 mL/min | ~98.5 | pH: ~9.6 | 91–96% | [155] |
| Separately Collected Human Urine | 4200–9200 | Pall ring-packed tower (surface area: 360 m2/m3) | ~25 kg/h | ~100 | Urine flow: ~90 L/h; pH: ~9.25 | 83–96.8% | [156] |
| Digestate and Centrate from Anaerobic Digestion | 950–4700 | Column with perforated trays, 11 levels, vacuum system | ~4.5 | ~81 | Vacuum: ~0.75 m H2O | 90–98% | [157] |
| Ammonia-Rich Wastewater | 5000–20,000 | Rotating packed bed (RPB); compact | ~0.18 kg/kg | ~135 (steam), ~55 (liquid) | RPM: ~750; pH > 11 | 95–98.8% | [150] |
| Parameter | Thermophilic System with Membranes | Conventional Mesophilic BNR |
|---|---|---|
| Operating Temperature | 50–65 °C | 20–37 °C |
| Microbial Activity | Dominated by thermophilic bacteria | Dominated by mesophilic bacteria |
| Ammonia Volatilization | Enhanced at high temperatures | Minimal |
| Nitrification Efficiency | Partial or limited due to thermal inhibition | High under optimal conditions |
| Sludge Production | Reduced due to enhanced biodegradation | Higher sludge yield |
| Membrane Integration Benefits | Biomass retention, nitrogen concentration | Less commonly used |
| Energy Demand | Higher due to heating | Lower but requires aeration |
| Pathogen Removal | High due to thermophilic conditions | Moderate |
| Wastewater Type | Reactor Design | Process Conditions | Additives | Ref. | |
|---|---|---|---|---|---|
| Wastewater containing nitrates; 0.05 M NaNO3 | Teflon chamber divided by Nafion 117 cation-exchange membrane; Bi cathode (2 cm2), Pt anode (6 cm2) | Potential: from −1.8 to −2.6 V vs. Ag/AgCl; constant helium flow (12 mL/min) | 0.4 M NaHCO3; 0.4 M Na2CO3 | Selectivity to N2: 58–65%; total Faradaic efficiency: 18–79% | [200] |
| Synthetic nitrate solution; 100 mg/L NO3− | Undivided cylindrical acrylic reactor, 400 mL volume; Cu–Zn cathode (62.2% Cu, 37.8% Zn), Ti/IrO2–Pt anode | Current: 40 mA/cm2; initial pH: 3.0, 6.5 or 12.0; duration: 300 min | 0.50 g/L NaCl; 0.5 g/L Na2SO4, | Removal of NO3−–N from 100 mg/L to 9.7 mg/L, no NH3 or NO2− detected | [201] |
| Synthetic nitrate solution; 2–10 mM NaNO3 | Rotating disk electrode with single-crystal copper; Cu (100) and Cu (111) single-crystal electrodes | Current: dependent on potential (+0.15 to −0.5 V vs. RHE), 50 mV/s scanning | 0.1 M HClO4; 0.1 M NaOH | Cu (100): higher activity for reduction to NH2OH in alkaline pH | [202] |
| Industrial wastewater; 200 mg/L NO3− | Reactor divided by Nafion 117 membrane; cathode: carbon with biofilm, anode: graphite | Voltage: −0.6 V; temperature: 30 °C; duration: 5 h | Na2CO3 buffer | 96% nitrate removal, minimal NH3 production | [203] |
| Industrial wastewater with high nitrate concentration; up to 85,000 mg/L NO3− | Various technologies: ER, EC, ED; electrodes: Cu, Sn, Fe, Al, BDD | Voltages: from −1.1 V to 15 V; pH: from 3 to 12 | NaCl; SO42−; NaOH | Efficiency up to 100% depending on technology | [204] |
| Agro-industrial and synthetic wastewater; 27 g/L NO3− | Two-chamber reactor with cation-exchange membrane; BDD cathodes and anodes | Current: 300–750 A/m2; temperature: 20–34 °C; low pH | pH = 1.5 | Nitrate reduced to <50 mg/L, no NH3 or NO2− | [205] |
| Wastewater Type | Reactor Design | Process Conditions | Additives | Ref. | |
|---|---|---|---|---|---|
| Dyeing wastewater; 161.2 ± 2.0 mg-N/L | Electrochemical cell, 0.4 L; anode: Ti/PbO2, cathode: Ti, electrode gap 20 mm | Current density: 20 mA/cm2; flow: 300 mL/min; pH: 8.3; temperature: 30 °C | 1.0 g/L NaCl | 100% in 60 min; N2 selectivity: 88.3% | [209] |
| Coking wastewater; NH3-N: 10 mg/L; TOC: 78 mg/L | Batch cell, 250 mL, controlled temperature; anode: Ti/BDD; cathode: stainless steel, electrode gap 10 mm | Current density: 20–60 mA/cm2; temperature: 30–60 °C | 0.2 M Na2SO4 | Complete removal under neutral and alkaline conditions | [210] |
| Simulated wastewater; NH3-N: 2000 mg/L | Non-diaphragm electrolysis cell, 0.5 L; anode and cathode: graphite, electrode gap 40 mm | Current density: 90 mA/cm2; pH 10; time: 8 h | 8000 mg/L NaCl | Reduction from 2000 to 280 mg/L (86%) | [212] |
| Synthetic and anaerobic digester effluent; NH3-N: 900 mg/L (synthetic); 560 mg/L (effluent) | Electrochemical cell, 570 mL, airflow 20 L/min; anode: Ti/RuO2-IrO2, cathode: porous titanium, floating cathode configuration | Current density: 5 mA/cm2; pH: 6.77; time: 8 h | 0.2 g/L NaCl | Up to 216 mg NH3-N/L/h (synthetic); 110 mg NH3-N/L/h (anaerobic effluent) | [213] |
| Municipal and synthetic wastewater; NH3-N: 40 mg/L (synthetic); 60 mg/L (municipal) | Acrylic cell, 300 mL; anode: Ti/RuO2-Pt; cathode: Ti, electrode gap 1 cm | Current density: 20 mA/cm2; time: 30 min | 0.5 g/L NaCl | 88.3% (municipal); 87.5% (simulated) | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasiński, S.; Kowal, P.; Czerwionka, K. Advanced Technologies for Nitrogen Removal and Recovery from Municipal and Industrial Wastewater. Materials 2025, 18, 1422. https://doi.org/10.3390/ma18071422
Kasiński S, Kowal P, Czerwionka K. Advanced Technologies for Nitrogen Removal and Recovery from Municipal and Industrial Wastewater. Materials. 2025; 18(7):1422. https://doi.org/10.3390/ma18071422
Chicago/Turabian StyleKasiński, Sławomir, Przemysław Kowal, and Krzysztof Czerwionka. 2025. "Advanced Technologies for Nitrogen Removal and Recovery from Municipal and Industrial Wastewater" Materials 18, no. 7: 1422. https://doi.org/10.3390/ma18071422
APA StyleKasiński, S., Kowal, P., & Czerwionka, K. (2025). Advanced Technologies for Nitrogen Removal and Recovery from Municipal and Industrial Wastewater. Materials, 18(7), 1422. https://doi.org/10.3390/ma18071422








