1. Introduction
With the rapid development of the global economy, carbon emissions continue to rise, making renewable energy generation methods such as wind and solar power increasingly prioritized by countries worldwide. However, due to the inherent intermittency and unpredictability of wind and solar power, their large-scale integration into the power grid inevitably exerts substantial impacts, leading to a continuous decline in system rotational inertia and increasing the difficulty of power balancing. This has sharply raised the demand for energy storage systems. Nevertheless, the initial investment costs for large-scale electrochemical energy storage systems are high, and under current return-on-investment models, cost recovery often takes several years. Therefore, to effectively enhance the reliability of energy storage asset valuation, mitigate investment risks, and avoid the rapid degradation of storage system lifespan and potential safety issues, it is crucial to diagnose and optimize the health status of large-scale energy storage systems and establish predictive models to advance the energy storage industry.
However, due to the complex internal microstructure of storage battery cells and various interfacial reactions, accurately assessing battery lifespan presents significant challenges. Researchers have undertaken comprehensive investigations into the degradation mechanisms of the lithium ion battery lifespan, examining the phenomenon from both microscopic and macroscopic vantage points. These studies aim to precisely diagnose the SOH within energy storage systems and refine optimization strategies for enhanced performance and longevity.
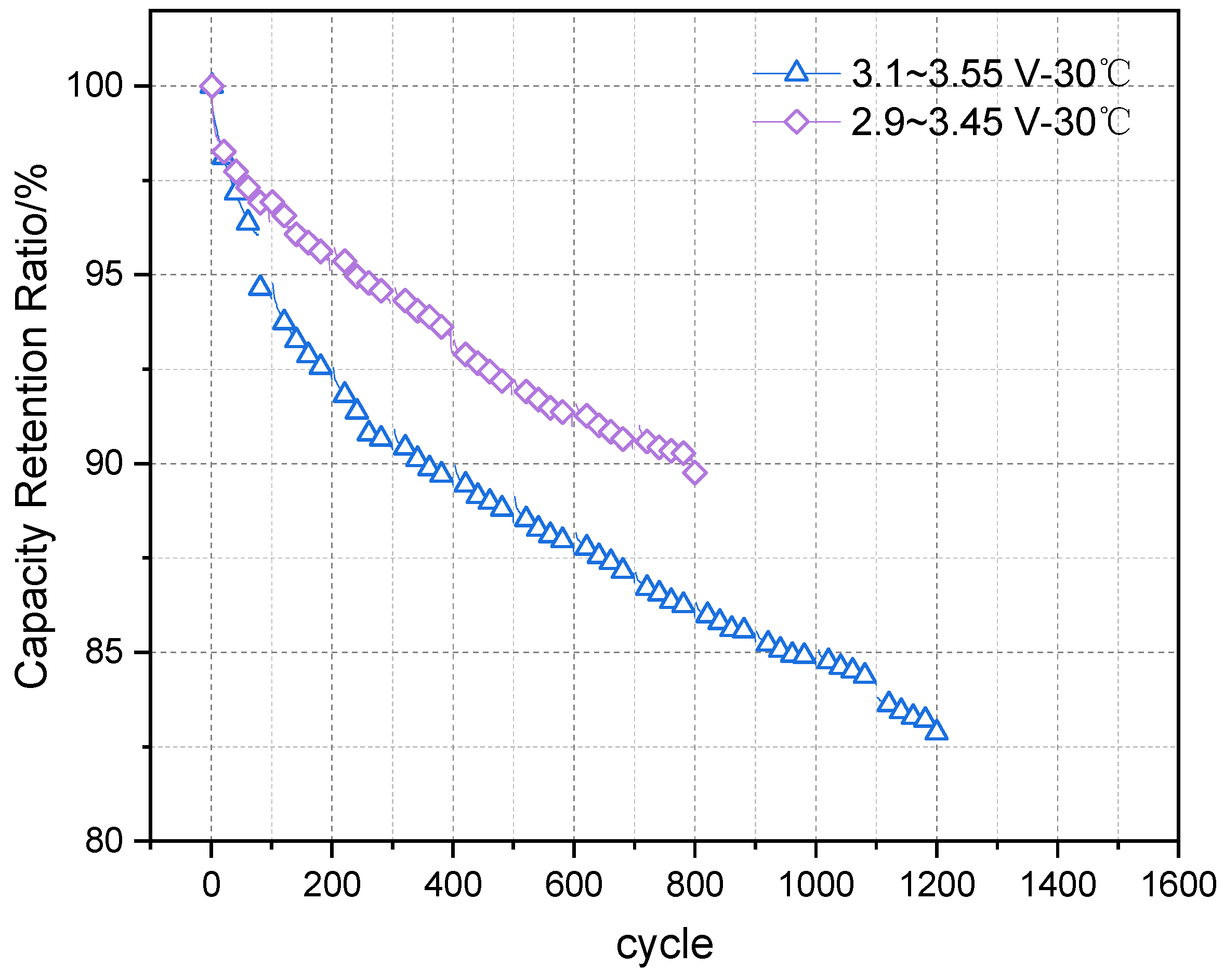
During the usage of lithium-ion batteries, lifespan degradation can be divided into two categories: calendar life and cycle life. In terms of calendar life, Dubarry et al. [
1] reviewed nearly 20 years of research on the calendar life of lithium ion batteries and compiled a comprehensive summary of the factors influencing the calendar life of batteries made from different materials. The findings indicate that the calendar life of almost all types of materials is closely related to the storage temperature and state of charge (SOC). At high SOC levels, the electrode surface potential is elevated, which accelerates side reactions with the electrolyte. High temperatures exacerbate irreversible side reactions, consuming active materials and active lithium within the battery, thus causing a loss of battery capacity.
For cycle life, the main influencing factors include cycling conditions, depth of discharge (DOD), and ambient temperature. The primary mechanisms affecting battery lifespan degradation focus on the loss of active material (LAM) in the electrodes and the loss of active lithium (LLI) [
2]. Based on the aforementioned studies, researchers have developed various battery aging models. These models are generally categorized into purely theoretical mechanism-based aging models and empirical aging models. Mechanism-based degradation models start from the micro-mechanisms of battery degradation and use various micro-level parameters to accurately characterize the battery state [
3,
4]. Empirical models, on the other hand, are constructed using actual cell test data, featuring fewer parameters and a relatively simpler structure [
5,
6].
Su et al. [
7] investigated the path dependence of the calendar life of ternary batteries under static and non-static conditions, and developed corresponding lifespan models. Through experiments, they compared calendar life degradation at 45 °C, 53 °C, and 60 °C across four SOC levels (40%, 60%, 80%, and 95%) under static storage and different storage paths. The study concluded that battery capacity degradation was independent of temperature and the SOC path, while the increase in internal resistance showed path dependence on SOC but was independent of the temperature path. Liu et al. [
8] proposed a physics-based battery remaining useful life (RUL) prediction method. Unlike traditional empirical models, this method considers three main battery degradation mechanisms: loss of active material in the positive electrode (LAMPE), loss of active material in the negative electrode (LAMNE), and loss of active lithium. Using the test results of voltage and capacity, the model calibrates degradation parameters with a half-cell model, and then fits the degradation parameters using empirical models and the least squares method. Compared to traditional capacity models, this method demonstrated higher accuracy in RUL prediction, particularly in the early stages of battery lifespan degradation, where the precision difference was more pronounced.
At present, research on the aging mechanism and modeling still faces two challenges: at the research level, (1) the time-varying characteristics of capacity attenuation have not yet established the evolution law framework under multi-stress coupling conditions [
9]; (2) the aging gradient distribution characteristics caused by multi-scale spatial heterogeneity of electrodes, cells, and modules need to be characterized by a three-dimensional characterization system [
10,
11]. Especially for hundreds of Ah commercial batteries, the phenomenon of non-uniform aging distribution caused by electrochemical–mechanical stress coupling has not formed a systematic research paradigm. In terms of modeling, (1) the existing mechanical–electrochemical coupling models are mostly limited to unipolar reaction path modeling such as the growth of the SEI film on the anode side [
12,
13], and lack the full-electrode co-evolution characterization of degradation mechanisms such as the phase transition of cathode-active materials. (2) It is difficult for the traditional model to effectively describe the aging distribution evolution law driven by multiple physical fields coupled with the temperature field, stress field, and concentration field under actual working conditions.
To address the aforementioned issues, this study innovatively introduced LAM and LLI attenuation mechanisms into the mechanism model, and successfully built a hundreds-of-Ah-energy storage battery life attenuation model, and based on the model simplification strategy, it has been fully applied and verified in the MWh-level energy storage power station. The systematic designs and experiments can be classified as the following three main aspects: constructing a mechanistic aging model for lithium iron phosphate/graphite (LFP/Graphite) batteries; simulating and verifying the results of the mechanistic aging model; and simplifying the mechanistic aging model to develop an engineering model. This provides theoretical support for optimizing lifespan control strategies.
In constructing the aging model for batteries, corresponding side reactions were incorporated into the typical mechanism model based on the identified degradation mechanisms. The cells were disassembled to obtain micro-level parameters of the battery. Additionally, using laboratory data from aging calibration experiments, a mechanistic aging model was developed. Through iterative simulation calculations, transient solutions for batteries with different cycle counts were performed, allowing for the determination of LAM and LLI within the battery.
Furthermore, by simulating the mechanism models under various operating conditions, the variation patterns of LAM and LLI can be obtained. Using data fitting methods, the functional forms dominated by different mechanisms were identified. These were then applied to simplify the mechanism model based on the matching relationship between the anode and the cathode, enabling the estimation of the battery’s state of health (SOH) and the development of an engineering model. Subsequently, the calculation results of the engineering model under complex conditions were compared with experimental data to validate the model’s accuracy. A sensitivity analysis of the factors affecting battery lifespan was conducted, and the model was ultimately applied to diagnose the health status of energy storage systems and optimize control strategies.
4. Simplification of the Mechanistic Model into the Engineering Model
Due to the lengthy computation time required for the mechanistic model, a single aging condition calculation typically takes several to tens of hours, making it challenging to apply this model directly for the rapid prediction and construction of energy storage system-level model. The mechanistic model is capable of handling complicated operating conditions including different variations in T, DOD, etc. However, in typical onsite application, the operating conditions are generally limited and regularized. Therefore, based on the mechanistic model, representative conditions were calculated to obtain internal aging information of the battery under different aging scenarios, enabling the development of a simplified engineering model.
4.1. Assumptions for Simplifying the Mechanistic Model
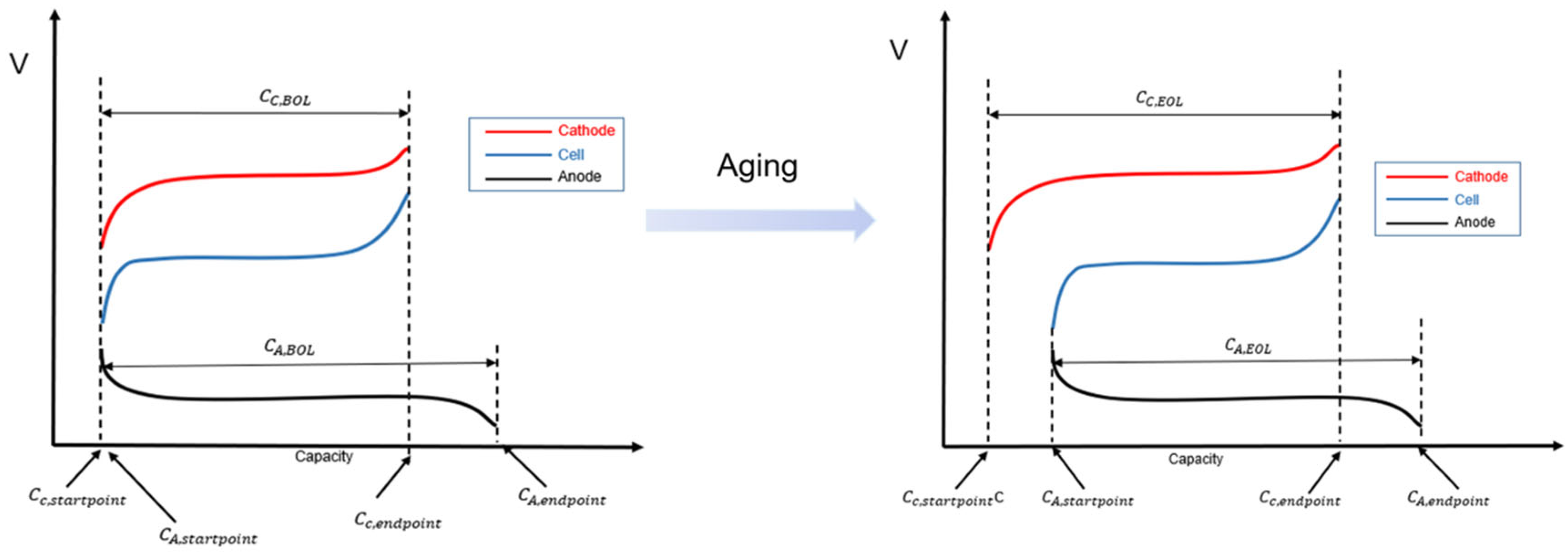
The foundation of the engineering model was to simplify the mechanistic model of the battery into two types of factors: LAM (loss of active material) and LLI (loss of lithium inventory). In different aging modes, the lithium intercalation range between the anode and cathode changes, ultimately leading to different battery capacity. First, certain assumptions need to be made regarding how LLI and LAM affect the relationship of lithium intercalation between the anode and cathode. Based on the characteristics of lithium ion batteries, the assumptions for the engineering model are as follows:
- (1)
Since the anode capacity is greater than the cathode capacity, the cathode capacity is used as a reference for normalization;
- (2)
LLI occurs during the charging process and only at the anode;
- (3)
LAM is caused by gas generation, so the LAM is the same for both the anode and cathode.
Based on these assumptions, the battery capacity model can be derived, as shown in
Figure 10.
where
is the initial anode capacity,
is the initial cathode capacity,
is the anode capacity after aging,
is the cathode capacity after aging,
is the position when the anode SOC = 0%,
is the position when the cathode SOC = 0%,
is the position when the anode SOC = 100%,
is the position when the cathode SOC = 100%,
is the normalized battery capacity,
is the loss of active material in the anode, and
is the loss of active material in the cathode.
The simplified battery capacity model above includes two core parameters: LLI and LAM. The variation in LLI is caused by SEI film growth and lithium plating in the mechanistic model, allowing for capacity loss due to SEI film growth and lithium plating side reactions to be calculated through model simulation.
is the capacity loss caused by LLI, represents the direction of thickness, and is the current density of the lithium plating reaction.
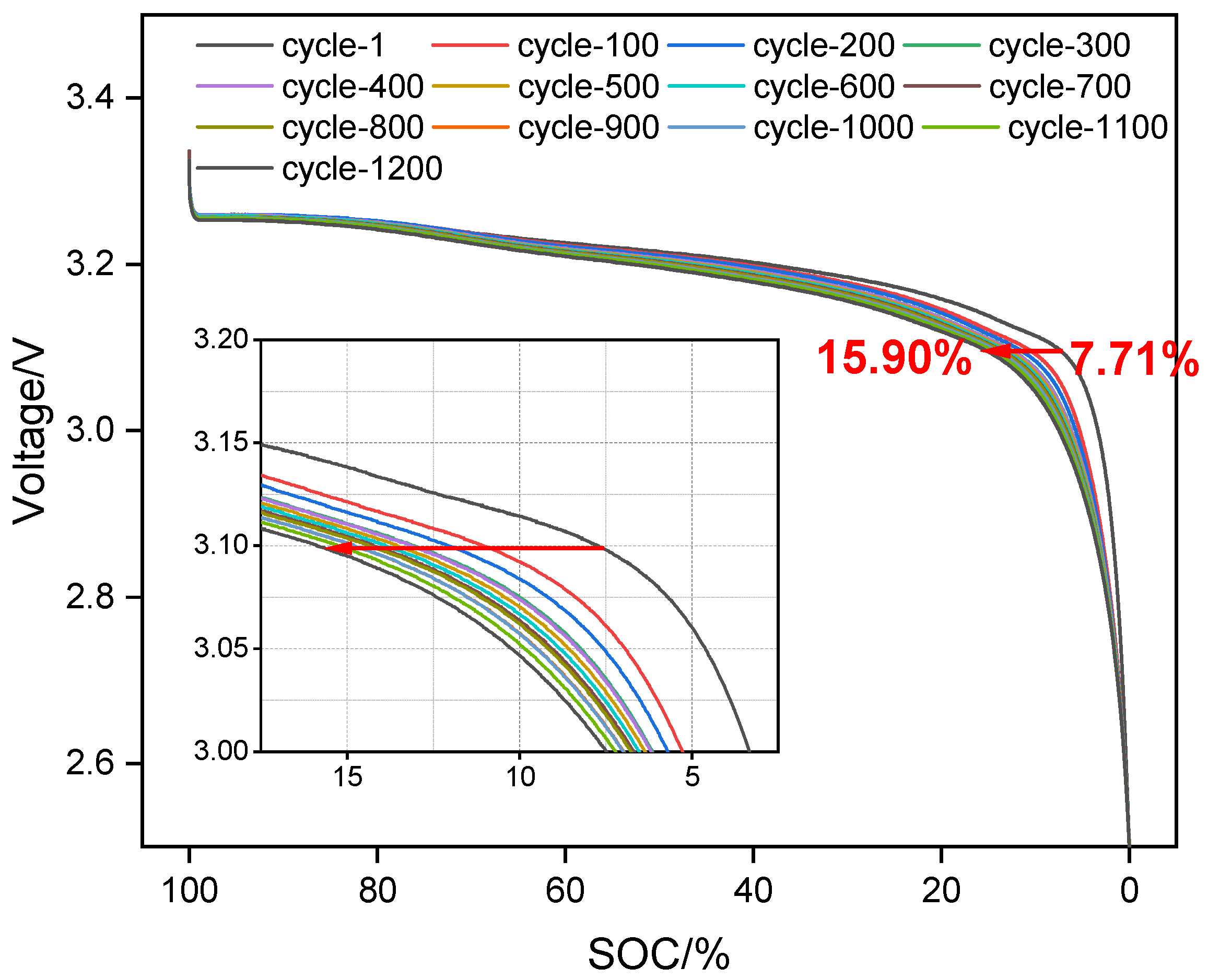
Based on the assumptions of the simplified mechanism model, LAM is primarily caused by gas generation and cannot be directly calculated through the model. However, since the overall capacity loss of the battery is mainly due to LLI and LAM, LAM can be indirectly obtained by calculating the difference between the total capacity loss of the battery and the capacity loss caused by LLI. Capacity calibration tests at 1/25C were conducted on batteries with different cycle counts to obtain their thermodynamic capacity
Qaging. The capacity loss due to LAM can be calculated as follows:
4.2. Data Fitting Results of LLI and LAM
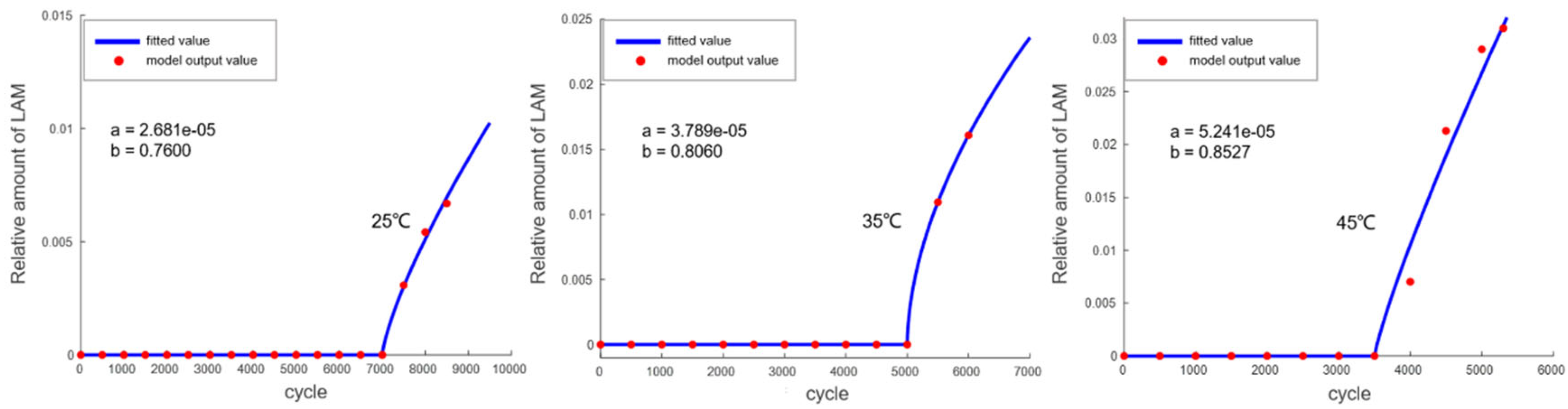
The establishment of the engineering model requires determining the trend of changes in LLI and LAM within the battery, which is obtained through calculations from the mechanism model. In the model, LLI and LAM are defined as follows: LLI is determined by calculating the SEI film growth and lithium plating amount on the particle surface, while LAM is obtained by the difference between the total thermodynamic capacity and LLI. First, the mechanism model was used to calculate the aging decay curves of the battery at 0.5P and 25 °C/35 °C/45 °C (where the calculation results of the mechanistic model at 25 °C and 45 °C were experimentally validated). The LLI values at 25 °C, 35 °C, and 45 °C were then obtained. Using the difference between the thermodynamic capacity of the battery measured at these three temperatures and the calculated LLI, LAM was determined. Finally, the resulting LLI and LAM variation curves were fitted with functions to derive a simplified engineering model.
The trend of LLI variation is shown in
Figure 11, and the fitted function is as follows:
The trend of LAM variation is shown in
Figure 12, and the fitted function is as follows (where
G represents the onset point of gas generation):
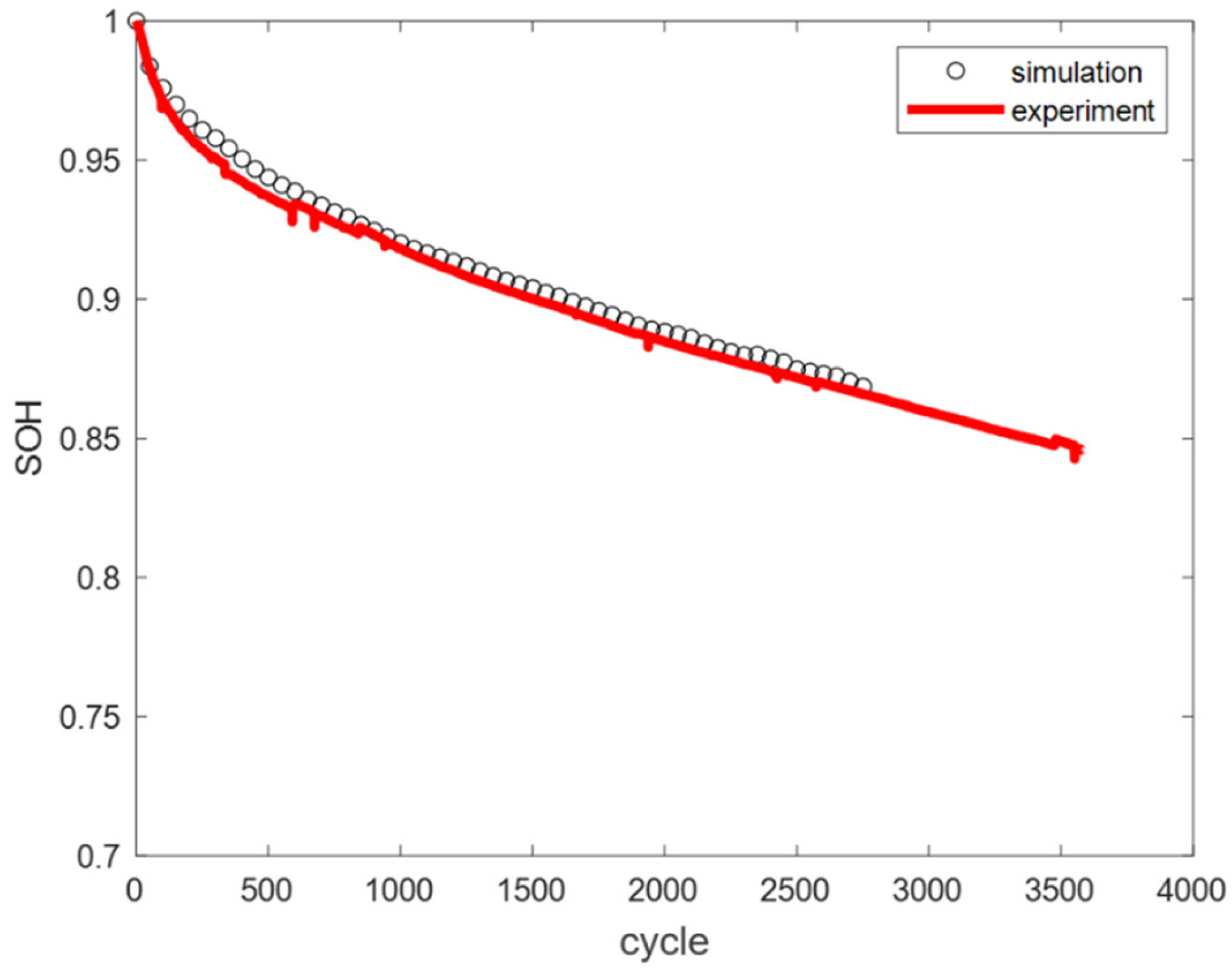
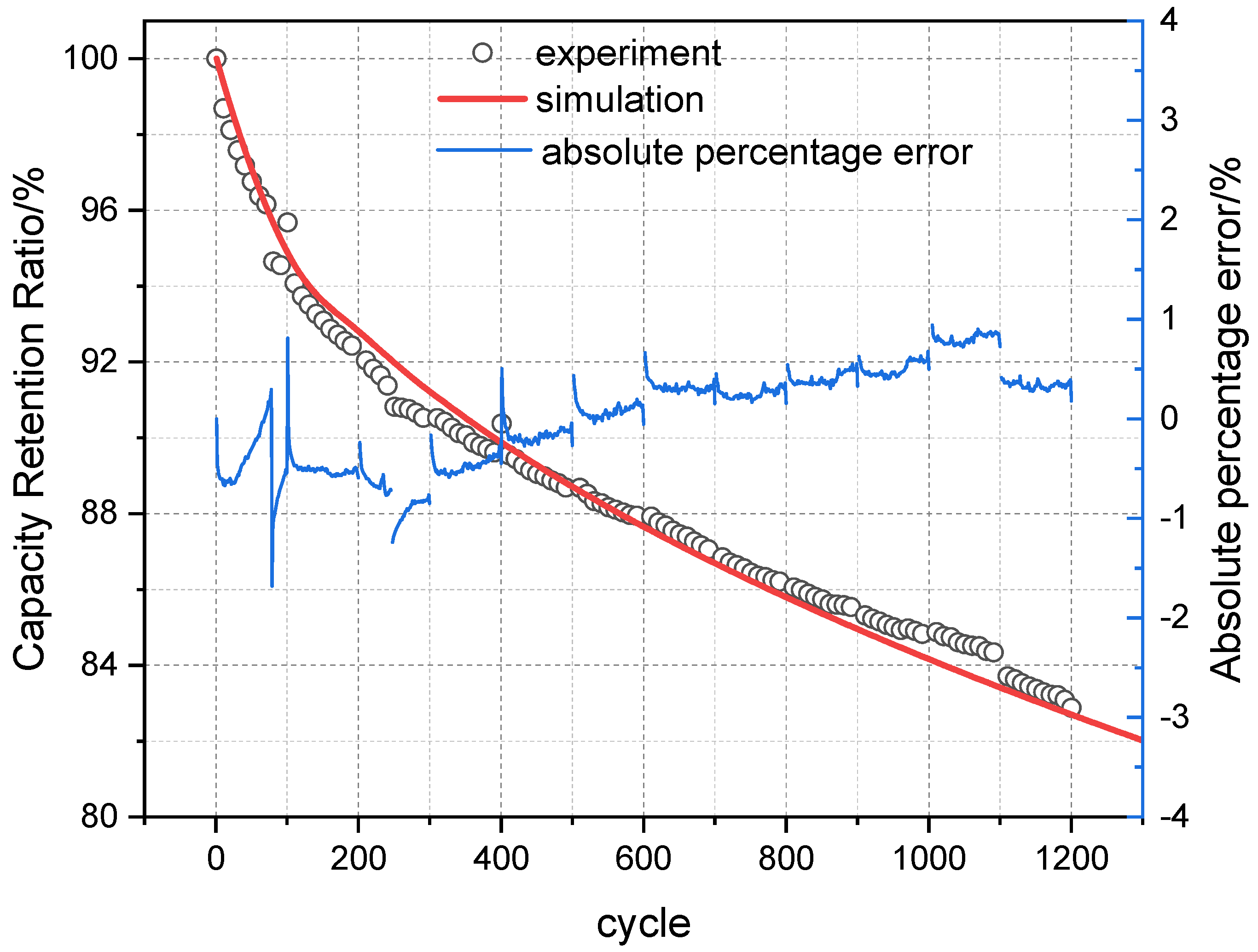
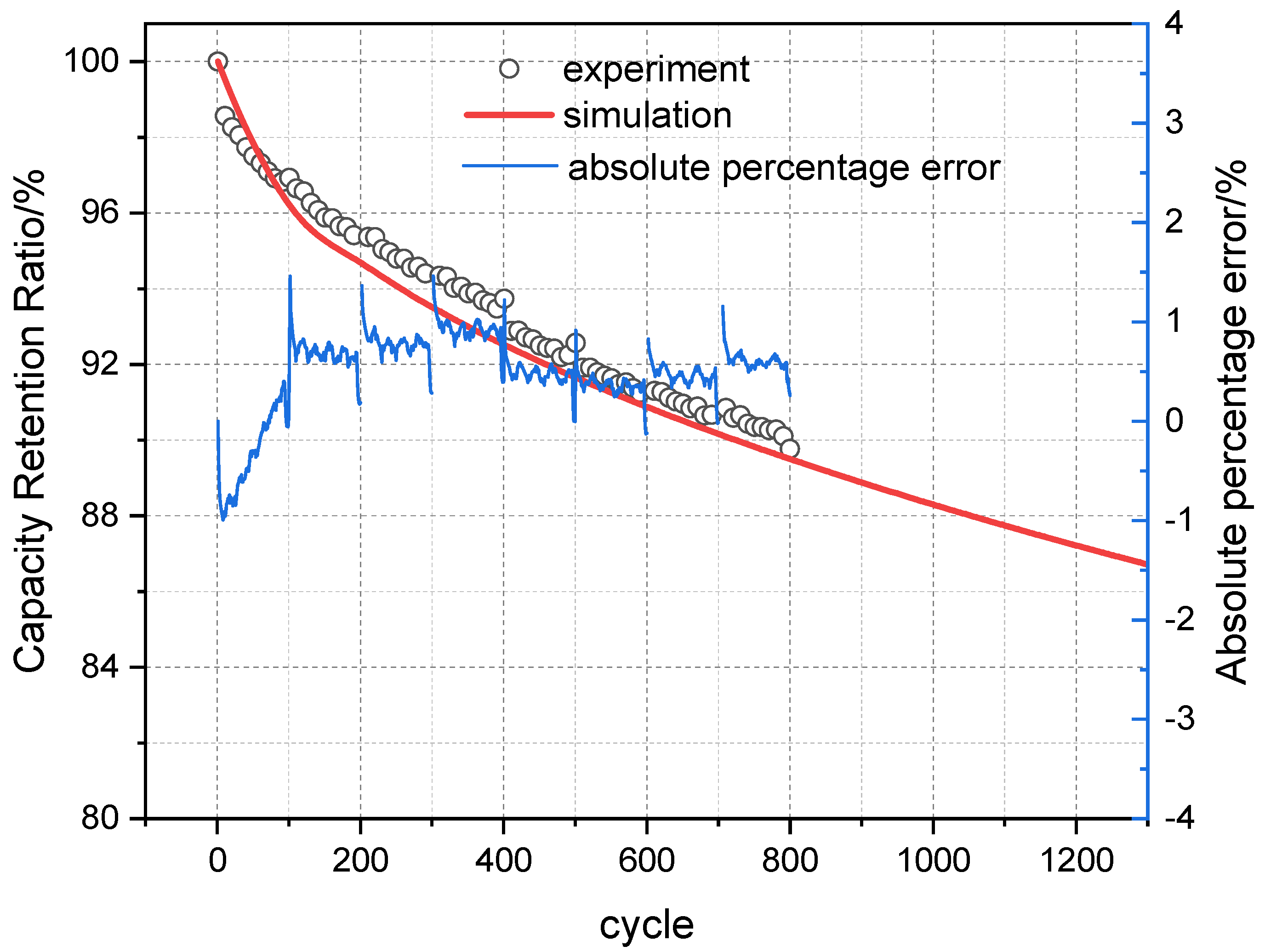
Based on the LLI and LAM fitting functions established through the above analysis, the calculated SOH decay values of the battery under three temperature conditions were compared with the values calculated by the mechanism model, as shown in
Figure 13. The results show a close match between the two, indicating that the determination of these fitting functions is effective.
Using the engineering model described above, assumptions can be made about the variation in coefficients in the fitting formula with operating conditions (such as temperature), thereby obtaining the battery decay patterns under different conditions and generating aging datasets for constructing a data-driven model. Here, temperature was used as an example to illustrate the generation process of the aging dataset required for the data-driven model.
The relationship between the parameters in the fitting formula and temperature is described by the Arrhenius equation:
where
is the reaction rate constant,
is the pre-exponential factor,
is the activation energy,
is the absolute temperature, and
is the molar gas constant.
As shown in
Figure 14, the coefficients of the LLI and LAM variation functions at 25 °C, 35 °C, and 45 °C were fitted using the Arrhenius equation to obtain the reaction coefficients a and b at different temperatures T.
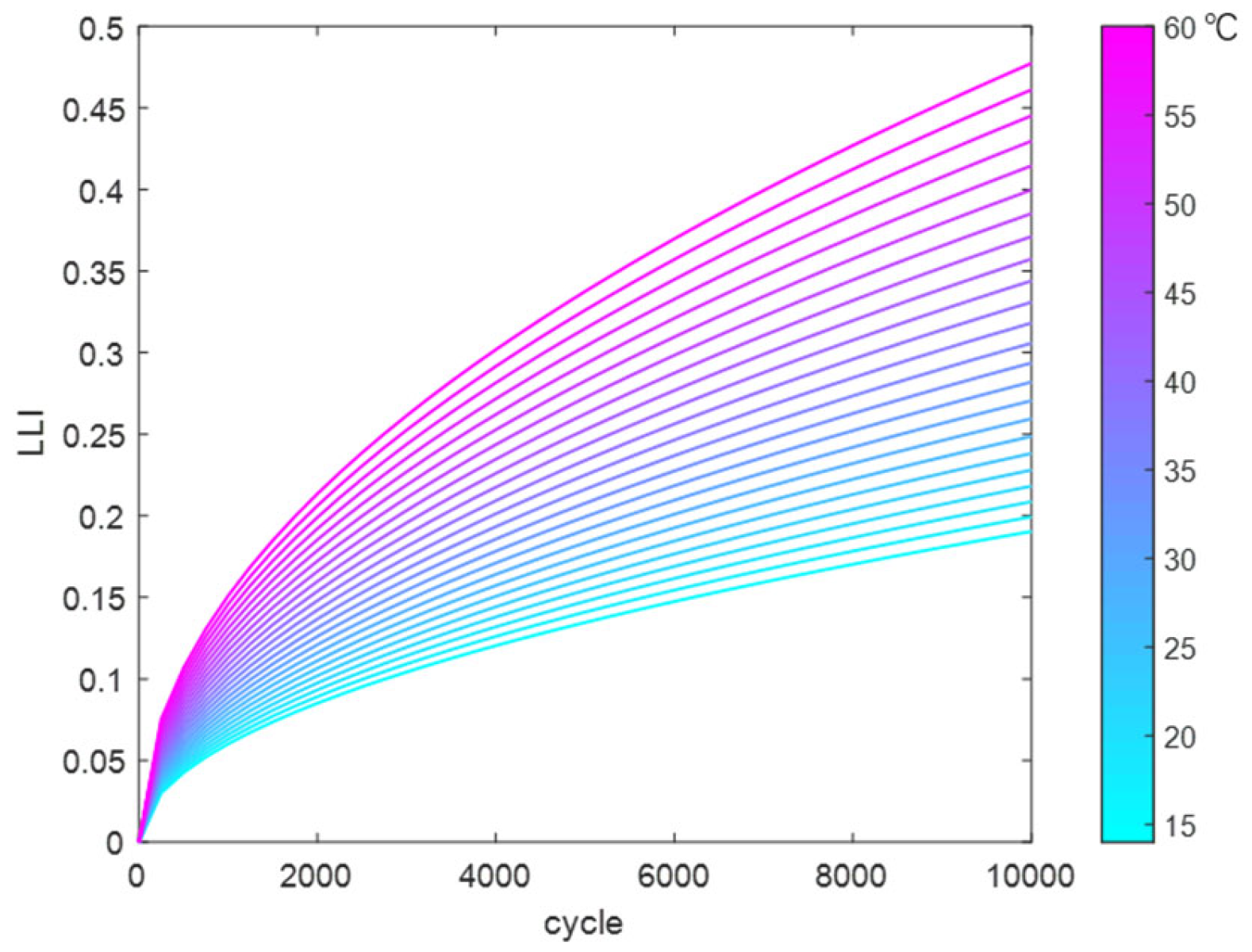
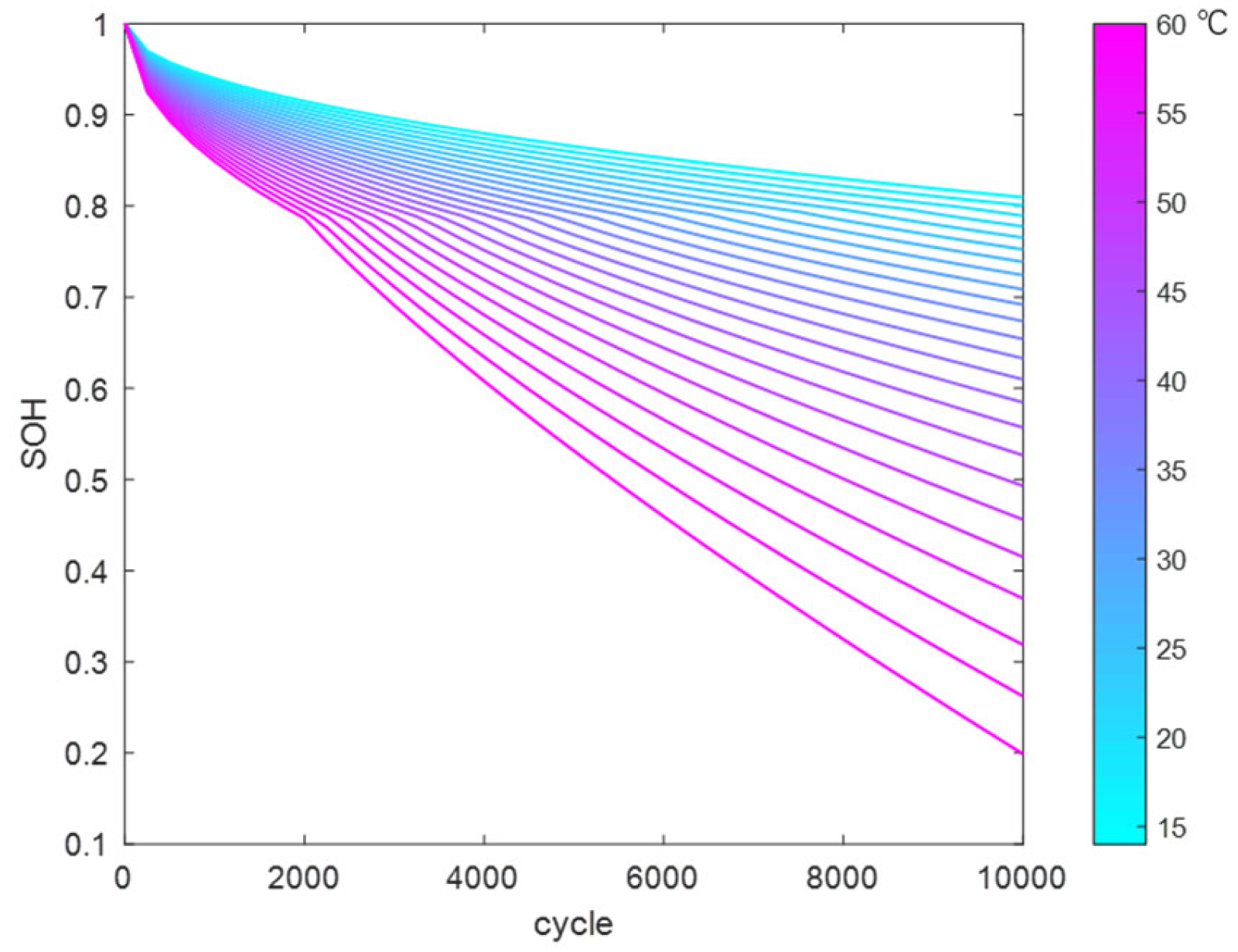
Using the above method, the reaction coefficients in the LLI fitting function were fitted and solved to obtain a temperature-dependent fitting function. This was used to calculate the trend of LLI variation with cycle count at different temperatures (14 °C to 60 °C), as shown in
Figure 15.
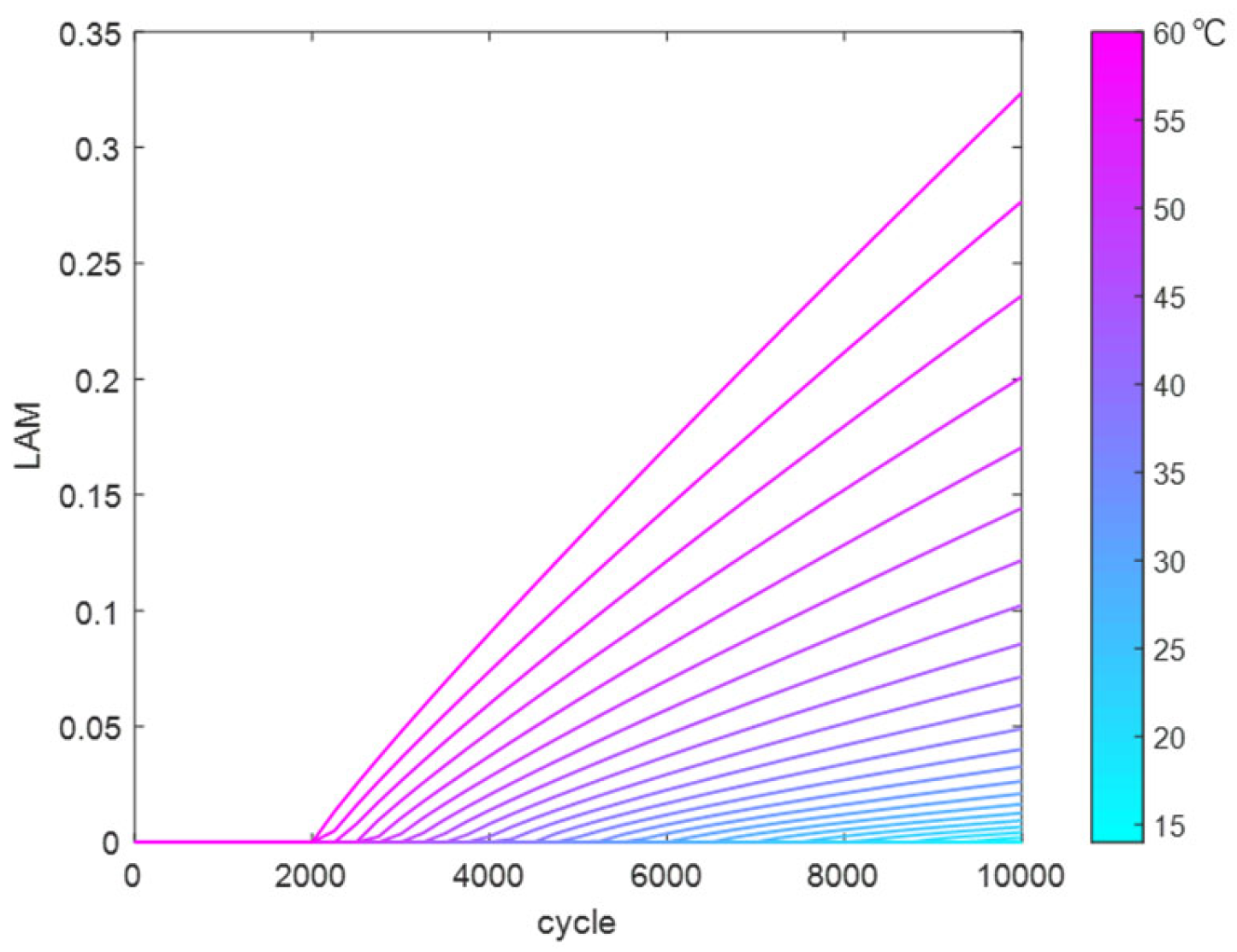
Similarly, the reaction coefficients in the LAM fitting function were fitted and solved to obtain a temperature-dependent fitting function. This was used to calculate the trend of LAM variation with cycle count at different temperatures (14 °C to 60 °C), as shown in
Figure 16.
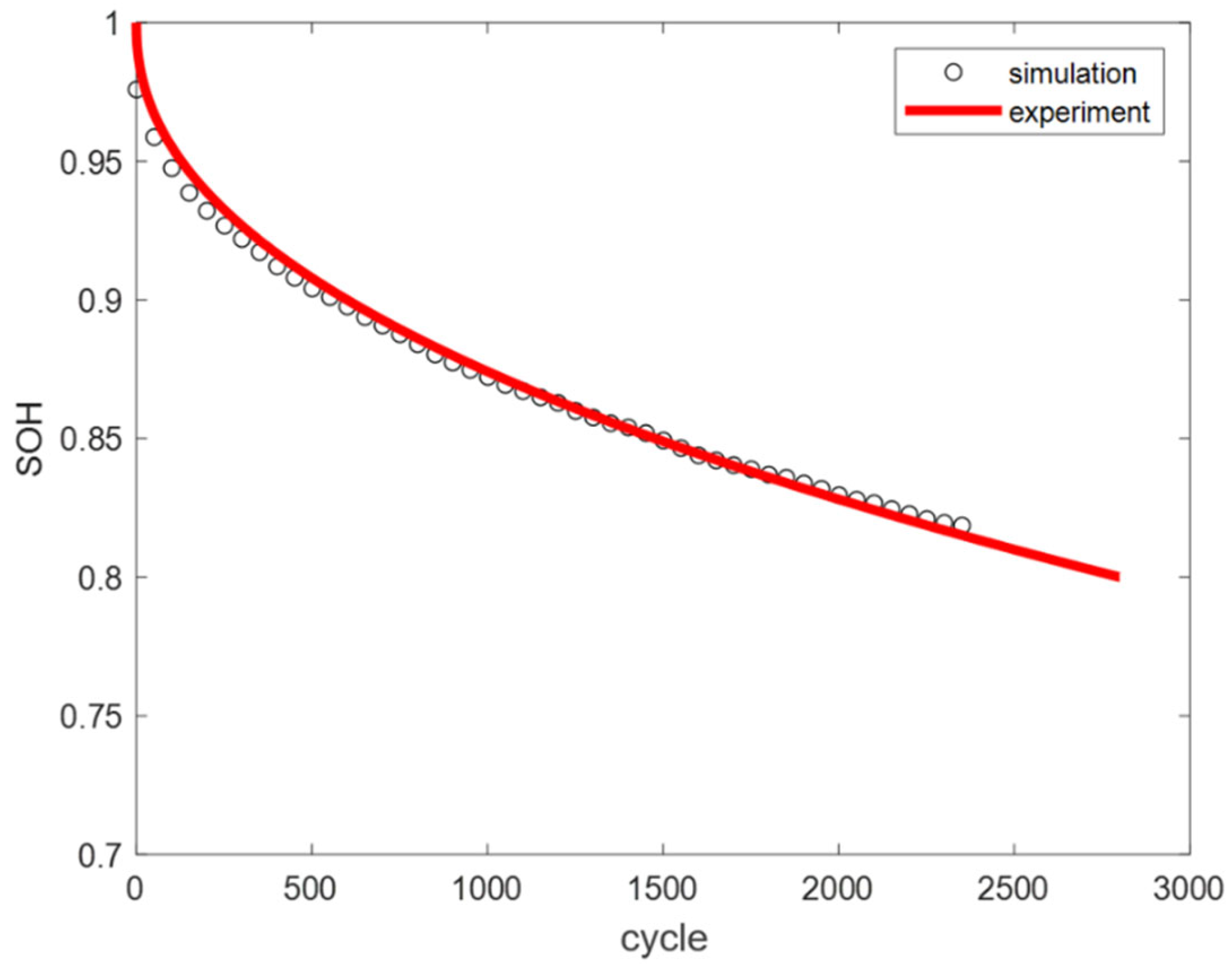
By substituting the LAM and LLI datasets into the model, the battery SOH variation curves under SEI film growth and gas generation mechanisms at different temperatures can be obtained, as shown in
Figure 17.
The engineering model established using the above method can calculate the capacity decay curve under different temperature conditions. Compared to the mechanism model, this model has only a few parameters, which significantly reduces computation time while ensuring model accuracy, making it suitable for rapid SOH calculations in practical engineering projects. The error propagation in the aging model primarily stems from the following three aspects: parameter identification, simplification of nonlinear dynamic characteristics, and limitations of data samples. To effectively reduce error accumulation, future research will integrate the interpretability of mechanistic models with the adaptability of data-driven models to conduct multi-model fusion studies. Additionally, based on identified key factors affecting lifespan degradation (such as temperature, DOD, and C-rate), more cross-orthogonal experiments will be designed to dynamically calibrate model parameters, thereby continuously enhancing the robustness and predictive accuracy of the models.
4.3. Validation of Engineering Model Under Onsite Operating Conditions
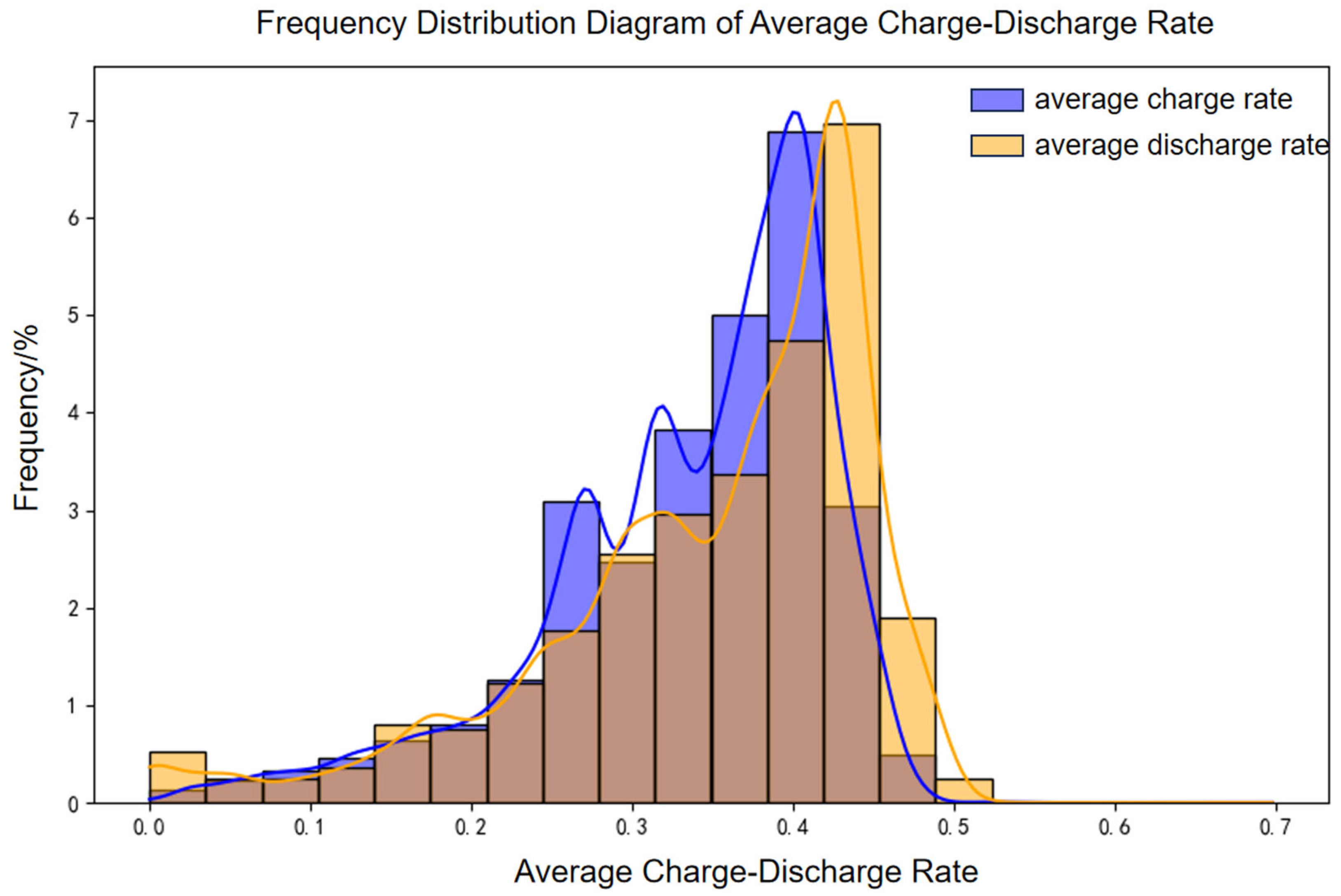
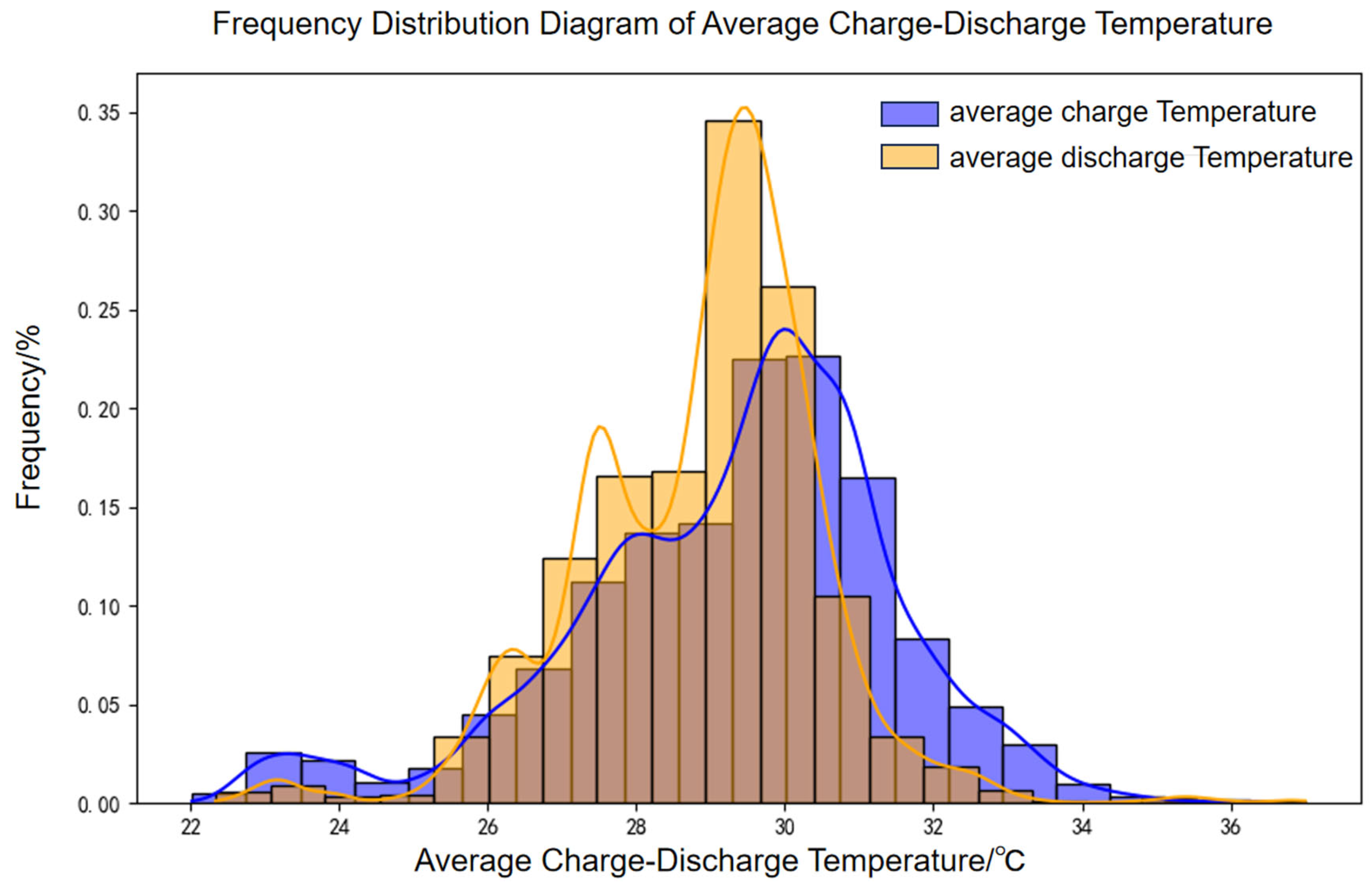
A 100 MW/200 MWh energy storage power station was selected to evaluate battery aging under real-world operating conditions using an established engineering model. The accuracy of the model was subsequently validated through measured data. Operational data from March 2022 to December 2024 were analyzed, with the distributions of charge–discharge rates and temperatures depicted in
Figure 18 and
Figure 19, respectively.
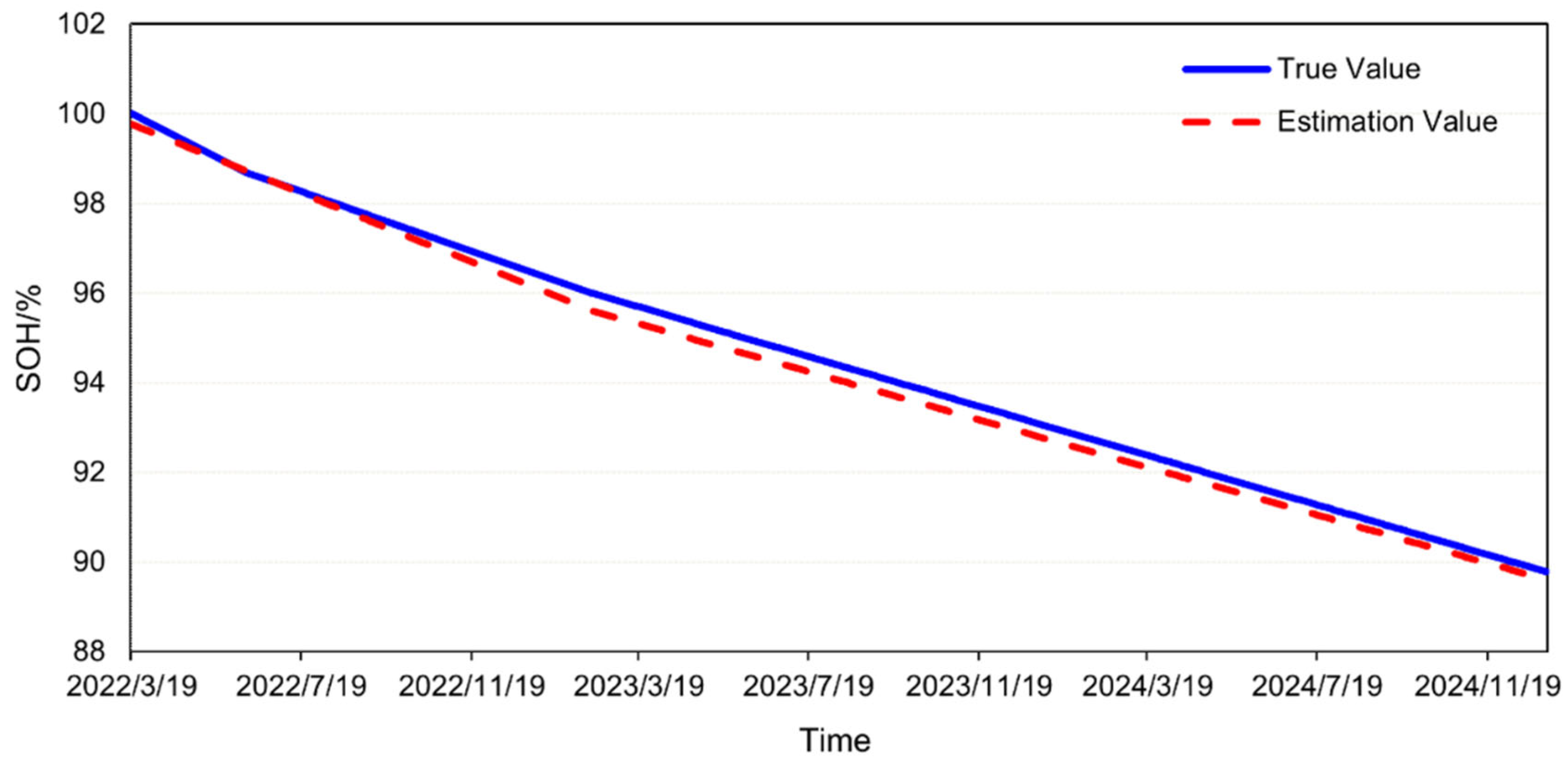
Under actual operating conditions, the measured SOH of the battery and the SOH calculated by the engineering model are compared in
Figure 20. As of 31 December 2024, the measured SOH of the battery was 89.78%, while the engineering model predicted an SOH of 89.61%, resulting in an error of −0.17%. These findings demonstrate that the engineering model exhibited high accuracy and was capable of reliably predicting battery aging under complex operational conditions. Under identical hardware configurations (Intel(R) Xeon(R) Platinum 8260 CPU @ 2.40 GHz 2.39 GHz, 1024 GB RAM, 8 × 3.84 TB SATA SSD), the computational time required for estimating the SOH of the battery using a mechanistic model was 8.12 h, whereas the engineering model accomplished the same task in a mere 10 s. This significant disparity in computational efficiency underscores the advantage of the engineering model, which not only expedites the SOH estimation process, but also facilitates the scalability of the model to system-level applications, thereby enhancing its potential for large-scale engineering deployment.
5. Conclusions
The mechanistic model in this study addresses the limitations of empirical LFP/graphite cell aging model in adequately accounting for side reactions on the cathode side. Based on the P2D model, it specifically models the three aging mechanisms unique to the LFP/Graphite system: SEI film growth, lithium plating, and gas generation. Under different temperature and DOD conditions, the average error between the simulation results of this model and actual measurements was within ±1%, demonstrating high accuracy and adaptability.
Additionally, to facilitate engineering applications of the model, a simplified engineering model was developed based on the matching relationship between the anode and cathode, consisting of two core parameters: LLI and LAM. This engineering model, grounded in the cell’s aging mechanisms, calculates and outputs LLI and LAM values under various conditions, followed by regression fitting and simplification. This approach significantly reduces the model parameters and computational load, making it suitable for large-scale engineering applications, and also provides a foundation for building model for energy storage systems. The validation of the engineering model within a 100 MW/200 MWh energy storage project revealed a minimal deviation of −0.17% from the actual State of Health (SOH) when the battery’s SOH had degraded to 89.78%. Compared to the mechanistic model, the computational time under the same hardware configuration was reduced from 8.12 h to 10 s. At the technology transformation level, it is expected to be divided into the following aspects: (1) industry adaptation approach: simplify the computing framework and align it with the industry-standard hardware and software ecosystem; (2) deployment case studies: work with MWh-class energy storage plants to pilot, and continuously validate to optimize model accuracy; (3) scalability analysis: evaluate the cost of cloud-based deployments and the analytics latency of million-battery databases.
In subsequent work, efforts will focus on developing a comprehensive energy storage system model based on the engineering model, which incorporates multiple battery cells connected in series and parallel. This model will be integrated with an auxiliary power consumption model of the thermal management system to establish a coupled energy efficiency–lifetime model. The aim is to accurately calculate and optimize the actual discharge capacity of large-scale energy storage systems, thereby enhancing the overall economic benefits of energy storage stations. This approach will provide a robust framework for improving the operational efficiency and profitability of energy storage facilities.