Preparation and Characterization of Co-Diamond Composite Coatings Obtained in a Single-Step Hybrid Electrophoretic Deposition Process
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
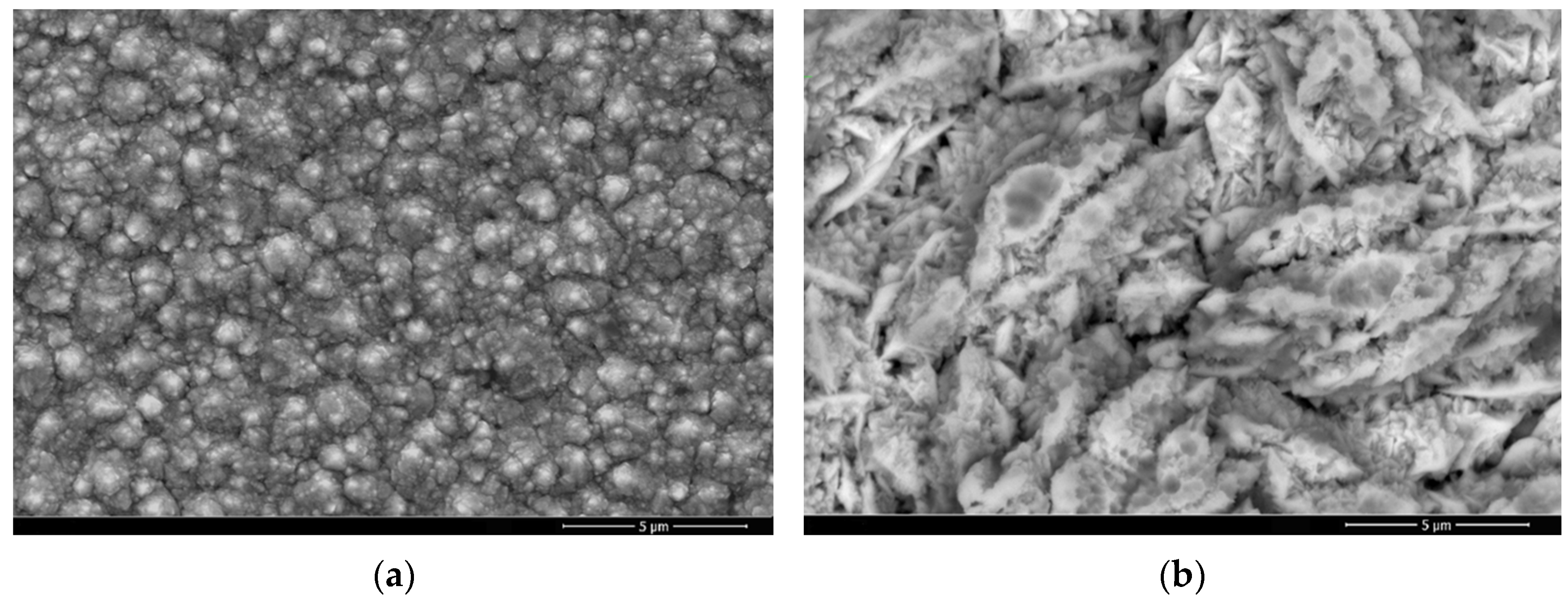
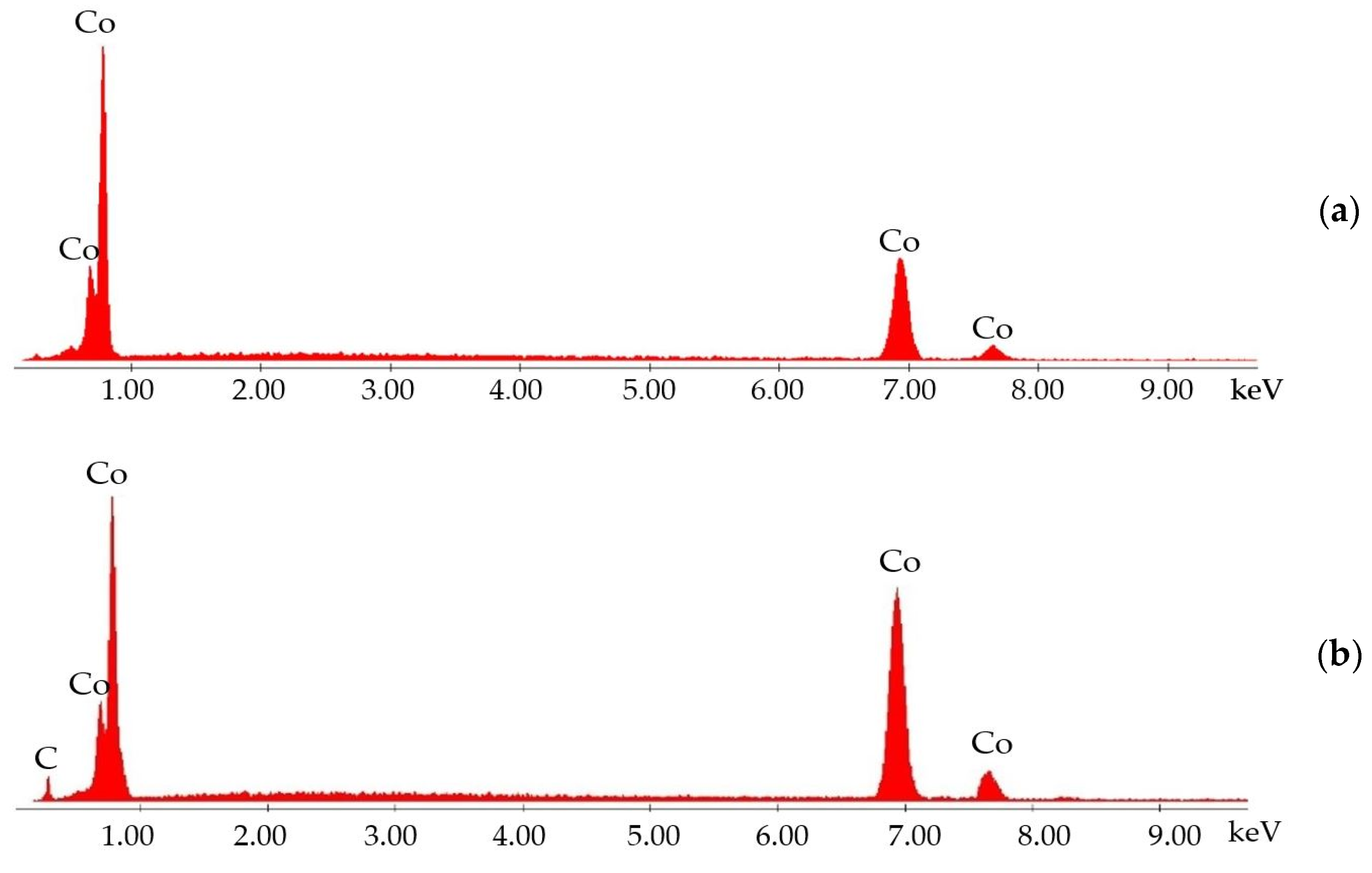
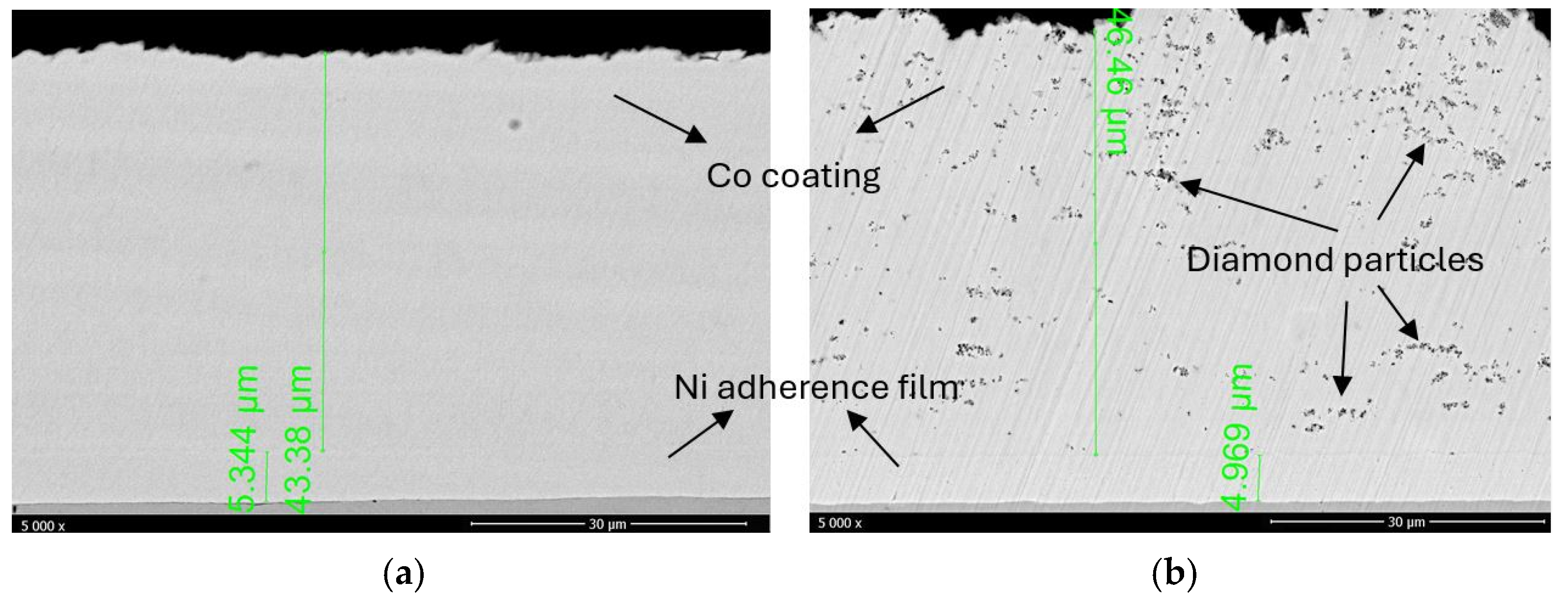
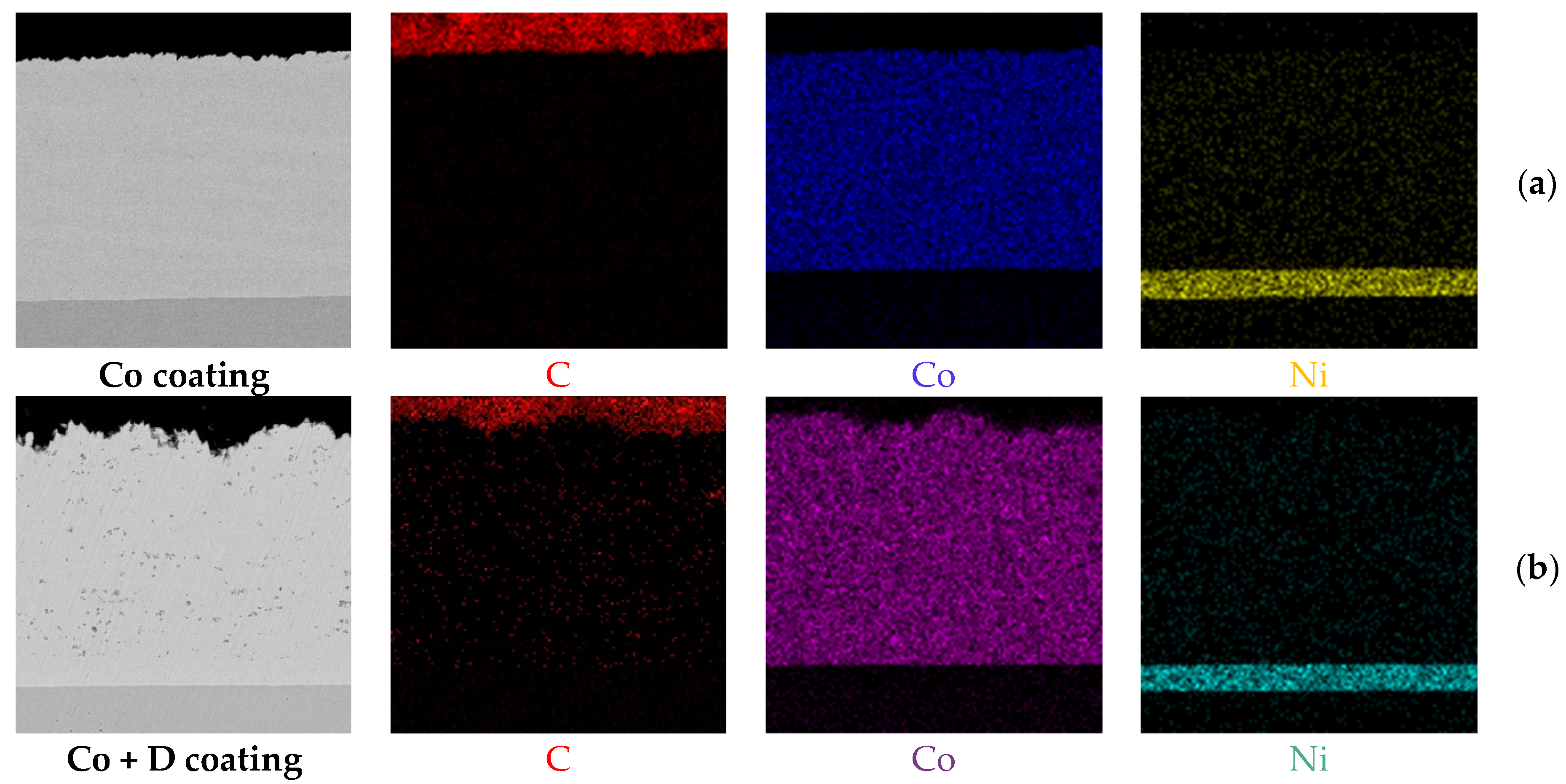
3.1. Microstructure and Chemical Composition
3.2. Corrosion Behavior
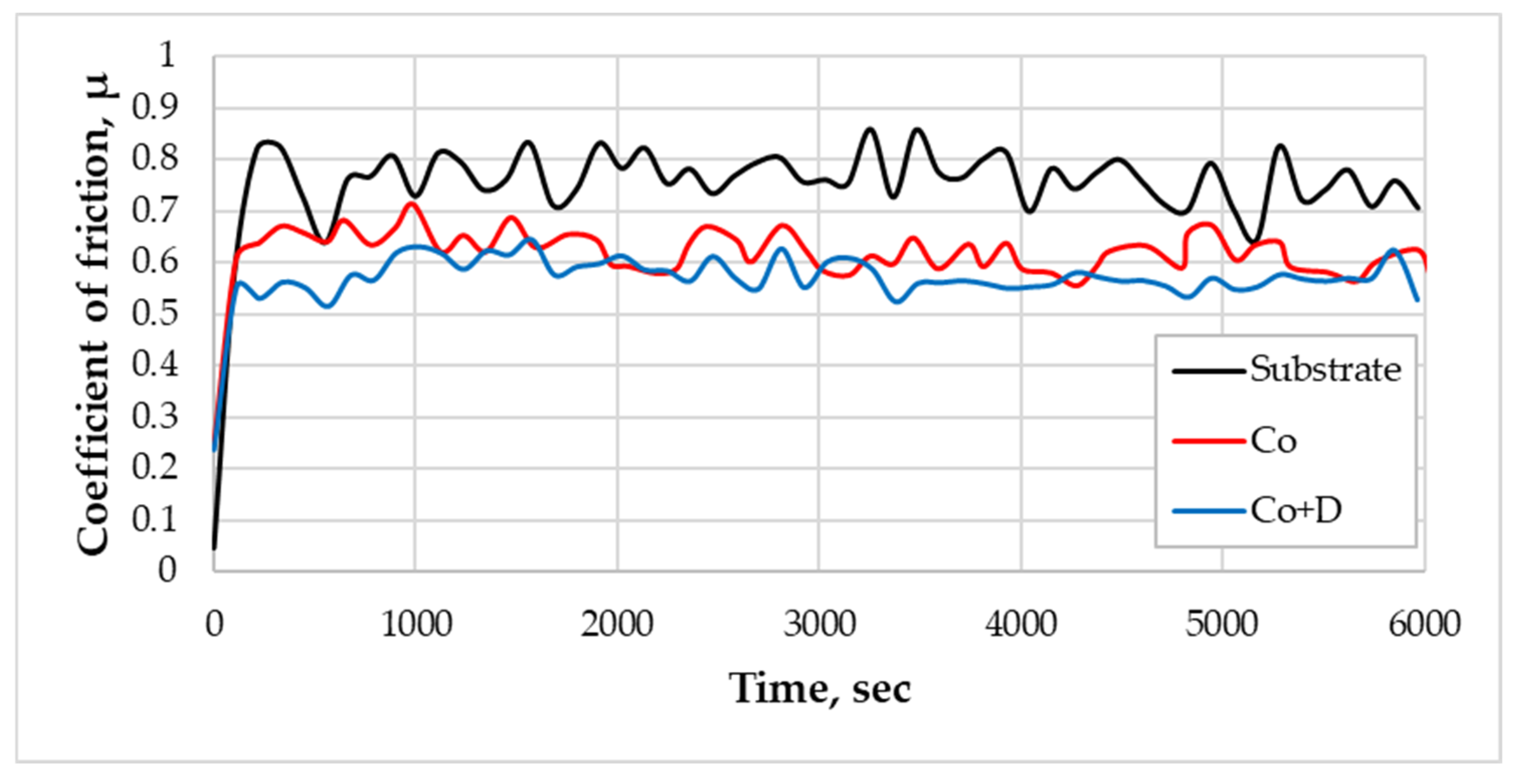
3.3. Hardness and Sliding Wear Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazumder, M. Global Impact of Corrosion: Occurrence, Cost and Mitigation. Glob. J. Eng. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Tyagi, R.; Mandal, A.; Das, A.; Tripathi, A.; Prakash, C.; Campilho, R.; Saxena, K. Electrical Discharge Coating a Potential Surface Engineering Technique: A State of the Art. Processes 2022, 10, 1971. [Google Scholar] [CrossRef]
- Farooq, S.A.; Raina, A.; Mohan, S.; Sing, R.A.; Jayalakshmi, S.; Haq, M.I.U. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Chua, M.H.; Ong, P.J.; Lee, J.J.C.; Chin, K.L.O.; Wang, S.; Kai, D.; Ji, R.; Kong, J.; Dong, Z.; et al. Recent Advances in Nanotechnology-Based Functional Coatings for the Built Environment. Mater. Today Adv. 2022, 15, 100270. [Google Scholar] [CrossRef]
- Jiang, X.; Zhuo, Y.; Wang, P.; Yang, M.; Liao, Y.; Chen, B. The Perspectives of Hydrophobic Coatings for Mitigating Icing on Atmospheric Structures. Coatings 2023, 13, 326. [Google Scholar] [CrossRef]
- Wu, P.; Xue, Z.; Yu, T.; Penkov, O.V. Transparent Self-Cleaning Coatings: A Review. Coatings 2023, 13, 1270. [Google Scholar] [CrossRef]
- Negut, I.; Albu, C.; Bita, B. Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings 2024, 14, 256. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, M.A.; Hossain, N.; Rana, M. A Review of the Tribological Behavior of Electrodeposited Cobalt (Co) Based Composite Coatings. Compos. Part C 2022, 9, 100307. [Google Scholar] [CrossRef]
- Al-Azzawi, A.; Baumli, P. Methods of Composite Coating: A Review. Mater. Sci. Eng. 2015, 40, 26–32. [Google Scholar]
- Askari, H.; Zbib, H.M.; Sun, X. Multiscale Modeling of Inclusions and Precipitation Hardening in Metal Matrix Composites: Application to Advanced High-Strength Steels. J. Nanomech. Micromech. 2013, 3, 24–33. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Tailor, S. Self-Lubricating Composite Coatings: A Review of Deposition Techniques and Material Advancement. Mater. Today Proc. 2023, (in press). [Google Scholar] [CrossRef]
- Anwar, S.; Khan, F.; Zhang, Y.; Caines, S. Zn Composite Corrosion Resistance Coatings: What Works and What Does Not Work? J. Loss Prev. Process Ind. 2021, 69, 104376. [Google Scholar] [CrossRef]
- Calderón, J.; Henao, J.; Calderón, M.G.A. Erosion–Corrosion Resistance of Ni Composite Coatings with Embedded SiC Nanoparticles. Electrochim. Acta 2014, 124, 190–198. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y.; Makhlouf, A.S.H. Chapter 24—Recent Advances in Nanocomposite Coatings for Corrosion Protection Applications. In Handbook of Nanoceramic and Nanocomposite Coatings and Materials; Makhlouf, A.S.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 515–549. [Google Scholar]
- Pane, S.; Gomez, E.; Valles, E. Enhanced Magnetism in Electrodeposited-Based CoNi Composites Containing High Percentage of Micron Hard-Magnetic Particles. Electrochem. Commun. 2007, 9, 1755–1760. [Google Scholar] [CrossRef]
- Pushpavanam, M.; Manikandan, H.; Ramanathan, K. Preparation and Characterization of Nickel–Cobalt-Diamond Electro-Composites by Sediment Co-Deposition. Surf. Coat. Technol. 2007, 201, 6372–6379. [Google Scholar] [CrossRef]
- Du, L.; Xu, B.; Dong, S.; Yang, H.; Tu, W. Study of Tribological Characteristics and Wear Mechanism of Nano-Particle Strengthened Nickel-Based Composite Coatings under Abrasive Contaminant Lubrication. Wear 2004, 257, 1058–1063. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Sun, X.Y.; Bai, X.L. Comparative Study on the Microstructure, Wear Behavior, and Corrosion Performance of Iron-Based and Cobalt-Based Coatings Fabricated by Laser Cladding. J. Mater. Eng. Perform. 2023, 33, 12398–12412. [Google Scholar] [CrossRef]
- Encalada, A.I.; Alidokht, S.A.; Sharifi, N.; Stoyanov, P.; Makowiec, M.; Moreau, C.; Chromik, R. Wear Behavior of HVOF Sprayed Cobalt-Based Composite Coatings Reinforced with Cr3C2. Wear 2024, 546–547, 205310. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Liu, G.; Qian, Y.; Xu, Y.; Xiang, D. Surface Modification of 42CrMo Steels: A Review from Wear and Corrosion Resistance. Coatings 2024, 14, 337. [Google Scholar] [CrossRef]
- Zhang, K.; Ju, H.; Xing, F.; Wang, W.; Li, Q.; Yu, X.; Liu, W. Microstructure and Properties of Composite Coatings by Laser Cladding Inconel 625 and Reinforced WC Particles on Non-Magnetic Steel. Opt. Laser Technol. 2023, 163, 109321. [Google Scholar] [CrossRef]
- Wood, R.J. Tribology of Thermal Sprayed WC–Co Coatings. Int. J. Refract. Met. Hard Mater. 2010, 28, 82–94. [Google Scholar] [CrossRef]
- Zhang, X.; Kornmeier, J.R.; Hofmann, M.; Langebeck, A.; Alameddin, S.; Alessio, R.P.; Fritzen, F.; Bunn, J.R.; Cabeza, S. Residual Stresses in Cu Matrix Composite Surface Deposits after Laser Melt Injection. Strain 2023, 59, e12457. [Google Scholar] [CrossRef]
- Mahdi, M.; Sanjabi, S. Vacuum Brazed Ni-Based Coating Reinforced with Core-Shell WC@Cu/Co-P. Surf. Coat. Technol. 2022, 448, 128920. [Google Scholar] [CrossRef]
- Kamburov, V.; Valkanov, S.; Mateev, V.; Marinova, I.; Dimitrova, R.; Nikolov, A.; Penyashki, T.; Kostadinov, G.; Kandeva, M. Microstructural Analysis and Electroresistance Investigation of Hardalloyed Electrospark-Deposited Coatings on TiAl6V4 Alloy. In Proceedings of the 12th National Conference with International Participation (ELECTRONICA), Sofia, Bulgaria, 27–28 May 2021; pp. 1–6. [Google Scholar]
- Doğan, F.; Duru, E.; Akbulut, H.; Aslan, S. How the Duty Cycle Affects Wear and Corrosion: A Parametric Study in the Ni–B–TiN Composite Coatings. Results Surf. Interfaces 2023, 11, 100112. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Yan, F.; Li, S.; Ren, C.; Yin, Z.; Li, A.; Li, H.; Ji, X.; Zheng, Y.; et al. An overview of graphene-based hydroxyapatite composites for orthopedic applications. Bioact. Mater. 2018, 3, 1–18. [Google Scholar] [CrossRef]
- Shi, L.; Sun, C.; Gao, P.; Zhou, F.; Liu, W. Mechanical Properties and Wear and Corrosion Resistance of Electrodeposited Ni–Co/SiC Nanocomposite Coating. Appl. Surf. Sci. 2006, 252, 3591–3599. [Google Scholar] [CrossRef]
- Allongue, P.; Cagnon, L.; Gomes, C.; Gundel, A.; Costa, V. Electrodeposition of Co and Ni/Au(111) Ultrathin Layers. Part I: Nucleation and Growth Mechanisms from In Situ STM. Surf. Sci. 2004, 557, 41–56. [Google Scholar] [CrossRef]
- Farrokhzad, M.; Saha, G.; Khan, T. Wear Performance of Co-Electrodeposited Cermet Coatings. Surf. Coat. Technol. 2013, 235, 75–85. [Google Scholar] [CrossRef]
- Pasha, A.; Rajaprakash, B.M. Functionally Graded Materials (FGM) Fabrication and Its Potential Challenges & Applications. Mater. Today Proc. 2022, 52, 413–418. [Google Scholar]
- Hu, X.; Qu, N. Preparation of Nickel-Cobalt/Carborundum Carbide Composite Coatings by Supergravity Field-Enhanced Electrodeposition. Chin. J. Mech. Eng. 2020, 33, 57. [Google Scholar] [CrossRef]
- Cârâc, G.; Bund, A.; Thiemig, D. Electrocodeposition and Characterization of Cobalt Lanthanide Oxides Composite Coatings. Surf. Coat. 2007, 2, 403–411. [Google Scholar] [CrossRef]
- Branzoi, F.; Mihai, A.; Zaki, M. Anticorrosion Protection of New Composite Coating for Cobalt-Based Alloy in Hydrochloric Acid Solution Obtained by Electrodeposition Methods. Coatings 2024, 14, 106. [Google Scholar] [CrossRef]
- Liu, C.; Su, F.; Liang, J. Producing Cobalt–Graphene Composite Coating by Pulse Electrodeposition with Excellent Wear and Corrosion Resistance. Appl. Surf. Sci. 2015, 351, 889–896. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Chen, C.; Liu, J.; Zhao, L.; Dai, J. Fabrication of Co-Based Coatings on Titanium Alloy by Laser Cladding with CeO₂ Addition. Mater. Manuf. Process. 2016, 31, 1461–1467. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhang, P.; Yu, Z.; Li, C.; Xu, P.; Lu, Y. Laser Cladding of Co-Based Alloy/TiC/CaF₂ Self-Lubricating Composite Coatings on Copper for Continuous Casting Mold. Surf. Coat. Technol. 2013, 232, 362–369. [Google Scholar] [CrossRef]
- Weng, F.; Yu, H.; Liu, J.; Chen, C.; Dai, J.; Zhao, Z. Microstructure and Wear Property of the Ti5Si3/TiC Reinforced Co-Based Coatings Fabricated by Laser Cladding on Ti-6Al-4V. Opt. Laser Technol. 2017, 92, 156–162. [Google Scholar] [CrossRef]
- Toosinezhad, A.; Alinezhadfar, M.; Mahdavi, S. Tribological Behavior of Cobalt/Graphene Composite Coatings. Ceram. Int. 2020, 46, 16886–16894. [Google Scholar] [CrossRef]
- Bapu, G.R.; Thiruchelvam, T. Electrodeposited Cobalt–Boron Nitride Composites. Bull. Electrochem. 2001, 17, 529–534. [Google Scholar]
- Toosinezhad, A.; Alinezhadfar, M.; Mahdavi, S. Cobalt/Graphene Electrodeposits: Characteristics, Tribological Behavior, and Corrosion Properties. Surf. Coat. Technol. 2020, 385, 125418. [Google Scholar] [CrossRef]
- ASTM G99-17; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. ASTM International: West Conshohocken, PA, USA, 2017.
- Sun, H.; Feng, X.; Zhu, Q. Study on Hardness and Corrosion Resistance of Co-Mo-P/ZrO2 Composite Coating Electrodeposited on Q345 Steel. Int. J. Electrochem. Sci. 2024, 19, 100586. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Taylor, B.J.; Paranthaman, M.; Thompson, J.R.; Sinclair, J.W. Microstructure and Magnetic Properties of Electrodeposited Cobalt Films. J. Mater. Sci. 2008, 43, 1644–1649. [Google Scholar] [CrossRef]
- Kothanam, N.; Harachai, K.; Hom-On, C.; Qin, J.; Boonyongmaneerat, Y.; Triroj, N.; Jaroenapibal, P. Enhanced Particle Incorporation for Co-Electrodeposited Ni–P/Diamond Coatings with a Pulse-Stirring Technique. Appl. Surf. Sci. Adv. 2023, 18, 100499. [Google Scholar] [CrossRef]


| Steel | C [%] | Mn [%] | P [%] | S [%] | N [%] | Cu [%] | Fe [%] |
|---|---|---|---|---|---|---|---|
| S235 | <0.17 | <1.4 | <0.035 | <0.035 | <0.12 | <0.55 | Bal. |
| Type of Coating | Electrolyte | Deposition Conditions |
|---|---|---|
| Ni interlayer | 125 g L−1 NiSO4 · 7H2O + 25 g L−1 NiCl2 · 6 H2O + 20 g L−1 H3BO3 pH = 4.5–5 | 50 mA cm−2 for 5 min, at 50 °C, using a 99.99% nickel soluble anode |
| Co coating | 300 g L−1 CoSO4· 6H2O + 50 g L−1 CoCl2 · 6H2O + 30 g L−1 H3BO3 pH = 4.5–5 | 50 mA cm−2 for 20 min, at 25 °C, using a graphite inert anode |
| Co + Diamond particles composite coating | 300 g L−1 CoSO4· 6H2O + 50 g L−1 CoCl2 · 6H2O + 30 g L−1 H3BO3 + 2.5 g L−1 diamond particles (0.5 μm) pH = 4.5–5 | 25 mA cm−2 for 40 min, at 25 °C, using a graphite inert anode |
| Sample | E [mV] vs. Ag/AgCl | icorr [µA cm−2] | Corr. rate [mm Year−1] |
|---|---|---|---|
| S235 steel substrate (Substrate) | −556 | 23.2 | 0.283 |
| Co-based coating (Co) | −446 | 2.4 | 0.058 |
| Co + Diamond particles composite coating (Co + D) | −325 | 3.8 | 0.083 |
| Sample | µmin | µaverg | µmax |
|---|---|---|---|
| S235 steel substrate (Substrate) | 0.096 | 0.751 | 0.859 |
| Co-based coating (Co) | 0.246 | 0.612 | 0.713 |
| Co + Diamond particle composite coating (Co + D) | 0.237 | 0.568 | 0.645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uțu, D.; Muntean, R.; Anghel, I.-M.; Hulka, I.; Uțu, I.-D. Preparation and Characterization of Co-Diamond Composite Coatings Obtained in a Single-Step Hybrid Electrophoretic Deposition Process. Materials 2025, 18, 1294. https://doi.org/10.3390/ma18061294
Uțu D, Muntean R, Anghel I-M, Hulka I, Uțu I-D. Preparation and Characterization of Co-Diamond Composite Coatings Obtained in a Single-Step Hybrid Electrophoretic Deposition Process. Materials. 2025; 18(6):1294. https://doi.org/10.3390/ma18061294
Chicago/Turabian StyleUțu, Diana, Roxana Muntean, Iasmina-Mădălina Anghel (Petculescu), Iosif Hulka, and Ion-Dragoș Uțu. 2025. "Preparation and Characterization of Co-Diamond Composite Coatings Obtained in a Single-Step Hybrid Electrophoretic Deposition Process" Materials 18, no. 6: 1294. https://doi.org/10.3390/ma18061294
APA StyleUțu, D., Muntean, R., Anghel, I.-M., Hulka, I., & Uțu, I.-D. (2025). Preparation and Characterization of Co-Diamond Composite Coatings Obtained in a Single-Step Hybrid Electrophoretic Deposition Process. Materials, 18(6), 1294. https://doi.org/10.3390/ma18061294









