Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis
Highlights
- Mg AZ31+SPF+HT extracts showed high biocompatibility and osteointegrative effects in the hMSC in vitro model.
- Mg AZ31+SPF+HT extracts induced the up-release of anti-fibrotic soluble factors in the hMSC in vitro models.
- Mg AZ31+SPF+HT extracts can modulate in vitro canonical and non-canonical osteogenic processes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. hMSC Viability (WST-1 Test)
2.3. DNAds Concentration (PicoGreen Assay)
2.4. RNA Extraction and Real-Time PCR
2.5. OsteoImage Mineralization Assay
2.6. Evaluation of Supernatant Soluble Factors
2.7. Conditional Medium Preparation
2.8. LC-MS/MS
2.9. Statistical Analysis
3. Results
3.1. Cell Viability Response to Mg AZ31+SPF and Mg AZ31+SPF+HT Extract Treatments
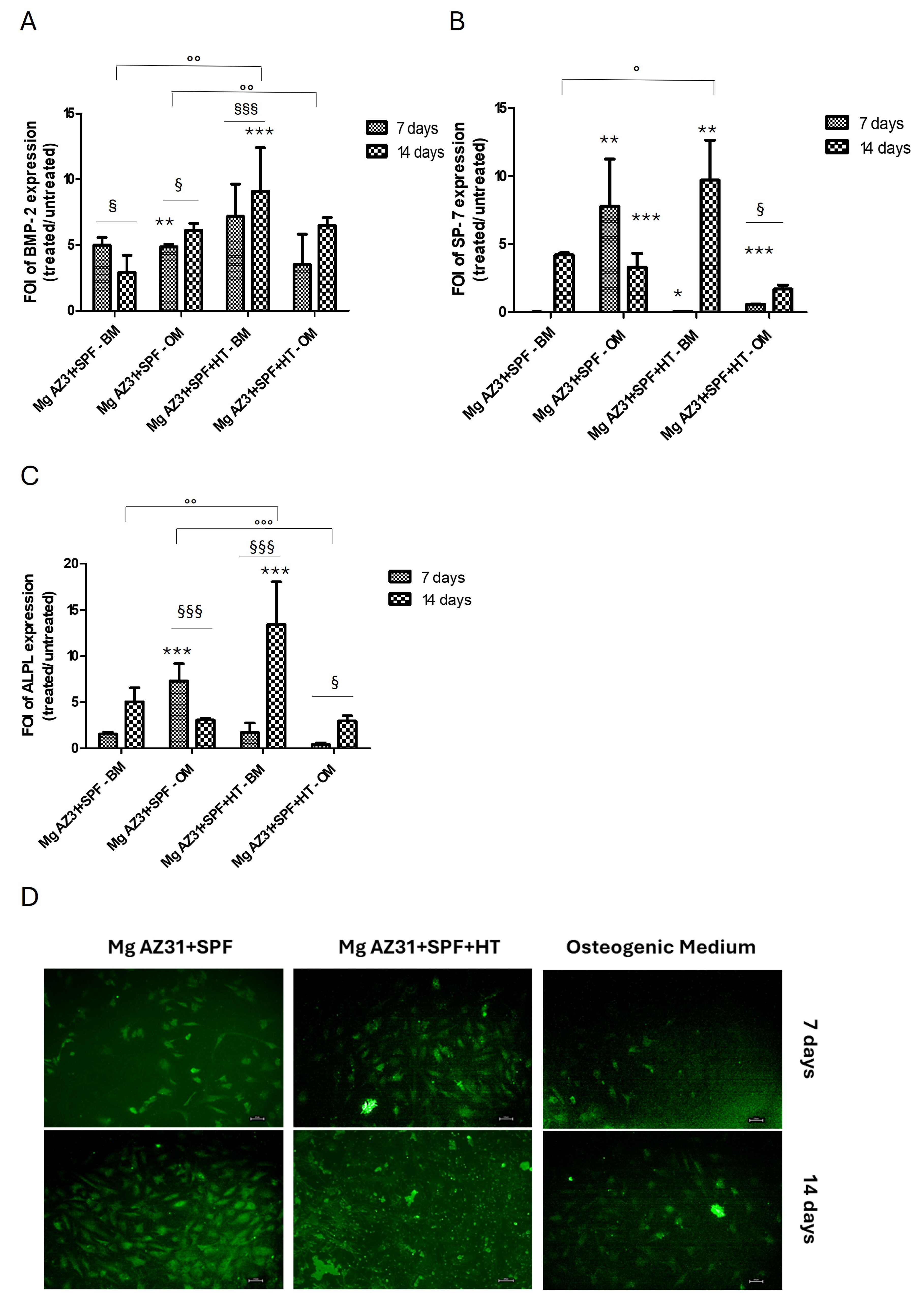
3.2. Osteoinductive Effects of Mg AZ31+SPF and Mg AZ31+SPF+HT on hMSCs
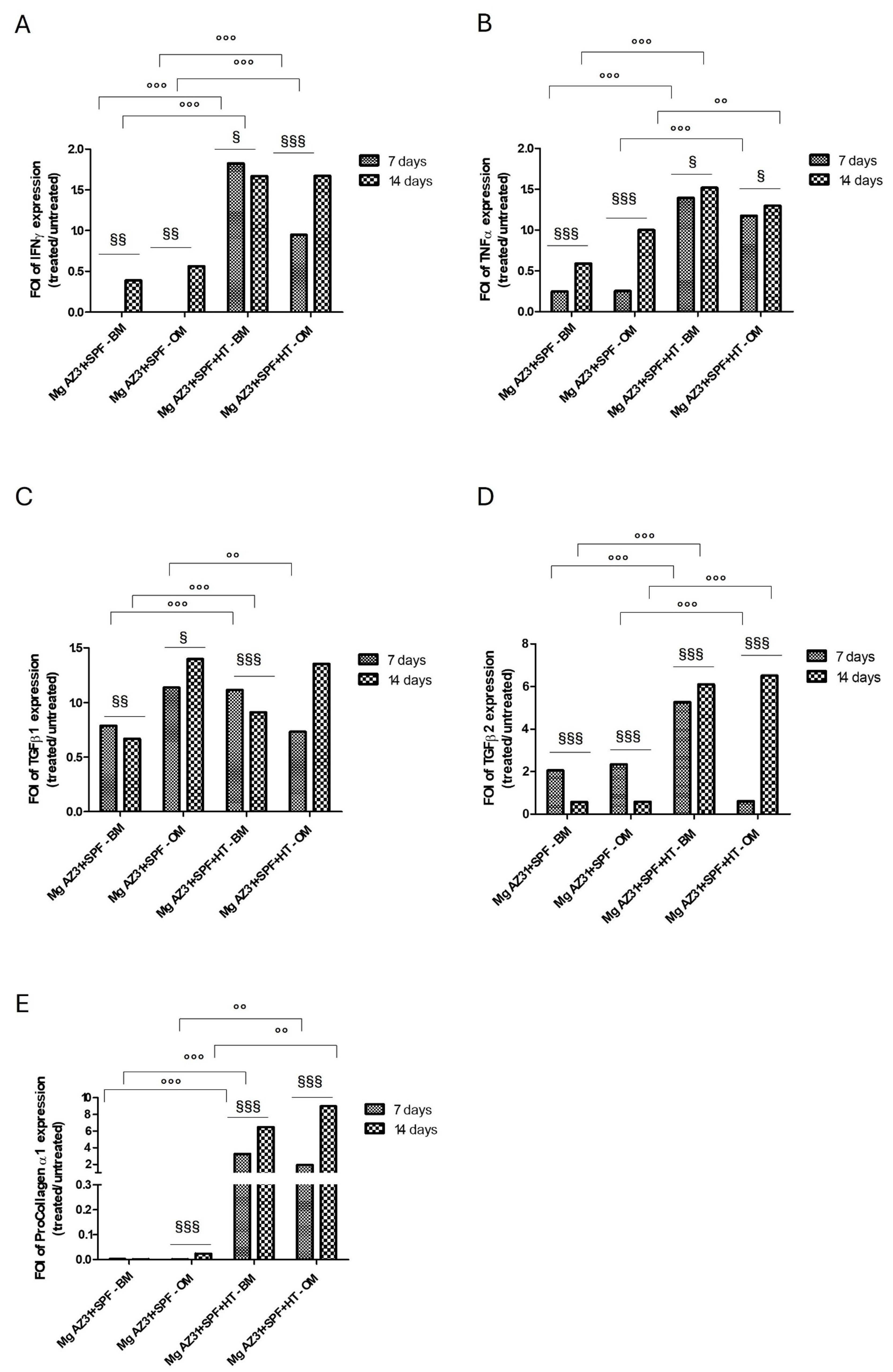
3.3. Analysis of Fibrotic Potential of Mg AZ31+SPF and Mg AZ31+SPF+HT Extracts on hMSC Supernatant Soluble Factors
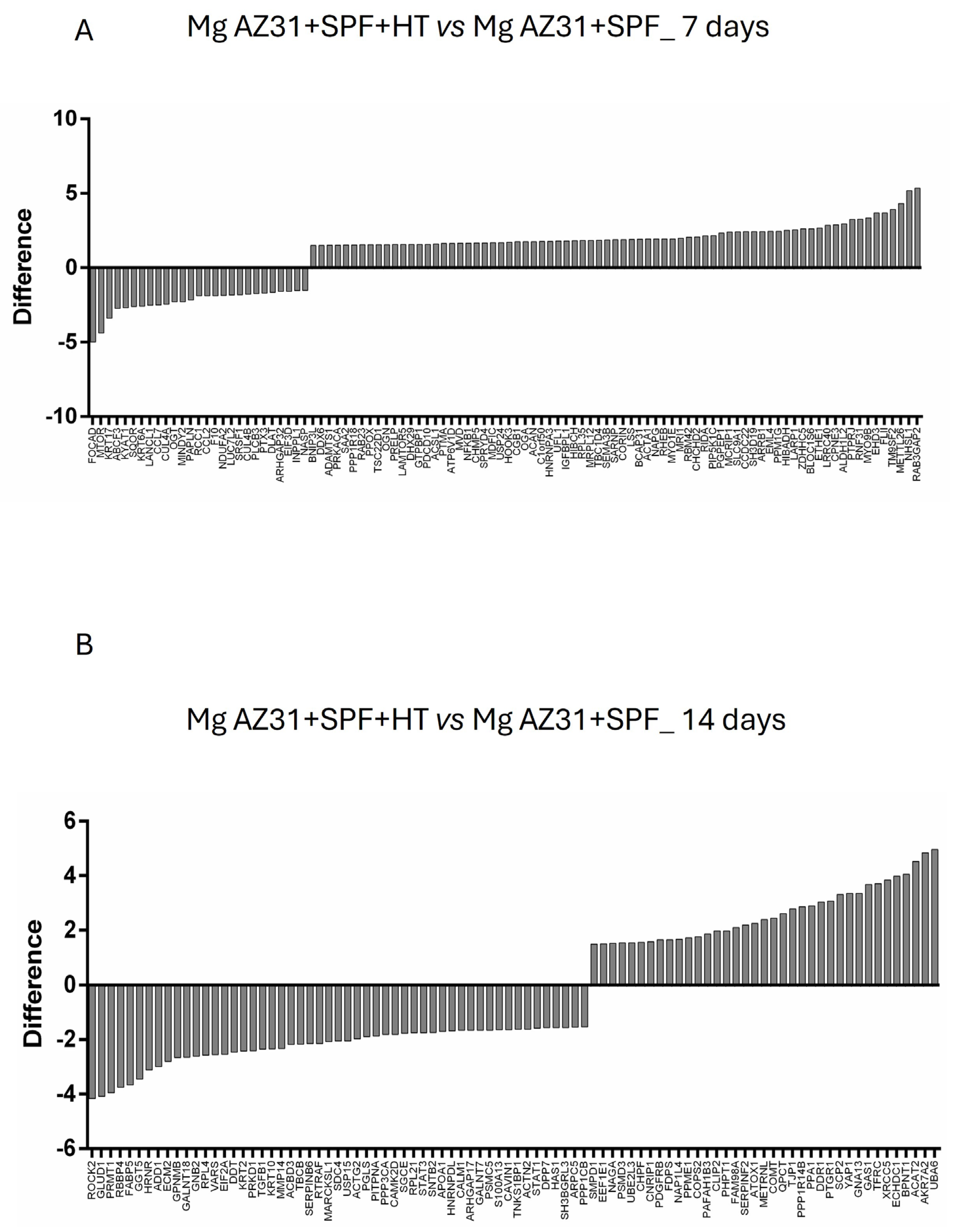
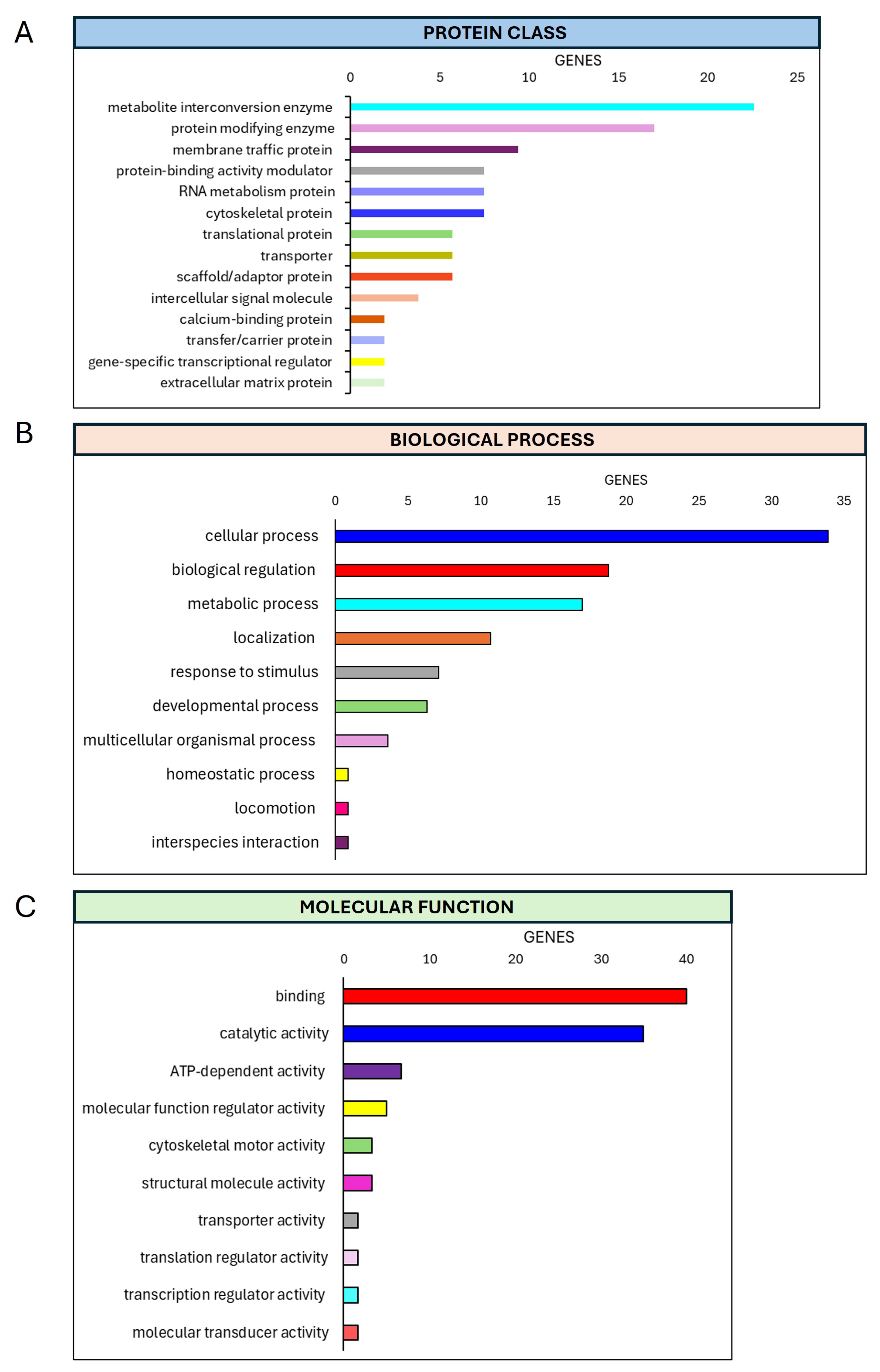
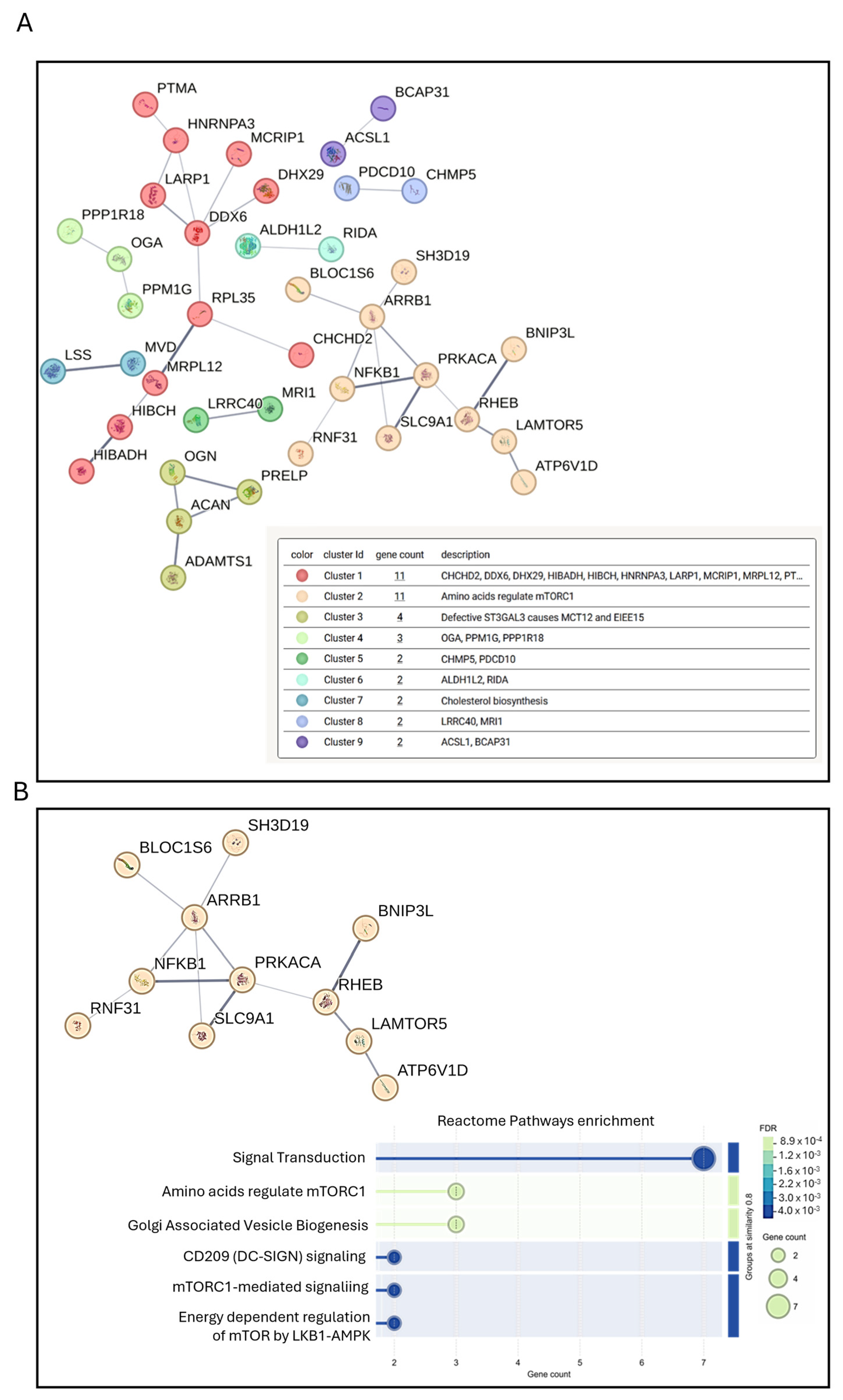
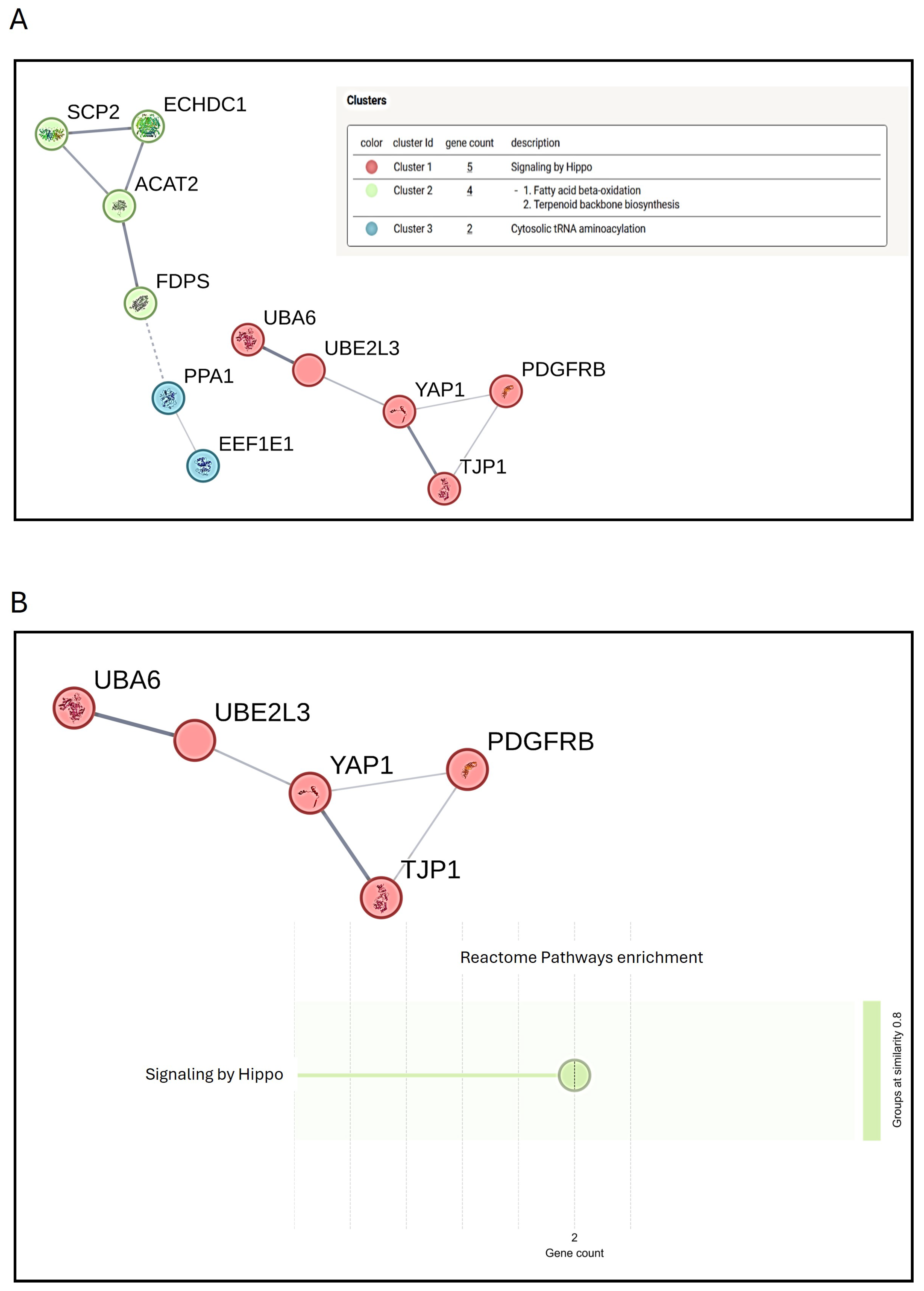
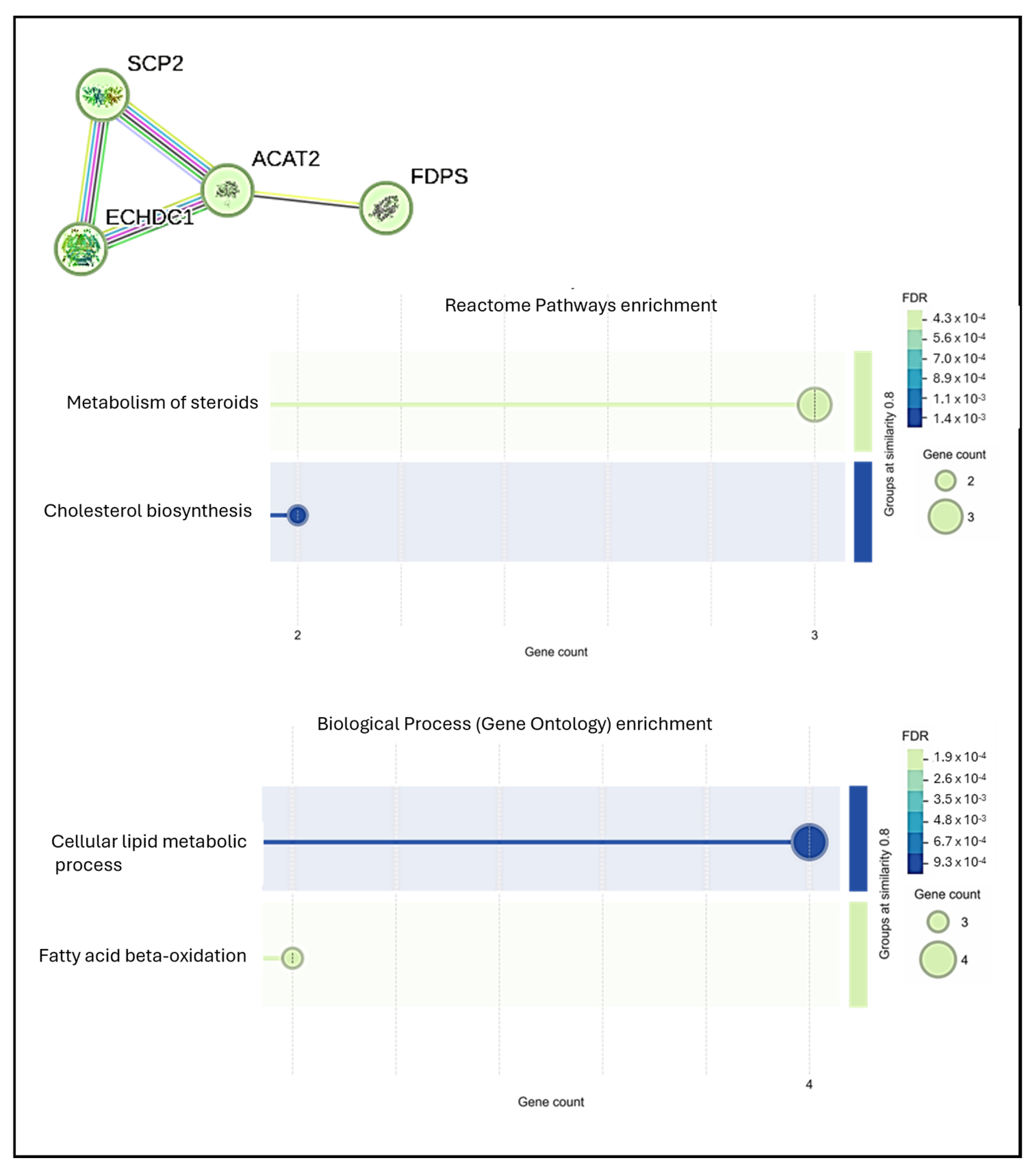
3.4. Preliminary Mass Spectrometric Investigation of hMSCs Treated with Mg AZ31+SPF or Mg AZ31+SPF+HT Extracts
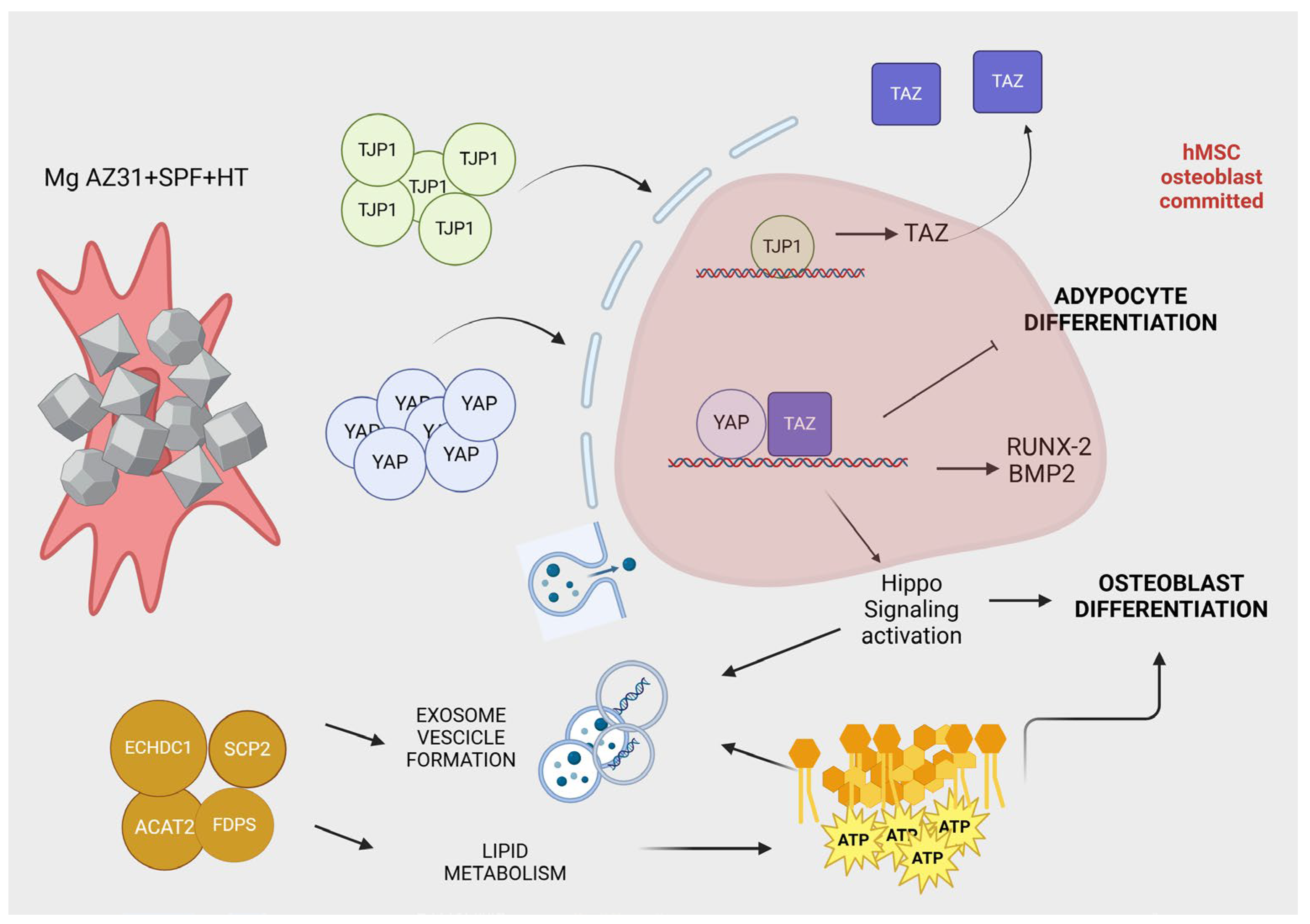
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xin, L.; Wen, Y.; Song, J.; Chen, T.; Zhai, Q. Bone Regeneration Strategies Based on Organelle Homeostasis of Mesenchymal Stem Cells. Front. Endocrinol. 2023, 14, 1151691. [Google Scholar] [CrossRef] [PubMed]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Xiong, Y.; Mi, B.B.; Lin, Z.; Hu, Y.Q.; Yu, L.; Zha, K.K.; Panayi, A.C.; Yu, T.; Chen, L.; Liu, Z.P.; et al. The Role of the Immune Microenvironment in Bone, Cartilage, and Soft Tissue Regeneration: From Mechanism to Therapeutic Opportunity. Mil. Med. Res. 2022, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Babczyk, P.; Tobiasch, E. Exosomes: A New Hope for Angiogenesis-Mediated Bone Regeneration. Int. J. Mol. Sci. 2024, 25, 5204. [Google Scholar] [CrossRef]
- Aboutalebianaraki, N.; Neal, C.J.; Seal, S.; Razavi, M. Biodegradable Mg-Sc-Sr Alloy Improves Osteogenesis and Angiogenesis to Accelerate Bone Defect Restoration. J. Funct. Biomater. 2022, 13, 261. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone Biomaterials and Interactions with Stem Cells. Bone Res. 2017, 5, 17059. [Google Scholar]
- Soleymani, S.; Naghib, S.M. 3D and 4D Printing Hydroxyapatite-Based Scaffolds for Bone Tissue Engineering and Regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Nasr Azadani, M.; Zahedi, A.; Bowoto, O.K.; Oladapo, B.I. A Review of Current Challenges and Prospects of Magnesium and Its Alloy for Bone Implant Applications. Prog. Biomater. 2022, 11, 1–26. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Yu, X.; Etim, I.P.; Ibrahim, M.; Yang, K. Mechanical Properties of Magnesium Alloys for Medical Application: A Review. J. Mech. Behav. Biomed. Mater. 2018, 87, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Giavaresi, G.; Bellavia, D.; De Luca, A.; Costa, V.; Raimondi, L.; Cordaro, A.; Sartori, M.; Terrando, S.; Toscano, A.; Pignatti, G.; et al. Magnesium Alloys in Orthopedics: A Systematic Review on Approaches, Coatings and Strategies to Improve Biocompatibility, Osteogenic Properties and Osteointegration Capabilities. Int. J. Mol. Sci. 2023, 25, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhi, P.; Liu, L.; Chang, J.; Liu, C.; Zhang, Q.; Zhou, J.; Liu, Z.; Fan, Y. Advances in the Study of Magnesium Alloys and Their Use in Bone Implant Material. Metals 2022, 12, 1500. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, W.; Liu, J.; Wang, L.; Tang, Y.; Wang, K. A Review on Magnesium Alloys for Biomedical Applications. Front. Bioeng. Biotechnol. 2022, 10, 953344. [Google Scholar] [CrossRef]
- Chen, L.; Yan, Z.; Qiu, T.; Zhu, J.; Liu, G.; Han, J.; Guo, C. Long-Term Temporospatial Complementary Relationship between Degradation and Bone Regeneration of Mg–Al Alloy. ACS Appl. Bio Mater. 2023, 6, 4703–4713. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Pouly, J.L.; Canis, M. Dose-Dependent pro- or Anti-Fibrotic Responses of Endometriotic Stromal Cells to Interleukin-1β and Tumor Necrosis Factor α. Sci. Rep. 2020, 10, 9467. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-Beta Signaling and the Fibrotic Response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Steele, H.; Cheng, J.; Willicut, A.; Dell, G.; Breckenridge, J.; Culberson, E.; Ghastine, A.; Tardif, V.; Herro, R. TNF Superfamily Control of Tissue Remodeling and Fibrosis. Front. Immunol. 2023, 14, 1219907. [Google Scholar] [CrossRef]
- Chakraborty, A.; Wang, C.; Hodgson-Garms, M.; Broughton, B.R.S.; Frith, J.E.; Kelly, K.; Samuel, C.S. Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Reverse Bleomycin-Induced Pulmonary Fibrosis and Related Lung Stiffness. Biomed. Pharmacother. 2024, 178, 117259. [Google Scholar] [CrossRef]
- Ghanbari, A.; Bordbar-Khiabani, A.; Warchomicka, F.; Sommitsch, C.; Yarmand, B.; Zamanian, A. PEO/Polymer Hybrid Coatings on Magnesium Alloy to Improve Biodegradation and Biocompatibility Properties. Surf. Interfaces 2023, 36, 102495. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, X.; Xie, Y.; Bai, L.; Guan, S. Fucoidan/Collagen Composite Coating on Magnesium Alloy for Better Corrosion Resistance and pro-Endothelialization Potential. Int. J. Biol. Macromol. 2024, 255, 128044. [Google Scholar] [CrossRef] [PubMed]
- Bahrampour, S.; Bordbar-Khiabani, A.; Siadati, M.H.; Gasik, M. Tuning Biomechanical Behavior and Biocompatibility of Mg–Zn–Ca Alloys by Mn3O4 Incorporated Plasma Electrolytic Oxidation Coatings. Ceram. Int. 2024, 50, 29703–29710. [Google Scholar] [CrossRef]
- Tatullo, M.; Piattelli, A.; Ruggiero, R.; Marano, R.M.; Iaculli, F.; Rengo, C.; Papallo, I.; Palumbo, G.; Chiesa, R.; Paduano, F.; et al. Functionalized Magnesium Alloys Obtained by Superplastic Forming Process Retain Osteoinductive and Antibacterial Properties: An In-Vitro Study. Dent. Mater. 2024, 40, 557–562. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Q.; Zhang, Y.-H.; Wu, G. Hydrothermal Synthesis of Protective Coating on Magnesium Alloy Using De-Ionized Water. Surf. Coat. Technol. 2012, 206, 2961–2966. [Google Scholar] [CrossRef]
- Mohammadi-Zerankeshi, M.; Zohrevand, M.; Alizadeh, R. Hydrothermal Coating of the Biodegradable Mg-2Ag Alloy. Metals 2023, 13, 1260. [Google Scholar] [CrossRef]
- Peng, F.; Li, H.; Wang, D.; Tian, P.; Tian, Y.; Yuan, G.; Xu, D.; Liu, X. Enhanced Corrosion Resistance and Biocompatibility of Magnesium Alloy by Mg–Al-Layered Double Hydroxide. ACS Appl. Mater. Interfaces 2016, 8, 35033–35044. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Liu, S.; Li, Z.; Zhang, L.; Wu, R.; Hou, L.; Zhang, M. Hydrothermal Synthesis of Protective Coating on Mg Alloy for Degradable Implant Applications. Coatings 2019, 9, 160. [Google Scholar] [CrossRef]
- De Luca, A.; Ruggiero, R.; Cordaro, A.; Marrelli, B.; Raimondi, L.; Costa, V.; Bellavia, D.; Aiello, E.; Pavarini, M.; Piccininni, A.; et al. Towards Accurate Biocompatibility: Rethinking Cytotoxicity Evaluation for Biodegradable Magnesium Alloys in Biomedical Applications. J. Funct. Biomater. 2024, 15, 382. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, H.; Martinez, D.C.; Shah, F.A.; Loo, A.J.; Emanuelsson, L.; Norlindh, B.; Willumeit-Römer, R.; Plocinski, T.; Swieszkowski, W.; Palmquist, A.; et al. Magnesium Implant Degradation Provides Immunomodulatory and Proangiogenic Effects and Attenuates Peri-Implant Fibrosis in Soft Tissues. Bioact. Mater. 2023, 26, 353–369. [Google Scholar] [CrossRef]
- Cusanno, A.; Guglielmi, P.; Sorgente, D.; Palumbo, G. Numerical/Experimental Investigation of the Effect of the Laser Treatment on the Thickness Distribution of a Magnesium Superplastically Formed Part. Adv. Manuf. 2024. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and Go Extraction Tips for Matrix-Assisted Laser Desorption/Ionization, Nanoelectrospray, and LC/MS Sample Pretreatment in Proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Huang, X.; Ebert, D.; Mills, C.; Guo, X.; Thomas, P.D. Protocol Update for Large-Scale Genome and Gene Function Analysis with the PANTHER Classification System (v.14.0). Nat. Protoc. 2019, 14, 703–721. [Google Scholar] [CrossRef]
- Thomas, P.D.; Ebert, D.; Muruganujan, A.; Mushayahama, T.; Albou, L.; Mi, H. PANTHER: Making Genome-scale Phylogenetics Accessible to All. Protein Sci. 2022, 31, 8–22. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the Evolution of Gene Function, and Other Gene Attributes, in the Context of Phylogenetic Trees. Nucleic Acids Res. 2012, 41, D377–D386. [Google Scholar] [CrossRef]
- Iacobescu, G.L.; Corlatescu, A.D.; Popa, M.; Iacobescu, L.; Cirstoiu, C.; Orban, C. Exploring the Implications of Golgi Apparatus Dysfunction in Bone Diseases. Cureus 2024, 16, e56982. [Google Scholar] [CrossRef]
- Costa, V.; Carina, V.; Fontana, S.; De Luca, A.; Monteleone, F.; Pagani, S.; Sartori, M.; Setti, S.; Faldini, C.; Alessandro, R.; et al. Osteogenic Commitment and Differentiation of Human Mesenchymal Stem Cells by Low-intensity Pulsed Ultrasound Stimulation. J. Cell. Physiol. 2018, 233, 1558–1573. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Chaya, A.; Liu, K.; Verdelis, K.; Sfeir, C. The Role of Magnesium Ions in Bone Regeneration Involves the Canonical Wnt Signaling Pathway. Acta Biomater. 2019, 98, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green Synthesis of Magnesium Oxide Nanoparticles and Its Applications: A Review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Bandeira, M.; Giovanela, M.; Roesch-Ely, M.; Devine, D.M.; Da Silva Crespo, J. Green Synthesis of Zinc Oxide Nanoparticles: A Review of the Synthesis Methodology and Mechanism of Formation. Sustain. Chem. Pharm. 2020, 15, 100223. [Google Scholar] [CrossRef]
- Costa, V.; Carina, V.; Raimondi, L.; De Luca, A.; Bellavia, D.; Conigliaro, A.; Salamanna, F.; Alessandro, R.; Fini, M.; Giavaresi, G. MiR-33a Controls hMSCS Osteoblast Commitment Modulating the Yap/Taz Expression Through EGFR Signaling Regulation. Cells 2019, 8, 1495. [Google Scholar] [CrossRef]
- Goldberg, M.T.; Han, Y.-P.; Yan, C.; Shaw, M.C.; Garner, W.L. TNF-α Suppresses α-Smooth Muscle Actin Expression in Human Dermal Fibroblasts: An Implication for Abnormal Wound Healing. J. Investig. Dermatol. 2007, 127, 2645–2655. [Google Scholar] [CrossRef]
- Yusop, N.; Moseley, R.; Waddington, R.J. Hyperglycemia Exerts Disruptive Effects on the Secretion of TGF-Β1 and Its Matrix Ligands, Decorin and Biglycan, by Mesenchymal Sub-Populations and Macrophages during Bone Repair. Front. Dent. Med. 2023, 4, 1200122. [Google Scholar] [CrossRef]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming Growth Factor Beta Family: Insight into the Role of Growth Factors in Regulation of Fracture Healing Biology and Potential Clinical Applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef]
- Szulc, P. Bone Turnover: Biology and Assessment Tools. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef]
- Knecht, R.S.; Bucher, C.H.; Van Linthout, S.; Tschöpe, C.; Schmidt-Bleek, K.; Duda, G.N. Mechanobiological Principles Influence the Immune Response in Regeneration: Implications for Bone Healing. Front. Bioeng. Biotechnol. 2021, 9, 614508. [Google Scholar] [CrossRef]
- Chen, J.; Long, F. mTOR Signaling in Skeletal Development and Disease. Bone Res. 2018, 6, 1. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone Marrow Stromal/Stem Cell-Derived Extracellular Vesicles Regulate Osteoblast Activity and Differentiation In Vitro and Promote Bone Regeneration In Vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Cox, S.C.; Azoidis, I.; McGuinness, A.J.A.; Cooke, M.; Heaney, L.M.; Davis, E.T.; Jones, S.W.; Grover, L.M. Osteoblast-Derived Vesicle Protein Content Is Temporally Regulated During Osteogenesis: Implications for Regenerative Therapies. Front. Bioeng. Biotechnol. 2019, 7, 92. [Google Scholar]
- Ansari, S.; de Wildt, B.W.M.; Vis, M.A.M.; de Korte, C.E.; Ito, K.; Hofmann, S.; Yuana, Y. Matrix Vesicles: Role in Bone Mineralization and Potential Use as Therapeutics. Pharmaceuticals 2021, 14, 289. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Weiss, S.J. Snail/Slug-YAP/TAZ Complexes Cooperatively Regulate Mesenchymal Stem Cell Function and Bone Formation. Cell Cycle 2017, 16, 399–405. [Google Scholar] [CrossRef]
- Seo, E.; Basu-Roy, U.; Gunaratne, P.H.; Coarfa, C.; Lim, D.S.; Basilico, C.; Mansukhani, A. SOX2 Regulates YAP1 to Maintain Stemness and Determine Cell Fate in the Osteo-Adipo Lineage. Cell Rep. 2013, 3, 2075–2087. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Huang, T.; Wang, B.; Xiang, L. Hippo-YAP/TAZ Signaling in Osteogenesis and Macrophage Polarization: Therapeutic Implications in Bone Defect Repair. Genes Dis. 2023, 10, 2528–2539. [Google Scholar] [CrossRef]
- Wang, S.H.; Lee, S.P.; Yang, C.W.; Lo, C.M. Surface Modification of Biodegradable Mg-Based Scaffolds for Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation. Materials 2021, 14, 441. [Google Scholar] [CrossRef]
- Nandy, A.; Helderman, R.C.M.; Thapa, S.; Jayapalan, S.; Richards, A.; Narayani, N.; Czech, M.P.; Rosen, C.J.; Rendina-Ruedy, E. Lipolysis Supports Bone Formation by Providing Osteoblasts with Endogenous Fatty Acid Substrates to Maintain Bioenergetic Status. Bone Res. 2023, 11, 62. [Google Scholar] [CrossRef]
- Neuman, W.F.; Neuman, M.W.; Brommage, R. Aerobic Glycolysis in Bone: Lactate Production and Gradients in Calvaria. Am. J. Physiol. 1978, 234, C41–C50. [Google Scholar] [CrossRef]
- Adamek, G.; Felix, R.; Guenther, H.L.; Fleisch, H. Fatty Acid Oxidation in Bone Tissue and Bone Cells in Culture. Characterization and Hormonal Influences. Biochem. J. 1987, 248, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on Clinical Applications of Magnesium-Based Orthopaedic Implants: A Review from Clinical Translational Perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef] [PubMed]


| Gene | ID QuantiTect Primer Assay | Gene Globe |
|---|---|---|
| ALPL | Hs_ALPL_1_SG | QT00012957 |
| SP7 | Hs_SP7_1_SG | QT00213514 |
| BMP2 | Hs_BMP2_1_SG | QT00012544 |
| b-ACTIN | Hs_ACTB_1_SG | QT00095431 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, V.; Raimondi, L.; Scilabra, S.D.; Pinto, M.L.; Bellavia, D.; De Luca, A.; Guglielmi, P.; Cusanno, A.; Cattini, L.; Pulsatelli, L.; et al. Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis. Materials 2025, 18, 1254. https://doi.org/10.3390/ma18061254
Costa V, Raimondi L, Scilabra SD, Pinto ML, Bellavia D, De Luca A, Guglielmi P, Cusanno A, Cattini L, Pulsatelli L, et al. Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis. Materials. 2025; 18(6):1254. https://doi.org/10.3390/ma18061254
Chicago/Turabian StyleCosta, Viviana, Lavinia Raimondi, Simone Dario Scilabra, Margot Lo Pinto, Daniele Bellavia, Angela De Luca, Pasquale Guglielmi, Angela Cusanno, Luca Cattini, Lia Pulsatelli, and et al. 2025. "Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis" Materials 18, no. 6: 1254. https://doi.org/10.3390/ma18061254
APA StyleCosta, V., Raimondi, L., Scilabra, S. D., Pinto, M. L., Bellavia, D., De Luca, A., Guglielmi, P., Cusanno, A., Cattini, L., Pulsatelli, L., Pavarini, M., Chiesa, R., & Giavaresi, G. (2025). Effect of Hydrothermal Coatings of Magnesium AZ31 Alloy on Osteogenic Differentiation of hMSCs: From Gene to Protein Analysis. Materials, 18(6), 1254. https://doi.org/10.3390/ma18061254










