Superplastic Deformation Behavior and Microstructural Evolution of Electroformed Nickel Foils Determined by Thermomechanical Analysis
Abstract
1. Introduction
2. Experimental
2.1. Electroforming of Nanocrystalline Ni Foils
2.2. Thermal Behavior and Superplasticity Analysis
3. Results and Discussion
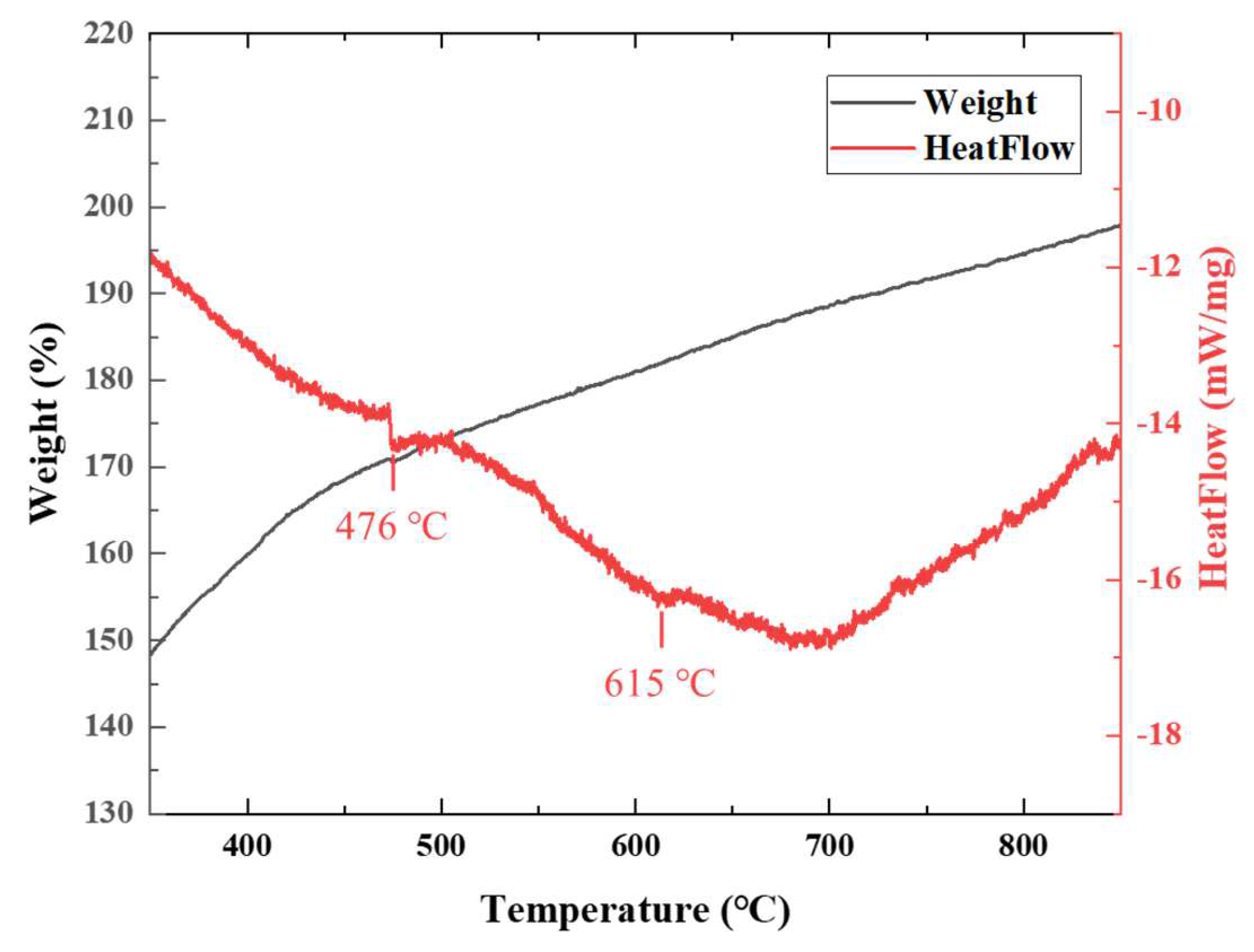
3.1. Thermal Behavior Analysis
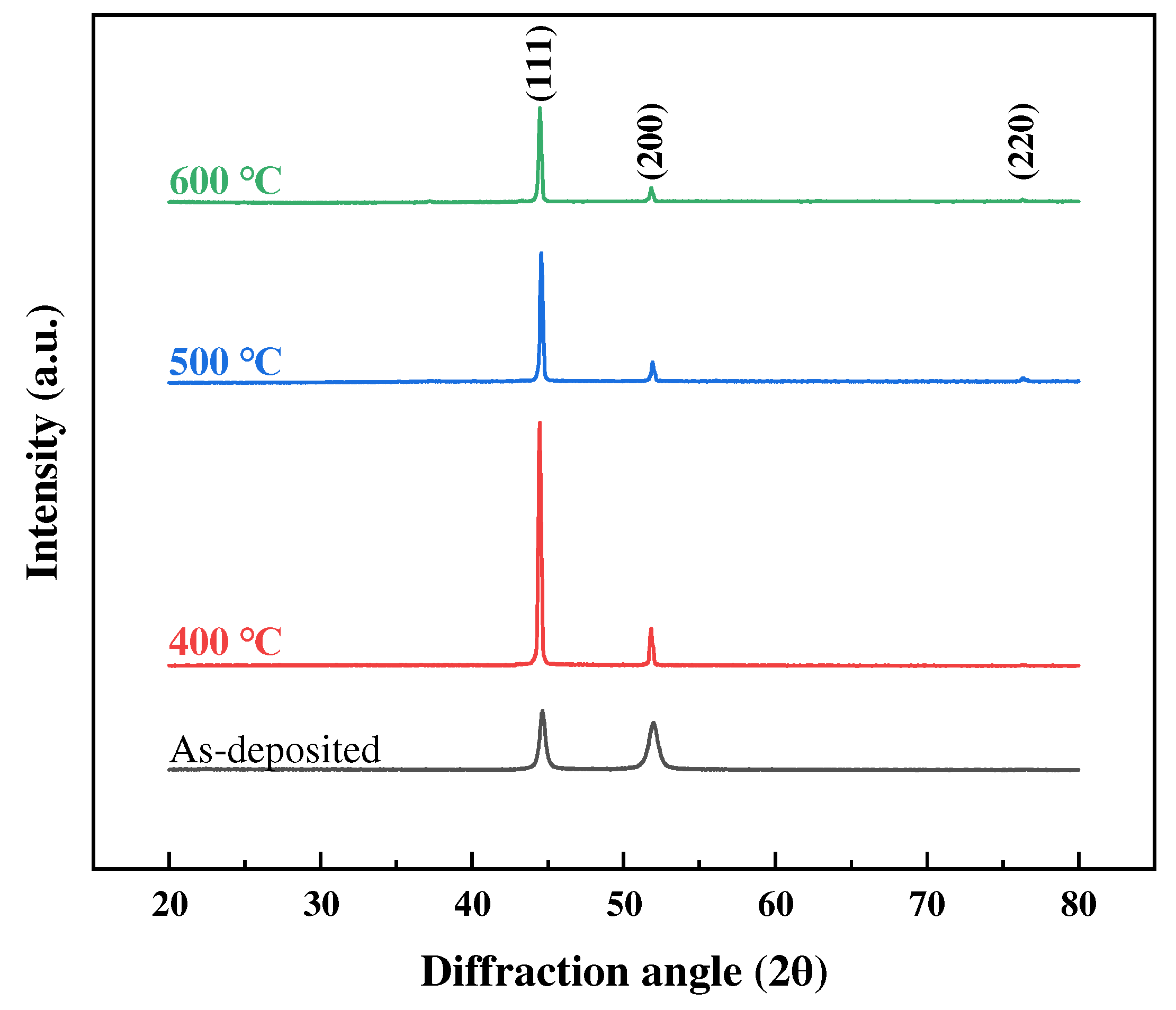
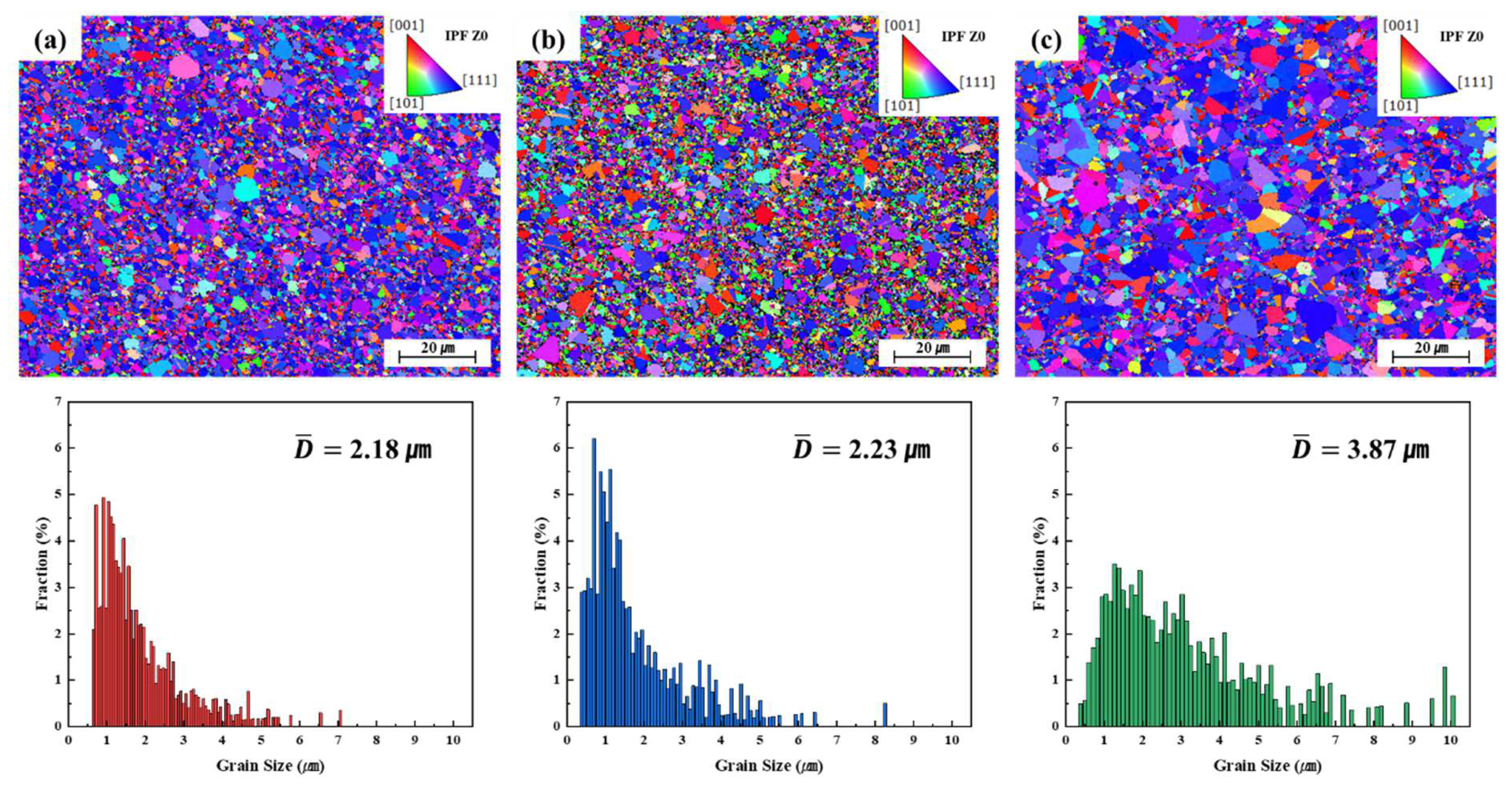
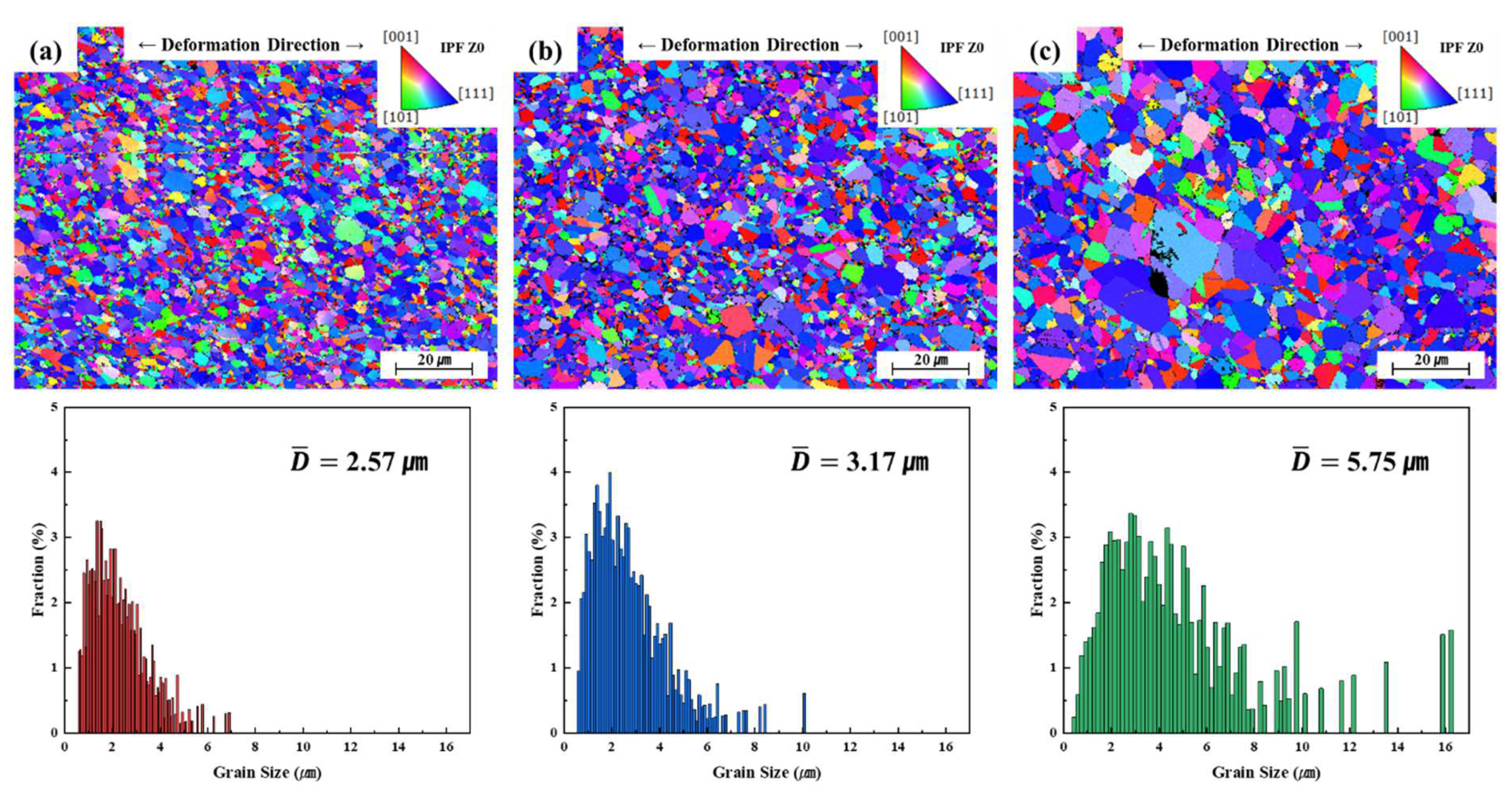
3.2. Grain Growth and Microstructural Evolution
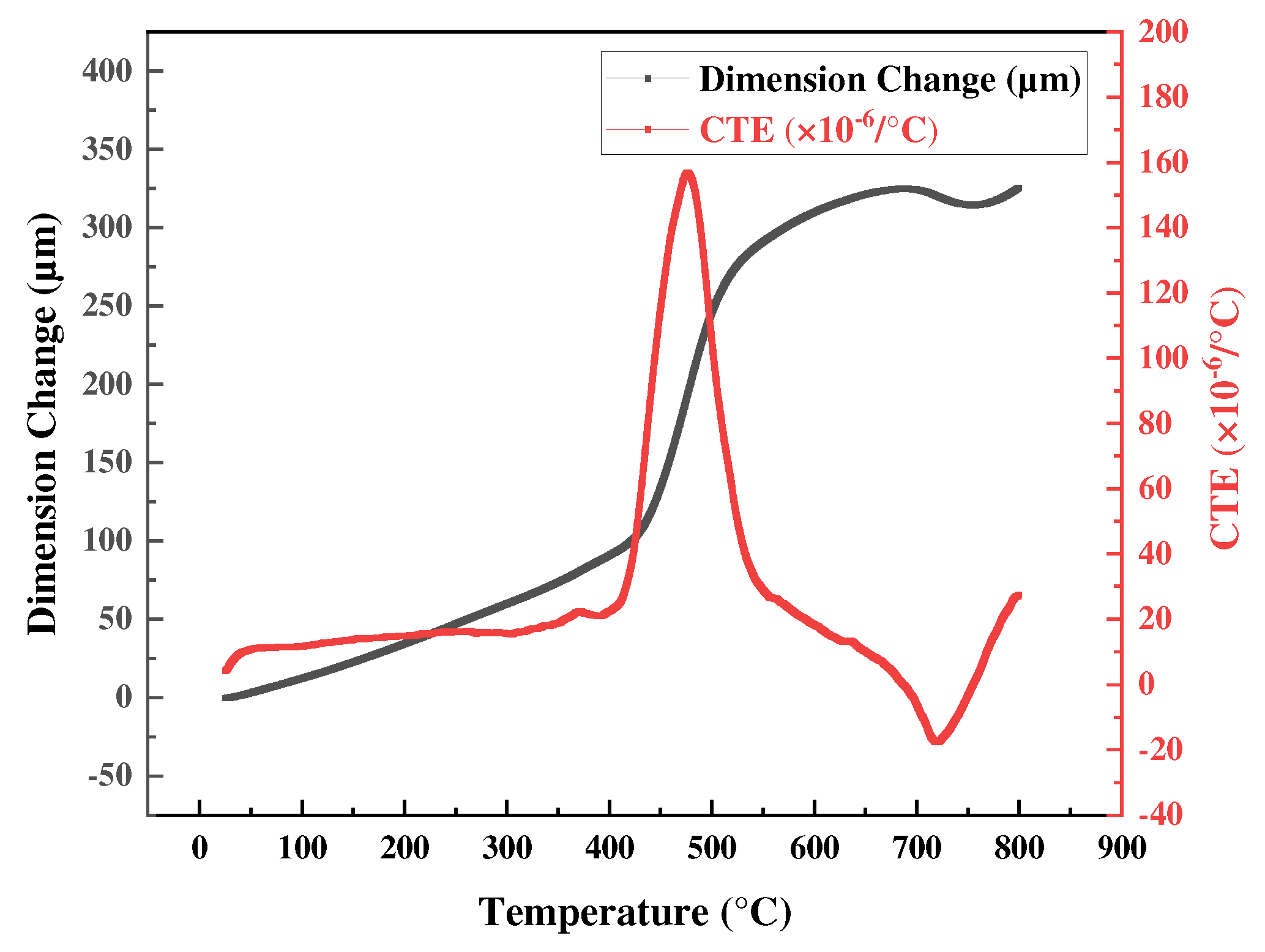
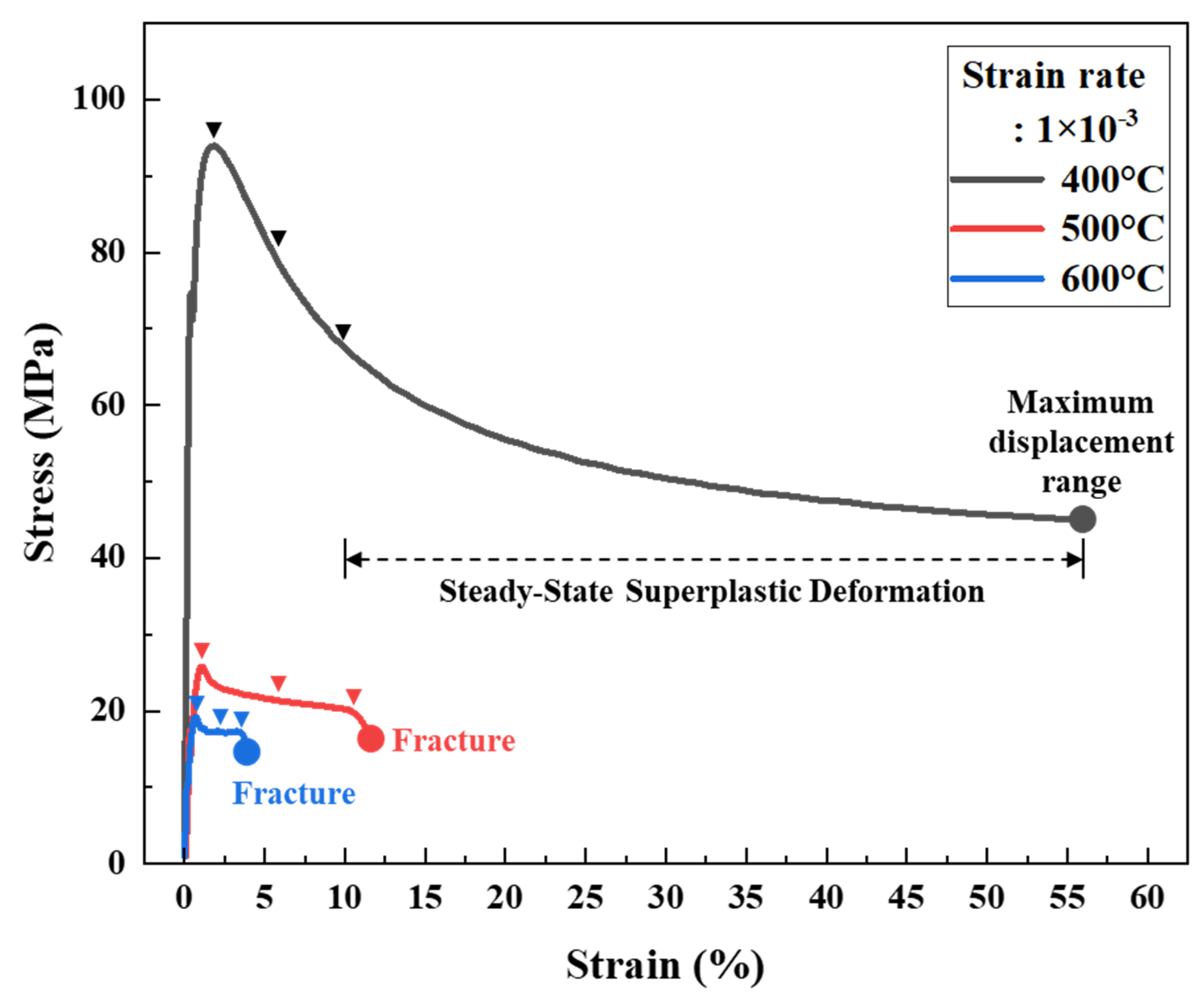
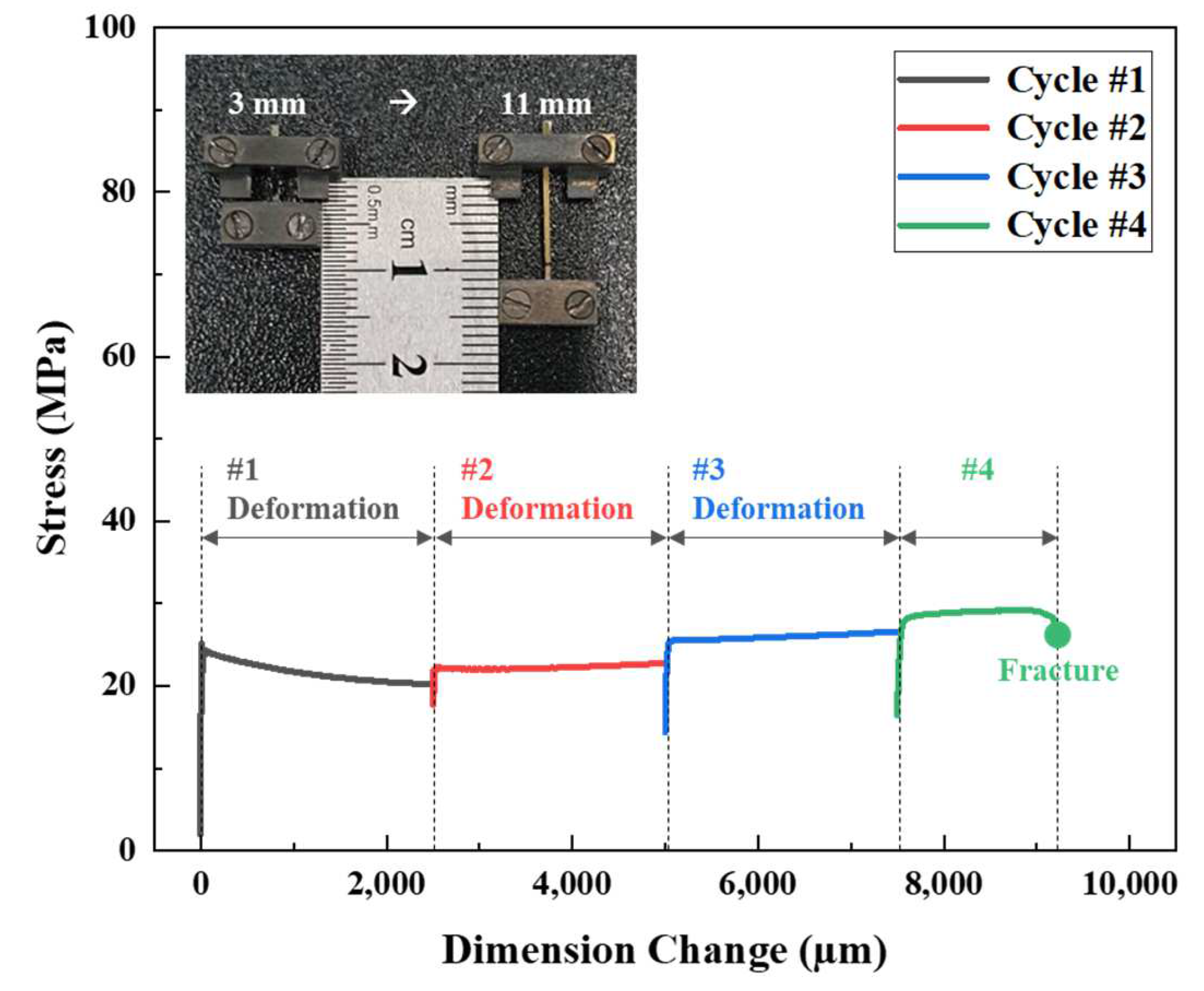
3.3. Superplasticity Analysis Using TMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sherby, O.D. Advances in superplasticity and in superplastic materials. ISIJ Int. 1989, 29, 698–716. [Google Scholar] [CrossRef]
- Padmanabhan, K.A.; Prabu, S.B.; Mulyukov, R.R.; Nazarov, A.; Imayev, R.M.; Chowdhury, S.G. Superplasticity: Common Basis for a Near-Ubiquitous Phenomenon; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Motallebi, R.; Savaedi, Z.; Mirzadeh, H. Superplasticity of high-entropy alloys: A review. Arch. Civ. Mech. Eng. 2022, 22, 20. [Google Scholar] [CrossRef]
- Kawasaki, M.; Langdon, T.G. The contribution of severe plastic deformation to research on superplasticity. Mater. Trans. 2019, 60, 1123–1130. [Google Scholar] [CrossRef]
- Masuda, H.; Sato, E. Diffusional and dislocation accommodation mechanisms in superplastic materials. Acta Mater. 2020, 197, 235–252. [Google Scholar] [CrossRef]
- Giuliano, G. (Ed.) Superplastic Forming of Advanced Metallic Materials: Methods and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Nguyen, N.T.C.; Asghari-Rad, P.; Sathiyamoorthi, P.; Zargaran, A.; Lee, C.S.; Kim, H.S. Ultrahigh high-strain-rate superplasticity in a nanostructured high-entropy alloy. Nat. Commun. 2020, 11, 2736. [Google Scholar] [CrossRef]
- Sorgente, D. Superplasticity and superplastic forming. Metals 2021, 11, 946. [Google Scholar] [CrossRef]
- Barnes, A.J. Superplastic forming 40 years and still growing. J. Mater. Eng. Perform. 2007, 16, 440–454. [Google Scholar] [CrossRef]
- Han, J.; Kang, S.H.; Lee, S.J.; Kawasaki, M.; Lee, H.J.; Ponge, D.; Lee, Y.K. Superplasticity in a lean Fe-Mn-Al steel. Nat. Commun. 2017, 8, 751. [Google Scholar] [CrossRef]
- Bhatta, L.; Pesin, A.; Zhilyaev, A.P.; Tandon, P.; Kong, C.; Yu, H. Recent development of superplasticity in aluminum alloys: A review. Metals 2020, 10, 77. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Kaibyshev, O.A.; Astanin, V.V.; Emaletdinov, A.K. The nature of grain boundary sliding and the superplastic flow. Phys. Status Solidi (A) 1983, 78, 439–448. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.V.; Yakovtseva, O.A.; Irzhak, A.V. The role of grain boundary sliding and intragranular deformation mechanisms for a steady stage of superplastic flow for Al–Mg-based alloys. Mater. Sci. Eng. A 2022, 833, 142524. [Google Scholar] [CrossRef]
- Kaneyama, G.; Koto, H.; Takigawa, Y. Effect of Boron and Tungsten Addition on Superplasticity and Grain Growth in Electrodeposited Nickel Alloys. Mater. Trans. 2022, 63, 1001–1005. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Liu, F.; Nie, Y.; Jiang, L. Superplastic behavior of a powder metallurgy superalloy during isothermal compression. J. Mater. Sci. Technol. 2019, 35, 2591–2599. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Liu, C.; Ruan, J.; Huang, H.; Zhou, X.; Jiang, L. Hot deformation behavior, superplasticity and microstructure evolution of a new hot isostatic pressed nickel-based superalloy. Mater. Sci. Eng. A 2024, 891, 145997. [Google Scholar] [CrossRef]
- Al-Hammadi, R.A.; Zhang, R.; Cui, C.; Zhou, Z.; Zhou, Y. Effects of temperature on superplastic and fracture behaviors of a Ni-Co-based superalloy. J. Alloys Compd. 2023, 958, 170524. [Google Scholar] [CrossRef]
- Wu, L.H.; Zhang, H.; Zeng, X.H.; Xue, P.; Xiao, B.L.; Ma, Z.Y. Achieving superior low temperature and high strain rate superplasticity in submerged friction stir welded Ti-6Al-4V alloy. Sci. China Mater. 2018, 61, 417–423. [Google Scholar] [CrossRef]
- Wang, H.; Koenigsmann, K.; Zhang, S.; Li, Y.; Liu, H.; Liu, H.; Yang, K. Extraordinary superplasticity at low homologous temperature and high strain rate enabled by a multiphase nanocrystalline network. Int. J. Plast. 2023, 168, 103694. [Google Scholar] [CrossRef]
- Liang, J.W.; Shen, Y.F.; Misra, R.D.K.; Liaw, P.K. High strength-superplasticity combination of ultrafine-grained ferritic steel: The significant role of nanoscale carbides. J. Mater. Sci. Technol. 2021, 83, 131–144. [Google Scholar] [CrossRef]
- Demirtas, M.; Purcek, G. Room temperature superplaticity in fine/ultrafine grained materials subjected to severe plastic deformation. Mater. Trans. 2019, 60, 1159–1167. [Google Scholar] [CrossRef]
- Li, H.Q.; Ebrahimi, F. An investigation of thermal stability and microhardness of electrodeposited nanocrystalline nickel-21% iron alloys. Acta Mater. 2003, 51, 3905–3913. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kashikura, Y. Grain growth and mechanical properties of electrodeposited nanocrystalline nickel–4.4 mass% phosphorus alloy. Mater. Sci. Eng. A 2003, 358, 76–83. [Google Scholar] [CrossRef]
- Wang, G.F.; Chan, K.C.; Zhang, K.F. Low temperature superplasticity of nanocrystalline electrodeposited Ni–Co alloy. Scr. Mater. 2006, 54, 765–770. [Google Scholar] [CrossRef]
- Lee, M.; Han, Y.; Yim, T.H. The Effect of Heat Treatment on the Thermal Expansion Behavior of Electroformed Nano-crystalline Fe-42 wt% Ni Alloy. J. Korean Inst. Surf. Eng. 2014, 47, 293–296. [Google Scholar] [CrossRef]
- McFadden, S.X.; Zhilyaev, A.P.; Mishra, R.S.; Mukherjee, A.K. Observations of low-temperature superplasticity in electrodeposited ultrafine grained nickel. Mater. Lett. 2000, 45, 345–349. [Google Scholar] [CrossRef]
- Dalla Torre, F.; Spätig, P.; Schäublin, R.; Victoria, M. Deformation behaviour and microstructure of nanocrystalline electrodeposited and high pressure torsioned nickel. Acta Mater. 2005, 53, 2337–2349. [Google Scholar] [CrossRef]
- Prasad, M.J.N.V.; Chokshi, A.H. Extraordinary high strain rate superplasticity in electrodeposited nano-nickel and alloys. Scr. Mater. 2010, 63, 136–139. [Google Scholar] [CrossRef]
- Prasad, M.J.N.V.; Chokshi, A.H. Microstructural stability and superplasticity in an electrodeposited nanocrystalline Ni–P alloy. Acta Mater. 2011, 59, 4055–4067. [Google Scholar] [CrossRef]
- Schüler, K.; Philippi, B.; Weinmann, M.; Marx, V.M.; Vehoff, H. Effects of processing on texture, internal stresses and mechanical properties during the pulsed electrodeposition of nanocrystalline and ultrafine-grained nickel. Acta Mater. 2013, 61, 3945–3955. [Google Scholar] [CrossRef]
- Demir, M.; Kanca, E.; Karahan, I.H. Effect of saccharin addition on formation, wear and corrosion resistance of electrodeposited Ni-Cr coatings. Mater. Sci.-Pol. 2023, 41, 111–125. [Google Scholar] [CrossRef]
- McFadden, S.X.; Mukherjee, A.K. Sulfur and superplasticity in electrodeposited ultrafine-grained Ni. Mater. Sci. Eng. A 2005, 395, 265–268. [Google Scholar] [CrossRef]
- Prasad, M.J.N.V.; Chokshi, A.H. Superplasticity in electrodeposited nanocrystalline nickel. Acta Mater. 2010, 58, 5724–5736. [Google Scholar] [CrossRef]
- Soni, S.K.; Sheldon, B.W.; Hearne, S.J. Origins of saccharin-induced stress reduction based on observed fracture behavior of electrodeposited Ni films. J. Mater. Sci. 2014, 49, 1399–1407. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Huang, X. Effect of Saccharin on the process and properties of nickel electrodeposition from sulfate electrolyte. Int. J. Metall. Mater. Eng 2016, 2, 123. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, B.; Dolai, S.; Seshu, K.S.; Prakash, A.; Gollapudi, S. Combined effect of sodium lauryl sulphate and saccharin on microstructure and corrosion performance of electrodeposited nickel prepared from modified watts bath. Metall. Mater. Trans. A 2021, 52, 1913–1926. [Google Scholar] [CrossRef]
- Maizza, G.; Eom, H.; Lee, M.; Yim, T.H.; Nakagawa, E.; Pero, R.; Ohmura, T. Mechanical and fracture behaviour of the three-scale hierarchy structure in as-deposited and annealed nanocrystalline electrodeposited Ni–Fe alloys. J. Mater. Sci. 2019, 54, 13378–13393. [Google Scholar] [CrossRef]
- Maizza, G.; Pero, R.; Kaciulis, S.; Bolli, E.; Eom, H.; Lee, M.; Yim, T.H. Correlation between the bath composition and nanoporosity of DC-electrodeposited Ni-Fe alloy. Surf. Interface Anal. 2020, 52, 907–913. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, Z.; Zhang, H.; Lian, J. Enhanced tensile ductility in an electrodeposited nanocrystalline copper. J. Mater. Res. 2008, 23, 2238–2244. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, K. Superplastic properties of Al2O3/Ni-Mn nanocomposite fabricated by electrodeposition. J. Mater. Sci. Technol. 2010, 26, 625–628. [Google Scholar] [CrossRef]
- Farrell, M.; Drabek, M.; Qarni, M.J. A method for the systematic assessment of lubricant performance during superplastic sheet forming: Verfahren zur systematischen Bewertung der Schmierstoffleistung bei der superplastischen Blechumformung. Mater. Werkst. 2017, 48, 976–982. [Google Scholar] [CrossRef]
- Nagayama, T.; Yamamoto, T.; Nakamura, T.; Mizutani, Y. Fabrication of low CTE metal masks by the Invar Fe-Ni alloy electroforming process for large and fine pitch OLED displays. ECS Trans. 2013, 50, 117. [Google Scholar] [CrossRef]
- Kang, N.Y.; Lee, J.H. Effects of bath composition and current density on the electrodeposition behavior of fe–ni invar alloy. Electron. Mater. Lett. 2023, 19, 503–509. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, N.; Gilchrist, M.; Fang, F. Advances in precision micro/nano-electroforming: A state-of-the-art review. J. Micromech. Microeng. 2020, 30, 103002. [Google Scholar] [CrossRef]
- McFadden, S.X. Superplasticity of Nanocrystalline Nickel Aluminide Alloy and Nanocrystalline Nickel. Ph.D. Thesis, University of California, Davis, CA, USA, 2001. [Google Scholar]
- Chan, K.C.; Wang, G.F.; Wang, C.L.; Zhang, K.F. Low temperature superplastic gas pressure forming of electrodeposited Ni/SiCp nanocomposites. Mater. Sci. Eng. A 2005, 404, 108–116. [Google Scholar] [CrossRef]
- Chan, K.C.; Wang, C.L.; Wang, G.F.; Zhang, K.F. Superplastic deformation behavior of electrodeposited nickel matrix nanocomposite. In Materials Science Forum; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2007; Volume 551, pp. 521–526. [Google Scholar]
- Dalla Torre, F.; Van Swygenhoven, H.; Victoria, M. Nanocrystalline electrodeposited Ni: Microstructure and tensile properties. Acta Mater. 2002, 50, 3957–3970. [Google Scholar] [CrossRef]
- Kolonits, T.; Jenei, P.; Tóth, B.G.; Czigány, Z.; Gubicza, J.; Péter, L.; Bakonyi, I. Characterization of defect structure in electrodeposited nanocrystalline Ni films. J. Electrochem. Soc. 2015, 163, D107. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of NiP alloy coatings: A review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Rashidi, A.M.; Amadeh, A. Effect of electroplating parameters on microstructure of nanocrystalline nickel coatings. J. Mater. Sci. Technol. 2010, 26, 82–86. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A Found. Adv. 2016, 72, 385–390. [Google Scholar] [CrossRef]
- Miranda, M.A.R.; Sasaki, J.M. The limit of application of the Scherrer equation. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, 54–65. [Google Scholar] [CrossRef]
- Ni, H.; Zhu, J.; Wang, Z.; Lv, H.; Su, Y.; Zhang, X. A brief overview on grain growth of bulk electrodeposited nanocrystalline nickel and nickel-iron alloys. Rev. Adv. Mater. Sci. 2019, 58, 98–106. [Google Scholar] [CrossRef]
- Julie, S.; Wasekar, N.P.; Parida, P.K.; Santra, S.; David, C.; Kamruddin, M. Effect of grain size on the thermal stability of electrodeposited nanocrystalline nickel: X-ray diffraction studies. Thin Solid Film. 2022, 745, 139114. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.F.; Hong, Y.; Liu, Y.N.; Hao, S.J.; Cui, L.S. Corrosion behavior of electrodeposited Ni with normal and bimodal grain size distribution. Trans. Nonferrous Met. Soc. China 2019, 29, 424–436. [Google Scholar]
- Darnbrough, J.E.; Christien, F.; Flewitt, P.E.J. Kinetics and dynamics of planar abnormal grain growth in nanocrystalline nickel. Acta Mater. 2017, 141, 67–74. [Google Scholar] [CrossRef]
- Jeng, Y.R.; Tsai, P.C.; Chiang, S.H. Effects of grain size and orientation on mechanical and tribological characterizations of nanocrystalline nickel films. Wear 2013, 303, 262–268. [Google Scholar] [CrossRef]
- Langdon, T.G. The physics of superplastic deformation. Mater. Sci. Eng. A 1991, 137, 1–11. [Google Scholar] [CrossRef]
- Horita, Z.; Furukawa, M.; Nemoto, M.; Barnes, A.J.; Langdon, T.G. Superplastic forming at high strain rates after severe plastic deformation. Acta Mater. 2000, 48, 3633–3640. [Google Scholar] [CrossRef]
- Kawasaki, M.; Langdon, T.G. Achieving superplastic properties in ultrafine-grained materials at high temperatures. J. Mater. Sci. 2016, 51, 19–32. [Google Scholar] [CrossRef]
- Mohamed, F.A. Creep and superplasticity: Evolution and rationalization. Adv. Eng. Mater. 2020, 22, 1900532. [Google Scholar] [CrossRef]
- Langdon, T.G. An analysis of flow mechanisms in high temperature creep and superplasticity. Mater. Trans. 2005, 46, 1951–1956. [Google Scholar] [CrossRef]
- Langdon, T.G. A Lifetime of Research in Creep, Superplasticity, and Ultrafine-Grained Materials. Adv. Eng. Mater. 2020, 22, 1900442. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Chauhan, M. Interpretation of the creep behavior of nanocrystalline Ni in terms of dislocation accommodated boundary sliding. Metall. Mater. Trans. A 2006, 37, 3555–3567. [Google Scholar] [CrossRef]


| Electrodeposition Parameters | Value | |
|---|---|---|
| Electrolyte composition (g/L) | Ni(SO3NH2)2·4H2O | 180 |
| NiCl2·6H2O | 20 | |
| H3BO3 | 30 | |
| C7H5NO3S | 1 | |
| CH3(CH2)11OSO3Na | 0.1 | |
| Temperature (°C) | 50 | |
| pH | 4 | |
| Current density (mA/cm2) | 60 | |
| Cathode/Anode | Stainless steel 304L/Nickel S-Rounds in Ti basket | |
| Agitation | Paddle agitator, Overflow circulation | |
| Condition | Phase Analysis | 2 θ (Deg) | FWHM | Grain Size (nm) |
|---|---|---|---|---|
| As-deposited | FCC (111) | 44.596 | 0.412 | 21.78 |
| FCC (200) | 51.907 | 0.699 | 13.21 | |
| FCC (220) | 76.570 | 0.400 | 26.45 | |
| Annealed at 400 °C | FCC (111) | 44.432 | 0.157 | 57.31 |
| FCC (200) | 51.790 | 0.150 | 61.54 | |
| FCC (220) | 76.280 | 0.140 | 75.41 | |
| Annealed at 500 °C | FCC (111) | 44.548 | 0.155 | 57.97 |
| FCC (200) | 51.905 | 0.167 | 55.30 | |
| FCC (220) | 76.349 | 0.194 | 54.45 | |
| Annealed at 600 °C | FCC (111) | 44.454 | 0.153 | 58.55 |
| FCC (200) | 51.799 | 0.155 | 59.55 | |
| FCC (220) | 76.300 | 0.161 | 65.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Kim, H.; Ahn, J. Superplastic Deformation Behavior and Microstructural Evolution of Electroformed Nickel Foils Determined by Thermomechanical Analysis. Materials 2025, 18, 1365. https://doi.org/10.3390/ma18061365
Lee M, Kim H, Ahn J. Superplastic Deformation Behavior and Microstructural Evolution of Electroformed Nickel Foils Determined by Thermomechanical Analysis. Materials. 2025; 18(6):1365. https://doi.org/10.3390/ma18061365
Chicago/Turabian StyleLee, Minsu, Hohyeong Kim, and Jinho Ahn. 2025. "Superplastic Deformation Behavior and Microstructural Evolution of Electroformed Nickel Foils Determined by Thermomechanical Analysis" Materials 18, no. 6: 1365. https://doi.org/10.3390/ma18061365
APA StyleLee, M., Kim, H., & Ahn, J. (2025). Superplastic Deformation Behavior and Microstructural Evolution of Electroformed Nickel Foils Determined by Thermomechanical Analysis. Materials, 18(6), 1365. https://doi.org/10.3390/ma18061365






