Advances in High-Temperature Non-Metallocene Catalysts for Polyolefin Elastomers
Abstract
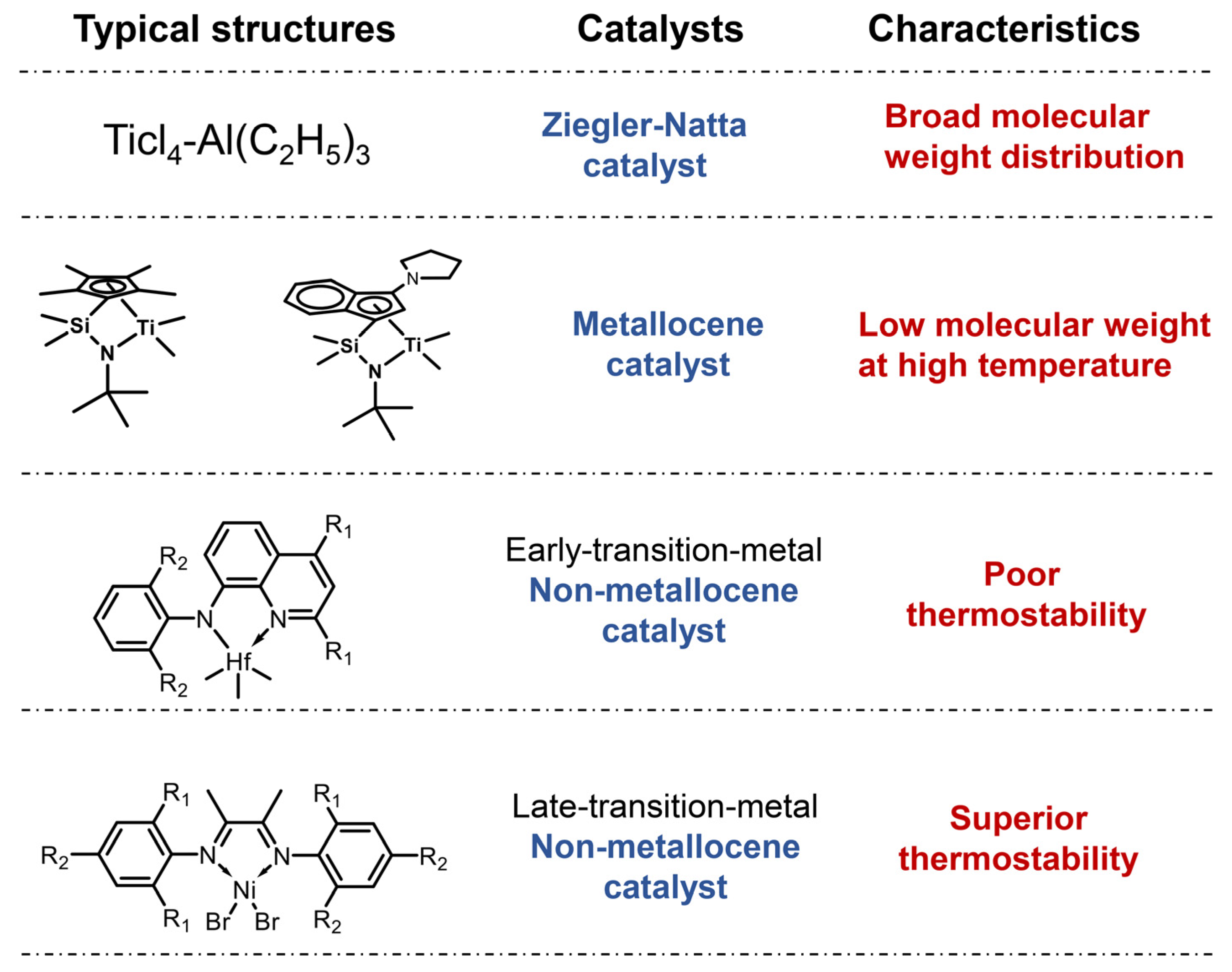
1. Introduction
2. N,N-Bidentate Ligands
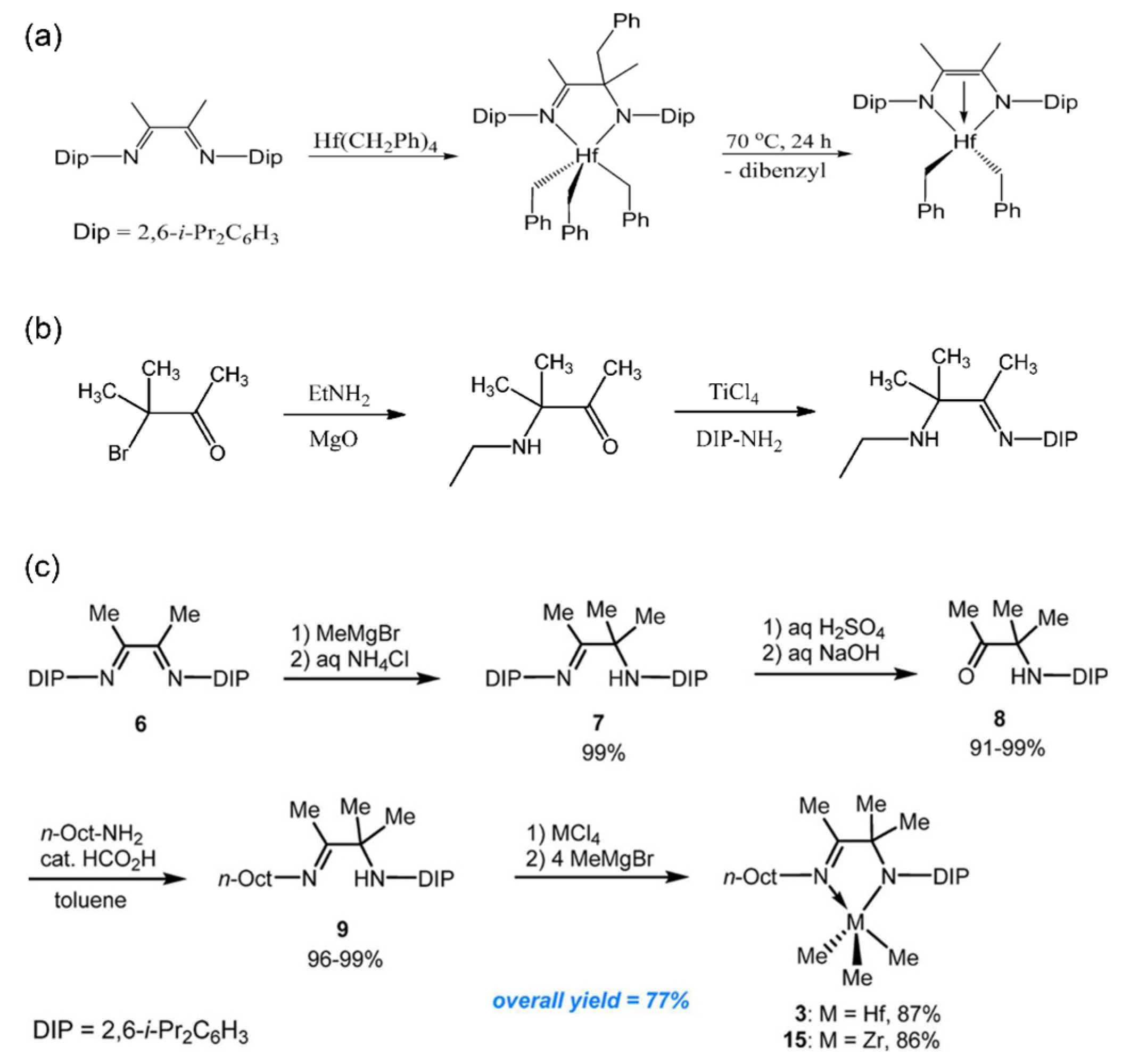
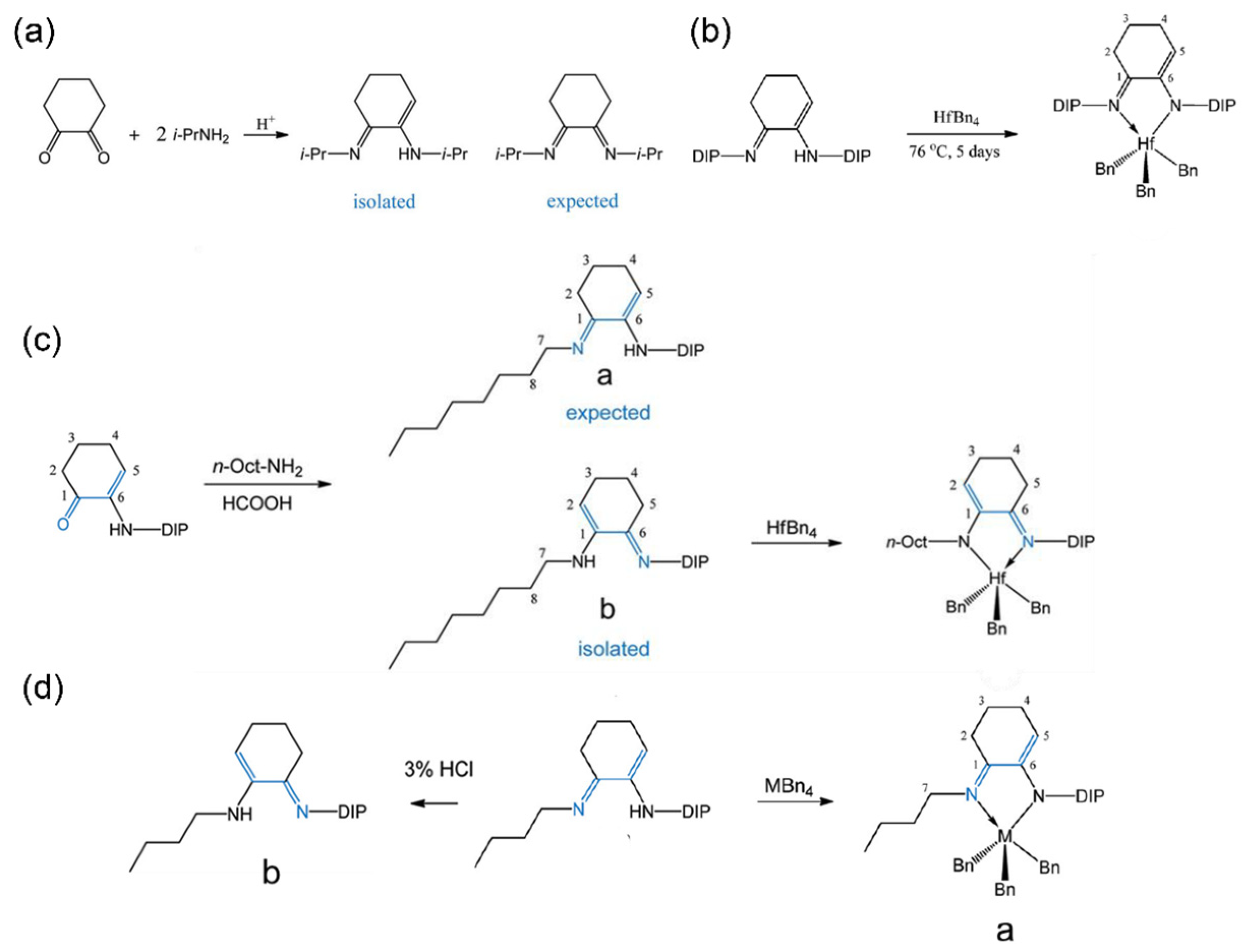
2.1. Imino-amido Ligands
2.2. Imino-enamido Ligand
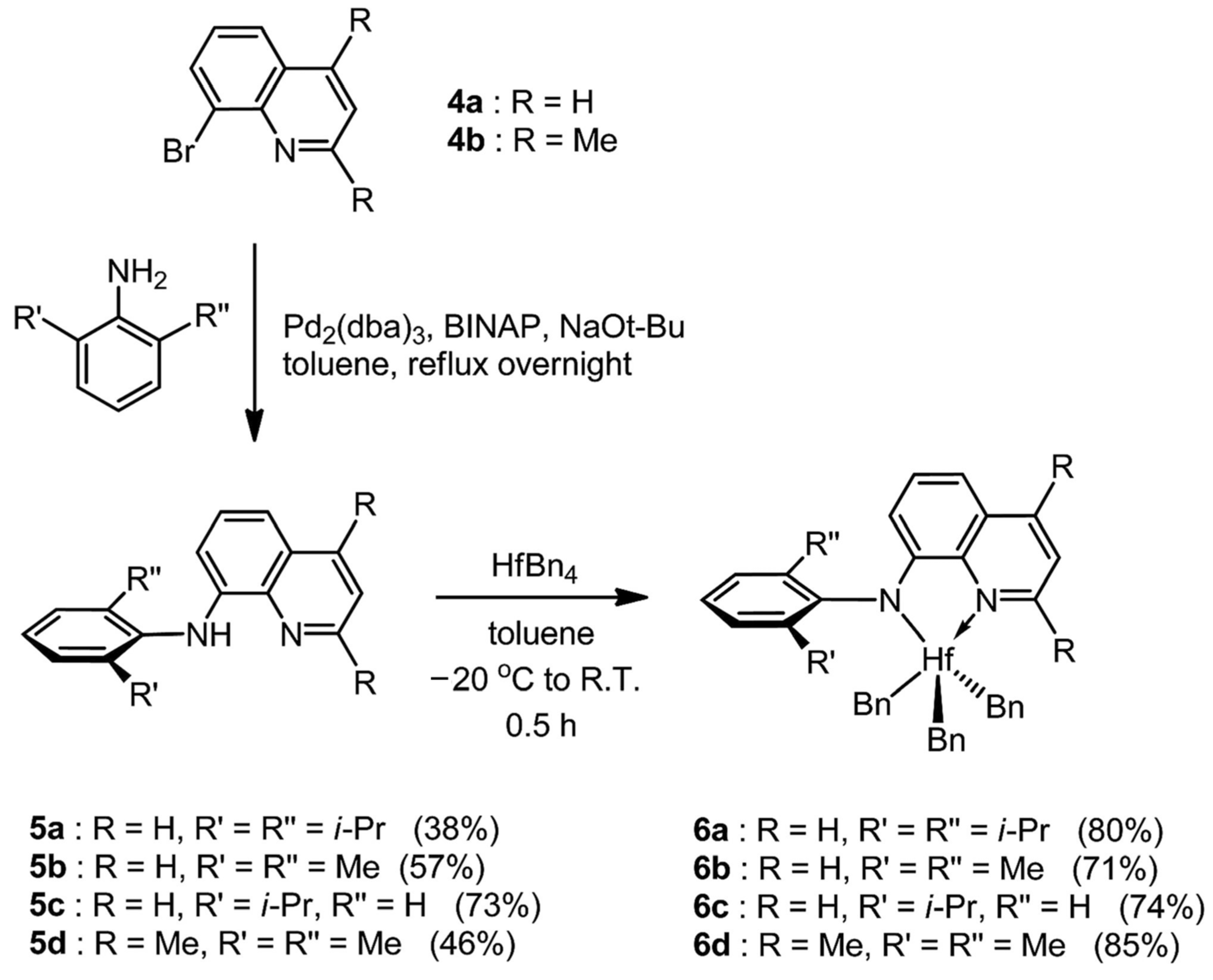
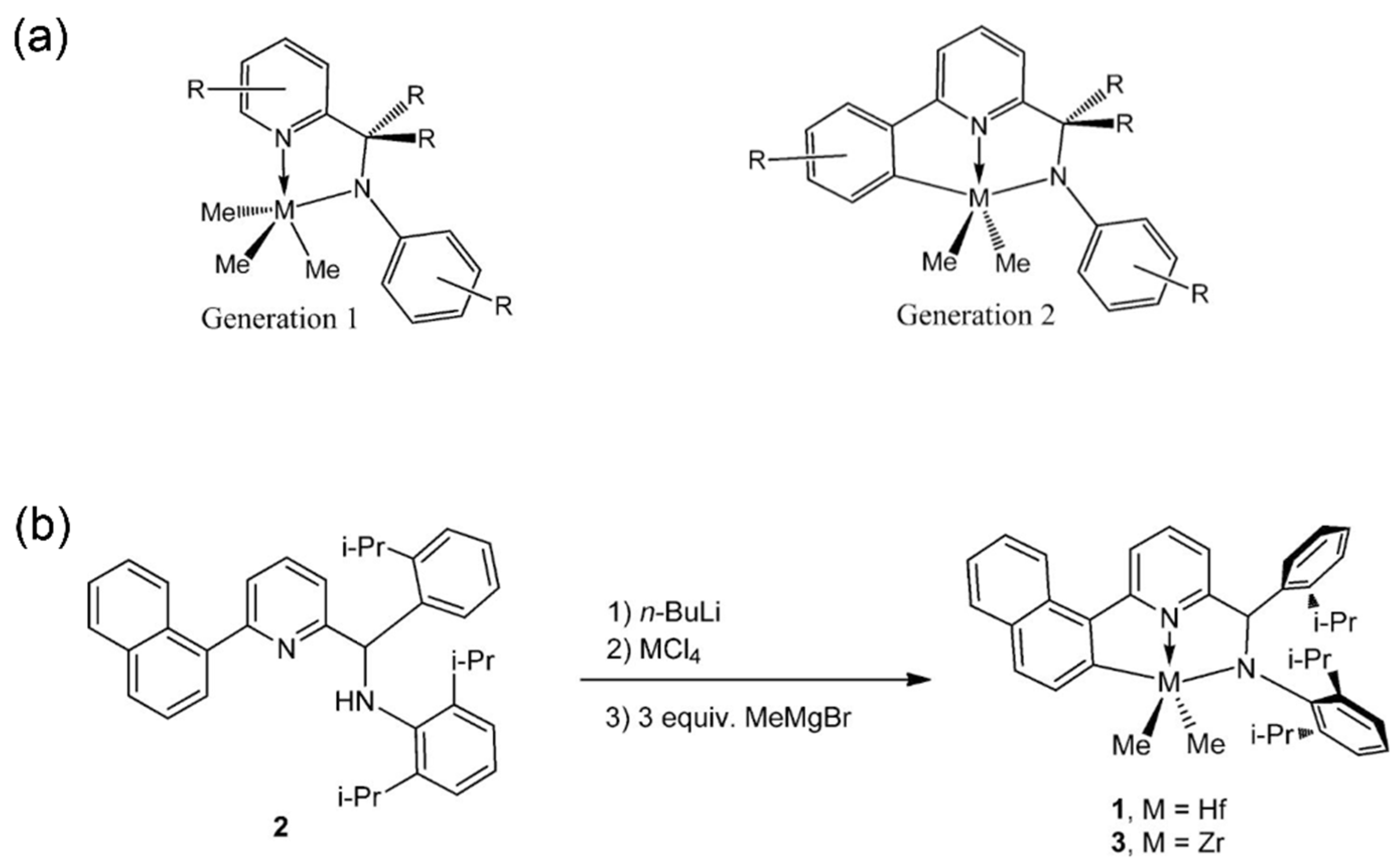
2.3. Amido-quinoline Ligand
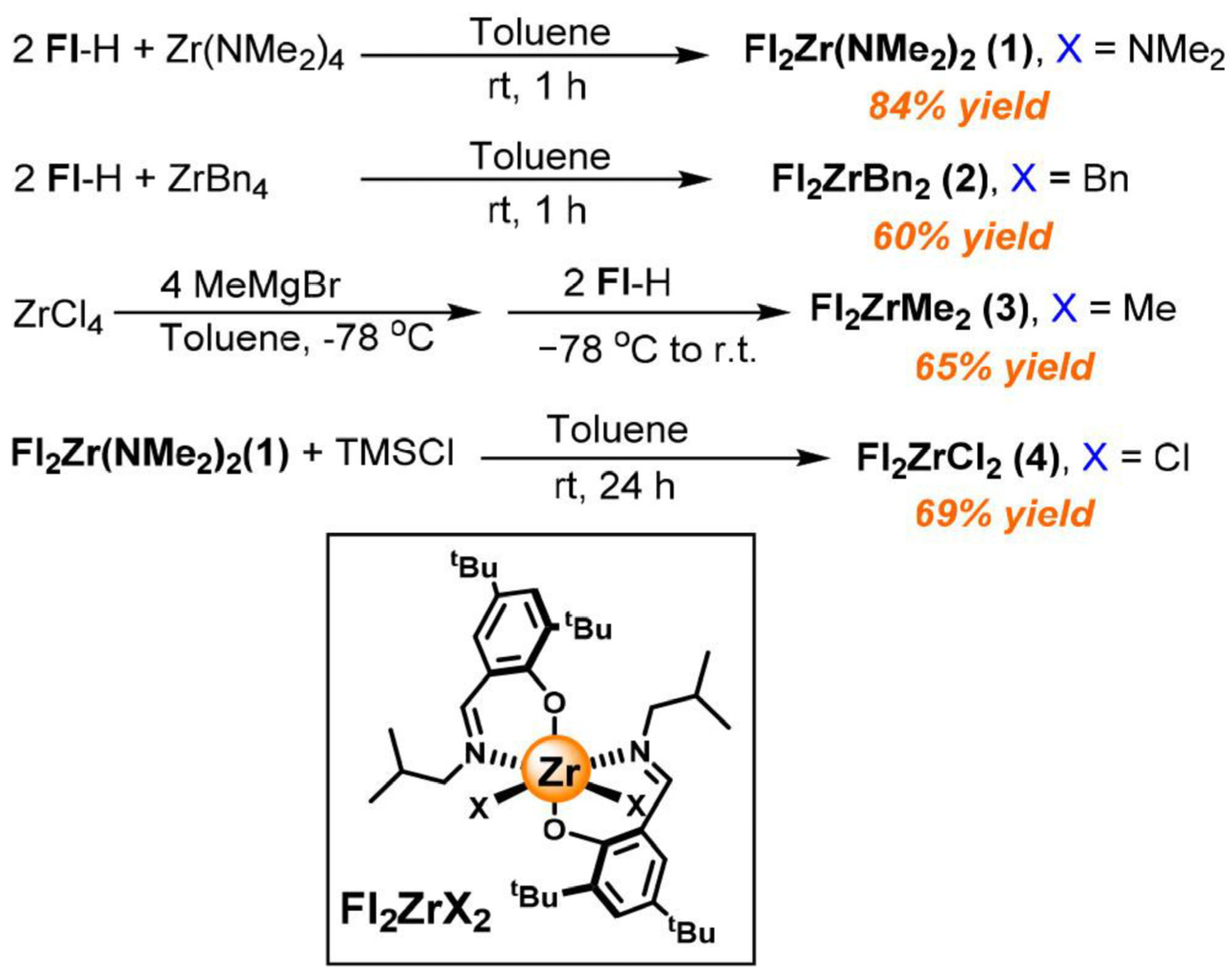
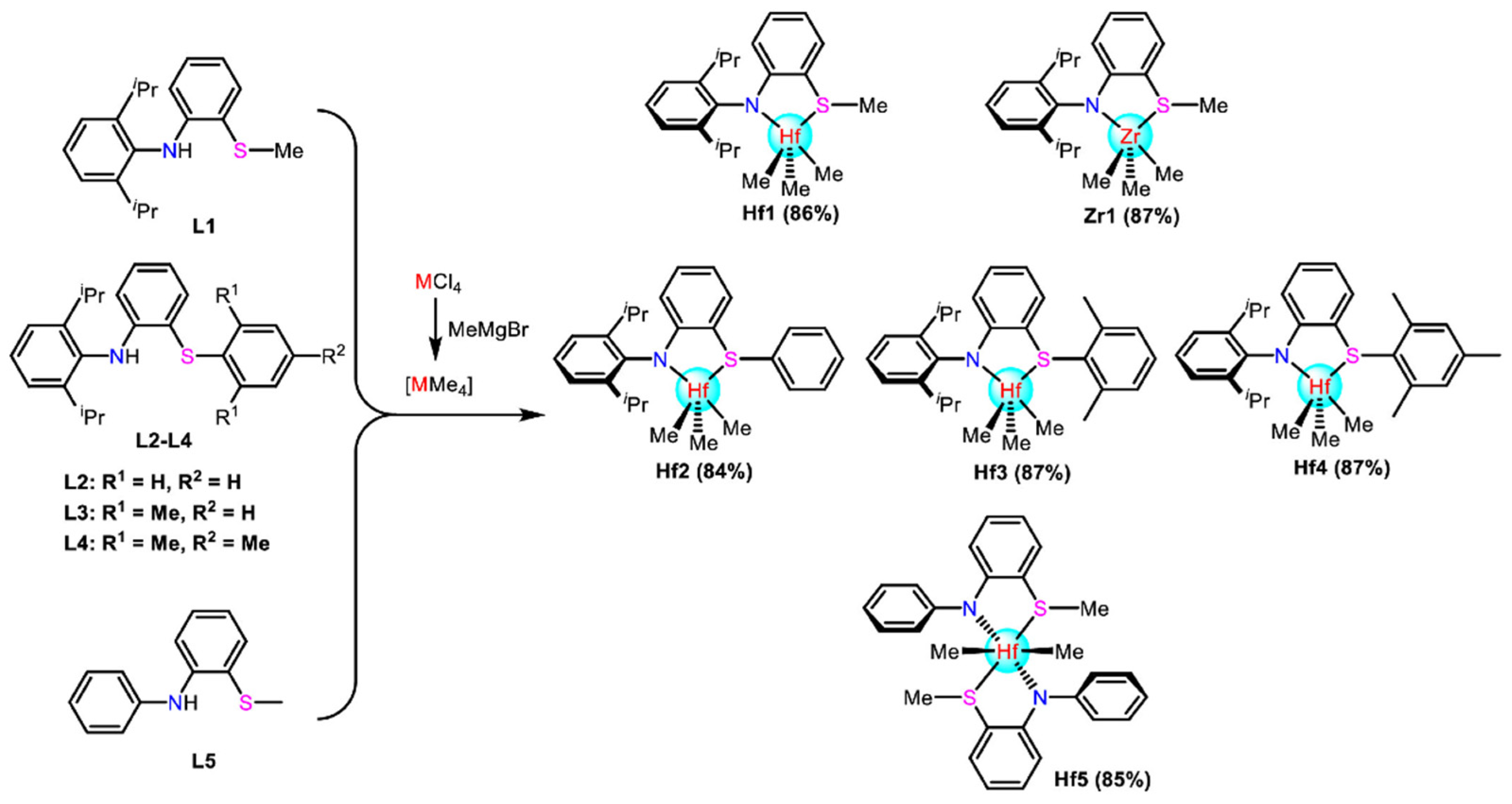
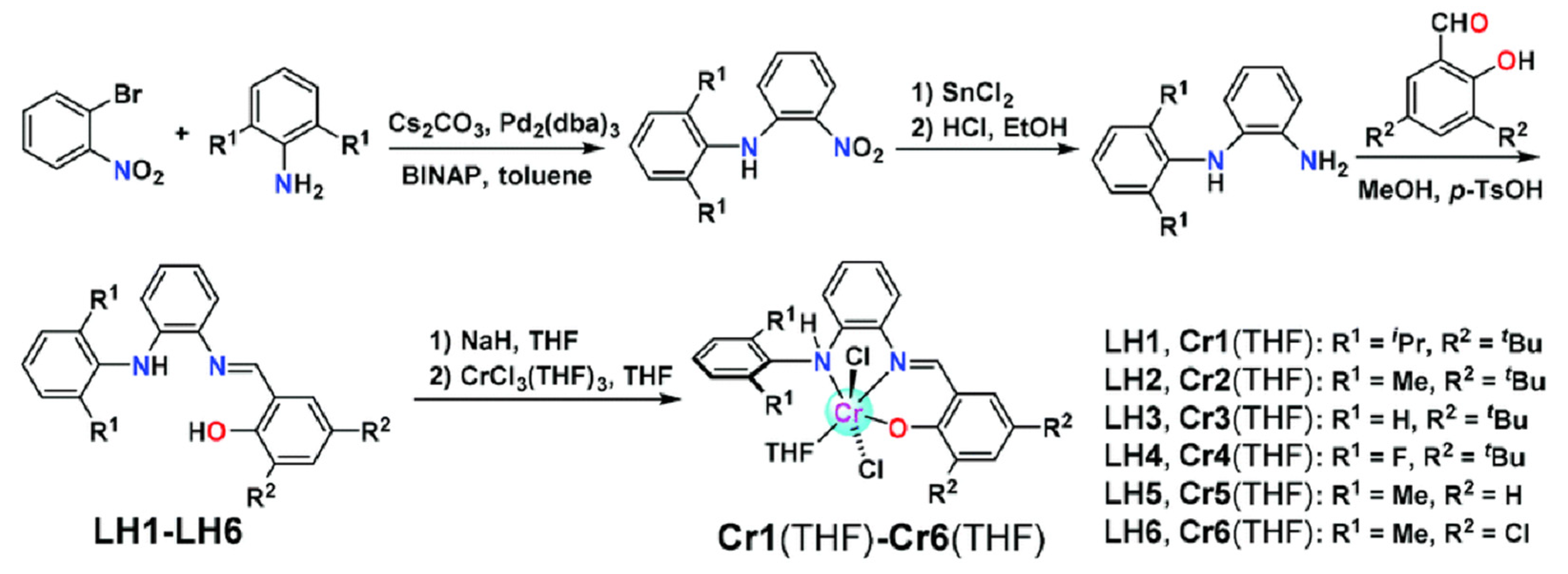
3. N,O and N,S-Bidentate Ligands
4. Tridentate Ligands
4.1. Phenoxy-imine-amino Ligands
4.2. Phenoxy-imine-quinoline Ligands
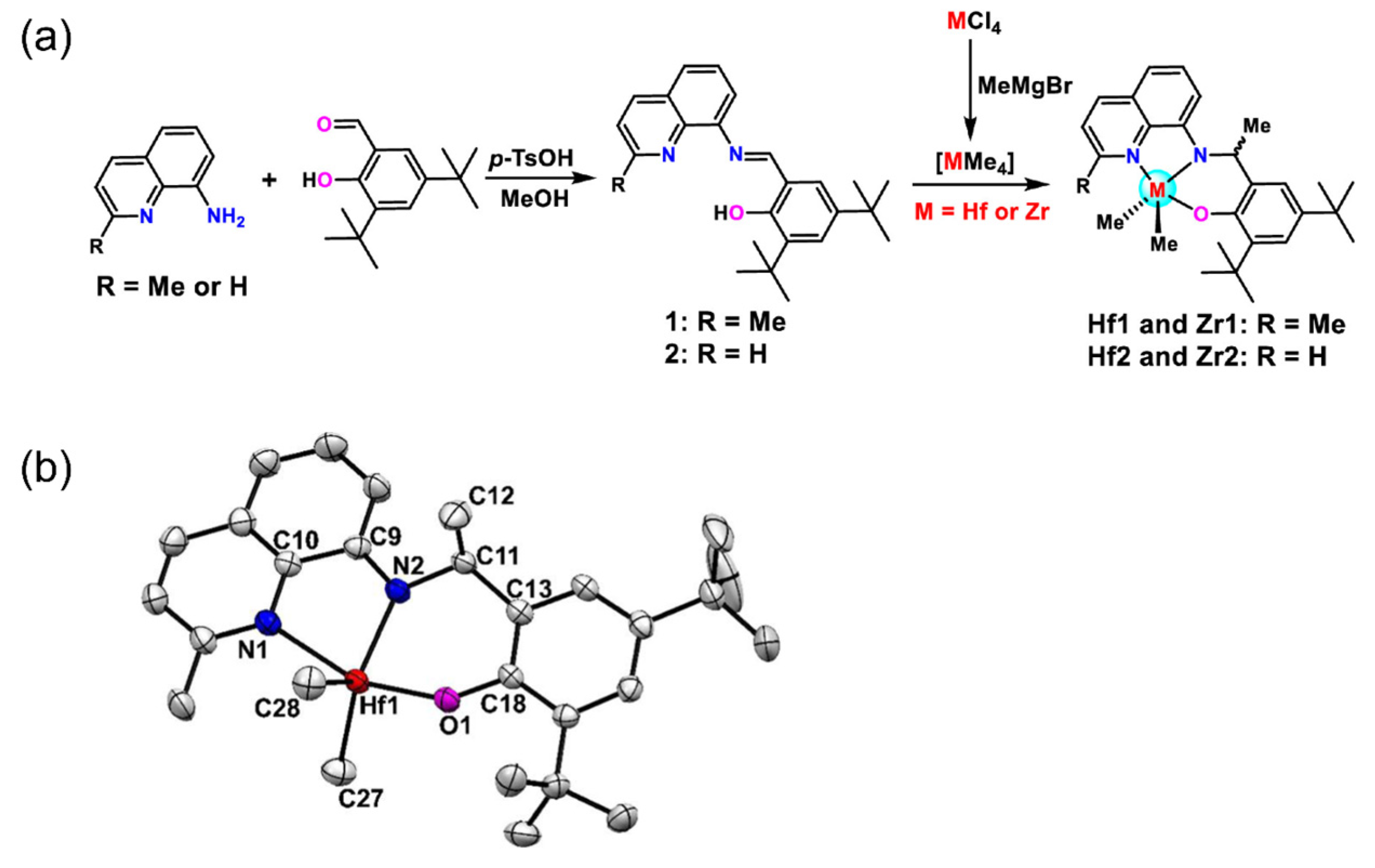
4.3. Quinoline-imine-thioether Ligands
4.4. Pyridine-amino Ligands
5. Influence of Metal Coordination
6. Outlook
- Explore new catalysts: The growing demand for POE products has led to an increased need for customized catalysts with tailored properties for various application scenarios, while maintaining the basic principles: ease of synthesis, cost-effectiveness, and environmental friendliness. Notably, the development cost of new catalysts can be significantly reduced by integrating virtual screening and machine learning technologies.
- Improve quinoline-based catalysts: The potential of high-thermostability quinolone derivatives has not yet been fully exploited. For further industrial applications, the low insertion rate of quinoline-based tridentate catalysts needs to be addressed immediately.
- Clarify structure–performance relationship: At present, the correlation between catalytic structure and performance remains insufficiently understood. Despite well-recognized electronic effects and steric hindrance capable of tuning the behavior of catalysts, it works only when comparing catalysts of the same types. There is still an absence of a structure–performance basis guiding the development of new catalyst skeletons. The lack of in-depth understanding of the catalytic mechanism constrains the improvement of current catalysts and the design of next-generation catalysts.
- Optimize costs: Further attention must be paid to the fabrication costs of catalysts with applicable value. Currently, most non-metallocene catalysts remain confined to laboratory research, with only a limited number employed in proprietary in-plant applications under confidentiality agreements. The absence of commercial pricing data presents challenges for comprehensive cost–benefit analysis. However, drawing parallels with the commercialization trajectory of metallocene catalysts, excessive production costs would inevitably diminish their market penetration potential. This requires intensive consideration from catalyst design to synthesis route selection and production landing: at the laboratory level, designing streamlined synthesis routes with reduced step counts and simpler reaction conditions, and at the industrial scale, optimizing the reaction and developing purification systems to improve the yield.
7. Conclusions
- (a)
- N,N-Bidentate catalysts (including imine-amino, imine-enamine, and quinoline-imine) are derived from diimine catalysts. In these complexes, an imine nitrogen atom participates in the metal center via coordination interactions, while the other nitrogen forms a covalent bond with metal. The incorporation of covalent bonding significantly enhances thermal stability. And the relatively open coordination geometry facilitates copolymer monomer insertion, endowing N,N-bidentate catalysts with performance metrics comparable to metallocene catalysts (imine-enamine). Notably, the synthesis conditions required for ligand preparation confer advantages in terms of industrial scale-up and cost-effectiveness, contrasting sharply with the strict anhydrous/oxygen-free environments typically mandated for metallocene ligand synthesis. Among these catalysts, quinoline-imine, although compromised in catalytic activity, deserves further attention as its high-temperature-resistant features allow higher efficiency of mass and heat transfer in industrial production.
- (b)
- N,O/S-Bidentate ligands, structurally characterized by phenoxy-imine or thioether-amine motifs, originate from FI catalyst derivatives. These systems generally display suboptimal thermal stability, catalytic activity, and copolymer monomer incorporation capabilities. Consequently, they are predominantly employed in combination with other catalysts for the production of OBC.
- (c)
- Tridentate ligands typically combine N,N-bidentate and N,O/S-bidentate, creating sterically crowded metal centers that severely restrict copolymer monomer insertion. This structural limitation confines their primary application to LLDPE production. A special case is the pyridine-amino type of catalysts, which achieve copolymerization capabilities equivalent to imine-enamine catalysts. Despite exhibiting polymerization activity one order of magnitude lower than imine-enamine systems, their unique synergy with FI catalysts in OBC manufacturing has attracted significant research attention.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| POE | Polyolefin elastomer |
| Z-N | Ziegler–Natta |
| MAO | Methylaluminoxane |
| Cp2ZrCl2 | dichlorodicyclopentadienyl zirconium |
| CGC | Constrained geometry catalyst |
| Cp | Cyclopentadienyl |
| EPOE | Ethylene-based polyolefin elastomer |
| OBC | Olefin block copolymer |
| PE | Polymer dispersity index |
| iPr | Isopropyl |
| Me | Methyl |
| PE | Polyethylene |
| FI | Phenoxy-imine |
| O4 | Four-oxygen coordination structure |
| M | Metal |
| LLDPE | Linear low-density polyethylene |
| THF | Tetrahydrofuran |
| GPC | Chromatography |
| TMA | Trimethylaluminum |
| COC | Copolymers of cycloolefin |
References
- Zanchin, G.; Leone, G. Polyolefin thermoplastic elastomers from polymerization catalysis: Advantages, pitfalls and future challenges. Prog. Polym. Sci. 2021, 113, 101342. [Google Scholar] [CrossRef]
- Zou, C.; Si, G.F.; Chen, C.L. A general strategy for heterogenizing olefin polymerization catalysts and the synthesis of polyolefins and composites. Nat. Commun. 2022, 13, 1954. [Google Scholar] [CrossRef]
- Gao, W.Q.; Chang, Y.L.; Zhou, Q.M.; Wang, Q.Y.; Lim, K.H.; Wang, D.L.; Hu, J.J.; Wang, W.J.; Li, B.G.; Liu, P.W. Chemical recycling of polyolefin waste: From the perspective of efficient pyrolysis reactors. Front. Chem. Sci. Eng. 2024, 18, 147. [Google Scholar] [CrossRef]
- Chung, T.C. Synthesis of functional polyolefin copolymers with graft and block structures. Prog. Polym. Sci. 2002, 27, 39–85. [Google Scholar] [CrossRef]
- Sun, M.H.; Xiao, Y.K.; Liu, K.; Yang, X.; Liu, P.W.; Jie, S.Y.; Hu, J.J.; Shi, S.B.; Wang, Q.Y.; Lim, K.H.; et al. Synthesis and characterization of polyolefin thermoplastic elastomers: A review. Can. J. Chem. Eng. 2023, 101, 4886–4906. [Google Scholar] [CrossRef]
- Shiraki, Y.; Saito, M.; Yamada, N.L.; Ito, K.; Yokoyama, H. Adhesion to untreated polyethylene and polypropylene by needle–like polyolefin crystals. Macromolecules 2023, 56, 2429–2436. [Google Scholar] [CrossRef]
- Wang, X.Y.; Gao, Y.S.; Tang, Y. Sustainable developments in polyolefin chemistry: Progress, challenges, and outlook. Prog. Polym. Sci. 2023, 143, 101713. [Google Scholar] [CrossRef]
- Qiao, J.L.; Guo, M.F.; Wang, L.S.; Liu, D.B.; Zhang, X.F.; Yu, L.Q.; Song, W.B.; Liu, Y.Q. Recent advances in polyolefin technology. Polym. Chem. 2011, 2, 1611–1623. [Google Scholar] [CrossRef]
- Stürzel, M.; Mihan, S.; Mülhaupt, R. From multisite polymerization catalysis to sustainable materials and all–polyolefin composites. Chem. Rev. 2016, 116, 1398–1433. [Google Scholar] [CrossRef]
- Thakur, A.K.; Gupta, S.K.; Chaudhari, P. Slurry–phase ethylene polymerization processes: A review on multiscale modeling and simulations. Rev. Chem. Eng. 2022, 38, 539–568. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhou, T.; Li, Y.Q.; Lu, X.; Guan, Y.B.; Cao, Y.C.; Cao, G.P. Microenvironment modulation of metal–organic frameworks (MOFs) for coordination olefin oligomerization and (co)polymerization. Small 2023, 19, 2205898. [Google Scholar] [CrossRef] [PubMed]
- Atan, M.F.; Hussain, M.A.; Abbasi, M.R.; Khan, M.J.H.; Patah, M.F.A. Advances in mathematical modeling of gas–phase olefin polymerization. Processes 2019, 7, 67. [Google Scholar] [CrossRef]
- Chen, L.Y.; Yang, H.J.; Sun, W.H. Advances of olefin polymerization in aqueous solutions. Prog. Chem. 2003, 15, 401–408. [Google Scholar]
- Pladis, P.; Kanellopoulos, V.; Chatzidoukas, C.; Kiparissides, C. Effect of reaction conditions and catalyst design on the rheological properties of polyolefins produced in gas–phase olefin polymerization reactors. Macromol. Theory Simul. 2008, 17, 478–487. [Google Scholar] [CrossRef]
- Kaminsky, W.; Müller, F.; Sperber, O. Comparison of olefin polymerization processes with metallocene catalysts. Macromol. Mater. Eng. 2005, 290, 347–352. [Google Scholar] [CrossRef]
- Wu, C.J.; Ren, M.Q.; Hou, L.P.; Qu, S.Z.; Li, X.W.; Zheng, C.; Chen, J.; Wang, W. Ethylene copolymerization with linear and end–cyclized olefins via a metallocene catalyst: Polymerization behavior and thermal Properties of copolymers. Engineering 2023, 30, 93–99. [Google Scholar] [CrossRef]
- Szot, W.; Chakraborty, D.; Bouyahyi, M.; Jasinska–Walc, L.; Duchateau, R. Functionalized polyolefins produced by post–metallocenes; High added value materials, but can they be produced efficiently? Macromolecules 2024, 57, 10996–11006. [Google Scholar] [CrossRef]
- Li, F.; Liu, W.F. Progress in the catalyst for ethylene/α–olefin copolymerization at high temperature. Can. J. Chem. Eng. 2023, 101, 4992–5019. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R. Development of group IV molecular catalysts for high temperature ethylene–α–plefin copolymerization reactions. Acc. Chem. Res. 2015, 48, 2004–2016. [Google Scholar] [CrossRef]
- Jiang, X.B.; He, A.H. Stereospecific polymerization of olefins with supported Ziegler—Natta catalysts. Polym. Int. 2014, 63, 179–183. [Google Scholar] [CrossRef]
- Hagen, H.; Boersma, J.; van Koten, G. Homogeneous vanadium–based catalysts for the Ziegler–Natta polymerization of α–olefins. Chem. Soc. Rev. 2002, 31, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.M.; Gupta, V.K. Progress in MgCl2 supported Ziegler–Natta catalyzed polyolefin products and applications. J. Polym. Res. 2021, 28, 45. [Google Scholar] [CrossRef]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler–Natta and metallocene catalysts on polyolefin structure, properties, and processing ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef] [PubMed]
- Sinn, H.; Kaminsky, W.; Vollmer, H.J.; Woldt, R. “Living polymers” on polymerization with extremely productive Ziegler catalysts. Angew. Chem. Int. Ed. 1980, 19, 390–392. [Google Scholar] [CrossRef]
- Kaminsky, W. Metallocene based polyolefin nanocomposites. Materials 2014, 7, 1995–2013. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.Z.; Fu, Z.S.; Fan, Z.Q. Immobilization of metallocene catalysts. Prog. Chem. 2014, 26, 737–748. [Google Scholar]
- Chen, C.L. Designing catalysts for olefin polymerization and copolymerization: Beyond electronic and steric tuning. Nat. Rev. Chem. 2018, 2, 6–14. [Google Scholar] [CrossRef]
- Wang, B.Q. Ansa–metallocene polymerization catalysts: Effects of the bridges on the catalytic activities. Coord. Chem. Rev. 2006, 250, 242–258. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Jin, G.X. Polymerized metallocene catalysts and late transition metal catalysts for ethylene polymerization. Coord. Chem. Rev. 2006, 250, 95–109. [Google Scholar] [CrossRef]
- Qu, S.Z.; Zhang, T.Y.; Wang, W. Olefin Polymerization with nitrogen–coordinated half–metallocene catalyst systems. Prog. Chem. 2019, 31, 929–938. [Google Scholar]
- Stevens, J.C.; Timmers, F.J.; Wilson, D.R.; Schmidt, G.F.; Nickias, P.N.; Rosen, R.K.; Knight, G.W.; Lai, S. Constrained Geometry Addition Polymerization Catalysts, Processes for Their Preparation, Precursors Therefor, Methods of Use, and Novel Polymers Formed Therewith. European Patent EP0416815B1, 19 October 1997. [Google Scholar]
- Canich, J.A.M.W.; Licciardi, G.F. Mono–Cp Heteroatom Containing Group IVB Transition Metal Complexes with MAO: Supported Catalyst for Olefin Polymerization. U.S. Patent US5057475, 15 October 1989. [Google Scholar]
- Klosin, J.; Kruper, W.J.; Nickias, P.N.; Roof, G.R.; De Waele, P.; Abboud, K.A. Heteroatom–substituted constrained–geometry complexes. Dramatic substituent effect on catalyst efficiency and polymer molecular weight. Organometallics 2001, 20, 2663–2665. [Google Scholar] [CrossRef]
- Miller, S.A.; Bercaw, J.E. Highly stereoregular syndiotactic polypropylene formation with metallocene catalysts via influence of distal ligand substituents. Organometallics 2004, 23, 1777–1789. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, E.J.; Jung, S.; Ha, J.J.; Seo, B.; Lee, B.Y.; Joung, U.G.; Joe, D.J. Transition Metal Complexes, Catalyst Compositions Containing the Same, and Olefin Polymerization Using the Catalyst Compositions. U.S. Patent US7538239, 12 May 2009. [Google Scholar]
- Braunschweig, H.; Breitling, F.M. Constrained geometry complexes—Synthesis and applications. Coord. Chem. Rev. 2006, 250, 2691–2720. [Google Scholar] [CrossRef]
- Wen, Z.; Wu, C.J.; Chen, J.; Qu, S.Z.; Li, X.W.; Wang, W. Homogeneous non–metallocene group 4 metals ligated with N,N bidentate ligand(s) for olefin polymerization. Polymers 2024, 16, 406. [Google Scholar] [CrossRef] [PubMed]
- Budagumpi, S.; Kim, K.H.; Kim, I. Catalytic and coordination facets of single–site non–metallocene organometallic catalysts with N–heterocyclic scaffolds employed in olefin polymerization. Coord. Chem. Rev. 2011, 255, 2785–2809. [Google Scholar] [CrossRef]
- Antonov, A.A.; Bryliakov, K.P. Post–metallocene catalysts for the synthesis of ultrahigh molecular weight polyethylene: Recent advances. Eur. Polym. J. 2021, 142, 110162. [Google Scholar] [CrossRef]
- Vittoria, A.; Kulyabin, P.S.; Antinucci, G.; Iashin, A.N.; Uborsky, D.V.; Cuthbert, E.N.T.; Budzelaar, P.H.M.; Voskoboynikov, A.Z.; Cipullo, R.; Ehm, C.; et al. The interplay of backbone stiffening and active pocket design in bis(phenolate–ether) Zr/Hf propene polymerization catalysts. ACS Catal. 2023, 13, 13151–13155. [Google Scholar] [CrossRef]
- Mishra, A.; Patil, H.R.; Gupta, V. Progress in propylene homo- and copolymers using advanced transition metal catalyst systems. New J. Chem. 2021, 45, 10577–10588. [Google Scholar] [CrossRef]
- Patil, A.O.; Hlatky, G.G. Beyond metallocenes: Next–generation polymerization catalysts. In Beyond Metallocenes: Next Generation Polymerization Catalysts; Patil, A.O., Hlatky, G.G., Eds.; Amer Chemical Soc: Washington, DC, USA, 2003; Volume 857, pp. 1–11. [Google Scholar]
- Ochedzan–Siodlak, W.; Dziubek, K. Metallocenes and post–metallocenes immobilized on ionic liquid–modified silica as catalysts for polymerization of ethylene. Appl. Catal. A—Gen. 2014, 484, 134–141. [Google Scholar] [CrossRef]
- Baier, M.C.; Zuideveld, M.A.; Mecking, S. Post–metallocenes in the industrial production of polyolefins. Angew. Chem. Int. Ed. 2014, 53, 9722–9744. [Google Scholar] [CrossRef]
- Konkol, M.; Okuda, J. Non–metallocene hydride complexes of the rare–earth metals. Coord. Chem. Rev. 2008, 252, 1577–1591. [Google Scholar] [CrossRef]
- Chen, Z.; Brookhart, M. Exploring ethylene/polar vinyl monomer copolymerizations using Ni and Pd α–diimine catalysts. Acc. Chem. Res. 2018, 51, 1831–1839. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Chen, C.L. Emerging palladium and nickel catalysts for copolymerization of olefins with polar monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Chen, C.L. A continuing legend: The Brookhart–type α–diimine nickel and palladium catalysts. Polym. Chem. 2019, 10, 2354–2369. [Google Scholar] [CrossRef]
- Qasim, M.; Bashir, M.S.; Iqbal, S.; Mahmood, Q. Recent advancements in α–diimine–nickel and –palladium catalysts for ethylene polymerization. Eur. Polym. J. 2021, 160, 110783. [Google Scholar] [CrossRef]
- Mahmood, Q.; Sun, W.H. N,N–chelated nickel catalysts for highly branched polyolefin elastomers: A survey. R. Soc. Open Sci. 2018, 5, 180367. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New pd(ii)–based and ni(ii)–based catalysts for polymerization of ethylene and alpha–olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Fang, J.; Sui, X.L.; Li, Y.G.; Chen, C.L. Synthesis of polyolefin elastomers from unsymmetrical –diimine nickel catalyzed olefin polymerization. Polym. Chem. 2018, 9, 4143–4149. [Google Scholar] [CrossRef]
- Guo, L.H.; Lian, K.B.; Kong, W.Y.; Xu, S.; Jiang, G.R.; Dai, S.Y. Synthesis of various branched ultra–high–molecular–weight polyethylenes using sterically hindered acenaphthene–based α–diimine Ni(II) catalysts. Organometallics 2018, 37, 2442–2449. [Google Scholar] [CrossRef]
- Gong, Y.F.; Li, S.K.; Gong, Q.; Zhang, S.J.; Liu, B.Y.; Dai, S.Y. Systematic investigations of ligand steric effects on α–diimine nickel catalyzed olefin polymerization and copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Guo, L.H.; Sun, W.T.; Li, S.K.; Xu, G.; Dai, S. Bulky yet flexible substituents in insertion polymerization with α–diimine nickel and palladium systems. Polym. Chem. 2019, 10, 4866–4871. [Google Scholar] [CrossRef]
- Dai, S.Y.; Li, S.K.; Xu, G.Y.; Wu, C.; Liao, Y.D.; Guo, L.H. Flexible cycloalkyl substituents in insertion polymerization with α–diimine nickel and palladium species. Polym. Chem. 2020, 11, 1393–1400. [Google Scholar] [CrossRef]
- De Waele, P.; Jazdzewski, B.A.; Klosin, J.; Murray, R.E.; Theriault, C.N.; Vosejpka, P.C.; Petersen, J.L. Synthesis of hafnium and zirconium imino–amido complexes from bis–imine ligands. A new family of olefin polymerization catalysts. Organometallics 2007, 26, 3896–3899. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Jazdzewski, B.A.; Klosin, J.; Kuhlman, R.L.; Theriault, C.N.; Welsh, D.M.; Abboud, K.A. Imino–amido Hf and Zr complexes: Synthesis, isomerization, and olefin polymerization. Organometallics 2011, 30, 251–262. [Google Scholar] [CrossRef]
- Zhang, C.M.; Pan, H.Q.; Klosin, J.; Tu, S.Y.; Jaganathan, A. Synthetic optimization and scale–up of imino–amido hafnium and zirconium olefin polymerization catalysts. Org. Process Res. Dev. 2015, 19, 1383–1391. [Google Scholar] [CrossRef]
- Figueroa, R.; Froese, R.D.; He, Y.Y.; Klosin, J.; Theriault, C.N.; Abboud, K.A. Synthesis of imino–enamido hafnium and zirconium complexes: A new family of olefin polymerization catalysts with ultrahigh–molecular–weight capabilities. Organometallics 2011, 30, 1695–1709. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Figueroa, R.; McCann, S.D.; Mort, D.; Klosin, J. Synthesis and scale–up of imino–enamido hafnium and zirconium olefin polymerization catalysts. Organometallics 2013, 32, 2963–2972. [Google Scholar] [CrossRef]
- Klosin, J.; Fontaine, P.P.; Figueroa, R.; McCann, S.D.; Mort, D. Preparation of new olefin polymerization precatalysts by facile derivatization of imino–enamido ZrMe3 and HfMe3 complexes. Organometallics 2013, 32, 6488–6499. [Google Scholar] [CrossRef]
- Szuromi, E.; Klosin, J.; Abboud, K.A. Aminotroponiminato hafnium and zirconium complexes: Synthesis and ethylene/1–octene copolymerization study. Organometallics 2011, 30, 4589–4597. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Klosin, J.; McDougal, N.T. Hafnium amidoquinoline complexes: Highly active olefin polymerization catalysts with ultrahigh molecular weight capacity. Organometallics 2012, 31, 6244–6251. [Google Scholar] [CrossRef]
- Invergo, A.M.; Liu, S.F.; Dicken, R.D.; Mouat, A.R.; Delferro, M.; Lohr, T.L.; Marks, T.J. How close is too close? Polymerization behavior and monomer–dependent reorganization of a bimetallic salphen organotitanium catalyst. Organometallics 2018, 37, 2429–2436. [Google Scholar] [CrossRef]
- Fontaine, P.P.; Ueligger, S.; Klosin, J.; Hazari, A.; Daller, J.; Hou, J.B. Development of improved amidoquinoline polyolefin catalysts with ultrahigh molecular weight capacity. Organometallics 2015, 34, 1354–1363. [Google Scholar] [CrossRef]
- Han, B.H.; Liu, Y.X.; Feng, C.Y.; Liu, S.F.; Li, Z.B. Development of group 4 metal complexes bearing fused–ring amido–trihydroquinoline ligands with improved high–temperature catalytic performance toward olefin (co)polymerization. Organometallics 2021, 40, 242–252. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, M.X.; Liu, S.F.; Li, Z.B. Synthesis of amido–quinoline hafnium and zirconium complexes with improved performances toward ethylene/1–octene copolymerization. Organometallics 2024, 43, 2472–2479. [Google Scholar] [CrossRef]
- Younkin, T.R.; Conner, E.F.; Henderson, J.I.; Friedrich, S.K.; Grubbs, R.H.; Bansleben, D.A. Neutral, single–component nickel (II) polyolefin catalysts that tolerate heteroatoms. Science 2000, 287, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hustad, P.D.; Coates, G.W. A new catalyst for highly syndiospecific living olefin polymerization: Homopolymers and block copolymers from ethylene and propylene. J. Am. Chem. Soc. 2001, 123, 5134–5135. [Google Scholar] [CrossRef]
- Matsui, S.; Mitani, M.; Saito, J.; Tohi, Y.; Makio, H.; Matsukawa, N.; Takagi, Y.; Tsuru, K.; Nitabaru, M.; Nakano, T.; et al. A family of zirconium complexes having two phenoxy–imine chelate ligands for olefin polymerization. J. Am. Chem. Soc. 2001, 123, 6847–6856. [Google Scholar] [CrossRef]
- Ishii, S.; Mitani, M.; Saito, J.; Matsuura, S.; Furuyama, R.; Fujita, T. Ethylene polymerization behavior of polymethylene–bridged bis (phenoxy–imine) Zr complexes. In Science and Technology in Catalysis 2002; Anpo, M., Onaka, M., Yamashita, H., Eds.; Kodansha Ltd.: Tokyo, Japan, 2003; Volume 145, pp. 49–54. [Google Scholar]
- Boussie, T.R.; Brümmer, O.; Diamond, G.M.; Goh, C.; Lapointe, A.M.; Leclerc, M.K.; Shoemaker, J.A. Bridged Bi–Aromatic Ligands, Catalysts, Processes for Polymerizing and Polymers Therefrom. U.S. Patent US6841502, 22 March 2005. [Google Scholar]
- Salata, M.R.; Marks, T.J. Catalyst nuclearity effects in olefin polymerization. enhanced activity and comonomer enchainment in ethylene plus olefin copolymerizations mediated by bimetallic group 4 phenoxyiminato catalysts. Macromolecules 2009, 42, 1920–1933. [Google Scholar] [CrossRef]
- Salata, M.R.; Marks, T.J. Synthesis, characterization, and marked polymerization selectivity characteristics of binuclear phenoxyiminato organozirconium catalysts. J. Am. Chem. Soc. 2008, 130, 12–13. [Google Scholar] [CrossRef]
- Khoshsefat, M.; Ma, Y.P.; Sun, W.H. Multinuclear late transition metal catalysts for olefin polymerization. Coord. Chem. Rev. 2021, 434, 213788. [Google Scholar] [CrossRef]
- Liu, S.F.; Xing, Y.H.; Zheng, Q.D.; Jia, Y.T.; Li, Z.B. Synthesis of anthracene–bridged dinuclear phenoxyiminato organotitanium catalysts with enhanced activity, thermal stability, and comonomer incorporation ability toward ethylene (co)polymerization. Organometallics 2020, 39, 3268–3274. [Google Scholar] [CrossRef]
- Xing, Y.H.; Xu, L.L.; Liu, S.F.; Li, Z.B. Dinuclear group 4 metal complexes bearing anthracene–bridged bifunctional amido–ether ligands: Remarkable metal effect and cooperativity toward ethylene/1–octene copolymerization. Inorg. Chem. 2023, 62, 2859–2869. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.S.; Christianson, M.D.; Wang, Y.; Chen, J.Z.; Marshall, S.; Klosin, J.; Lohr, T.L.; Marks, T.J. Unexpected precatalyst σ–ligand effects in phenoxyimine zr–catalyzed ethylene/1–octene copolymerizations. J. Am. Chem. Soc. 2019, 141, 7822–7830. [Google Scholar] [CrossRef]
- Xiang, S.; Zhang, J.B.; Liu, S.F.; Braunstein, P.; Li, Z.B. Aluminum complexes bearing aminoquinoline ligands with different pendant donors: Remarkable impact of hemilability on the ring–opening polymerization of ε–caprolactone. Macromolecules 2024, 57, 3604–3613. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.F.; Tao, W.J.; Sun, X.L.; Yang, X.H.; Tang, Y. Copolymerization of ethylene with functionalized olefins by ONX titanium complexes. Macromolecules 2013, 46, 2870–2875. [Google Scholar] [CrossRef]
- Yang, X.H.; Liu, C.R.; Wang, C.; Sun, X.L.; Guo, Y.H.; Wang, X.K.; Wang, Z.; Xie, Z.W.; Tang, Y. [O–NSR]TiCl3–catalyzed copolymerization of ethylene with functionalized olefins. Angew. Chem. Int. Ed. 2009, 48, 8099–8102. [Google Scholar] [CrossRef]
- Liu, C.H.; Feng, W.Z.; Liu, S.F.; Kan, Z.; Li, Z.B. Development of group iv metal complexes bearing thioether–amido ligands with enhanced high–temperature catalytic performance toward olefin copolymerization. Inorg. Chem. 2024, 63, 19676–19686. [Google Scholar] [CrossRef]
- Tian, J.L.; Zhang, X.W.; Liu, S.F.; Li, Z.B. Chromium complexes supported by NNO–tridentate ligands: An unprecedented activity with the requirement of a small amount of MAO. Polym. Chem. 2022, 13, 1852–1860. [Google Scholar] [CrossRef]
- Tian, J.L.; Feng, W.Z.; Liu, S.F.; Li, Z.B. Titanium complexes bearing NNO–tridentate ligands: Highly active olefin polymerization catalysts with great control on molecular weight and distribution. Chin. J. Chem. 2022, 40, 2785–2793. [Google Scholar] [CrossRef]
- Gao, Z.H.; Tian, J.L.; Han, Y.X.; Liu, S.F.; Li, Z.B. Zirconium and hafnium complexes bearing tridentate ONN–ligands: Extremely high activity toward ethylene (co)polymerization. Inorg. Chem. 2024, 63, 18137–18145. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.Z.; Liu, C.H.; Liu, S.F.; Li, Z.B. Hafnium and zirconium complexes bearing ONN–tridentate ligands and their catalytic properties toward olefin polymerization. Organometallics 2023, 42, 3466–3473. [Google Scholar] [CrossRef]
- Wang, D.Q.; Zhou, S.M.; Liu, Y.X.; Kang, X.H.; Liu, S.F.; Li, Z.B.; Braunstein, P. Controlling polyethylene molecular weights and distributions using chromium complexes supported by SNN–tridentate ligands. Macromolecules 2022, 55, 2433–2443. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhou, S.M.; Yang, W.Q.; Liu, S.F. Hafnium and zirconium complexes bearing SNN–ligands enhancing catalytic performances toward ethylene/1–octene copolymerization. Organometallics 2022, 41, 3724–3731. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic production of olefin block copolymers via chain shuttling polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef]
- Frazier, K.A.; Froese, R.D.; He, Y.Y.; Klosin, J.; Theriault, C.N.; Vosejpka, P.C.; Zhou, Z.; Abboud, K.A. Pyridylamido hafnium and zirconium complexes: Synthesis, dynamic behavior, and ethylene/1–octene and propylene polymerization reactions. Organometallics 2011, 30, 3318–3329. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Jiang, H.; Chen, A.; Zou, C. Bidentate pyridyl–amido hafnium catalysts for copolymerization of ethylene with 1–octene and norbornene. Eur. J. Inorg. Chem. 2023, 26, e202200725. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Ma, Z.S.; Li, C.; Zou, C. Designing easily accessible tridentate hafnium catalysts for ethylene/1–octene copolymerization. Polymer 2024, 294, 126699. [Google Scholar] [CrossRef]
- Naga, N.; Imanishi, Y. Copolymerization of ethylene and cyclopentene with zirconocene catalysts: Effect of ligand structure of zirconocenes. Macromol. Chem. Phys. 2002, 203, 159–165. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Vijayakrishna, K.; Mani, R. Copolymerization of ethylene and norbornene by zirconium complexes containing symmetrically tuned trianionic ligands. Polym. Bull. 2010, 65, 13–23. [Google Scholar] [CrossRef]
- Zhang, C.G.; Zhang, L.Y.; Li, H.Y.; Yu, S.Y.; Wang, Z.X. Differences between insertions of ethylene into metallocene and non-metallocene ethylene polymerization catalysts. J. Phys. Org. Chem. 2013, 26, 70–76. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Y.; Lan, Z.; You, Q.L.; Li, T.C.; Li, X.D.; Zhang, D.H.; Xie, G.Y. A Methylene-bridged salicylaldiminato tridentate ONS binuclear titanium complex for ethylene-norbornene copolymerization. J. Macromol. Sci. Part A-Pure Appl. Chem. 2018, 55, 489–495. [Google Scholar] [CrossRef]
- Wang, J.; Nan, F.; Guo, J.P.; Wang, J.; Shi, X.H.; Huang, H.B.; Huang, Q.G.; Yang, W.T. Copolymers of Ethylene and Vinyl Amino Acidic Ester with High Molecular Weight Prepared by Non-metallocene Catalysts. Catal. Lett. 2016, 146, 609–619. [Google Scholar] [CrossRef]
- Patel, N.; Valodkar, V.; Tembe, G. Recent developments in catalyst systems for selective oligomerization and polymerization of higher α-olefins. Polym. Chem. 2023, 14, 2542–2571. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Y.; Rodriguez-Delgado, A.; Chen, E.Y.X. Neutral Metallocene Ester Enolate and Non-Metallocene Alkoxy Complexes of Zirconium for Catalytic Ring-Opening Polymerization of Cyclic Esters. Organometallics 2008, 27, 5632–5640. [Google Scholar] [CrossRef]
- Capacchione, C.; Proto, A.; Ebeling, H.; Mülhaupt, R.; Möller, K.; Manivannan, R.; Spaniol, T.P.; Okuda, J. Non-metallocene catalysts for the styrene polymerization:: Isospecific group 4 metal bis(phenolate) catalysts. J. Mol. Catal. A-Chem. 2004, 213, 137–140. [Google Scholar] [CrossRef]
- Agrawal, D.; Shrivastava, Y.; De, S.K.; Singh, P.K. Synthesis of post-metallocene catalyst and study of its olefin polymerization activity at room temperature in aqueous solution followed by prediction of yield. J. Polym. Res. 2019, 26, 167. [Google Scholar] [CrossRef]
- Bialek, M.; Fryga, J. Copolymerization of Ethylene with Selected Vinyl Monomers Catalyzed by Group 4 Metal and Vanadium Complexes with Multidentate Ligands: A Short Review. Polymers 2021, 13, 4456. [Google Scholar] [CrossRef]
- Rishina, L.A.; Kissin, Y.V.; Lalayan, S.S.; Gagieva, S.C.; Tuskaev, V.A.; Krasheninnikov, V.G. Polymerization of alkenes with a post-metallocene catalyst containing a titanium complex with an oxyquinolinyl ligand. J. Polym. Sci. Pol. Chem. 2017, 55, 1844–1854. [Google Scholar] [CrossRef]
- Rishina, L.A.; Lalayan, S.S.; Gagieva, S.C.; Tuskaev, V.A.; Perepelitsyna, E.O.; Kissin, Y.V. Polymers of propylene and higher 1-alkenes produced with post-metallocene complexes containing a saligenin-type ligand. Polymer 2013, 54, 6526–6535. [Google Scholar] [CrossRef]
- Gagieva, S.C.; Kurmaev, D.A.; Tuskaev, V.A.; Khrustalev, V.N.; Churakov, A.V.; Golubev, E.K.; Sizov, A.I.; Zvukova, T.M.; Buzin, M.I.; Nikiforova, G.G.; et al. First example of cationic titanium (III) complexes with crown ether as catalysts for ethylene polymerization. Eur. Polym. J. 2022, 170, 111166. [Google Scholar] [CrossRef]
- Hayatifar, M.; Pampaloni, G.; Bernazzani, L.; Capacchione, C.; Kissin, Y.V.; Galletti, A.M.R. A new post-metallocene catalyst for alkene polymerization: Copolymerization of ethylene and 1-hexene with titanium complexes bearing N,N-dialkylcarbamato ligands. Polym. Int. 2014, 63, 560–567. [Google Scholar] [CrossRef]
- Baek, J.W.; Kwon, S.J.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Preparation of Half- and Post-Metallocene Hafnium Complexes with Tetrahydroquinoline and Tetrahydrophenanthroline Frameworks for Olefin Polymerization. Polymers 2019, 11, 1093. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Yamaguchi, T.; Takenaka, K. Catalytic Behavior of Half-Metallocene Non-Cp-Type Group 4 Metal Complexes (M=Zr, Hf) with a Cyclooctatetraenyl Dianion during Olefin Polymerization. Eur. J. Inorg. Chem. 2024, 27, e202300545. [Google Scholar] [CrossRef]
- Nakata, N.; Saito, Y.; Watanabe, T.; Ishii, A. Completely Isospecific Polymerization of 1-Hexene Catalyzed by Hafnium(IV) Dichloro Complex Incorporating with an OSSO -type Bis(phenolate) Ligand. Top. Catal. 2014, 57, 918–922. [Google Scholar] [CrossRef]
- Urciuoli, G.; Zaccaria, F.; Zuccaccia, C.; Cipullo, R.; Budzelaar, P.H.M.; Vittoria, A.; Ehm, C.; Macchioni, A.; Busico, V. Cocatalyst effects in Hf-catalysed olefin polymerization: Taking well-defined Al-alkyl borate salts into account. Dalton Trans. 2024, 53, 2286–2293. [Google Scholar] [CrossRef]
- Zhou, G.L.; Mu, H.L.; Jian, Z.B. A comprehensive picture on catalyst structure construction in palladium catalyzed ethylene (co)polymerizations. J. Catal. 2020, 383, 215–220. [Google Scholar] [CrossRef]
- Zheng, H.D.; Gao, H.Y. Noncovalent Interactions in Late Transition Metal-Catalyzed Polymerization of Olefins. Macromolecules 2024, 57, 6899–6913. [Google Scholar] [CrossRef]
- Song, Z.H.; Wang, S.C.; Gao, R.; Wang, Y.; Gou, Q.Q.; Zheng, G.; Feng, H.S.; Fan, G.Q.; Lai, J.J. Recent Advancements in Mechanistic Studies of Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Polymers 2023, 15, 4343. [Google Scholar] [CrossRef]
- Guo, L.H.; Liu, W.J.; Chen, C.L. Late transition metal catalyzed α-olefin polymerization and copolymerization with polar monomers. Mat. Chem. Front. 2017, 1, 2487–2494. [Google Scholar] [CrossRef]
- Deng, H.Y.; Zheng, H.D.; Gao, H.; Pei, L.X.; Gao, H.Y. Late Transition Metal Catalysts with Chelating Amines for Olefin Polymerization. Catalysts 2022, 12, 22. [Google Scholar] [CrossRef]
- Mao, G.L.; Jiang, Y.; Li, N.; Wang, Q.; Zheng, D.J.; Li, M.J.; Ning, Y.N. Recent progresses of late-transition metal complexes with nonsymmetric diimine ligands in ethylene polymerization and oligomerization. Chin. Sci. Bull. 2014, 59, 2505–2512. [Google Scholar] [CrossRef]
- Zohuri, G.H.; Seyedi, S.M.; Sandaroos, R.; Damavandi, S.; Mohammadi, A. Novel Late Transition Metal Catalysts Based on Iron: Synthesis, Structures and Ethylene Polymerization. Catal. Lett. 2010, 140, 160–166. [Google Scholar] [CrossRef]
- Liang, T.; Goudari, S.B.; Chen, C.L. A simple and versatile nickel platform for the generation of branched high molecular weight polyolefins. Nat. Commun. 2020, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.X.; Zhou, J.J.; Zhu, N.N.; Peng, D.; Chen, C.L. Heterogeneous Nickel Catalysts for the Synthesis of Ethylene-Based Polyolefin Elastomers. Macromolecules 2024, 57, 1080–1086. [Google Scholar] [CrossRef]
- Mitchell, N.E.; Long, B.K. Recent advances in thermally robust, late transition metal-catalyzed olefin polymerization. Polym. Int. 2019, 68, 14–26. [Google Scholar] [CrossRef]
- Yue, Q.; Gao, R.; Song, Z.H.; Gou, Q.Q. Recent Advancements in the Synthesis of Ultra-High Molecular Weight Polyethylene via Late Transition Metal Catalysts. Polymers 2024, 16, 1688. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, H.X.; Wang, K.T.; Wu, G.; Li, Y.B. Efficient Preparation of Cyclic Olefin Copolymers with Unreacted Double Bonds by Using Thermal Stable Non-Metallocene Vanadium Catalytic System. Macromol. Chem. Phys. 2019, 220, 1900008. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z.F.; Li, X.W.; Chen, J.; Qu, S.Z.; Wei, Y.M.; Wen, Z.; Wang, W. Copolymerization of Ethylene With Polar Monomers by Group 4 Catalysts. Polym. Rev. 2024, 2444674. [Google Scholar] [CrossRef]
- Chae, C.G.; Ryu, J.Y.; Ho, L.N.T.; Lee, W.; Kim, D.G.; Park, S.; Park, B.K.; Chung, T.M.; Kim, Y.S. Vinyl-addition copolymerization of norbornene and 1-octene catalyzed by a non-bridged half-titanocene with a tetrazole-phenoxy ligand. Polymer 2024, 295, 126679. [Google Scholar] [CrossRef]
- Bahena, E.N.; Schafer, L.L. From Stoichiometric to Catalytic E-H Functionalization by Non- Metallocene Zirconium Complexes?Recent Advances and Mechanistic Insights. ACS Catal. 2022, 12, 14934–14953. [Google Scholar] [CrossRef]
- Asim, W.; Waheeb, A.S.; Awad, M.A.; Kadhum, A.M.; Ali, A.; Mallah, S.H.; Iqbal, M.A.; Kadhim, M.M. Recent advances in the synthesis of zirconium complexes and their catalytic applications. J. Mol. Struct. 2022, 1250, 131925. [Google Scholar] [CrossRef]
- Eguchi, K.; Aoyagi, K.; Nakajima, Y.; Ando, W.; Sato, K.; Shimada, S. Synthesis and Structure of Metallocene-type Ti(III) Complexes, and Their Catalytic Activity towards Olefin Hydrosilylation Reactions. Chem. Lett. 2017, 46, 1262–1264. [Google Scholar] [CrossRef]
- Furayama, R.; Saito, J.; Ishii, S.; Mitani, M.; Matsui, S.; Tohi, Y.; Makio, H.; Matsukawa, N.; Tanaka, H.; Fujita, T. Ethylene and propylene polymerization behavior of a series of bis(phenoxy-imine)titanium complexes. J. Mol. Catal. A-Chem. 2003, 200, 31–42. [Google Scholar] [CrossRef]
- Li, T.C.; Lan, Z.; Xie, G.Y.; Luo, D.R.; Li, L.; Xiong, S.F.; Zhang, L.; Ouyang, L.P.; Zhang, A.Q. Binuclear Titanium Catalysts Based on Methylene-Bridged Tridentate Salicylaldiminato Ligands for Ethylene Homo- and Copolymerization. Catal. Lett. 2017, 147, 996–1005. [Google Scholar] [CrossRef]
- Huttenloch, M.E.; Dorer, B.; Rief, U.; Prosenc, M.H.; Schmidt, K.; Brintzinger, H.H. ansa-metallocene derivatives XXXIX biphenyl-bridged metallocene complexes of titanium, zirconium, and vanadium: Syntheses, crystal structures and enantioseparation. J. Organomet. Chem. 1997, 541, 219–232. [Google Scholar] [CrossRef]
- Xu, T.Q.; Gao, W.; Mu, Y.; Ye, L. Titanium(IV) complexes with monocyclopentadienyl and phenoxy-alkoxo ligands: Synthesis, structures and catalytic properties for ethylene polymerization. Polyhedron 2007, 26, 3357–3362. [Google Scholar] [CrossRef]
- Melillo, G.; Izzo, L.; Zinna, M.; Tedesco, C.; Oliva, L. Branching formation in the ethylene polymerization with meso ansa metallocene-based catalysts. Macromolecules 2002, 35, 9256–9261. [Google Scholar] [CrossRef]
- Murray, M.C.; Baird, M.C. Polymerization of 1-hexene catalyzed by Cp*TiMe3-B(C6F5)3: A mechanistic and structural study. Can. J. Chem. 2001, 79, 1012–1018. [Google Scholar] [CrossRef]
- Mattheis, C.; van der Zeijden, A.A.; Fröhlich, R. Monocyclopentadienyl and mono(2-methoxyethylcyclopentadienyl) zirconium methyl complexes. J. Organomet. Chem. 2000, 602, 51–58. [Google Scholar] [CrossRef]
- Kwon, S.J.; Baek, J.W.; Lee, H.J.; Kim, T.J.; Ryu, J.Y.; Lee, J.; Shin, E.J.; Lee, K.S.; Lee, B.Y. Preparation of Pincer Hafnium Complexes for Olefin Polymerization. Molecules 2019, 24, 1676. [Google Scholar] [CrossRef] [PubMed]
- Park, K.L.; Baek, J.W.; Moon, S.H.; Bae, S.M.; Lee, J.C.; Lee, J.; Jeong, M.S.; Lee, B.Y. Preparation of Pyridylamido Hafnium Complexes for Coordinative Chain Transfer Polymerization. Polymers 2020, 12, 1100. [Google Scholar] [CrossRef]
- Zaccaria, F.; Cipullo, R.; Correa, A.; Budzelaar, P.H.M.; Busico, V.; Ehm, C. Separating Electronic from Steric Effects in Ethene/α-Olefin Copolymerization: A Case Study on Octahedral ONNO Zr-Catalysts. Processes 2019, 7, 384. [Google Scholar] [CrossRef]
- Luciano, E.; Della Monica, F.; Buonerba, A.; Grassi, A.; Capacchione, C.; Milione, S. Polymerization of ethylene and propylene promoted by group 4 metal complexes bearing thioetherphenolate ligands. Polym. Chem. 2015, 6, 4657–4668. [Google Scholar] [CrossRef]
- Toda, T.; Kasahara, Y.; Iwasaki, J.; Wakatsuki, A.; Ohta, S.; Matano, Y.; Takenaka, K. A Zirconium Complex Bearing an NPN Tridentate Ligand Composed of Dibenzophosphole and Pyrrolide Moieties: Synthesis, Structure, and Ethylene-Polymerization Ability. Organometallics 2024, 44, 128–136. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Komarov, P.D.; Kostomarova, O.D.; Kolosov, N.A.; Ivchenko, P.V. MAO- and Borate-Free Activating Supports for Group 4 Metallocene and Post-Metallocene Catalysts of α-Olefin Polymerization and Oligomerization. Polymers 2023, 15, 3095. [Google Scholar] [CrossRef] [PubMed]
- Valdés, H.; Germán-Acacio, J.M.; van Koten, G.; Morales-Morales, D. Bimetallic complexes that merge metallocene and pincer-metal building blocks: Synthesis, stereochemistry and catalytic reactivity. Dalton Trans. 2022, 51, 1724–1744. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef]
- Kaminsky, W. Environmentally compatible polymerization of olefins by the use of metallocene catalysts. Chemosphere 2001, 43, 33–38. [Google Scholar] [CrossRef]

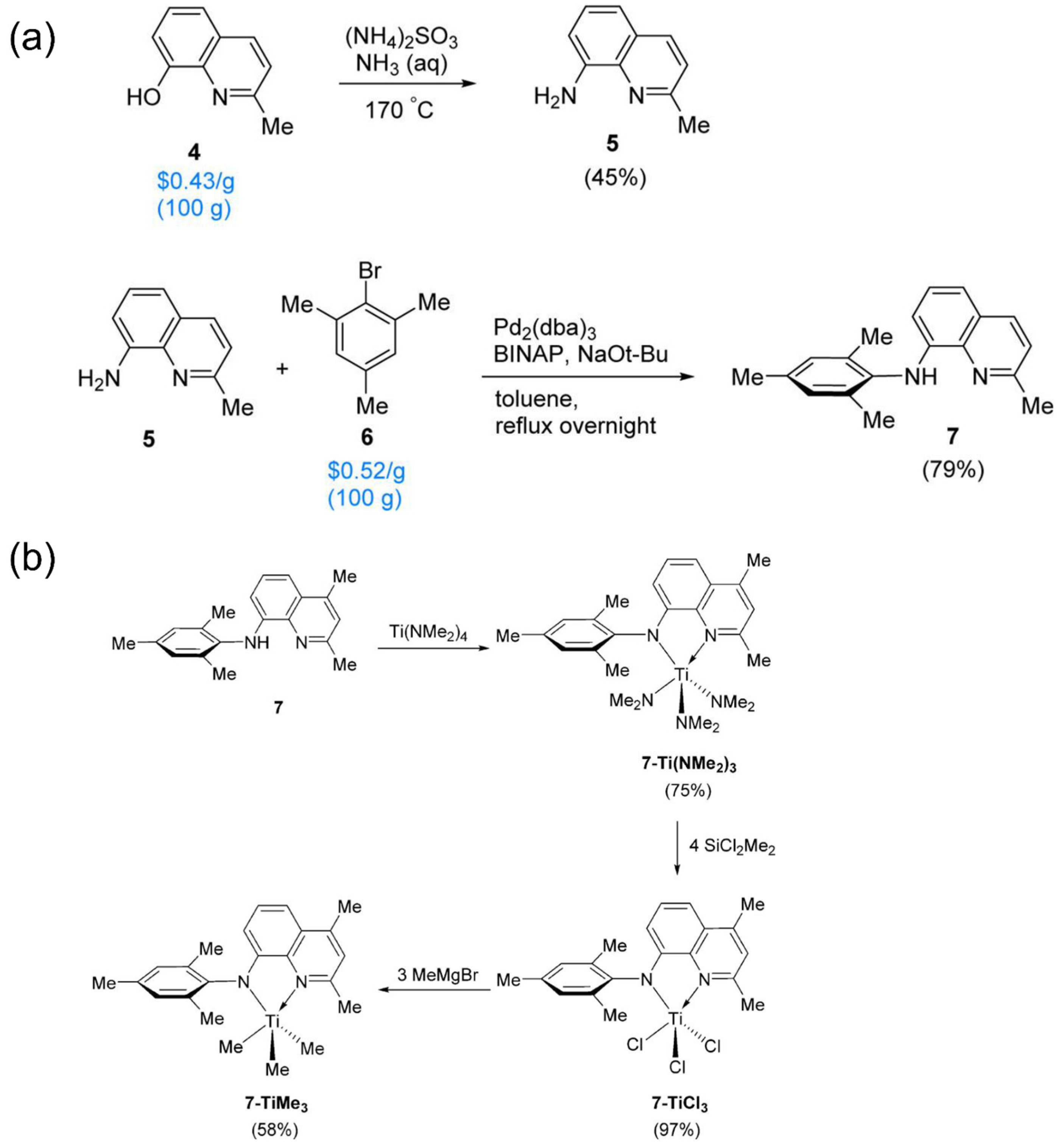
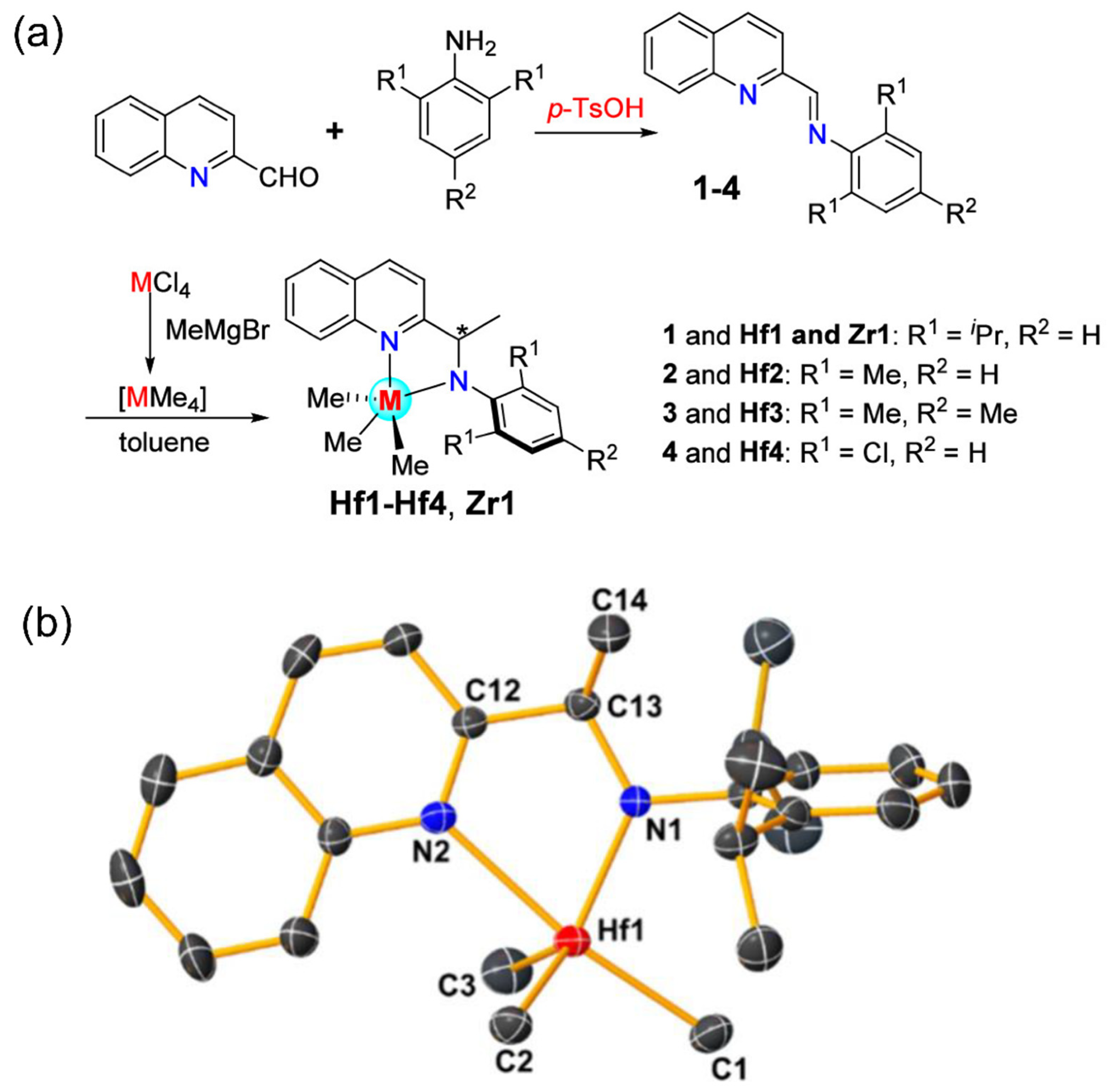
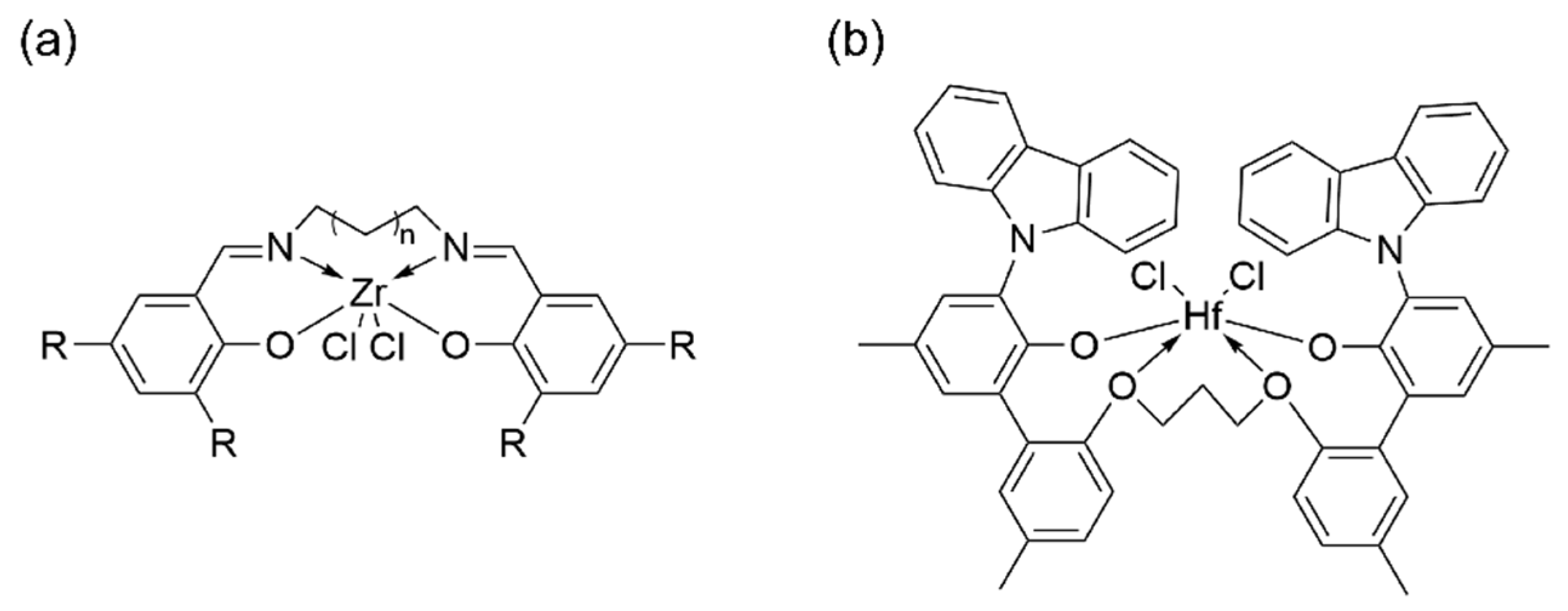
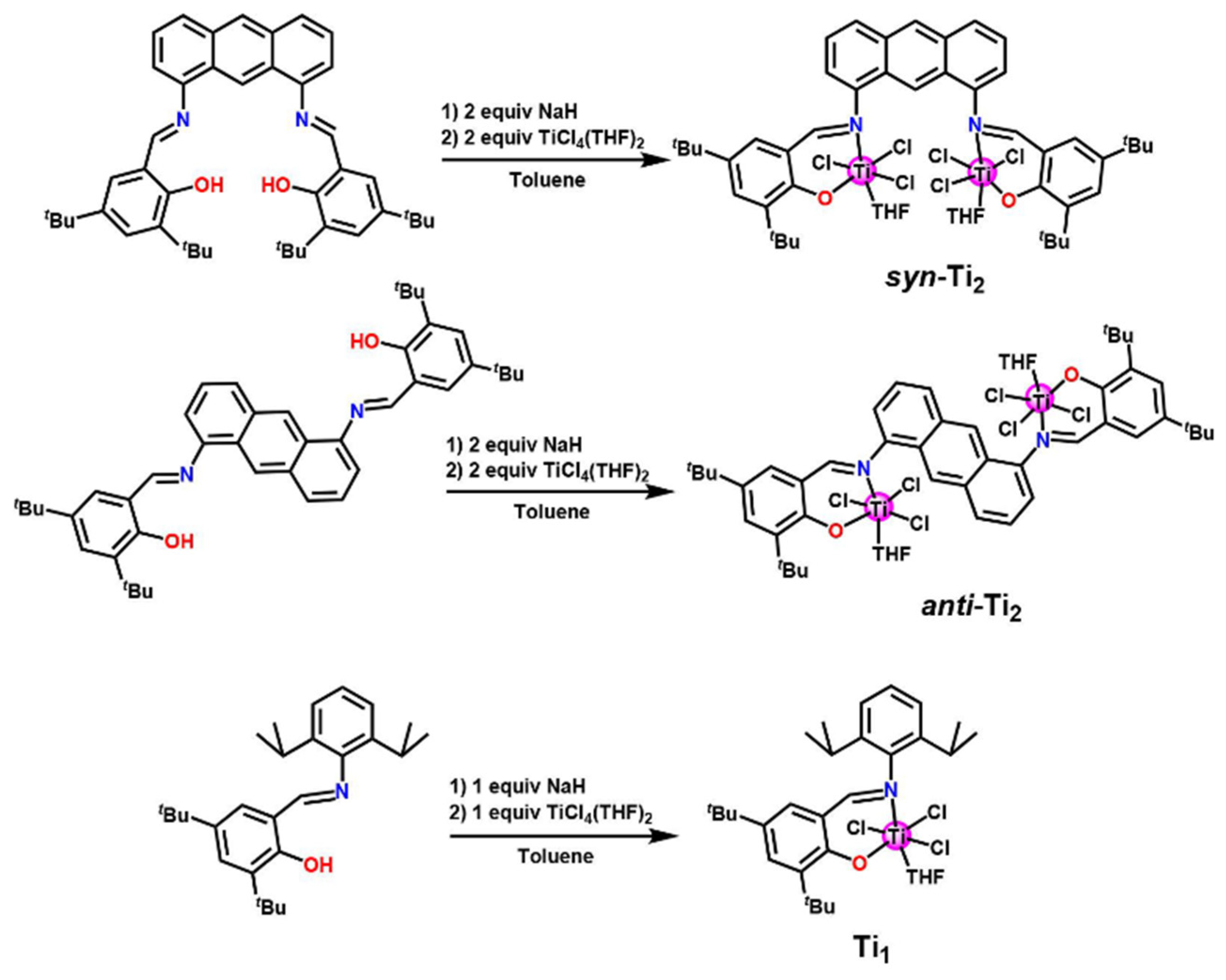

| Procatalyst | Octene Incorp (wt%) | Polymer (g) | Activity (g poly/mmol cat.) | MW (g/mol) | Mn (g/mol) | Polymer Dispersity Index | TC (°C) |
|---|---|---|---|---|---|---|---|
| Imino-amido | 9.8 | 18.3 | 24.339 | 390.690 | 124.180 | 3.27 | 108 |
| Imino-enamido | 18.3 | 23.5 | 31.352 | 640.130 | 145.290 | 4.16 | 87 |
| 6a | 13.4 | 19.2 | 25.599 | 280.300 | 100.500 | 2.79 | 95 |
| 6b | 16.5 | 30.9 | 41.165 | 403.610 | 116.760 | 3.46 | 89 |
| 6c | 6.9 | 10.6 | 14.094 | 159.800 | 54.580 | 2.93 | 122 |
| 6d | 14.9 | 28.3 | 37.715 | 632.200 | 212.500 | 2.97 | 93 |
| Catalysts | Route Numbers | Yield (%) | Activity (g/mmolcat.) a | Incorp Rate (wt%) b | Advantage | Application |
|---|---|---|---|---|---|---|
| Imino-amido [59] | 5 | 77 | 140,800 | 3.8/14.9 | High molecular weight | POE POE POE |
| Imino-enamido [61] | 6 | 57 | 195,500 | 7.0/14.8 | High activity, Mw, and incorp rate | |
| Amido-quinoline [66] | 4 | 85 | 55,417 | 13.3/25.3 | Thermally stable | |
| Phenoxy-imine-amino [87] | 5 | 85 | 67,500 | 0.29 | Multi-coordinable metal | LLDPE LLDPE LLDPE |
| Phenoxy-imine-quinoline [88] | 3 | 77 | 79,200 (100 °C) | 0.64 | Facile synthesis | |
| Quinoline-imine-thioether [90] | 4 | 79 | 22,110 (130 °C) | 1.7 | Thermally stable | |
| Pyridine-amino [92] | 6 | 78 | 13,267 | 12.1/15.6 | High incorp rate Product OBC | POE, OBC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, X.; Chen, S.; Shan, T. Advances in High-Temperature Non-Metallocene Catalysts for Polyolefin Elastomers. Materials 2025, 18, 1334. https://doi.org/10.3390/ma18061334
Wang C, Li X, Chen S, Shan T. Advances in High-Temperature Non-Metallocene Catalysts for Polyolefin Elastomers. Materials. 2025; 18(6):1334. https://doi.org/10.3390/ma18061334
Chicago/Turabian StyleWang, Cheng, Xin Li, Si Chen, and Tianyu Shan. 2025. "Advances in High-Temperature Non-Metallocene Catalysts for Polyolefin Elastomers" Materials 18, no. 6: 1334. https://doi.org/10.3390/ma18061334
APA StyleWang, C., Li, X., Chen, S., & Shan, T. (2025). Advances in High-Temperature Non-Metallocene Catalysts for Polyolefin Elastomers. Materials, 18(6), 1334. https://doi.org/10.3390/ma18061334





