Hardness Changes Due to the Morphological Evolution of Microstructural Phases in an As-Solidified Zn-Fe Alloy
Abstract
1. Introduction
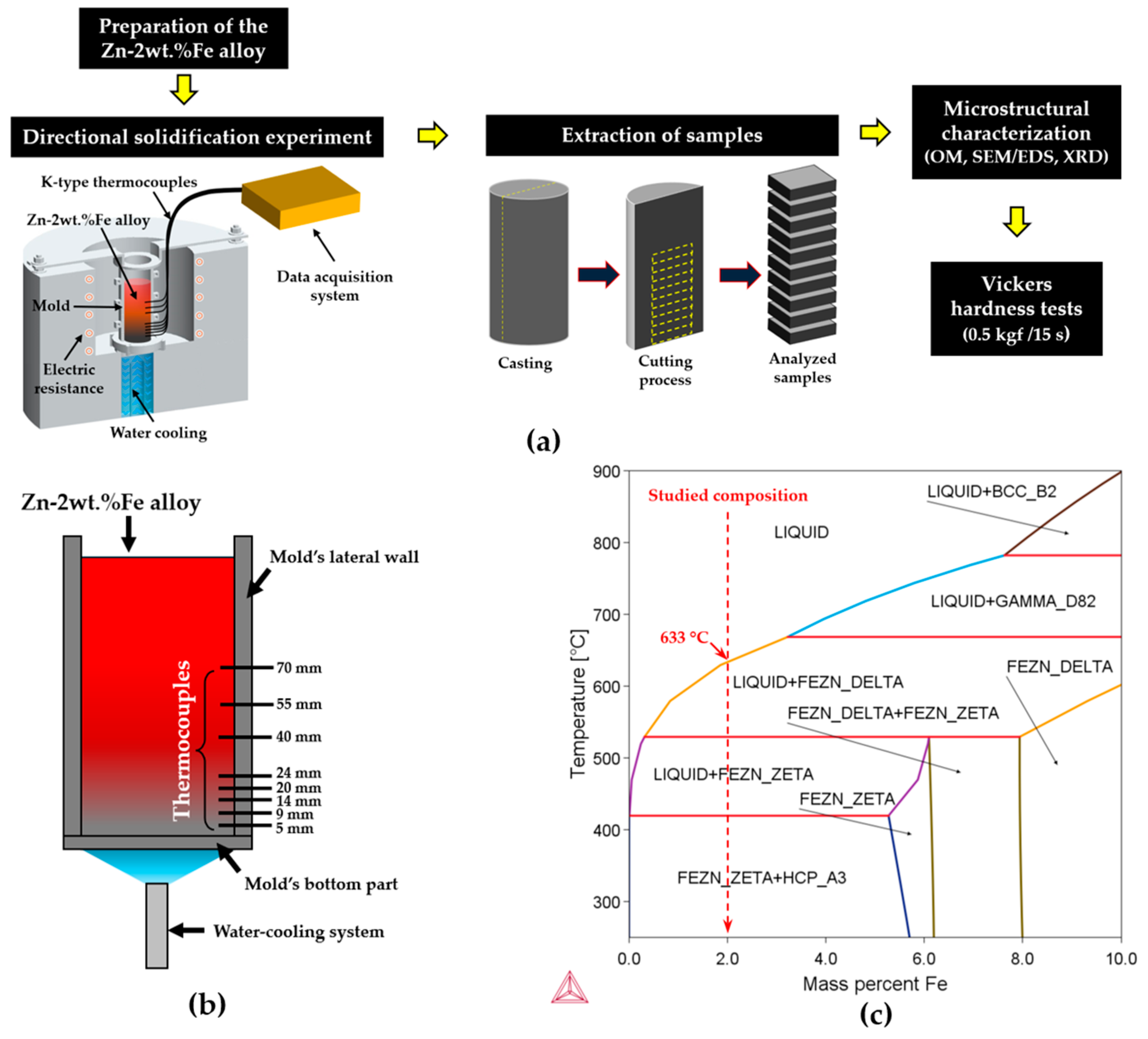
2. Materials and Methods
3. Results and Discussion
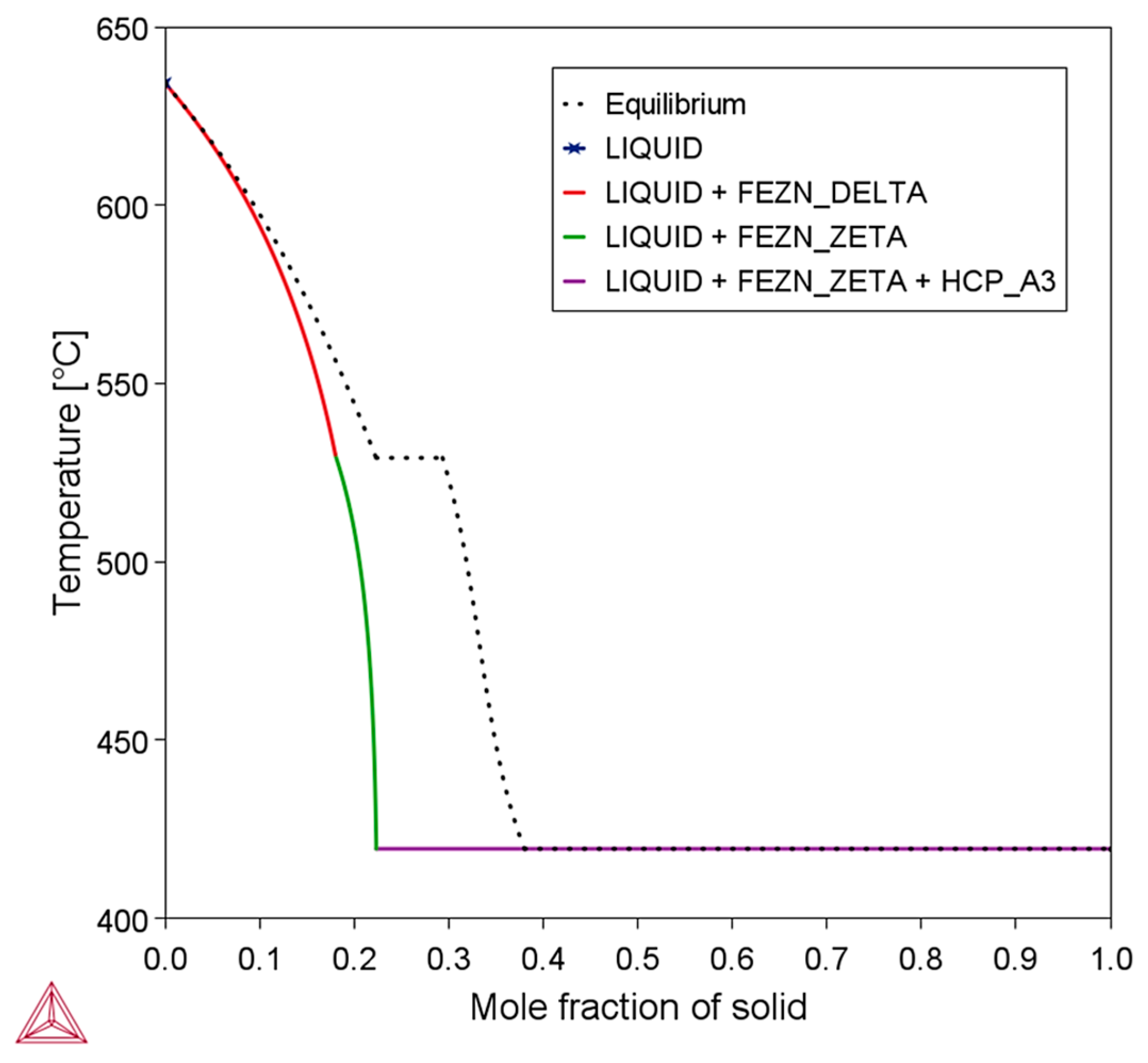
3.1. Zn-Fe Phase Diagram
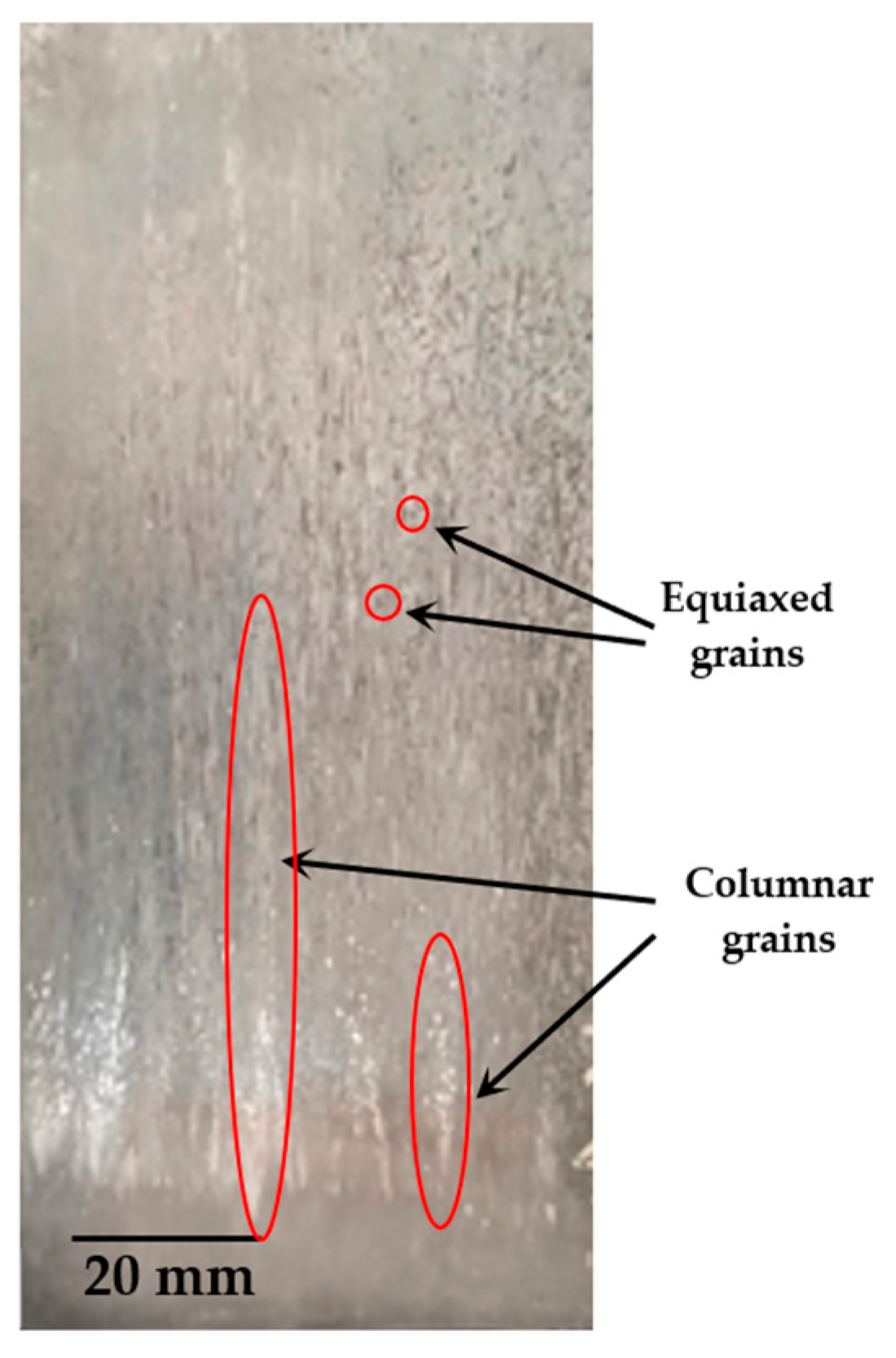
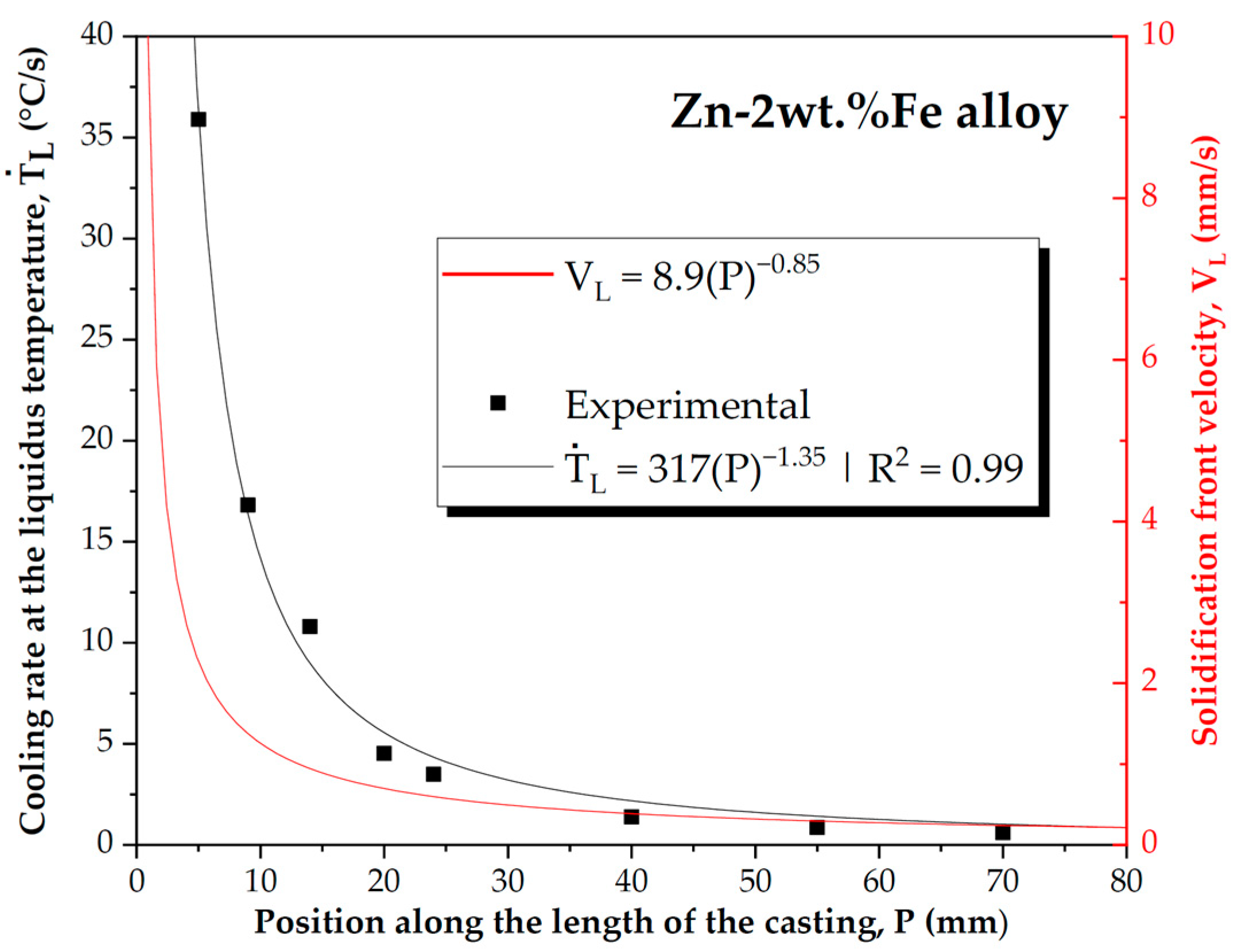
3.2. Solidification Microstructure and Thermal Parameters
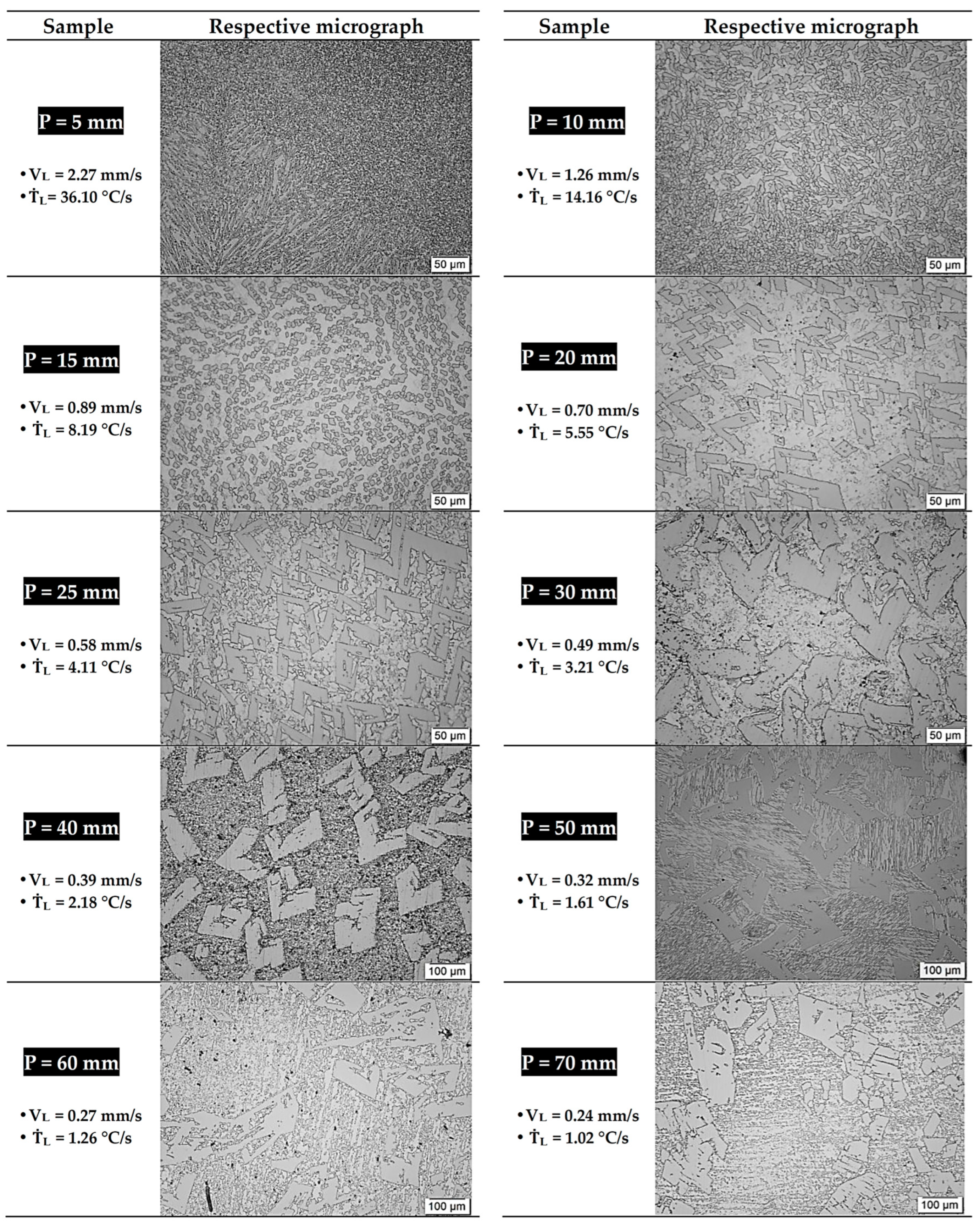
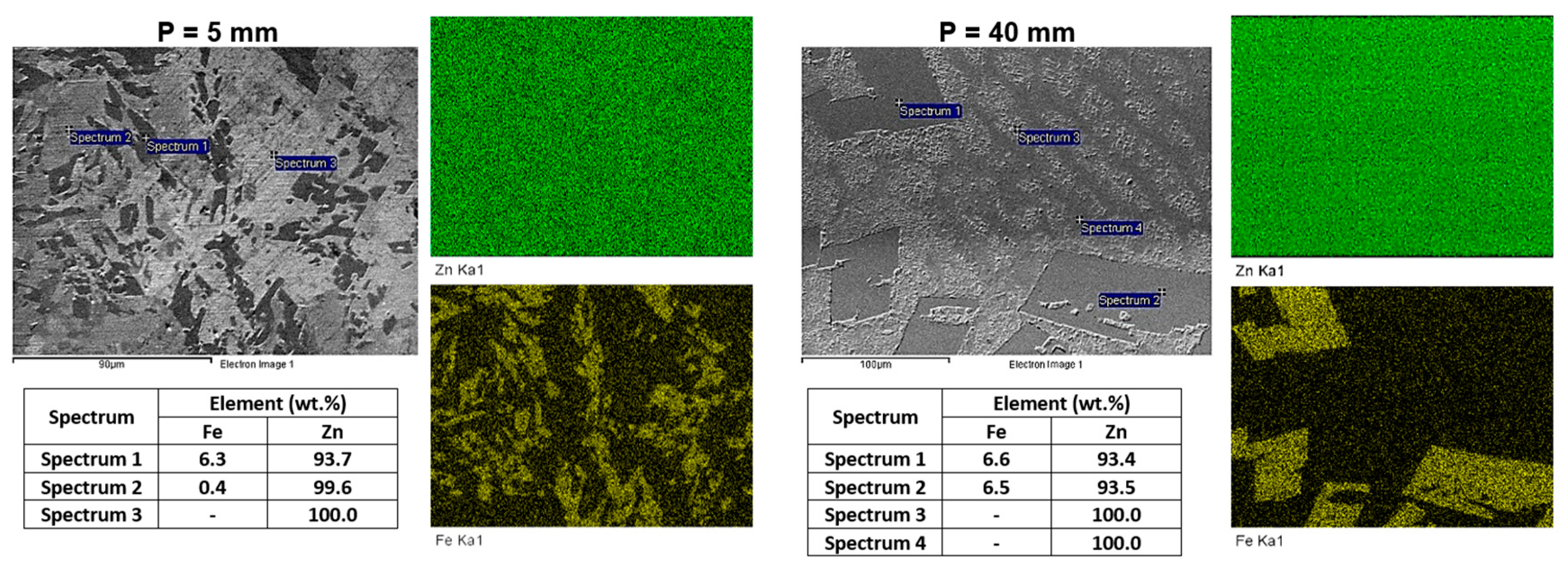
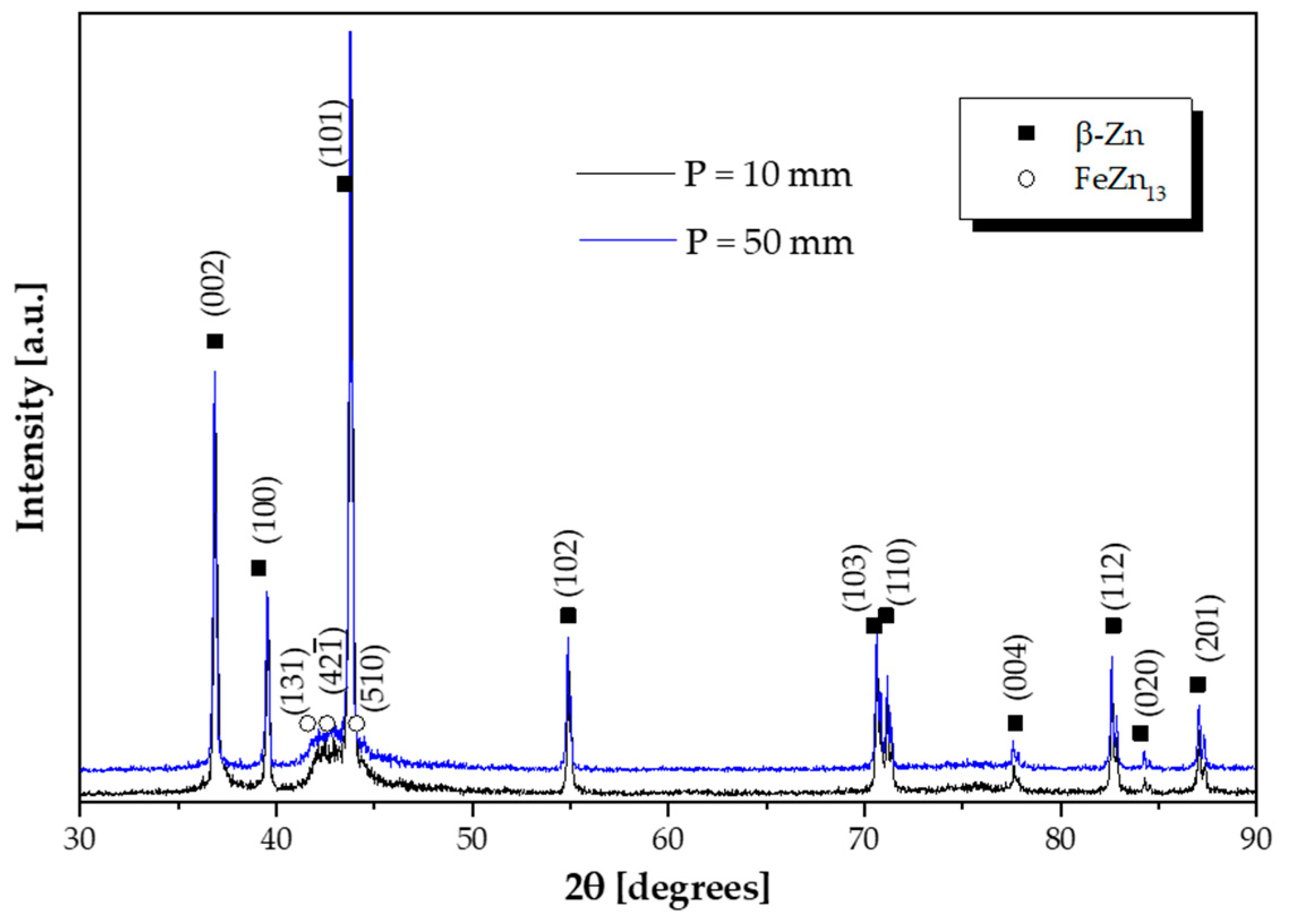
3.3. Microstructural Evolution
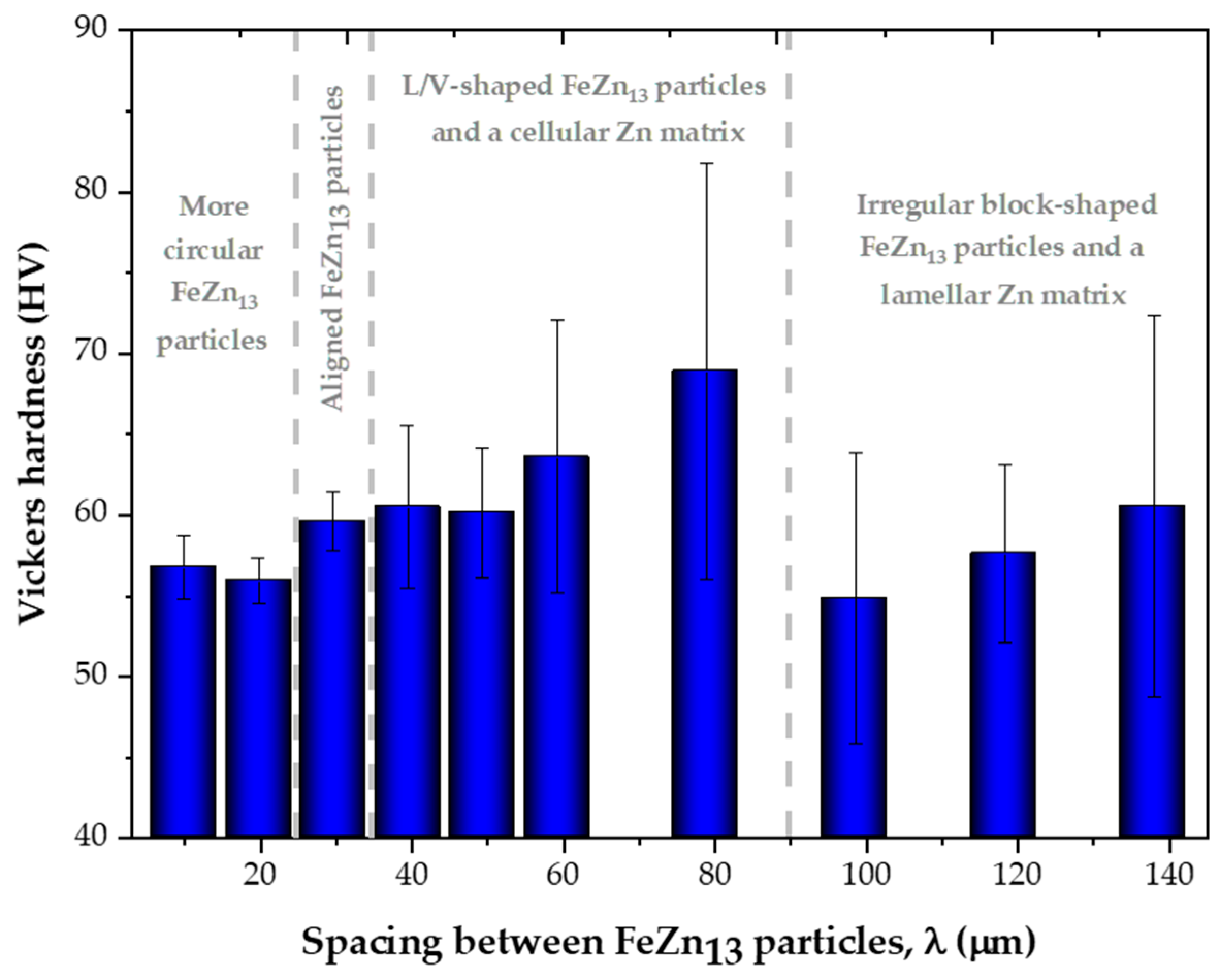
3.4. Influence of Microstructure on Hardness
4. Conclusions
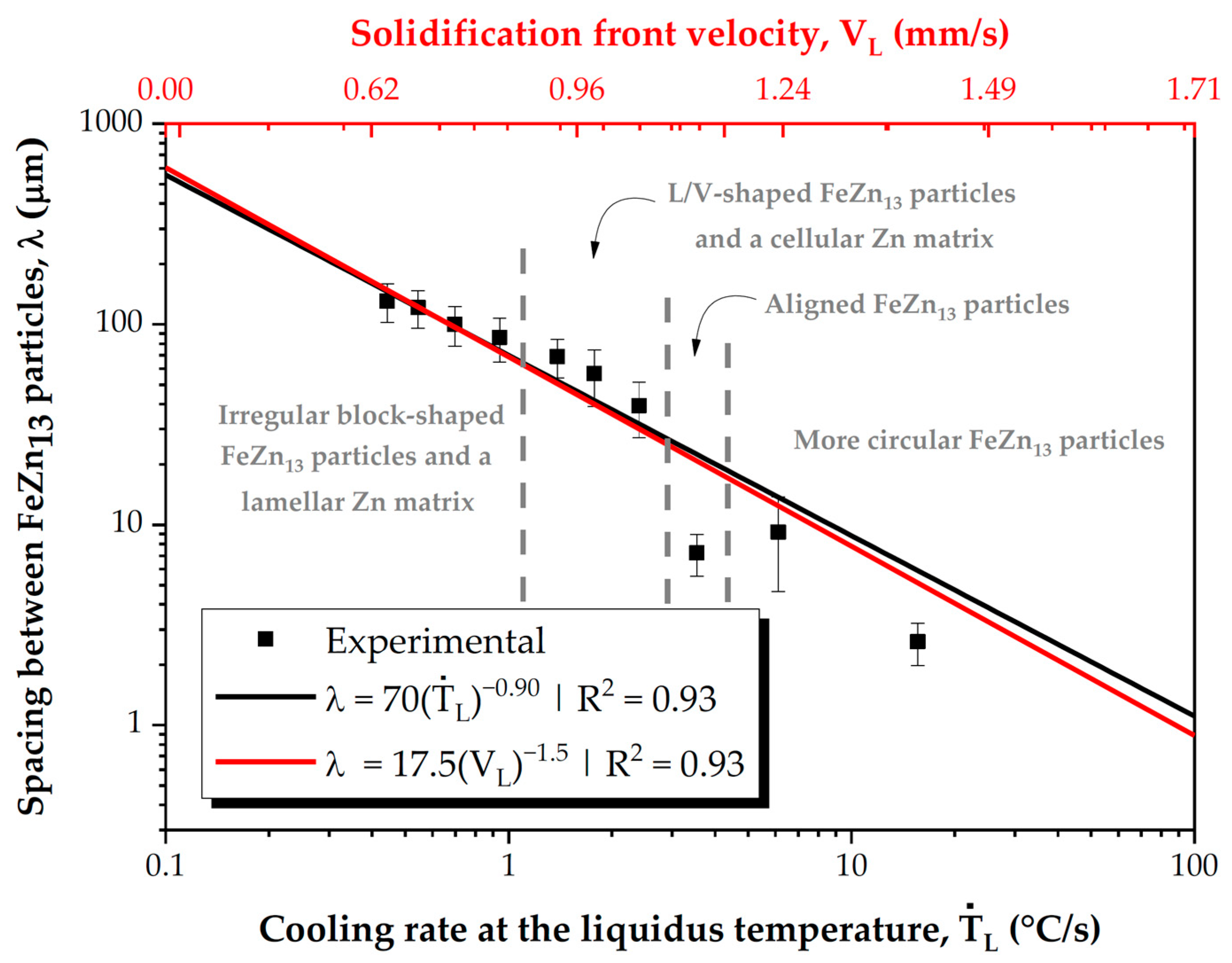
- The directional solidification experiment led to various cooling conditions along the length of the casting, allowing us to analyze the microstructural formation as a function of ṪL and VL ranges, which spanned from 1.02 to 36.10 °C/s and from 0.24 to 2.27 mm/s, respectively.
- The variations in the cooling conditions during solidification resulted in four different microstructural morphologies, corresponding to three morphological transitions as cooling decreased. The first transition caused the alignment of the FeZn13 particles. The second morphological transition involved the formation of FeZn13 particles with “V” or “L” profiles and the emergence of cellular morphology in the Zn-rich matrix. The third transition led to the development of a lamellar profile in the Zn-rich matrix.
- The morphologies of the microstructural phases were shown to play an important role in the hardness of Zn-2wt.%Fe, which could range from 56.4 to 63.3 HV, depending primarily on these morphologies. FeZn13 particles with slightly circular geometries favored low hardness levels. With the alignment of FeZn13 particles into more organized patterns, hardness slightly increased. V- and L-shaped FeZn13 particles increased the average hardness. FeZn13 particles with more complex forms, together with the Zn matrix, developed a progressively more pronounced lamellar profile, which induced a slight decrease in hardness.
- Identifying that hardness is sensitive to the microstructural phase morphologies of Zn-2wt.%Fe is a valuable insight for developing Zn-Fe alloys with improved properties. In terms of engineering applications, including bioabsorbable implants, the hardness values obtained here fall within the range reported in the literature.
- The outcomes of this work provide additional guidance for the development of Zn-Fe alloys and open new directions for future research, focusing on mechanical properties beyond hardness, such as tensile strength and fracture toughness, while also evaluating biodegradation and cytotoxicity under different processing conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Z.-Z.; Gao, X.-X.; Chen, H.-T.; Liu, X.-F.; Li, A.; Zhang, H.-J.; Wang, L.-N. Enhancement in Mechanical and Corrosion Resistance Properties of a Biodegradable Zn-Fe Alloy through Second Phase Refinement. Mater. Sci. Eng. C 2020, 116, 111197. [Google Scholar] [CrossRef] [PubMed]
- Pierson, D.; Edick, J.; Tauscher, A.; Pokorney, E.; Bowen, P.; Gelbaugh, J.; Stinson, J.; Getty, H.; Lee, C.H.; Drelich, J.; et al. A Simplified in Vivo Approach for Evaluating the Bioabsorbable Behavior of Candidate Stent Materials. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of Biodegradation of Biocompatable Magnesium Alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Vida, T.A.; Brito, C.; Lima, T.S.; Spinelli, J.E.; Cheung, N.; Garcia, A. Near-Eutectic Zn-Mg Alloys: Interrelations of Solidification Thermal Parameters, Microstructure Length Scale and Tensile/Corrosion Properties. Curr. Appl. Phys. 2019, 19, 582–598. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Goldman, J.; Drelich, J.; Aghion, E. The Suitability of Zn–1.3%Fe Alloy as a Biodegradable Implant Material. Metals 2018, 8, 153. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H.; Huang, L.; Tian, L.; et al. Development of Biodegradable Zn-1X Binary Alloys with Nutrient Alloying Elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Hercz, G.; Andress, D.L.; Nebeker, H.G.; Shinaberger, J.H.; Sherrard, D.J.; Coburn, J.W. Reversal of Aluminum-Related Bone Disease After Substituting Calcium Carbonate for Aluminum Hydroxide. Am. J. Kidney Dis. 1988, 11, 70–75. [Google Scholar] [CrossRef]
- Králová, Z.O.; Gorejová, R.; Oriňaková, R.; Petráková, M.; Oriňak, A.; Kupková, M.; Hrubovčáková, M.; Sopčák, T.; Baláž, M.; Maskaľová, I.; et al. Biodegradable Zinc-Iron Alloys: Complex Study of Corrosion Behavior, Mechanical Properties and Hemocompatibility. Prog. Nat. Sci. Mater. Int. 2021, 31, 279–287. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, D.; Xue, H.; Guan, R.; Liu, H. Microstructure of Biodegradable Zn-Fe Alloys and Mechanical and Corrosion Properties. JOM 2020, 72, 3661–3671. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In Vivo Performances of Pure Zn and Zn–Fe Alloy as Biodegradable Implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef]
- Yue, R.; Huang, H.; Ke, G.; Zhang, H.; Pei, J.; Xue, G.; Yuan, G. Microstructure, Mechanical Properties and in Vitro Degradation Behavior of Novel Zn-Cu-Fe Alloys. Mater. Charact. 2017, 134, 114–122. [Google Scholar] [CrossRef]
- Shi, Z.-Z.; Li, Z.-L.; Bai, W.-S.; Tuoliken, A.; Yu, J.; Liu, X.-F. (Fe, Mn)Zn13 Phase and Its Core-Shell Structure in Novel Biodegradable Zn-Mn-Fe Alloys. Mater. Des. 2019, 162, 235–245. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Ren, J.; Liu, C.; Hu, Y.; Yang, Y. Microstructure, Mechanical Property and Corrosion Behavior of Biomedical Zn-Fe Alloy Prepared by Low-Temperature Sintering. J. Alloys Compd. 2023, 934, 167812. [Google Scholar] [CrossRef]
- Su, Y.; Fu, J.; Lee, W.; Du, S.; Qin, Y.-X.; Zheng, Y.; Wang, Y.; Zhu, D. Improved Mechanical, Degradation, and Biological Performances of Zn–Fe Alloys as Bioresorbable Implants. Bioact. Mater. 2022, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, X.; Guo, P.; Zhang, Y.; Xu, D.; Pang, Y.; Song, Z.; Yang, L. Effects of Li Addition on the Properties of Biodegradable Zn–Fe–Li Alloy: Microstructure, Mechanical Properties, Corrosion Behavior, and Cytocompatibility. Mater. Today Commun. 2024, 39, 108661. [Google Scholar] [CrossRef]
- Bhat, R.S.; Balakrishna, M.K.; Parthasarathy, P.; Hegde, A.C. Structural Properties of Zn-Fe Alloy Coatings and Their Corrosion Resistance. Coatings 2023, 13, 772. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, J. Fabrication of Biodegradable Superhydrophobic Zn-Fe Coating on Ultra-Light Mg-Li Alloy. Surf. Coat. Technol. 2024, 486, 130677. [Google Scholar] [CrossRef]
- Soares, T.; Cruz, C.; Barros, A.; Garcia, A.; Cheung, N. Microstructure Growth Morphologies, Macrosegregation, and Microhardness in Bi–Sb Thermal Interface Alloys. Adv. Eng. Mater. 2020, 22, 1901592. [Google Scholar] [CrossRef]
- Ben Tzion-Mottye, L.; Ron, T.; Eliezer, D.; Aghion, E. The Effect of Mn on the Mechanical Properties and In Vitro Behavior of Biodegradable Zn-2%Fe Alloy. Metals 2022, 12, 1291. [Google Scholar] [CrossRef]
- Jung, H.; Mangelinck-Noël, N.; Nguyen-Thi, H.; Billia, B. Columnar to Equiaxed Transition during Directional Solidification in Refined Al-Based Alloys. J. Alloys Compd. 2009, 484, 739–746. [Google Scholar] [CrossRef]
- Gündüz, M.; Çadırlı, E. Directional Solidification of Aluminium–Copper Alloys. Mater. Sci. Eng. A 2002, 327, 167–185. [Google Scholar] [CrossRef]
- He, J.; Li, D.-W.; He, F.-L.; Liu, Y.-Y.; Liu, Y.-L.; Zhang, C.-Y.; Ren, F.; Ye, Y.-J.; Deng, X.-D.; Yin, D.-C. A Study of Degradation Behaviour and Biocompatibility of Zn—Fe Alloy Prepared by Electrodeposition. Mater. Sci. Eng. C 2020, 117, 111295. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Han, K.; Ohnuma, I.; Kainuma, R. Experimental Determination of Phase Diagram at 450 °C in the Zn–Fe–Al Ternary System. J. Alloys Compd. 2021, 854, 157163. [Google Scholar] [CrossRef]
- Han, K.; Ohnuma, I.; Okuda, K.; Kainuma, R. Experimental Determination of Phase Diagram in the Zn-Fe Binary System. J. Alloys Compd. 2018, 737, 490–504. [Google Scholar] [CrossRef]
- Arrighi, C.; Savall, C.; Cohendoz, S.; Grosseau-Poussard, J.-L.; Baissac, L.; Olivier, M.-G.; Creus, J. Optimization of the Morphology, Structure and Properties of High Iron Content Zn–Fe Coatings by Pulse Electrodeposition. Mater. Chem. Phys. 2021, 263, 124366. [Google Scholar] [CrossRef]
- Yang, H.; Jia, B.; Zhang, Z.; Qu, X.; Li, G.; Lin, W.; Zhu, D.; Dai, K.; Zheng, Y. Alloying Design of Biodegradable Zinc as Promising Bone Implants for Load-Bearing Applications. Nat. Commun. 2020, 11, 401. [Google Scholar] [CrossRef]
- Vida, T.A.; Silva, C.A.P.; Lima, T.S.; Cheung, N.; Brito, C.; Garcia, A. Tailoring Microstructure and Microhardness of Zn−1wt.%Mg−(0.5wt.%Mn, 0.5wt.%Ca) Alloys by Solidification Cooling Rate. Trans. Nonferrous Met. Soc. China 2021, 31, 1031–1048. [Google Scholar] [CrossRef]
- Ibrahim, A.; Magliulo, N.; Groben, J.; Padilla, A.; Akbik, F.; Abdel Hamid, Z. Hardness, an Important Indicator of Bone Quality, and the Role of Collagen in Bone Hardness. J. Funct. Biomater. 2020, 11, 85. [Google Scholar] [CrossRef]


| Metals | Zn | Fe | Mn | Al | Ni | Cu | Mg | Pb |
|---|---|---|---|---|---|---|---|---|
| Zn | Balance | - | - | - | - | - | - | <0.1 |
| Fe | - | Balance | - | - | 0.01 | 0.01 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, G.C.C.; de Paula, A.; Barros, A.; Garcia, A.; Cheung, N. Hardness Changes Due to the Morphological Evolution of Microstructural Phases in an As-Solidified Zn-Fe Alloy. Materials 2025, 18, 1311. https://doi.org/10.3390/ma18061311
de Sousa GCC, de Paula A, Barros A, Garcia A, Cheung N. Hardness Changes Due to the Morphological Evolution of Microstructural Phases in an As-Solidified Zn-Fe Alloy. Materials. 2025; 18(6):1311. https://doi.org/10.3390/ma18061311
Chicago/Turabian Stylede Sousa, Guilherme Calixto Carneiro, Andrei de Paula, Andre Barros, Amauri Garcia, and Noé Cheung. 2025. "Hardness Changes Due to the Morphological Evolution of Microstructural Phases in an As-Solidified Zn-Fe Alloy" Materials 18, no. 6: 1311. https://doi.org/10.3390/ma18061311
APA Stylede Sousa, G. C. C., de Paula, A., Barros, A., Garcia, A., & Cheung, N. (2025). Hardness Changes Due to the Morphological Evolution of Microstructural Phases in an As-Solidified Zn-Fe Alloy. Materials, 18(6), 1311. https://doi.org/10.3390/ma18061311







