A Review of Nanofiber Electrodes and the In Situ Exsolution of Nanoparticles for Solid Oxide Cells
Abstract
1. Introduction
2. Solid Oxide Fuel Cells and Solid Oxide Electrolysis Cells
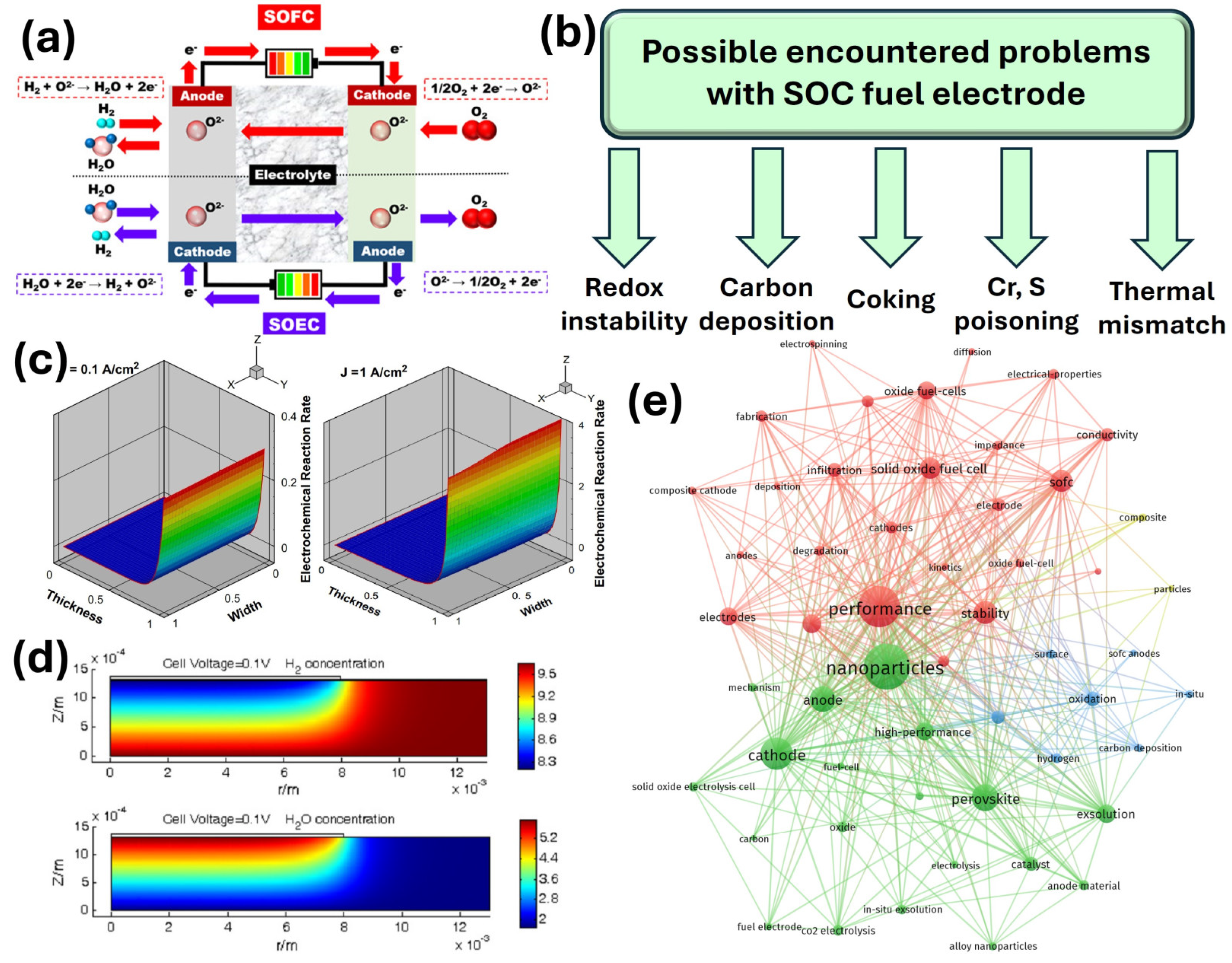
3. Fuel Electrodes Design for Solid Oxide Cells
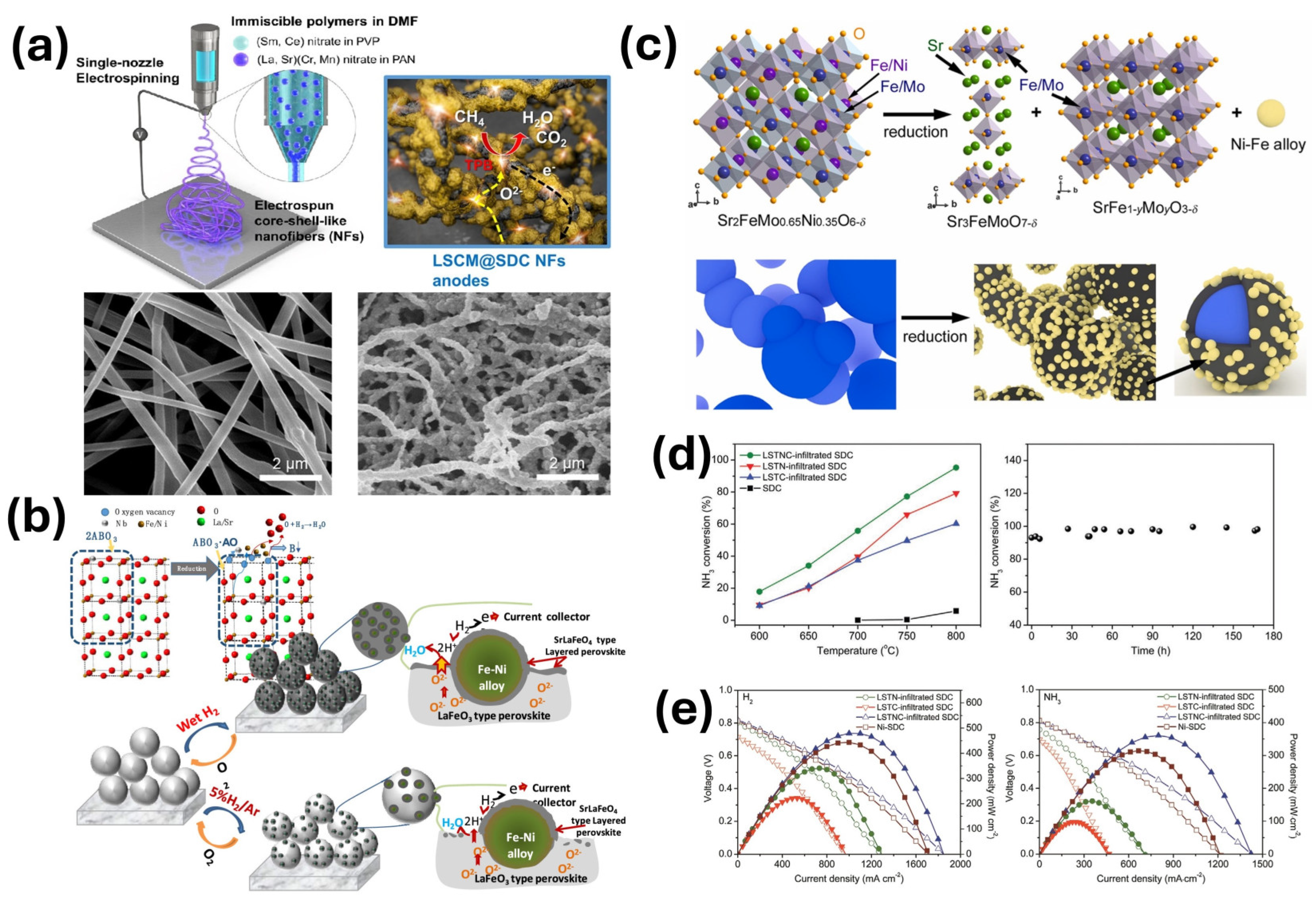
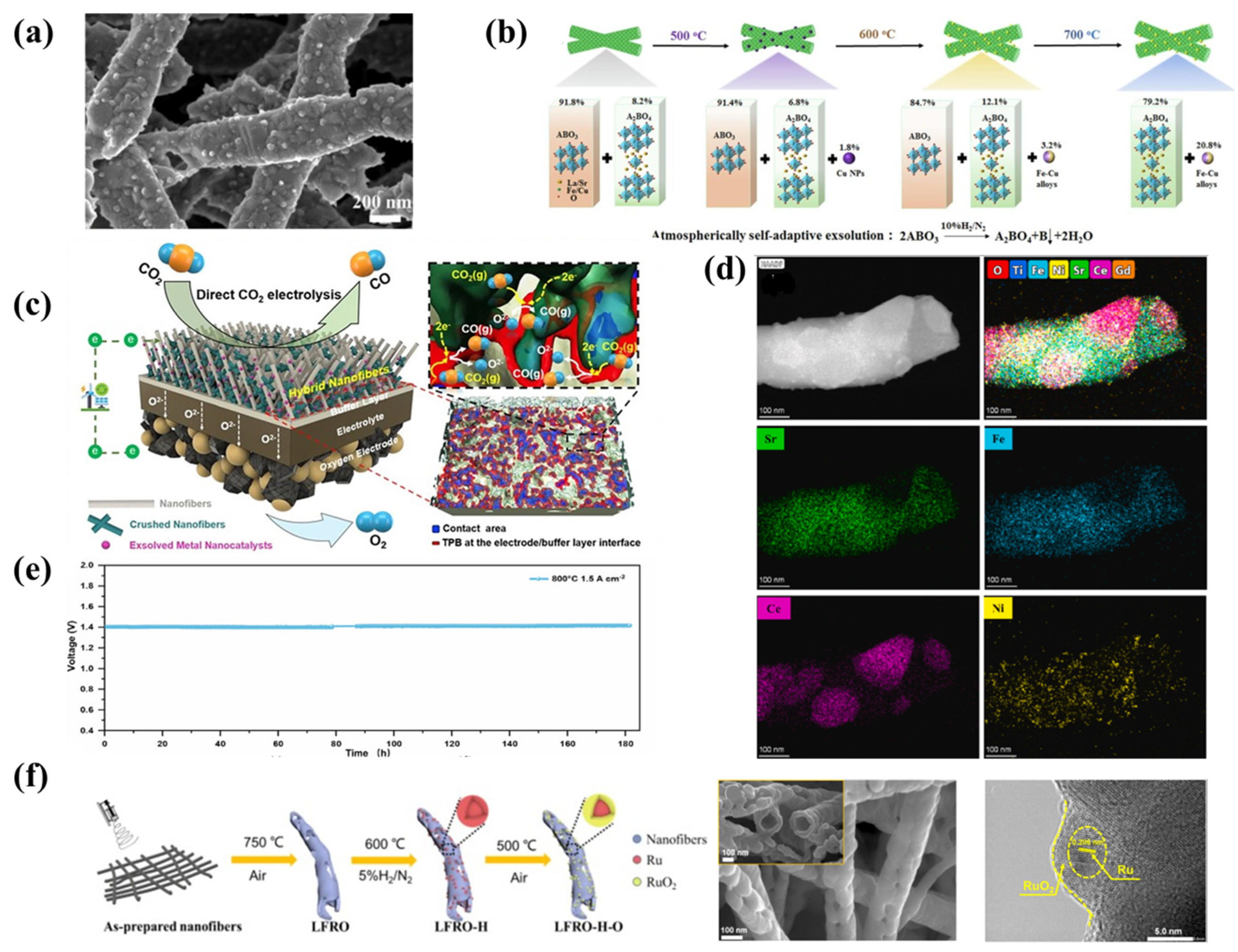
4. Nanofibers with In Situ Exsolution of Nanoparticles
4.1. In Situ Exsolution of Nanoparticles
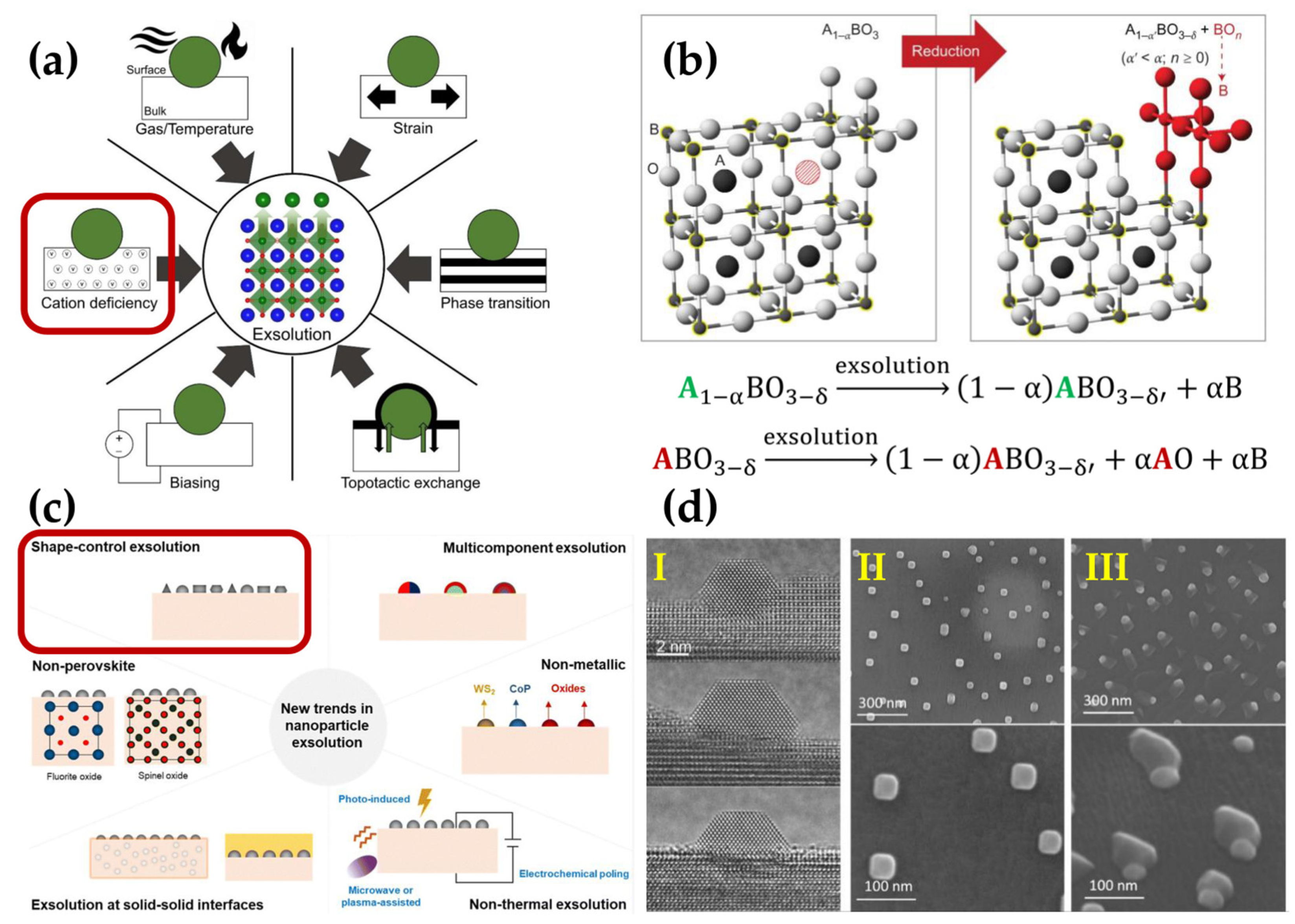

4.2. Multi-Elemental Nanocatalysts

| Catalyst | Crystal Structure | Synthesis Method | Material’s Catalytic Function | Reference |
|---|---|---|---|---|
| PdFeCoNiCu | Body-centered cubic | Colloidal synthesis (oil phase synthesis) | Hydrogen evolution reaction | [68] |
| NiFeCoCuPd (exsolved from LaFe0.7Ni0.1Co0.1Cu0.05Pd0.05O3 in reducing atmosphere) | Inhomogeneous metallic phase | Soft chemistry method (similar to the citric acid sol–gel-type Pechini method) | Catalytic dry methane reforming | [48,74] |
| Pt18Ni26Fe15Co14Cu27 | Face-centered cubic | One-pot oil phase synthesis | Hydrogen evolution reaction; methanol oxidation reaction | [65] |
| NiCoCuFeMn | Crystal metal alloys | Co-precipitation method | Steam reforming of methane | [70] |
| Fe0.1Co0.35Ni0.35Cu0.1Mo0.1 | Face-centered cubic | Reducing the corresponding oxide powder synthesized by sol-gel method | Cathode material in SOEC to co-electrolysis of H2O and CO2 toward syngas; anode material in SOFC | [64] |
| FeCoNiCuX (X = Al, Mo) | Face-centered cubic and Body-centered cubic | Mechanical alloying method in Ar atmosphere | SOFC anode, fueled by H2, CH4, and CO2 | [69] |
| Ni9.75Co13Cu16.25Fe16.25Mn9.75 | Face-centered cubic | Co-precipitation method | SOFC for steam reforming of methane | [70] |
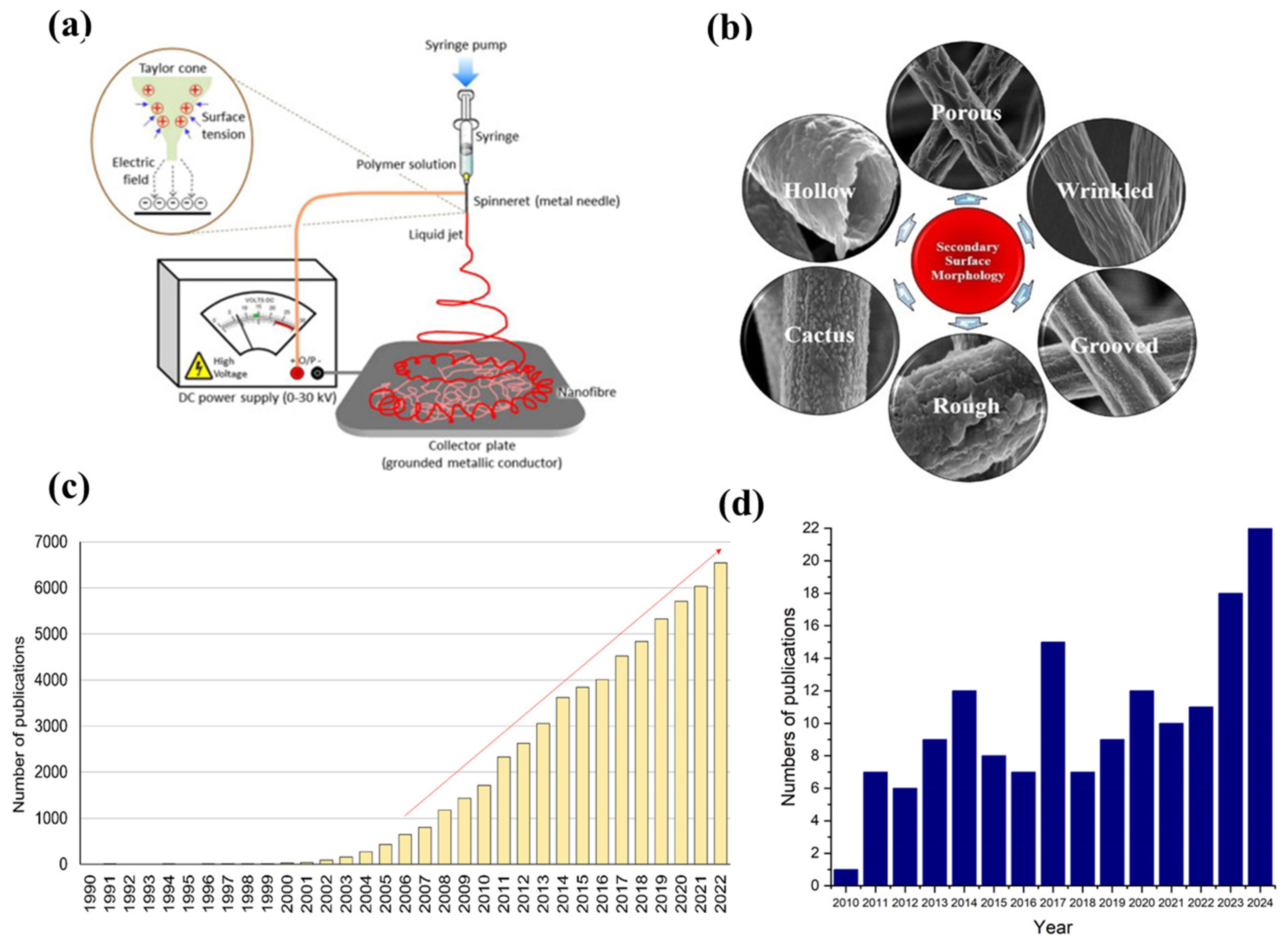
4.3. Nanofiber Electrodes for Solid Oxide Cells
4.4. Nanofibers with In Situ Exsolved Nanoparticles
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA. World Electricity Consumption 1990–2019. Energy Statistics Data Browser. 2022. Available online: https://www.iea.org/reports/electricity-information-overview/electricity-consumption (accessed on 7 March 2025).
- Fossil Fuels: The Dirty Facts. Natural Resources Defense Council. Available online: https://www.nrdc.org/stories/fossil-fuels-dirty-facts (accessed on 7 March 2025).
- The Sources and Solutions: Fossil Fuels. United States Environmental Protection Agency. 2022. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2021 (accessed on 7 March 2025).
- Bertand, S. Fact Sheet|Climate, Environmental, and Health Impacts of Fossil Fuels. Environmental and Energy Study Institute 2021. Available online: https://www.eesi.org/papers/view/fact-sheet-climate-environmental-and-health-impacts-of-fossil-fuels-2021 (accessed on 7 March 2025).
- BloomEnergy Customer Datasheet. 2020. Available online: https://www.bloomenergy.com/customer/honda/ (accessed on 7 March 2025).
- Meng, N.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. Prospect of Proton Exchange Membrane Fuel Cells for Transportation. In Proceedings of the World Renewable Energy Congress VIII, Denver, CO, USA, 29 August–3 September 2004. [Google Scholar]
- Kamlungsua, K.; Su, P.-C.; Chan, S.H. Hydrogen Generation Using Solid Oxide Electrolysis Cells. Fuel Cells 2020, 20, 644–649. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and Innovative Solid Oxide Electrolysis Cell Materials: A Short Review. Renew. Sustain. Energy Rev. 2021, 148, 111322. [Google Scholar] [CrossRef]
- Wolf, S.E.; Winterhalder, F.E.; Vibhu, V.; de Haart, L.G.J.; Guillon, O.; Eichel, R.A.; Menzler, N.H. Solid Oxide Electrolysis Cells—Current Material Development and Industrial Application. J. Mater. Chem. A 2023, 11, 17977–18028. [Google Scholar] [CrossRef]
- Shaikh, S.P.S.; Muchtar, A.; Somalu, M.R. A Review on the Selection of Anode Materials for Solid-Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2015, 51, 1–8. [Google Scholar] [CrossRef]
- Yue, W.; Li, Y.; Zheng, Y.; Wu, T.; Zhao, C.; Zhao, J.; Geng, G.; Zhang, W.; Chen, J.; Zhu, J.; et al. Enhancing Coking Resistance of Ni/YSZ Electrodes: In Situ Characterization, Mechanism Research, and Surface Engineering. Nano Energy 2019, 62, 64–78. [Google Scholar] [CrossRef]
- Yokokawa, H.; Horita, T.; Sakai, N.; Yamaji, K.; Brito, M.E.; Xiong, Y.P.; Kishimoto, H. Thermodynamic Considerations on Cr Poisoning in SOFC Cathodes. Solid State Ion. 2006, 177, 3193–3198. [Google Scholar] [CrossRef]
- Chiang, L.K.; Liu, H.C.; Shiu, Y.H.; Lee, C.H.; Lee, R.Y. Thermo-Electrochemical and Thermal Stress Analysis for an Anode-Supported SOFC Cell. Renew. Energy 2008, 33, 2580–2588. [Google Scholar] [CrossRef]
- Hussain, M.M.; Li, X.; Dincer, I. A Numerical Investigation of Modeling an SOFC Electrode as Two Finite Layers. Int. J. Hydrogen Energy 2009, 34, 3134–3144. [Google Scholar] [CrossRef]
- Dong, S.K.; Jung, W.N.; Rashid, K.; Kashimoto, A. Design and Numerical Analysis of a Planar Anode-Supported SOFC Stack. Renew. Energy 2016, 94, 637–650. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N.; Li, C. Numerical Modeling of an Anode-Supported SOFC Button Cell Considering Anodic Surface Diffusion. J. Power Sources 2007, 164, 639–648. [Google Scholar] [CrossRef]
- Wang, R.; Wang, T.; Ma, Y.; Wei, T.; Ye, Z.; Chen, B.; Dong, D. Control of Carbon Deposition over Methane-Fueled SOFCs through Tuning the O/C Ratio at the Anode/Electrolyte Interface. J. Power Sources 2022, 544, 231854. [Google Scholar] [CrossRef]
- Aslannejad, H.; Barelli, L.; Babaie, A.; Bozorgmehri, S. Effect of Air Addition to Methane on Performance Stability and Coking over NiO-YSZ Anodes of SOFC. Appl. Energy 2016, 177, 179–186. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. A Review on the Status of Anode Materials for Solid Oxide Fuel Cells. Mater. Sci. Eng. A 2003, 362, 228–239. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Senthil Kumar, S.; Aruna, S.T. Properties and Development of Ni/YSZ as an Anode Material in Solid Oxide Fuel Cell: A Review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Liu, M.; Lynch, M.E.; Blinn, K.; Alamgir, F.M.; Choi, Y. The Demand for Clean, Secure, and Sustainable Energy Sources Has Stimulated Great Interest in Fuel Cells: Devices That Convert. Mater. Today 2011, 14, 534–546. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Yan, M. A Review on the Preparation of Thin-Film YSZ Electrolyte of SOFCs by Magnetron Sputtering Technology. Sep. Purif. Technol. 2022, 298, 121627. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Shipilova, A.V.; Ionov, I.V.; Kovalchuk, A.N.; Rabotkin, S.V.; Oskirko, V.O. Magnetron-Sputtered YSZ and CGO Electrolytes for SOFC. J. Electron. Mater. 2016, 45, 3921–3928. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, J.; Ma, Y.; Sun, J.; Zhu, L. Electrical Properties of an A-Site Ba-Doped LSGM Ceramic Electrolyte for IT-SOFC. Ceram. Int. 2025, in press. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox Behaviour, Chemical Compatibility and Electrochemical Performance of Sr2MgMoO6−δ as SOFC Anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Zhou, X.; Xu, N.; Xie, Z.; Chen, N. Electrical Conductivity and Structural Stability of La-Doped SrTiO3 with A-Site Deficiency as Anode Materials for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 7913–7918. [Google Scholar] [CrossRef]
- Huang, Y.H.; Dass, R.I.; King, Z.L.; Goodenough, J.B. Double Perovskites as Anode Materials for Solid-Oxide Fuel Cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Irvine, J.T.S. A Redox-Stable Efficient Anode for Solid-Oxide Fuel Cells. Nat. Mater. 2003, 2, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Cho, H.-J.; Kim, J.; Kang, J.-Y.; Seo, J.; Kim, J.H.; Jeong, S.J.; Lim, D.-K.; Kim, I.; Jung, W. Nanofiber Composites as Highly Active and Robust Anodes for Direct-Hydrocarbon Solid Oxide Fuel Cells. ACS Nano 2022, 16, 14517–14526. [Google Scholar] [CrossRef]
- Park, B.H.; Choi, G.M. Ex-Solution of Ni Nanoparticles in a La0.2Sr0.8Ti1−xNixO3−δ Alternative Anode for Solid Oxide Fuel Cell. Solid State Ion. 2014, 262, 345–348. [Google Scholar] [CrossRef]
- Zhu, T.; Troiani, H.; Mogni, L.V.; Santaya, M.; Han, M.; Barnett, S.A. Exsolution and Electrochemistry in Perovskite Solid Oxide Fuel Cell Anodes: Role of Stoichiometry in Sr(Ti,Fe,Ni)O3. J. Power Sources 2019, 439, 227077. [Google Scholar] [CrossRef]
- Thommy, L.; Benamira, M.; Jardiel, T.; Günes, V.; Joubert, O.; Caldes, M.T. Ru Exsolution in Substituted La0.75Sr0.25Cr0.5Mn0.5O3−δ Compound as Anode Material for IT-SOFCs. Mater. Chem. Phys. 2021, 268, 124724. [Google Scholar] [CrossRef]
- Wu, X.; Yu, Y.; Chen, Y.; Li, L.; Ma, Z.F.; Yin, Y.M. Construction of Multifunctional Nanoarchitectures in One Step on a Composite Fuel Catalyst through in Situ Exsolution of La0.5Sr0.5Fe0.8Ni0.1Nb0.1O3. ACS Appl. Mater. Interfaces 2020, 12, 34890–34900. [Google Scholar] [CrossRef]
- Xia, C.; Li, Z.; Wang, S.; Beshiwork, B.A.; Lin, B. Recent Progress on Efficient Perovskite Ceramic Anodes for High-Performing Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2024, 62, 331–344. [Google Scholar] [CrossRef]
- Song, Y.; Li, H.; Xu, M.; Yang, G.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Infiltrated NiCo Alloy Nanoparticle Decorated Perovskite Oxide: A Highly Active, Stable, and Antisintering Anode for Direct-Ammonia Solid Oxide Fuel Cells. Small 2020, 16, 2001859. [Google Scholar] [CrossRef]
- Kousi, K.; Tang, C.; Metcalfe, I.S.; Neagu, D. Emergence and Future of Exsolved Materials. Small 2021, 17, 2006479. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Saccoccio, M.; Lu, Z.; Ciucci, F. From Material Design to Mechanism Study: Nanoscale Ni Exsolution on a Highly Active A-Site Deficient Anode Material for Solid Oxide Fuel Cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Kwon, O.; Joo, S.; Choi, S.; Sengodan, S.; Kim, G. Review on Exsolution and Its Driving Forces in Perovskites. J. Phys. Energy 2020, 2, 042001. [Google Scholar] [CrossRef]
- Belotti, A.; Wang, Y.; Curcio, A.; Liu, J.; Quattrocchi, E.; Pepe, S.; Ciucci, F. The Influence of A-Site Deficiency on the Electrochemical Properties of (Ba0.95La0.05)1−xFeO3−δ as an Intermediate Temperature Solid Oxide Fuel Cell Cathode. Int. J. Hydrogen Energy 2022, 47, 1229–1240. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Ménard, H.; Irvine, J.T.S. In Situ Growth of Nanoparticles through Control of Non-Stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Lopez-Garcia, A.; Delgado-Galicia, B.; Serra, J.M. New Trends in Nanoparticle Exsolution. Chem. Commun. 2024, 60, 7987–8007. [Google Scholar] [CrossRef] [PubMed]
- Neagu, D.; Kyriakou, V.; Roiban, I.L.; Aouine, M.; Tang, C.; Caravaca, A.; Kousi, K.; Schreur-Piet, I.; Metcalfe, I.S.; Vernoux, P.; et al. In Situ Observation of Nanoparticle Exsolution from Perovskite Oxides: From Atomic Scale Mechanistic Insight to Nanostructure Tailoring. ACS Nano 2019, 13, 12996–13005. [Google Scholar] [CrossRef]
- Zheng, K.; Lach, J.; Czaja, P.; Gogacz, M.; Czach, P.; Brzoza-Kos, A.; Winiarz, P.; Luo, J. Designing High-Performance Quasi-Symmetrical Solid Oxide Cells with a Facile Chemical Modification Strategy for Sr2Fe2−xWxO6−δ Ferrites Electrodes with in Situ Exsolution of Nanoparticles. J. Power Sources 2023, 587, 233707. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Liu, M.; Tadé, M.O.; Shao, Z. Progress and Prospects in Symmetrical Solid Oxide Fuel Cells with Two Identical Electrodes. Adv. Energy Mater. 2015, 5, 1500188. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Zhang, M.; Du, Z.; Guan, W.; Singhal, S.C.; Świerczek, K. Boosting the Electrode Reaction Kinetics of SSOFCs by the Synergistic Effect of Nanoparticle Codecoration on Both the Cathode and Anode. Chem. Mater. 2023, 35, 499–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmauss, T.A.; Barnett, S.A.; Neagu, D.; Irvine, J.T.S.; Wang, J.; Yildiz, B.; Opitz, A.K. Roadmap on Exsolution for Energy Applications. J. Phys. Energy 2023, 5, 032001. [Google Scholar]
- Lv, H.; Lin, L.; Zhang, X.; Song, Y.; Matsumoto, H.; Zeng, C.; Ta, N.; Liu, W.; Gao, D.; Wang, G.; et al. In Situ Investigation of Reversible Exsolution/Dissolution of CoFe Alloy Nanoparticles in a Co-Doped Sr2Fe1.5Mo0.5O6−δ Cathode for CO2 Electrolysis. Adv. Mater. 2020, 32, 1906193. [Google Scholar] [CrossRef] [PubMed]
- Najimu, M.; Jo, S.; Gilliard-AbdulAziz, K.L. Co-Exsolution of Ni-Based Alloy Catalysts for the Valorization of Carbon Dioxide and Methane. Acc. Chem. Res. 2023, 56, 3132–3141. [Google Scholar] [CrossRef]
- Hu, H.; Li, M.; Min, H.; Zhou, X.; Li, J.; Wang, X.; Lu, Y.; Ding, X. Enhancing the Catalytic Activity and Coking Tolerance of the Perovskite Anode for Solid Oxide Fuel Cells through In Situ Exsolution of Co–Fe Nanoparticles. ACS Catal. 2022, 12, 828–836. [Google Scholar] [CrossRef]
- Xu, M.; Cao, R.; Qin, H.; Zhang, N.; Yan, W.; Liu, L.; Irvine, J.T.S.; Chen, D. Exsolved Materials for CO2 Reduction in High-Temperature Electrolysis Cells. Mater. Rep. Energy 2023, 3, 100198. [Google Scholar] [CrossRef]
- Vera, E.; Trillaud, V.; Metaouaa, J.; Aouine, M.; Boreave, A.; Burel, L.; Roiban, I.L.; Steyer, P.; Vernoux, P. Comparative Study of Exsolved and Impregnated Ni Nanoparticles Supported on Nanoporous Perovskites for Low-Temperature CO Oxidation. ACS Appl. Mater. Interfaces 2024, 16, 7219–7231. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, M.R.; Luo, J.L. In Situ Exsolved Metal Nanoparticles: A Smart Approach for Optimization of Catalysts. Chem. Mater. 2020, 32, 5424–5441. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-Socketed Nickel Particles with Enhanced Coking Resistance Grown in Situ by Redox Exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Ding, D.; Liu, M.; Chen, F.; Xia, C. Fabrication and Modification of Solid Oxide Fuel Cell Anodes via Wet Impregnation/Infiltration Technique. J. Power Sources 2013, 237, 243–259. [Google Scholar] [CrossRef]
- Neagu, D.; Papaioannou, E.I.; Ramli, W.K.W.; Miller, D.N.; Murdoch, B.J.; Ménard, H.; Umar, A.; Barlow, A.J.; Cumpson, P.J.; Irvine, J.T.S.; et al. Demonstration of Chemistry at a Point through Restructuring and Catalytic Activation at Anchored Nanoparticles. Nat. Commun. 2017, 8, 1855. [Google Scholar] [CrossRef]
- Lv, H.; Wang, S.; Shen, Y.; Zhang, X.; Song, Y.; Li, R.; Ta, N.; Liu, T.; Wang, G.; Bao, X. Iron-Triggered Exsolution of FeNi Alloy Nanoparticles via Topotactic Cation Exchange on Pr0.7Sr0.3Cr0.9Ni0.1O3−δ Perovskite for CO2 Electrolysis. Next Energy 2023, 1, 100024. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jeong, H.; Won, B.R.; Jeon, H.; Park, C.H.; Park, D.; Kim, Y.; Lee, S.; Myung, J.H. Nanoparticle Exsolution on Perovskite Oxides: Insights into Mechanism, Characteristics and Novel Strategies. Nano-Micro Lett. 2024, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Han Kim, Y.; Jeong, H.; Park, C.H.; Won, B.R.; Jeon, H.; Taek Lee, K.; Myung, J.H. Exsolution of Phase-Separated Nanoparticles via Trigger Effect toward Reversible Solid Oxide Cell. Appl. Energy 2022, 323, 119615. [Google Scholar] [CrossRef]
- Steiger, P.; Burnat, D.; Madi, H.; Mai, A.; Holzer, L.; Van Herle, J.; Kröcher, O.; Heel, A.; Ferri, D. Sulfur Poisoning Recovery on a Solid Oxide Fuel Cell Anode Material through Reversible Segregation of Nickel. Chem. Mater. 2019, 31, 748–758. [Google Scholar] [CrossRef]
- Napolitano, F.; Soldati, A.L.; Geck, J.; Lamas, D.G.; Serquis, A. Electronic and Structural Properties of La0.4Sr0.6Ti1−yCoyO3±δ Electrode Materials for Symmetric SOFC Studied by Hard X-Ray Absorption Spectroscopy. Int. J. Hydrogen Energy 2013, 38, 8965–8973. [Google Scholar] [CrossRef]
- Santaya, M.; Jiménez, C.E.; Arce, M.D.; Carbonio, E.A.; Toscani, L.M.; Garcia-Diez, R.; Knop-Gericke, A.; Mogni, L.V.; Bär, M.; Troiani, H.E. Exsolution versus Particle Segregation on (Ni,Co)-Doped and Undoped SrTi0.3Fe0.7O3−δ Perovskites: Differences and Influence of the Reduction Path on the Final System Nanostructure. Int. J. Hydrogen Energy 2023, 48, 38842–38853. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, S. High-Entropy Materials for Catalysis: A New Frontier. Sci. Adv. 2021, 7, eabg1600. [Google Scholar] [CrossRef]
- Hou, S.; Ma, X.; Shu, Y.; Bao, J.; Zhang, Q.; Chen, M.; Zhang, P.; Dai, S. Self-Regeneration of Supported Transition Metals by a High Entropy–Driven Principle. Nat. Commun. 2021, 12, 5363. [Google Scholar] [CrossRef]
- Tong, J.; Ni, N.; Zhou, B.; Yang, C.; Reddy, K.M.; Tu, H.; Liu, Y.; Tan, Z.; Xiang, L.; Li, H.; et al. Toward High CO Selectivity and Oxidation Resistance Solid Oxide Electrolysis Cell with High-Entropy Alloy. ACS Catal. 2024, 14, 2897–2907. [Google Scholar] [CrossRef]
- Li, H.; Han, Y.; Zhao, H.; Qi, W.; Zhang, D.; Yu, Y.; Cai, W.; Li, S.; Lai, J.; Huang, B.; et al. Fast Site-to-Site Electron Transfer of High-Entropy Alloy Nanocatalyst Driving Redox Electrocatalysis. Nat. Commun. 2020, 11, 5437. [Google Scholar] [CrossRef]
- Kumar Katiyar, N.; Biswas, K.; Yeh, J.-W.; Sharma, S.; Sekhar Tiwary, C. A Perspective on the Catalysis Using the High Entropy Alloys. Nano Energy 2021, 88, 106261. [Google Scholar] [CrossRef]
- Wang, B.; Yao, Y.; Yu, X.; Wang, C.; Wu, C.; Zou, Z. Understanding the Enhanced Catalytic Activity of High Entropy Alloys: From Theory to Experiment. J. Mater. Chem. A 2021, 9, 19410–19438. [Google Scholar] [CrossRef]
- Zhang, D.; Shi, Y.; Zhao, H.; Qi, W.; Chen, X.; Zhan, T.; Li, S.; Yang, B.; Sun, M.; Lai, J.; et al. The Facile Oil-Phase Synthesis of a Multi-Site Synergistic High-Entropy Alloy to Promote the Alkaline Hydrogen Evolution Reaction. J. Mater. Chem. A 2021, 9, 889–893. [Google Scholar] [CrossRef]
- Chen, D.; Huan, Y.; Ma, G.; Ma, M.; Wang, X.; Xie, X.; Leng, J.; Hu, X.; Wang, T. High-Entropy Alloys FeCoNiCuX (X = Al, Mo)-Ce0.8Sm0.2O2 as High-Performance Solid Oxide Fuel Cell Anodes. ACS Appl. Energy Mater. 2023, 6, 1076–1084. [Google Scholar] [CrossRef]
- Lee, K.X.; Hu, B.; Dubey, P.K.; Anisur, M.R.; Belko, S.; Aphale, A.N.; Singh, P. High-Entropy Alloy Anode for Direct Internal Steam Reforming of Methane in SOFC. Int. J. Hydrogen Energy 2022, 47, 38372–38385. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, Z.; Di, H.; Bai, Y.; Yang, G.; Medvedev, D.A.; Luo, Z.; Wang, W.; Zhou, W.; Ran, R.; et al. High-Entropy Materials for Solid Oxide Cells: Synthesis, Applications, and Prospects. J. Energy Chem. 2025, 75, 268–296. [Google Scholar] [CrossRef]
- Cui, M.; Yang, C.; Hwang, S.; Yang, M.; Overa, S.; Dong, Q.; Yao, Y.; Brozena, A.H.; Cullen, D.A.; Chi, M.; et al. Multi-Principal Elemental Intermetallic Nanoparticles Synthesized via a Disorder-to-Order Transition. Sci. Adv. 2022, 8, eabm4322. [Google Scholar] [CrossRef]
- Xu, M.; Jeon, Y.; Naden, A.; Kim, H.; Kerherve, G.; Payne, D.J.; Shul, Y.G.; Irvine, J.T.S. Synergistic Growth of Nickel and Platinum Nanoparticles via Exsolution and Surface Reaction. Nat. Commun. 2024, 15, 48455. [Google Scholar] [CrossRef]
- Shah, S.; Xu, M.; Pan, X.; Gilliard-AbdulAziz, K.L. Complex Alloy and Heterostructure Nanoparticles Derived from Perovskite Oxide Precursors for Catalytic Dry Methane Reforming. ACS Appl. Nano Mater. 2022, 5, 17476–17481. [Google Scholar] [CrossRef]
- Mayyas, M.; Kalantar-Zadeh, K.; Mousavi, M.; Ghasemian, M.B.; Abbasi, R.; Li, H.; Christoe, M.J.; Han, J.; Wang, Y.; Zhang, C.; et al. Pulsing Liquid Alloys for Nanomaterials Synthesis. ACS Nano 2020, 14, 14070–14079. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Liu, Z.; Gu, Y.; Bi, L. Applications of Electrospun Nanofibers in Solid Oxide Fuel Cells—A Review. J. Alloys Compd. 2023, 937, 168288. [Google Scholar] [CrossRef]
- Desta, H.G.; Gebreslassie, G.; Zhang, J.; Lin, B.; Zheng, Y.; Zhang, J. Enhancing Performance of Lower-Temperature Solid Oxide Fuel Cell Cathodes through Surface Engineering. Prog. Mater. Sci. 2025, 147, 101353. [Google Scholar] [CrossRef]
- Parbey, J.; Wang, Q.; Yu, G.; Zhang, X.; Li, T.; Andersson, M. Progress in the Use of Electrospun Nanofiber Electrodes for Solid Oxide Fuel Cells: A Review. Rev. Chem. Eng. 2020, 36, 879–931. [Google Scholar] [CrossRef]
- Aruna, S.T.; Balaji, L.S.; Kumar, S.S.; Prakash, B.S. Electrospinning in Solid Oxide Fuel Cells—A Review. Renew. Sustain. Energy Rev. 2017, 67, 673–682. [Google Scholar] [CrossRef]
- Zaarour, B.; Zhu, L.; Jin, X. A Review on the Secondary Surface Morphology of Electrospun Nanofibers: Formation Mechanisms, Characterizations, and Applications. Chem. Sel. 2020, 5, 1335–1348. [Google Scholar] [CrossRef]
- Sarkar, A.; Bidu, J.M.; Panda, J.; Kwon, Y.J.; Bak, S.; Cho, K.Y.; Byun, S.; Cheong, J.Y. Applications of Electrospinning for Fuel Cell and Electrolysis Cell Applications in Hydrogen Technologies. Energy Rev. 2025, 4, 100119. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Gumeci, C.; Parrondo, J.; Hussain, A.M.; Thompson, D.; Dale, N. Praseodymium Based Double-Perovskite Cathode Nanofibers for Intermediate Temperature Solid Oxide Fuel Cells (IT-SOFC). Int. J. Hydrogen Energy 2021, 46, 31798–31806. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, L.; Yue, Z.; Dong, D.; Ai, N.; Jiang, S.P.; Zhao, D.; Wang, X.; Shao, Y.; Chen, K. Facile Approach for Improving the Interfacial Adhesion of Nanofiber Air Electrodes of Reversible Solid Oxide Cells. ACS Appl. Mater. Interfaces 2023, 15, 8120–8127. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, J.; Luo, Y.; Wang, Y.; Zhang, X.; Chen, X.; Liu, T.; Ding, M. Robust Cobalt-Free Perovskite Type Electrospun Nanofiber Cathode for Efficient Electrochemical Carbon Dioxide Reduction Reaction. J. Power Sources 2023, 587, 233705. [Google Scholar] [CrossRef]
- Chang, C.-L.; Hsu, C.-S.; Huang, J.-B.; Hsu, P.-H.; Hwang, B.-H. Preparation and Characterization of SOFC Cathodes Made of SSC Nanofibers. J. Alloys Compd. 2015, 620, 233–239. [Google Scholar] [CrossRef]
- Zhi, M.; Lee, S.; Miller, N.; Menzler, N.H.; Wu, N. An Intermediate-Temperature Solid Oxide Fuel Cell with Electrospun Nanofiber Cathode. Energy Environ. Sci. 2012, 5, 7066–7071. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, D.; Zhu, X.; Wang, N.; Chen, R.; Wang, B. New SOFC Cathode: 3D Core–Shell-Structured La0.6Sr0.4Co0.2Fe0.8O3−δ@PrO2−δ Nanofibers Prepared by Coaxial Electrospinning. ACS Appl. Energy Mater. 2022, 5, 11178–11190. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Guan, K.; Wei, Z.; Zhang, X.; Meng, J.; Liu, X.; Meng, J. La0.6Sr0.4Co0.2Fe0.8O3−δ/CeO2 Heterostructured Composite Nanofibers as a Highly Active and Robust Cathode Catalyst for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 26830–26841. [Google Scholar] [CrossRef]
- Sanna, C.; Zhang, W.; Costamagna, P.; Holtappels, P. Synthesis and Electrochemical Characterization of La0.6Sr0.4Co0.2Fe0.8O3−δ/Ce0.9Gd0.1O1.95 Co-Electrospun Nanofiber Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 13818–13831. [Google Scholar] [CrossRef]
- Zhao, E.; Jia, Z.; Zhao, L.; Xiong, Y.; Sun, C.; Brito, M.E. One-Dimensional La0.8Sr0.2Co0.2Fe0.8O3−δ/Ce0.8Gd0.2O1.9 Nanocomposite Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. J. Power Sources 2012, 219, 133–139. [Google Scholar] [CrossRef]
- Chou, C.-C.; Huang, C.-F.; Yeh, T.-H. Characterization and Catalytic Activity of La0.6Sr0.4Co0.2Fe0.8O3−δ–Yttria Stabilized Zirconia Electrospun Nano-Fiber as a Cathode Catalyst. Ceram. Int. 2013, 39, S549–S553. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Ding, Z.; Yang, G.; Xiao, X.; Peng, M.; Wu, M.; Li, J.; Liu, J.; Alodhayb, A.; et al. Nanoscale Intertwined Biphase Nanofiber as Active and Durable Air Electrode for Solid Oxide Electrochemical Cells. ACS Sustain. Chem. Eng. 2023, 11, 8592–8602. [Google Scholar] [CrossRef]
- Zhao, E.; Liu, X.; Liu, L.; Huo, H.; Xiong, Y. Effect of La0.8Sr0.2Co0.2Fe0.8O3−δ Morphology on the Performance of Composite Cathodes. Prog. Nat. Sci. Mater. Int. 2014, 24, 24–30. [Google Scholar] [CrossRef]
- Li, D.; Jin, Y.; Liu, C.; Fu, M.; Zong, X.; Xiong, Y. Effect of Optimal GDC Loading on Long-Term Stability of La0.8Sr0.2Co0.2Fe0.8O3−δ/Gd0.2Ce0.8O1.9 Composite Cathodes. Ceram. Int. 2021, 47, 6591–6596. [Google Scholar] [CrossRef]
- Zhao, E.; Ma, C.; Yang, W.; Xiong, Y.; Li, J.; Sun, C. Electrospinning La0.8Sr0.2Co0.2Fe0.8O3−δ Tubes Impregnated with Ce0.8Gd0.2O1.9 Nanoparticles for an Intermediate Temperature Solid Oxide Fuel Cell Cathode. Int. J. Hydrogen Energy 2013, 38, 6821–6829. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Xie, C.; Wang, Y.; Li, H.; Ding, X. Hetero-Structured Composite Cathodes by Electrospinning with High CO2-Poisoning Tolerance for Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2024, 50, 1137–1146. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In Situ Exsolution of Co/CoOx Core–Shell Nanoparticles on Double Perovskite Porous Nanotubular Webs: A Synergistically Active Catalyst for Soot Efficient Oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, J.; Zong, Z.; Fu, L.; Lian, Z.; Ni, C.; Wang, J.; Wan, Y.; Wu, K. A Mesoporous Catalytic Fiber Architecture Decorated by Exsolved Nanoparticles for Reversible Solid Oxide Cells. J. Power Sources 2020, 468, 228349. [Google Scholar] [CrossRef]
- Zou, L.; Tian, Y.; Pu, J.; Chi, B. Smart Utilization of In-Situ Exsolution Occurring in Ag Doped PrBa0.5Sr0.5Co2O5+δ Nanofiber Air Electrode of Intermediate Temperature Reversible Solid Oxide Cells. J. Alloys Compd. 2022, 900, 163540. [Google Scholar] [CrossRef]
- Song, Y.; Yin, Y.-M.; Li, L.; Chen, Z.; Ma, Z.-F. A Self-Assembled Dual-Phase Composite as a Precursor of High-Performance Anodes for Intermediate Temperature Solid Oxide Fuel Cells. Chem. Commun. 2018, 54, 12341–12344. [Google Scholar] [CrossRef]
- Huang, J.; Su, T.; Zhao, H.; Li, F.; Chiu, T.-W.; Singh, M.; Wu, Q.; Fan, L. Nano and Phase Engineering of Fe–Cu Alloy Exsolved Perovskite Oxide-Based Hetero-Catalysts for Efficient Oxygen Evolution Reaction. Fuel 2024, 356, 129479. [Google Scholar] [CrossRef]
- Xu, M.; Cao, R.; Wu, S.; Lee, J.; Chen, D.; Irvine, J.T.S. Nanoparticle Exsolution via Electrochemical Switching in Perovskite Fibers for Solid Oxide Fuel Cell Electrodes. J. Mater. Chem. A 2023, 11, 13007–13015. [Google Scholar] [CrossRef]
- Akhmadjonov, A.; Bae, K.T.; Lee, K.T. Novel Perovskite Oxide Hybrid Nanofibers Embedded with Nanocatalysts for Highly Efficient and Durable Electrodes in Direct CO2 Electrolysis. Nano-Micro Lett. 2024, 16, 93. [Google Scholar] [CrossRef]
- Wang, T.; Sun, N.; Wang, R.; Dong, D.; Wei, T.; Wang, Z. In-Situ Dual-Exsolved Nanometal Anchoring on Heterogeneous Composite Nanofiber Using as SOEC Cathode for Direct and Highly Efficient CO2 Electrolysis. J. Power Sources 2025, 626, 235821. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, J.; Zhou, L.; Yang, J.; Liu, Z.; Wu, K.; Zhao, H.; Wang, J.; Wu, K. Facile Fabrication of Exsolved Nanoparticle-Decorated Hollow Ferrite Fibers as Active Electrocatalyst for Oxygen Evolution Reaction. Chem. Eng. J. 2021, 418, 129422. [Google Scholar] [CrossRef]
- Wei, T.; Wang, J.; Jia, Y.; Harimoto, T. A Fibrous Perovskite Nanomaterial with Exsolved Ni–Cu Metal Nanoparticles as an Effective Composite Catalyst for External Steam Reforming of Liquid Alcohols. Crystals 2023, 13, 1594. [Google Scholar] [CrossRef]
- Xie, X.; Ma, Y.; Wang, T.; Gao, Y.; Li, J.; Zhang, T.; Ye, Z.; Dong, D. Nanofibrous Perovskite Ceramics with In-Situ Exsolved Ni3Fe Alloy Nanoparticles for Catalytic CO2 Methanation. J. Alloys Compd. 2024, 970, 172490. [Google Scholar] [CrossRef]
- Yin, C.; Yang, J.; Feng, J.; Sun, Y.; Liu, Z.; Wang, J.; Cui, J.; Xue, Z.; Zhang, L.; Zhou, Y.; et al. Tailoring the Reversible Phase Transition of Perovskite Nanofiber Electrodes for High-Performance and Durable Reversible Solid Oxide Cells. Nano-Micro Lett. 2025, 17, 150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lach, J.; Gogacz, M.; Winiarz, P.; Ling, Y.; Zhou, M.; Zheng, K. A Review of Nanofiber Electrodes and the In Situ Exsolution of Nanoparticles for Solid Oxide Cells. Materials 2025, 18, 1272. https://doi.org/10.3390/ma18061272
Lach J, Gogacz M, Winiarz P, Ling Y, Zhou M, Zheng K. A Review of Nanofiber Electrodes and the In Situ Exsolution of Nanoparticles for Solid Oxide Cells. Materials. 2025; 18(6):1272. https://doi.org/10.3390/ma18061272
Chicago/Turabian StyleLach, Jakub, Michał Gogacz, Piotr Winiarz, Yihan Ling, Mingjiong Zhou, and Kun Zheng. 2025. "A Review of Nanofiber Electrodes and the In Situ Exsolution of Nanoparticles for Solid Oxide Cells" Materials 18, no. 6: 1272. https://doi.org/10.3390/ma18061272
APA StyleLach, J., Gogacz, M., Winiarz, P., Ling, Y., Zhou, M., & Zheng, K. (2025). A Review of Nanofiber Electrodes and the In Situ Exsolution of Nanoparticles for Solid Oxide Cells. Materials, 18(6), 1272. https://doi.org/10.3390/ma18061272









