Performance Assessment of Graphene Oxide as a Protective Coating for Historical Stone
Abstract
1. Introduction
2. Materials and Methods
2.1. Stone Samples
2.2. Graphene Oxide Synthesis and Application
2.2.1. Synthesis
2.2.2. Application
2.3. Characterisation Methods
3. Results and Discussion
3.1. Characterization of Stone Substrate Specimens
3.2. Preliminary Experiments and Analysis of GO Coatings on Glass Slides
3.3. Application of GO Suspensions on Stone Substrates
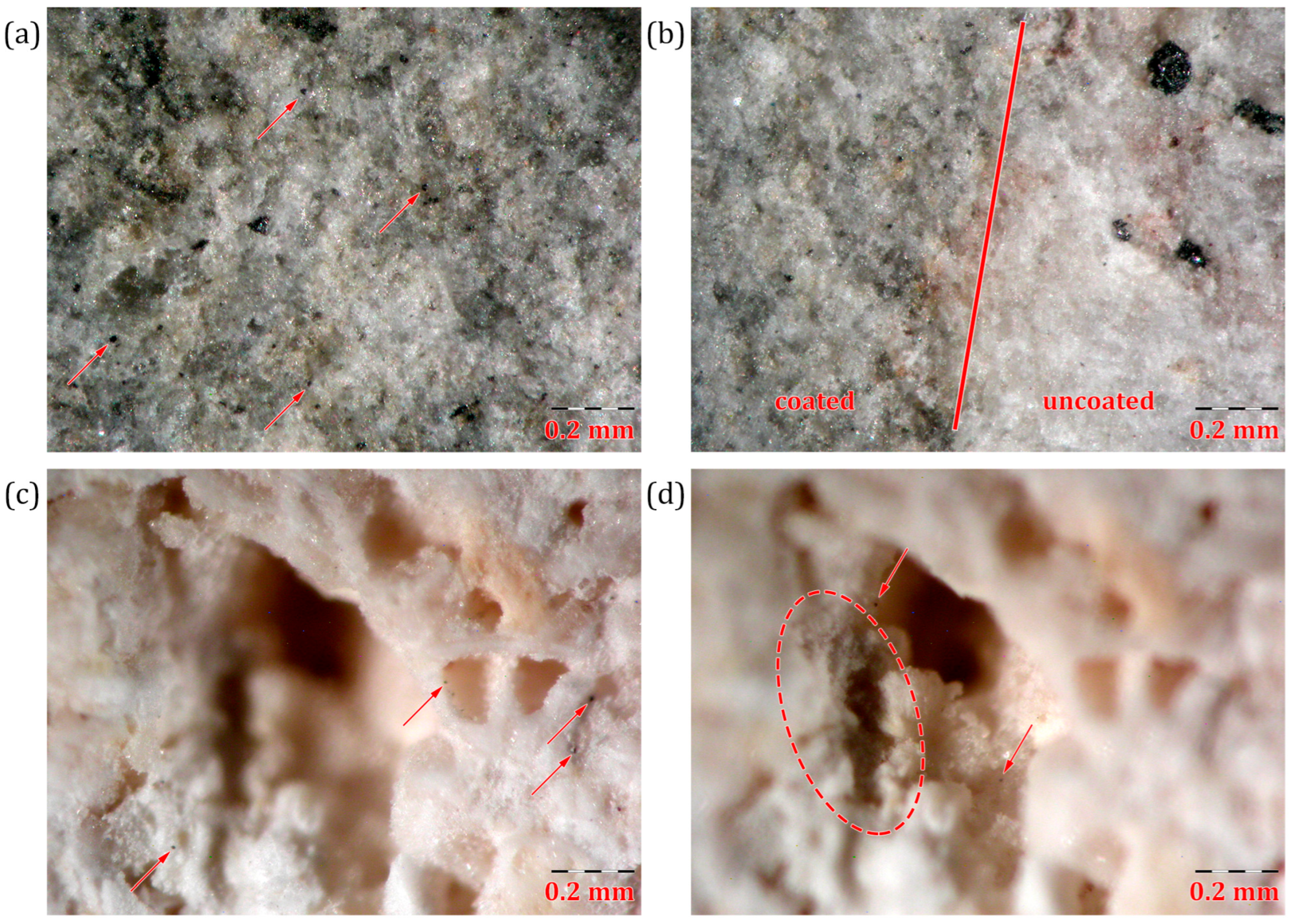
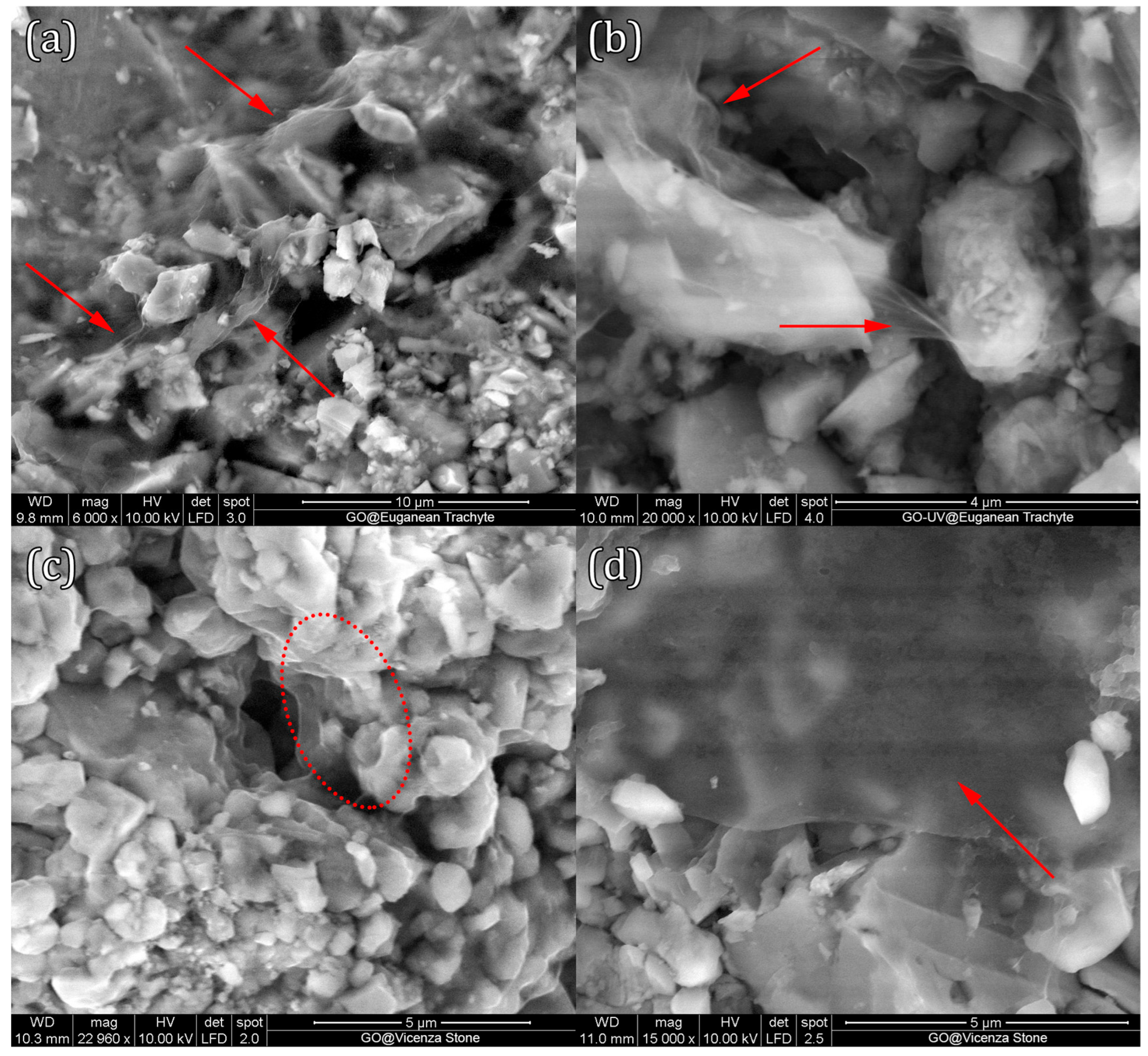
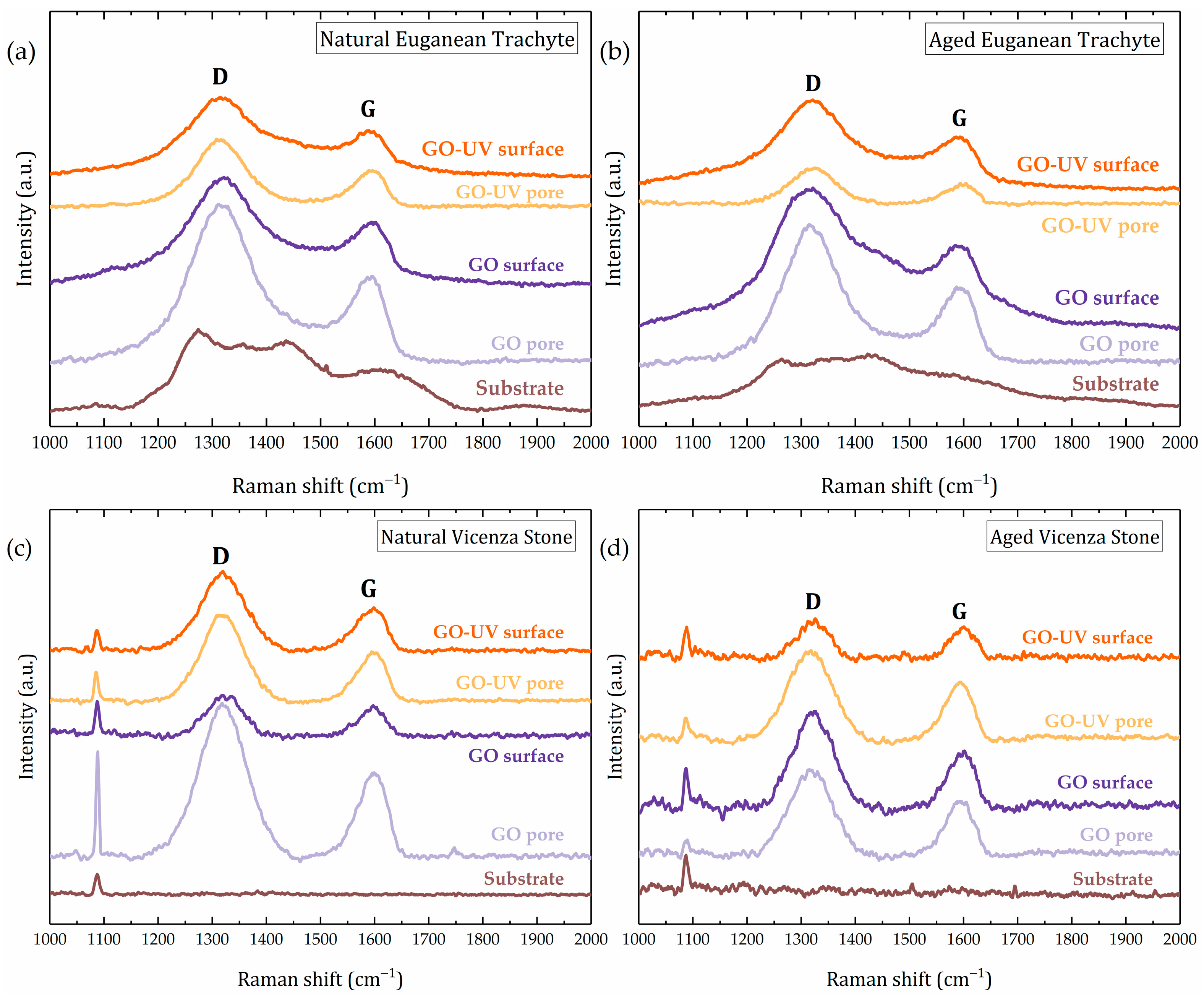
3.3.1. Surface Analysis of GO Coatings on Stone
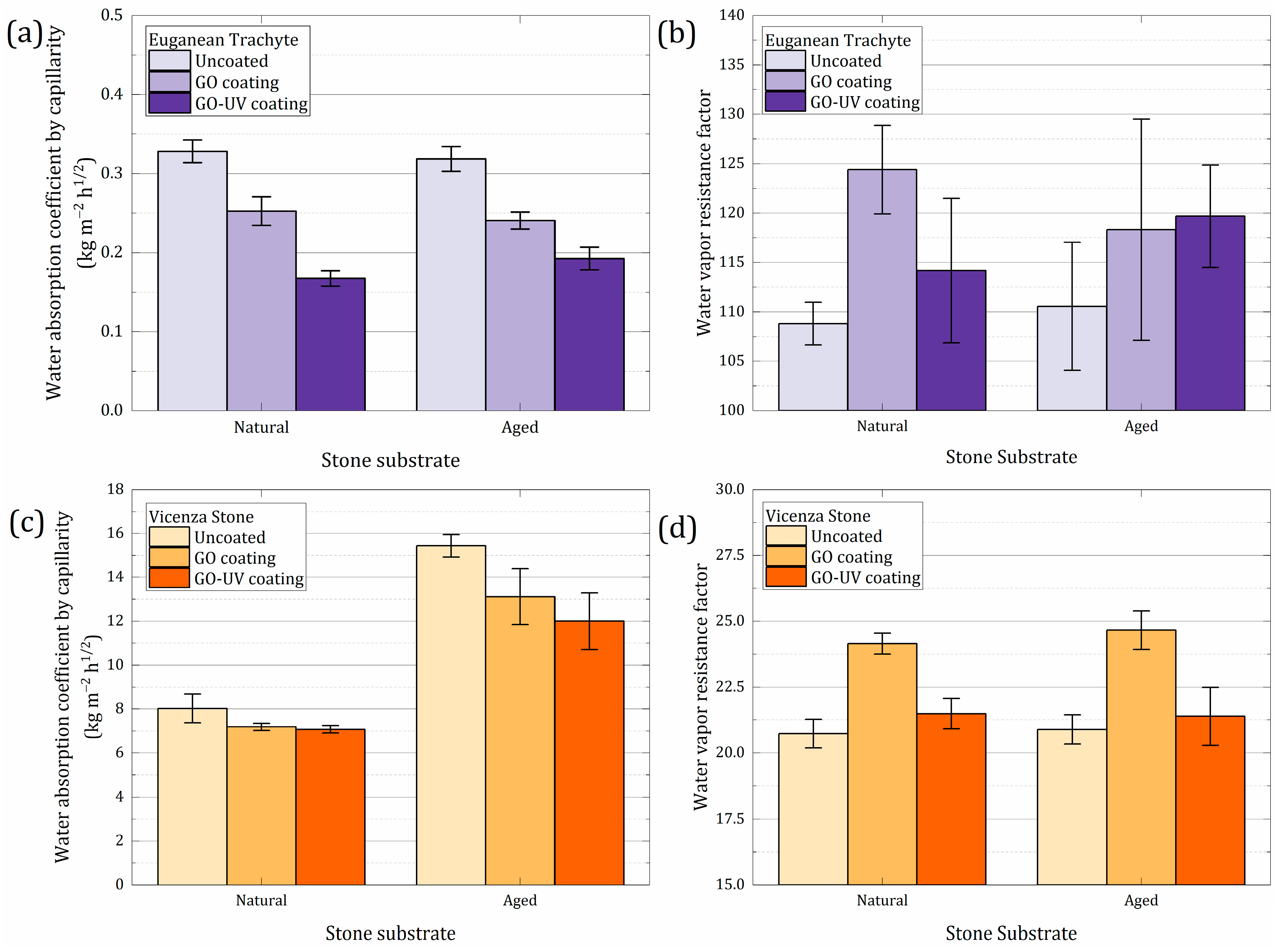
3.3.2. Evaluation of Hygric Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Steiger, M.; Charola, A.E.; Sterflinger, K. Weathering and Deterioration. In Stone in Architecture: Properties, Durability; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin, Heidelberg, Germany, 2014; pp. 225–316. [Google Scholar] [CrossRef]
- Siegesmund, S.; Snethlage, R. (Eds.) Stone in Architecture: Properties, Durability; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Basu, S.; Orr, S.A.; Aktas, Y.D. A Geological Perspective on Climate Change and Building Stone Deterioration in London: Implications for Urban Stone-Built Heritage Research and Management. Atmosphere 2020, 11, 788. [Google Scholar] [CrossRef]
- Fatorić, S.; Seekamp, E. Are cultural heritage and resources threatened by climate change? A systematic literature review. Clim. Chang. 2017, 142, 227–254. [Google Scholar] [CrossRef]
- Sesana, E.; Gagnon, A.S.; Ciantelli, C.; Cassar, J.; Hughes, J.J. Climate change impacts on cultural heritage: A literature review. WIREs Clim. Chang. 2021, 12, e710. [Google Scholar] [CrossRef]
- Gherardi, F. Current and Future Trends in Protective Treatments for Stone Heritage. In Conserving Stone Heritage: Traditional and Innovative Materials and Techniques; Gherardi, F., Maravelaki, P.N., Eds.; Cultural Heritage Science; Springer International Publishing: Cham, Switzerland, 2022; pp. 137–176. [Google Scholar] [CrossRef]
- Gherardi, F.; Maravelaki, P.N. (Eds.) Conserving Stone Heritage: Traditional and Innovative Materials and Techniques; Cultural Heritage Science; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Charola, A.E. Water-Repellent Treatments for Building Stones: A Practical Overview. APT Bull. J. Preserv. Technol. 1995, 26, 10–17. [Google Scholar] [CrossRef]
- Snethlage, R. Stone Conservation. In Stone in Architecture: Properties, Durability; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 415–550. [Google Scholar] [CrossRef]
- Daniele, V.; Taglieri, G.; Quaresima, R. The nanolimes in Cultural Heritage conservation: Characterisation and analysis of the carbonatation process. J. Cult. Herit. 2008, 9, 294–301. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Mater. De Construcción 2017, 67, 107. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Hosseini, M. Superhydrophobic Coatings for the Protection of Natural Stone. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Toniolo, L.; Gherardi, F. The Protection of Marble Surfaces: The Challenge to Develop Suitable Nanostructured Treatments. In Advanced Materials for the Conservation of Stone; Hosseini, M., Karapanagiotis, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 57–78. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.-M.; Grigorescu, R.M.; Iancu, L.; Andrei, E.R. Nanomaterials Used in Conservation and Restoration of Cultural Heritage: An Up-to-Date Overview. Materials 2020, 13, 2064. [Google Scholar] [CrossRef]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Ferrari, A.M.; Pini, M.; Neri, P.; Bondioli, F. Nano-TiO2 Coatings for Limestone: Which Sustainability for Cultural Heritage? Coatings 2015, 5, 232. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Kariper, İ.A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Shao, J.-J.; Lv, W.; Yang, Q.-H. Self-Assembly of Graphene Oxide at Interfaces. Adv. Mater. 2014, 26, 5586–5612. [Google Scholar] [CrossRef]
- Cotet, L.C.; Magyari, K.; Todea, M.; Dudescu, M.C.; Danciu, V.; Baia, L. Versatile self-assembled graphene oxide membranes obtained under ambient conditions by using a water–ethanol suspension. J. Mater. Chem. A 2017, 5, 2132–2142. [Google Scholar] [CrossRef]
- Alrammouz, R.; Podlecki, J.; Vena, A.; Garcia, R.; Abboud, P.; Habchi, R.; Sorli, B. Highly porous and flexible capacitive humidity sensor based on self-assembled graphene oxide sheets on a paper substrate. Sens. Actuators B Chem. 2019, 298, 126892. [Google Scholar] [CrossRef]
- Zhong, F.; He, Y.; Wang, P.; Chen, C.; Lin, Y.; Wu, Y.; Chen, J. Self-assembled graphene oxide-graphene hybrids for enhancing the corrosion resistance of waterborne epoxy coating. Appl. Surf. Sci. 2019, 488, 801–812. [Google Scholar] [CrossRef]
- Chuquitarqui, A.; Cotet, L.C.; Baia, M.; György, E.; Magyari, K.; Barbu-Tudoran, L.; Baia, L.; Díaz-González, M.; Fernández-Sánchez, C.; Perez Del Pino, A. New fabrication method for producing reduced graphene oxide flexible electrodes by using a low-power visible laser diode engraving system. Nanotechnology 2020, 31, 325402. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent development of graphene oxide based forward osmosis membrane for water treatment: A critical review. Desalination 2020, 491, 114452. [Google Scholar] [CrossRef]
- Ding, R.; Li, W.; Wang, X.; Gui, T.; Li, B.; Han, P.; Tian, H.; Liu, A.; Wang, X.; Liu, X.; et al. A brief review of corrosion protective films and coatings based on graphene and graphene oxide. J. Alloy. Compd. 2018, 764, 1039–1055. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.A.; Ansari, K.R.; Saleh, T.A. Graphene and graphene oxide as new class of materials for corrosion control and protection: Present status and future scenario. Prog. Org. Coat. 2020, 147, 105741. [Google Scholar] [CrossRef]
- Kumar, S.S.A.; Bashir, S.; Ramesh, K.; Ramesh, S. New perspectives on Graphene/Graphene oxide based polymer nanocomposites for corrosion applications: The relevance of the Graphene/Polymer barrier coatings. Prog. Org. Coat. 2021, 154, 106215. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Natarajan, B.; Eksik, O.; Ali Shojaee, S.; Lucca, D.A.; Ren, W.; Cheng, H.-M.; Koratkar, N. Superiority of Graphene over Polymer Coatings for Prevention of Microbially Induced Corrosion. Sci. Rep. 2015, 5, 13858. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Wang, M.-H.; Li, Q.; Li, X.; Liu, Y.; Fan, L.-Z. Effect of oxygen-containing functional groups in epoxy/reduced graphene oxide composite coatings on corrosion protection and antimicrobial properties. Appl. Surf. Sci. 2018, 448, 351–361. [Google Scholar] [CrossRef]
- Arun, T.; Ray, D.K.; Gupta, V.P.; Panda, S.S.; Sahoo, P.K.; Ghosh, J.; Sengupta, P.; Satyam, P.V. Surface protection coating material for controlling the decay of major construction stone. AIP Conf. Proc. 2017, 1832, 080017. [Google Scholar] [CrossRef]
- Galvagno, E.; Tartaglia, E.; Stratigaki, M.; Tossi, C.; Marasco, L.; Menegazzo, F.; Zanardi, C.; Omenetto, F.; Coletti, C.; Traviglia, A.; et al. Present Status and Perspectives of Graphene and Graphene-related Materials in Cultural Heritage. Adv. Funct. Mater. 2024, 34, 2313043. [Google Scholar] [CrossRef]
- González-Campelo, D.; Fernández-Raga, M.; Gómez-Gutiérrez, Á.; Guerra-Romero, M.I.; González-Domínguez, J.M. Extraordinary Protective Efficacy of Graphene Oxide over the Stone-Based Cultural Heritage. Adv. Mater. Interfaces 2021, 8, 2101012. [Google Scholar] [CrossRef]
- Martínez-García, R.; González-Campelo, D.; Fraile-Fernández, F.J.; Castañón, A.M.; Caldevilla, P.; Giganto, S.; Ortiz-Marqués, A.; Zelli, F.; Calvo, V.; González-Domínguez, J.M.; et al. Performance Study of Graphene Oxide as an Antierosion Coating for Ornamental and Heritage Dolostone. Adv. Mater. Technol. 2023, 8, 2300486. [Google Scholar] [CrossRef]
- Rodríguez, I.; Ortiz, A.; Caldevilla, P.; Giganto, S.; Búrdalo, G.; Fernández-Raga, M. Comparison between the Effects of Normal Rain and Acid Rain on Calcareous Stones under Laboratory Simulation. Hydrology 2023, 10, 79. [Google Scholar] [CrossRef]
- Búrdalo-Salcedo, G.; Rodríguez, I.; Fernández-Raga, M.; Fernández-Raga, S.; Rodríguez-Fernández, C.; González-Domínguez, J.M. Adaptation of a Standard Method for Water Absorption Testing of Stone Materials: The Case of a Hydrophilic Protective Coating. Materials 2023, 16, 4228. [Google Scholar] [CrossRef]
- Antolín-Rodríguez, A.; Merino-Maldonado, D.; Rodríguez-González, Á.; Fernández-Raga, M.; González-Domínguez, J.M.; Juan-Valdés, A.; García-González, J. Statistical Study of the Effectiveness of Surface Application of Graphene Oxide as a Coating for Concrete Protection. Coatings 2023, 13, 213. [Google Scholar] [CrossRef]
- Nishina, Y. Mass Production of Graphene Oxide Beyond the Laboratory: Bridging the Gap Between Academic Research and Industry. ACS Nano 2024, 18, 33264–33275. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Jia, P.-P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.-B.; Cui, Z.-S.; Wei, D.-P.; Shi, H.-F.; Pei, D.-S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Gao, Y.; Fang, Z. Integrating metabolic analysis with biological endpoints provides insight into nanotoxicological mechanisms of graphene oxide: From effect onset to cessation. Carbon 2016, 109, 65–73. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Soares, E.S.; De Jesus, M.B.; Ceragioli, H.J.; Irazusta, S.P.; Batista, A.G.; Vinolo, M.A.R.; Júnior, M.R.M.; Da Cruz-Höfling, M.A. Reduced graphene oxide: Nanotoxicological profile in rats. J. Nanobiotechnology 2016, 14, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Zhang, X.; Liao, W. Environmental transformation of graphene oxide in the aquatic environment. Chemosphere 2021, 262, 127885. [Google Scholar] [CrossRef]
- Serrano-Luján, L.; Víctor-Román, S.; Toledo, C.; Sanahuja-Parejo, O.; Mansour, A.E.; Abad, J.; Amassian, A.; Benito, A.M.; Maser, W.K.; Urbina, A. Environmental impact of the production of graphene oxide and reduced graphene oxide. SN Appl. Sci. 2019, 1, 179. [Google Scholar] [CrossRef]
- Novelo, F.J.X.; Pareja-Rodríguez, R.; Martínez-Flores, R.; Gattorno, G.R. Synthesis of graphene oxide from post-consumer PET bottles by a one-step thermal treatment in air atmosphere for removal of methylene blue. J. Environ. Chem. Eng. 2024, 12, 112244. [Google Scholar] [CrossRef]
- Park, W.K.; Yoon, Y.; Kim, S.; Choi, S.Y.; Yoo, S.; Do, Y.; Jung, S.; Yoon, D.H.; Park, H.; Yang, W.S. Toward Green Synthesis of Graphene Oxide Using Recycled Sulfuric Acid via Couette–Taylor Flow. ACS Omega 2017, 2, 186–192. [Google Scholar] [CrossRef]
- Franzoni, E.; Sassoni, E.; Scherer, G.W.; Naidu, S. Artificial weathering of stone by heating. J. Cult. Herit. 2013, 14, e85–e93. [Google Scholar] [CrossRef]
- Siegesmund, S.; Török, Á. Building Stones. In Stone in Architecture: Properties, Durability; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 11–95. [Google Scholar] [CrossRef]
- Salvini, S.; Coletti, C.; Maritan, L.; Massironi, M.; Pieropan, A.; Spiess, R.; Mazzoli, C. Petrographic characterization and durability of carbonate stones used in UNESCO World Heritage Sites in northeastern Italy. Environ. Earth Sci. 2023, 82, 49. [Google Scholar] [CrossRef]
- Di Benedetto, C.; Cappelletti, P.; Favaro, M.; Graziano, S.; Langella, A.; Calcaterra, D.; Colella, A. Porosity as key factor in the durability of two historical building stones: Neapolitan Yellow Tuff and Vicenza Stone. Eng. Geol. 2015, 193, 310–319. [Google Scholar] [CrossRef]
- Scrivano, S.; Gaggero, L.; Aguilar, J.G. An Experimental Investigation of the Effects of Grain Size and Pore Network on the Durability of Vicenza Stone. Rock Mech. Rock Eng. 2019, 52, 2935–2948. [Google Scholar] [CrossRef]
- Germinario, L.; Siegesmund, S.; Maritan, L.; Mazzoli, C. Petrophysical and mechanical properties of Euganean trachyte and implications for dimension stone decay and durability performance. Environ. Earth Sci. 2017, 76, 739. [Google Scholar] [CrossRef]
- Germinario, L.; Hanchar, J.M.; Sassi, R.; Maritan, L.; Cossio, R.; Borghi, A.; Mazzoli, C. New petrographic and geochemical tracers for recognizing the provenance quarry of trachyte of the Euganean Hills, northeastern Italy. Geoarchaeology 2018, 33, 430–452. [Google Scholar] [CrossRef]
- Siegesmund, S.; Dürrast, H. Physical and Mechanical Properties of Rocks. In Stone in Architecture: Properties, Durability; Siegesmund, S., Snethlage, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 97–224. [Google Scholar] [CrossRef]
- Graue, B.; Siegesmund, S.; Oyhantcabal, P.; Naumann, R.; Licha, T.; Simon, K. The effect of air pollution on stone decay: The decay of the Drachenfels trachyte in industrial, urban, and rural environments—A case study of the Cologne, Altenberg and Xanten cathedrals. Environ. Earth Sci. 2013, 69, 1095–1124. [Google Scholar] [CrossRef]
- Costinas, C.; Salagean, C.A.; Cotet, L.C.; Baia, M.; Todea, M.; Magyari, K.; Baia, L. Insights into the Stability of Graphene Oxide Aqueous Dispersions. Nanomaterials 2022, 12, 4489. [Google Scholar] [CrossRef]
- EN 15803:2009; Conservation of Cultural Property—Test Methods—Determination of Water Vapour permeability (δp). CEN (European Committee for Standardization): Brussels, Belgium, 2009.
- EN 15801:2009; Conservation of Cultural Property—Test Methods—Determination of Capillarity Water Absorption. CEN (European Committee for Standardization): Brussels, Belgium, 2009.
- EN 15802:2010; Conservation of Cultural Property—Test Methods—Determination of Static Contact Angle. CEN (European Committee for Standardization): Brussels, Belgium, 2010.
- Zendri, E.; Falchi, L.; Izzo, F.C.; Morabito, Z.M.; Driussi, G. A review of common NDTs in the monitoring and preservation of historical architectural surfaces. Int. J. Archit. Herit. 2017, 11, 987–1004. [Google Scholar] [CrossRef]
- Dai-Yang, L.; Pengou, M.; Tcheumi, H.L.; Nguihdama, D.; Wahabou, A.; Ngatchou, B.N.; Wamba, O.R.T.; Kouamo, H.T.; Nanseu-Njiki, C.P.; Ngameni, E. Physico-Chemical and Electrochemical Characterizations of a Trachyte in the Far North Region of Cameroon for Possible Electroanalytical and Environmental Purposes. J. Inorg. Organomet. Polym. 2021, 31, 542–551. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Guo, L.; Xia, H.; Chen, Q.-D.; Feng, J.; Sun, H.-B. Photoreduction of Graphene Oxides: Methods, Properties, and Applications. Adv. Opt. Mater. 2014, 2, 10–28. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Fuente, E.; Menéndez, J.A.; Díez, M.A.; Suárez, D.; Montes-Morán, M.A. Infrared Spectroscopy of Carbon Materials: A Quantum Chemical Study of Model Compounds. J. Phys. Chem. B 2003, 107, 6350–6359. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2009, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Dimiev, A.; Kosynkin, D.V.; Alemany, L.B.; Chaguine, P.; Tour, J.M. Pristine Graphite Oxide. J. Am. Chem. Soc. 2012, 134, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.d.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Fujimoto, H. Theoretical X-ray scattering intensity of carbons with turbostratic stacking and AB stacking structures. Carbon 2003, 41, 1585–1592. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- de Barros, N.G.; Neto, A.C.G.; Vaccioli, K.B.; Angulo, H.R.V.; Silva, L.G.d.A.e.; Toffoli, S.M.; Valera, T.S. Graphene Oxide: A Comparison of Reduction Methods. C 2023, 9, 73. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by Divalent Ions—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Raidongia, K.; Shao, J.; Yang, Q.-H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2014, 7, 166–170. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable barrier films and protective coatings based on reduced graphene oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Liu, L.; Meng, F.; Cui, Y.; Wang, F. Modification of graphene and graphene oxide and their applications in anticorrosive coatings. J. Coat. Technol. Res. 2021, 18, 311–331. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Zhang, Q.; Lin, J.; Sagoe-Crentsil, K.; Duan, W. Dispersion of silane-functionalized GO and its reinforcing effects in cement composites. J. Build. Eng. 2021, 43, 103228. [Google Scholar] [CrossRef]
- Qamar, S.; Ramzan, N.; Aleem, W. Graphene dispersion, functionalization techniques and applications: A review. Synth. Met. 2024, 307, 117697. [Google Scholar] [CrossRef]
- Schifano, E.; Cavallini, D.; De Bellis, G.; Bracciale, M.P.; Felici, A.C.; Santarelli, M.L.; Sarto, M.S.; Uccelletti, D. Antibacterial Effect of Zinc Oxide-Based Nanomaterials on Environmental Biodeteriogens Affecting Historical Buildings. Nanomaterials 2020, 10, 335. [Google Scholar] [CrossRef]
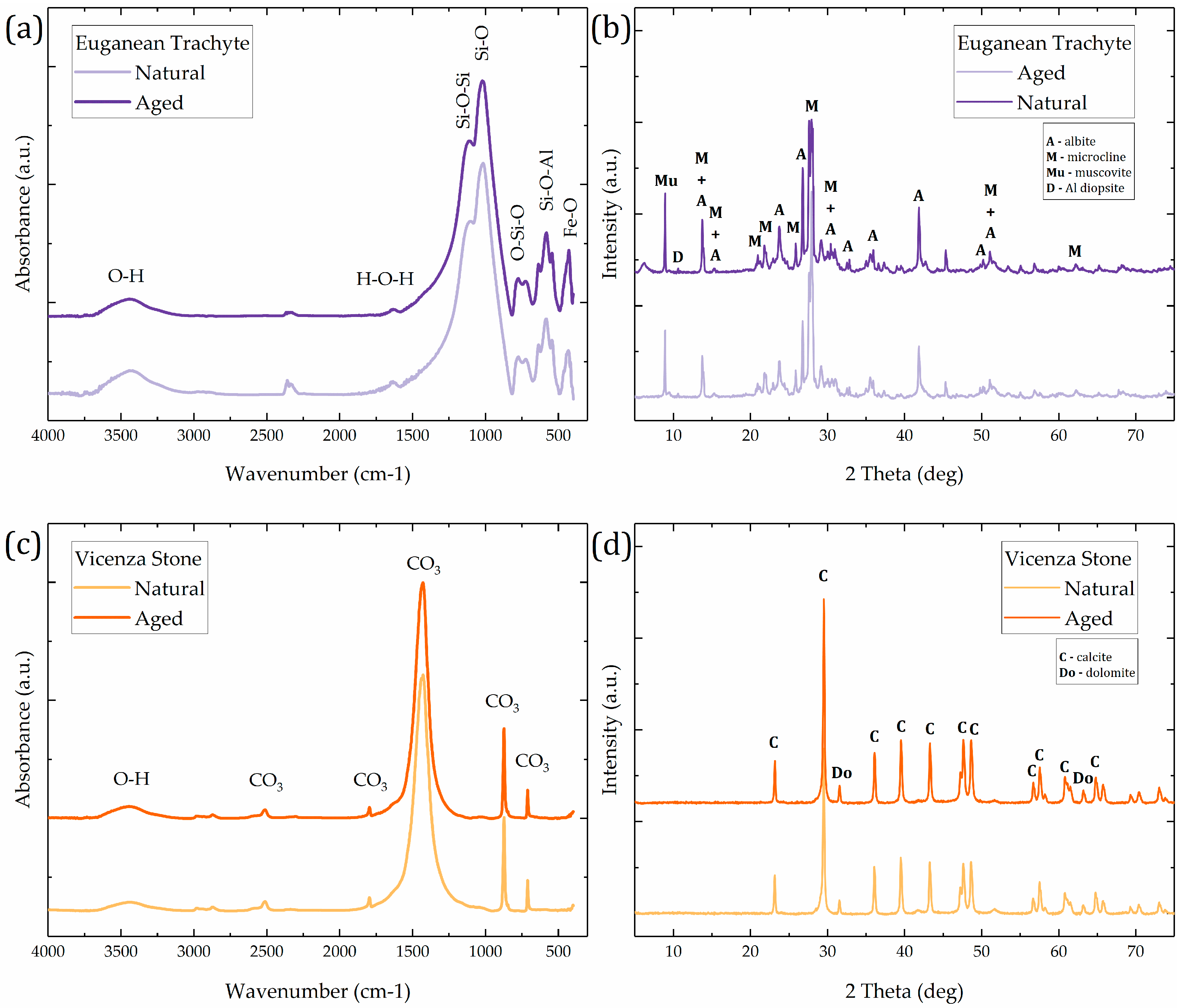
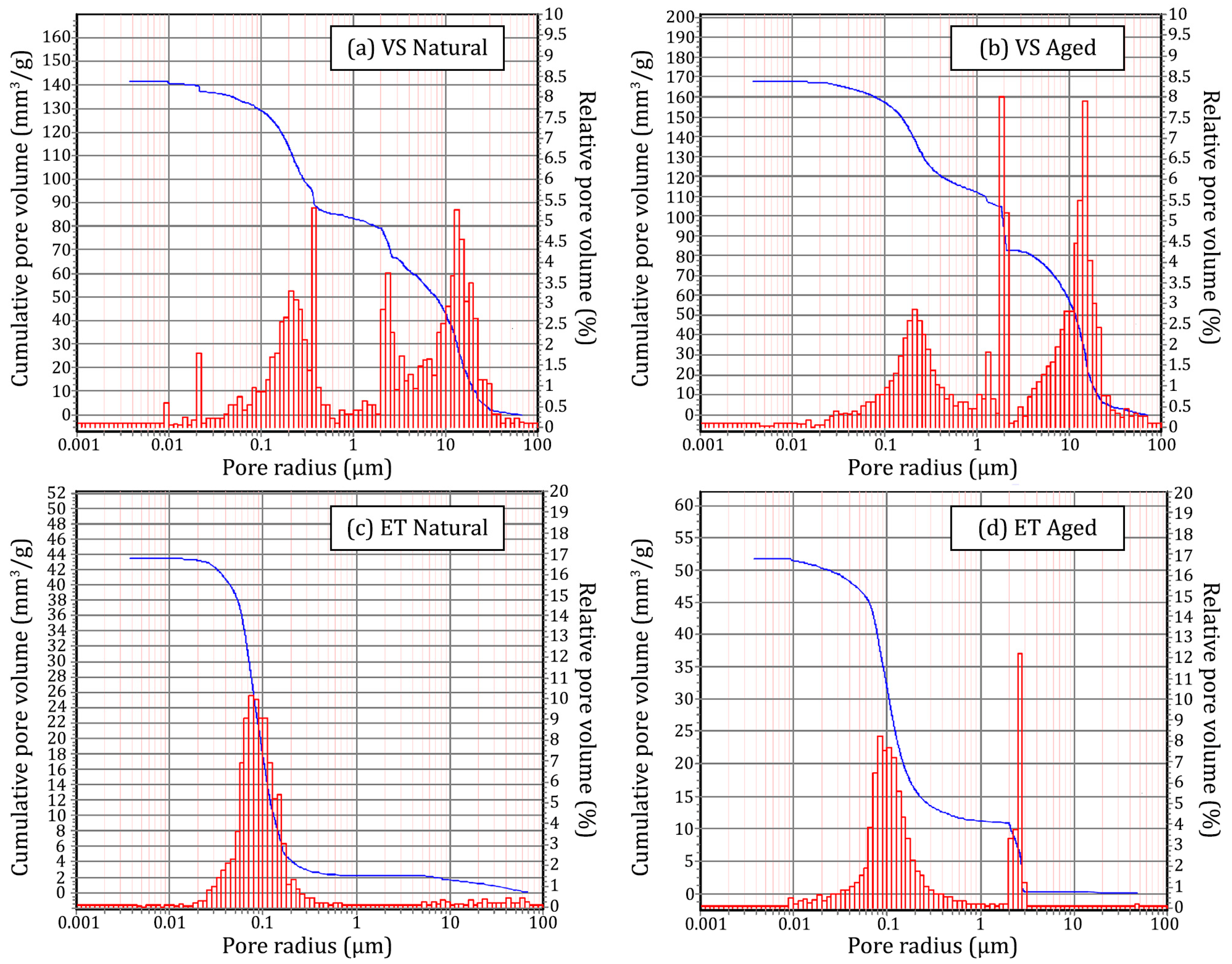

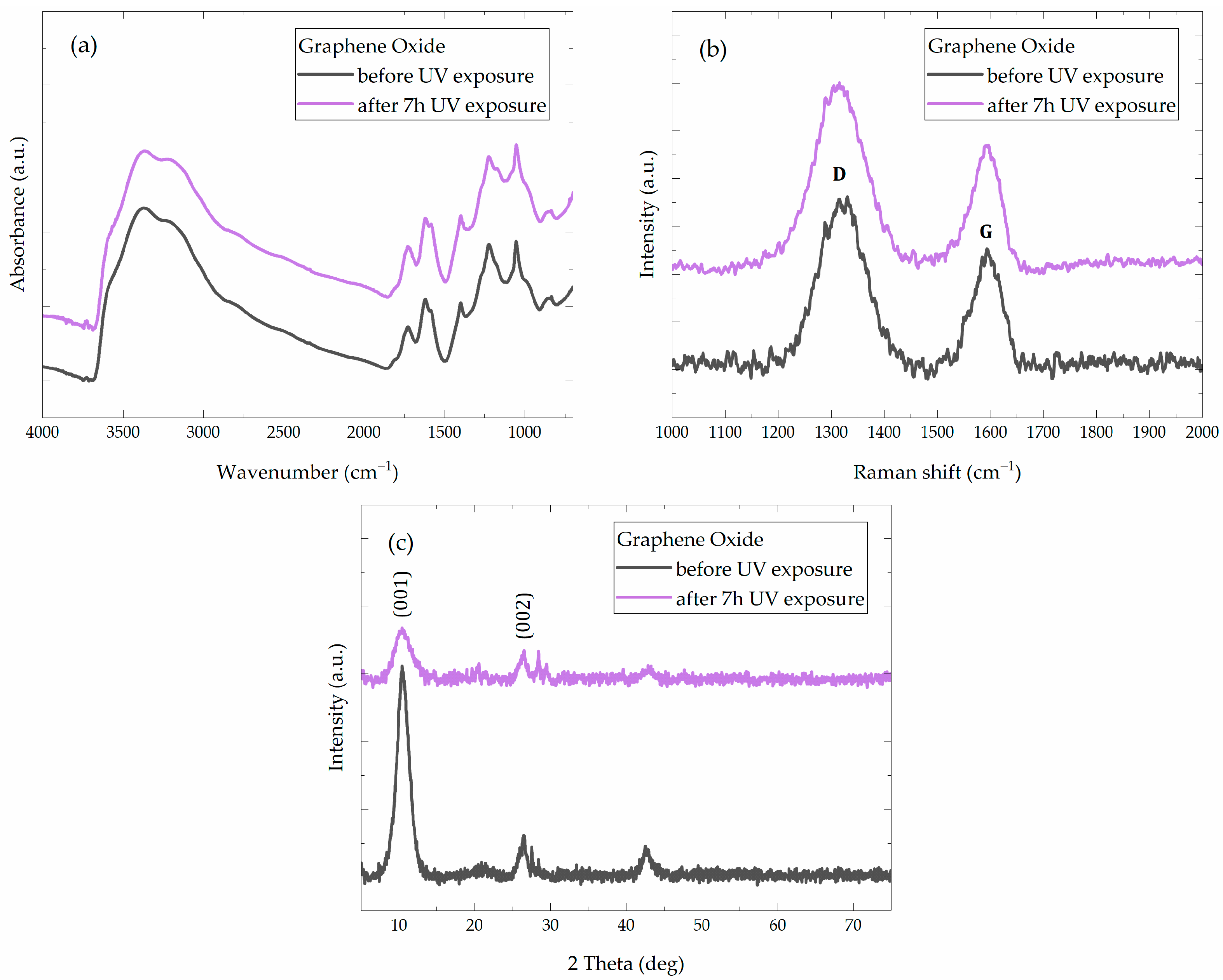
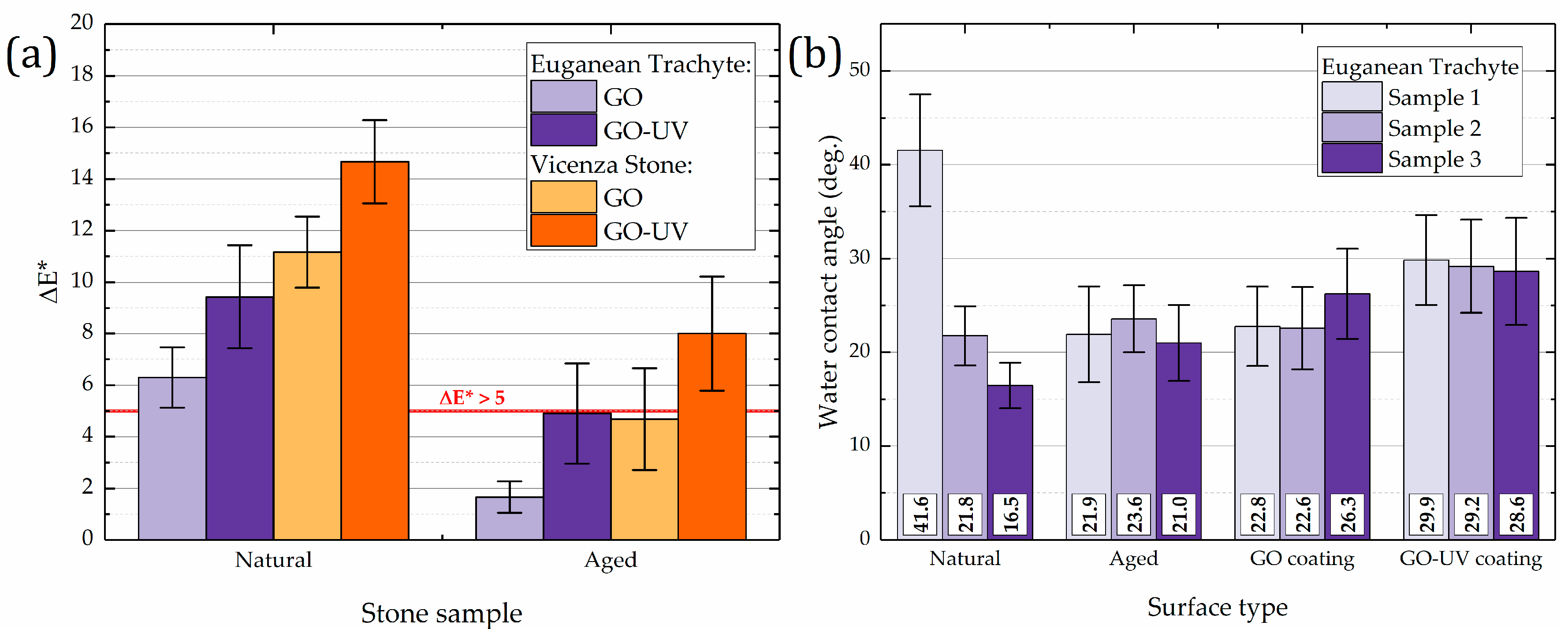
| Sample | Bulk Density (g/cm3) | Apparent Density (g/cm3) | Open Porosity (vol. %) | Mean Pore Radius (µm) | Median Pore Radius (µm) | Total Pore Volume (mm3/g) |
|---|---|---|---|---|---|---|
| ET Natural | 2.3506 | 2.6185 | 10.23 | 0.0792 | 0.0888 | 43.52 |
| ET Aged | 2.4154 | 2.7608 | 12.51 | 0.0942 | 0.1183 | 51.79 |
| VS Natural | 1.8004 | 2.4167 | 25.50 | 0.2216 | 2.4753 | 141.63 |
| VS Aged | 1.9459 | 2.8902 | 32.67 | 0.3421 | 2.0806 | 167.90 |
| Sample | Coating | Surface Feature | ID/IG Ratio | FWHM D Band (cm−1) | FWHM G Band (cm−1) |
|---|---|---|---|---|---|
| GO | reference | 1.47 | 104 | 64 | |
| GO–UV | reference | 1.48 | 113 | 63 | |
| VS Natural | GO | pore | 1.79 | 105 | 63 |
| surface | 1.44 | 77 | 57 | ||
| GO–UV | pore | 1.78 | 92 | 62 | |
| surface | 1.80 | 101 | 67 | ||
| VS Aged | GO | pore | 1.56 | 90 | 57 |
| surface | 1.72 | 86 | 56 | ||
| GO–UV | pore | 1.56 | 94 | 57 | |
| surface | 1.22 | 66 | 51 |
| Sample | Coating | Water Vapor Resistance Factor (Wet-Cup [65]) | Variation Against Uncoated Sample | Water Absorption Coefficient by Capillarity (kg/m2 h1/2 [66]) | Variation Against Uncoated Sample |
|---|---|---|---|---|---|
| ET Natural | uncoated | 108.81 ± 2.15 | 0.328 ± 0.014 | ||
| GO | 124.40 ± 4.47 | +14.3% | 0.253 ± 0.018 | −23.0% | |
| GO–UV | 114.18 ± 7.32 | +4.9% | 0.167 ± 0.010 | −49.0% | |
| ET Aged | uncoated | 110.56 ± 6.49 | 0.319 ± 0.016 | ||
| GO | 118.31 ± 11.20 | +7.0% | 0.241 ± 0.011 | −24.5% | |
| GO–UV | 119.68 ± 5.18 | +8.2% | 0.193 ± 0.014 | −39.5% | |
| VS Natural | uncoated | 20.73 ± 0.54 | 8.027 ± 0.658 | ||
| GO | 24.15 ± 0.40 | +16.5% | 7.186 ± 0.160 | −10.5% | |
| GO–UV | 21.49 ± 0.58 | +3.7% | 7.073 ± 0.164 | −11.9% | |
| VS Aged | uncoated | 20.89 ± 0.55 | 15.433 ± 0.508 | ||
| GO | 24.66 ± 0.73 | +18.0% | 13.118 ± 1.276 | −15.0% | |
| GO–UV | 21.39 ± 1.10 | +2.4% | 11.998 ± 1.295 | −22.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costinas, C.; Cotet, L.C.; Baia, L.; Habra, N.E.; Nodari, L.; Tomasin, P. Performance Assessment of Graphene Oxide as a Protective Coating for Historical Stone. Materials 2025, 18, 1243. https://doi.org/10.3390/ma18061243
Costinas C, Cotet LC, Baia L, Habra NE, Nodari L, Tomasin P. Performance Assessment of Graphene Oxide as a Protective Coating for Historical Stone. Materials. 2025; 18(6):1243. https://doi.org/10.3390/ma18061243
Chicago/Turabian StyleCostinas, Codrut, Liviu Cosmin Cotet, Lucian Baia, Naida El Habra, Luca Nodari, and Patrizia Tomasin. 2025. "Performance Assessment of Graphene Oxide as a Protective Coating for Historical Stone" Materials 18, no. 6: 1243. https://doi.org/10.3390/ma18061243
APA StyleCostinas, C., Cotet, L. C., Baia, L., Habra, N. E., Nodari, L., & Tomasin, P. (2025). Performance Assessment of Graphene Oxide as a Protective Coating for Historical Stone. Materials, 18(6), 1243. https://doi.org/10.3390/ma18061243








