Experimental Assessment of Multiple Properties of Mycelium-Based Composites with Sewage Sludge and Bagasse
Highlights
- A low-carbon footprint natural binder were proposed to prepare lightweight backfill materials.
- The mycelial growth biological compatibility for the production of mycelium-based lightweight soil utilizing sewage sludge have been thoroughly analyzed.
- The mechanical properties, and thermal conductivity of the mycelium-based lightweight soil met the requirements for the backfill purposes physico-mechanical and thermal properties of the sewage sludge-MBCs were investigated.
- Sewage sludge increased the density and compressive strength of the MLWS owing to the filling effect and strengthening effect of inorganic constituents.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
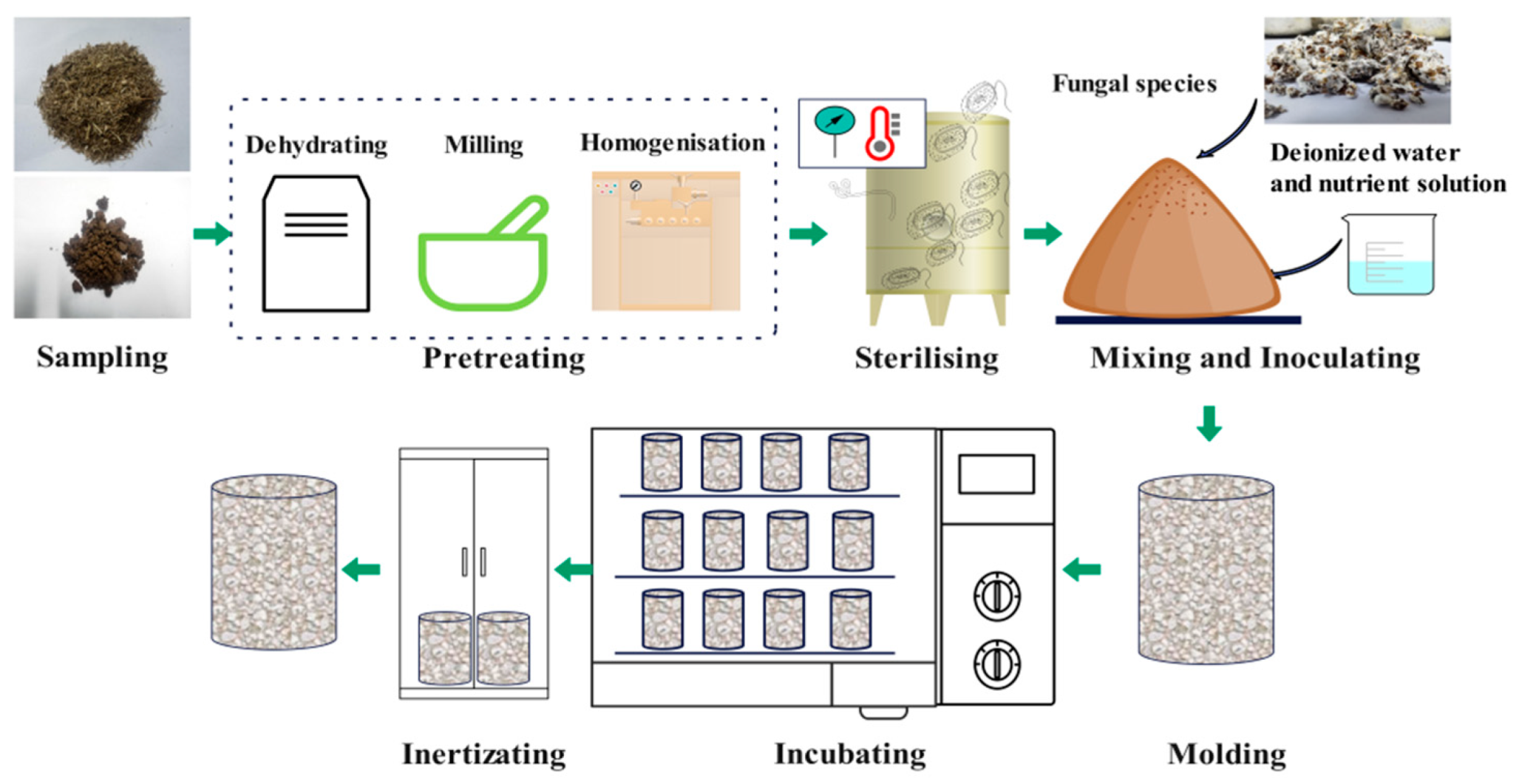
2.2. Sample Preparation
2.2.1. Preparation of Substrates
2.2.2. Preparation of MBCs
2.3. Testing and Characterization
2.3.1. Appearance, Density, and Volume Shrinkage
2.3.2. Compressive Strength Test
2.3.3. Thermal Conductivity Test
2.3.4. FTIR Test
2.3.5. SEM Test
2.3.6. TG Test
3. Results and Discussion
3.1. Appearance
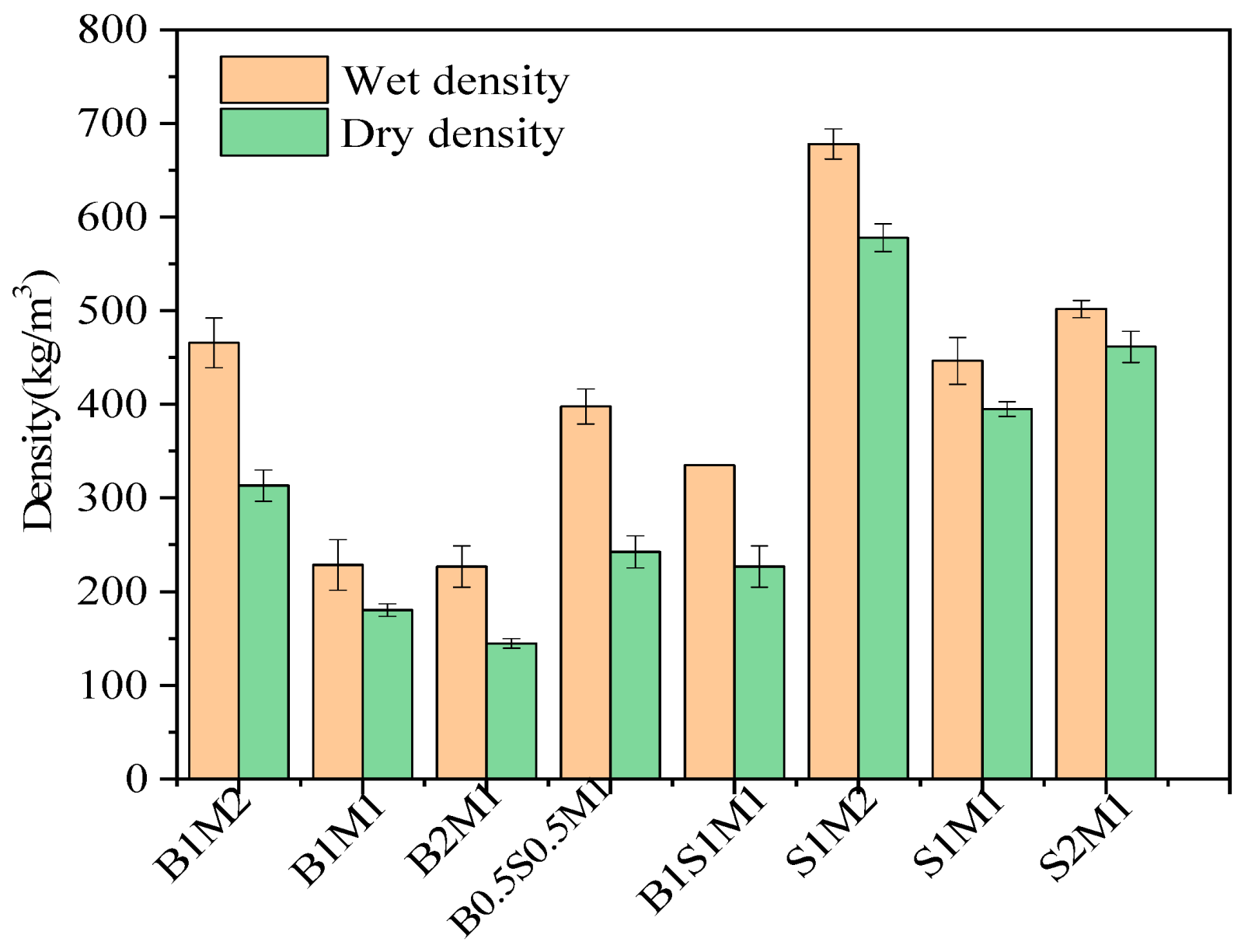
3.2. Density
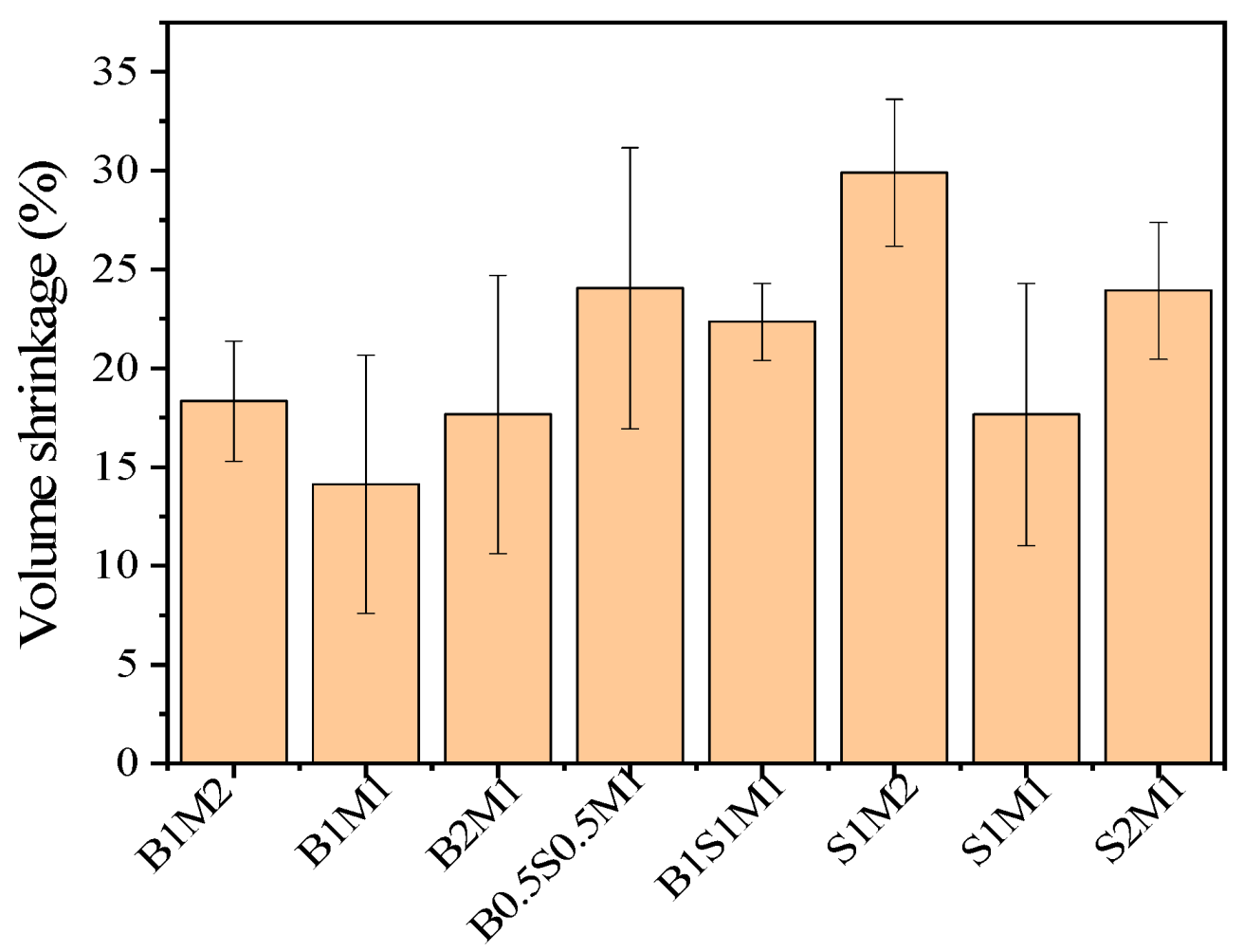
3.3. Volume Shrinkage
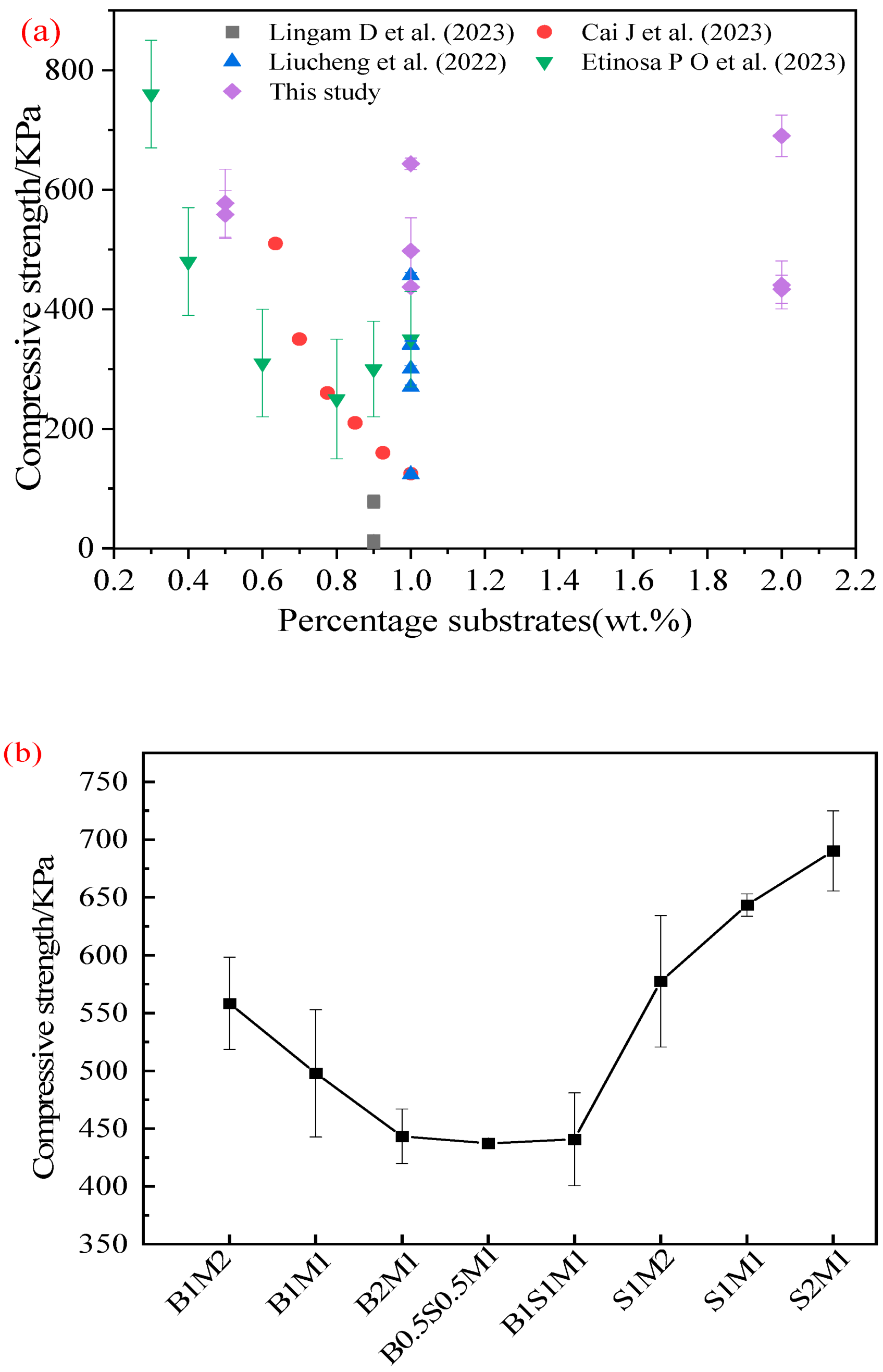
3.4. Compressive Strength
3.5. Thermal Conductivity
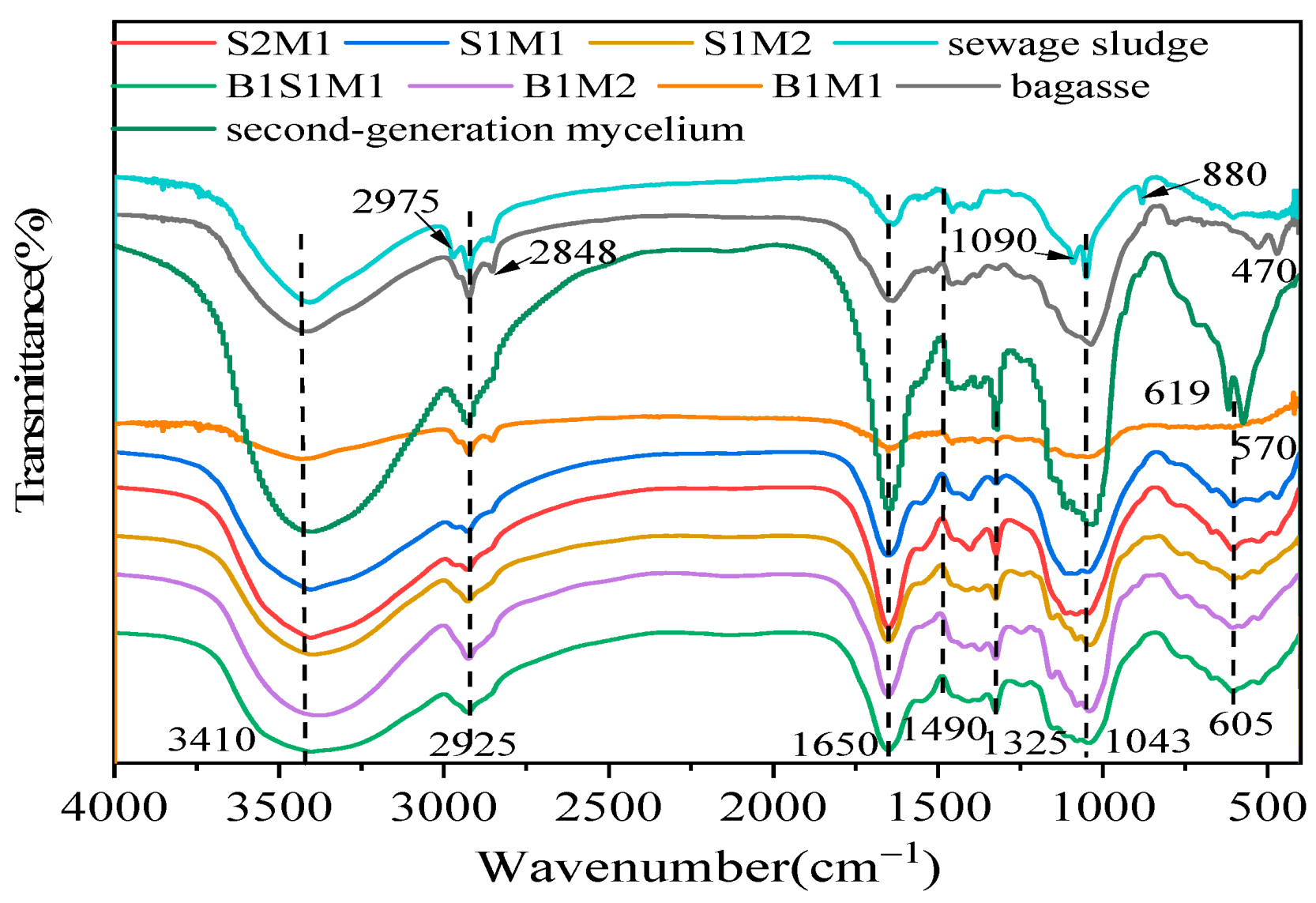
3.6. The FT-IR Analysis
3.7. SEM Analysis
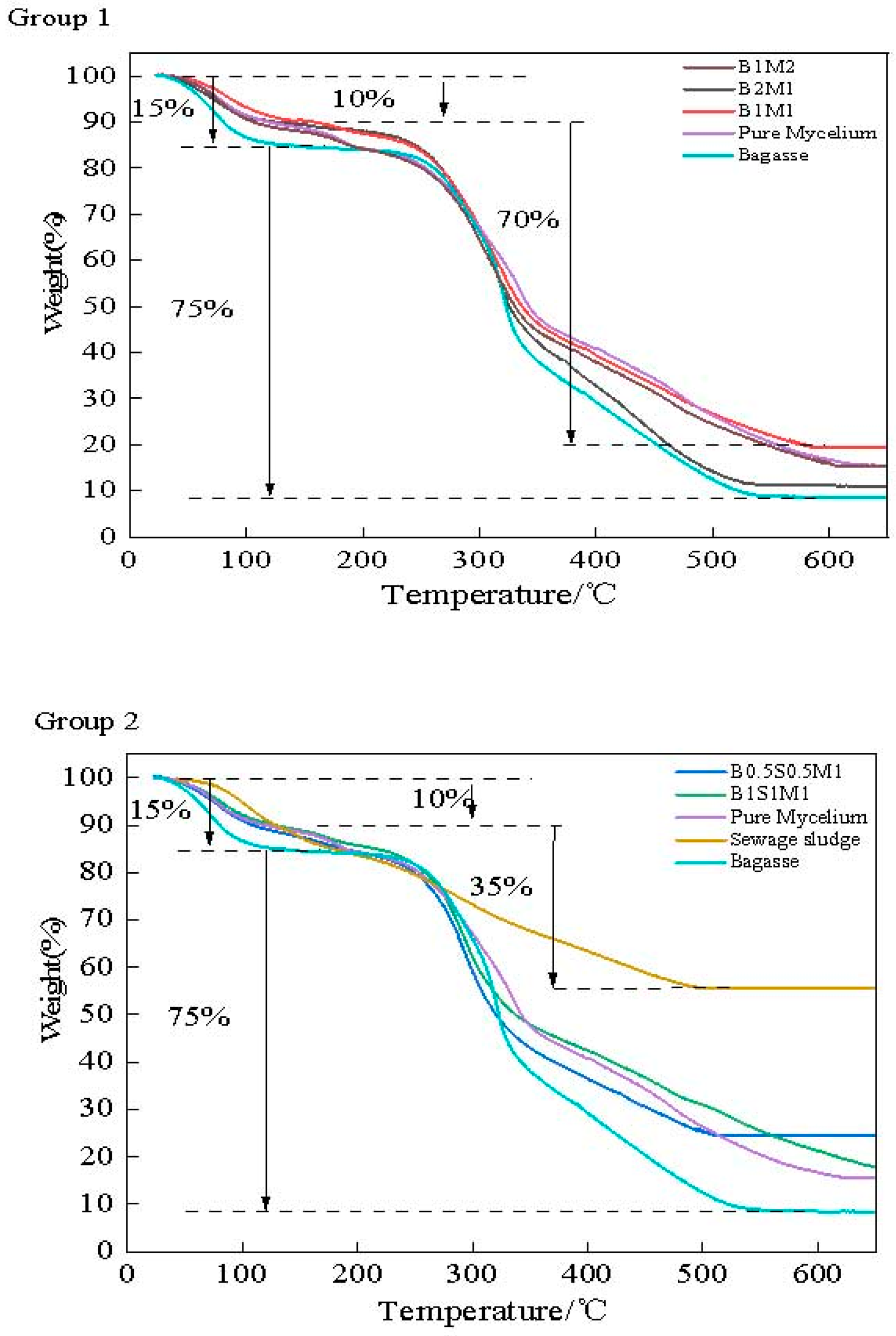
3.8. TGA Analysis
4. Conclusions
- (1)
- The results proved that the ready-made mycelium grown on the SS substrate bound together to form a mycelial network on day 10. The fungi had good biological compatibility with the SS. The density of the resulting MBCs increased with increasing SS content.
- (2)
- Owing to the filling effect and strengthening effect of SS, the compressive strengths of the MBCs increased significantly with increasing SS proportion. The compressive strength of the MBC prepared with an SS–ready-made mycelium ratio of 2:1 reached 690.20 KPa.
- (3)
- The thermal conductivity of the MBCs was comparable to those of the foamed lightweight soil used in highways. However, the mycelium as a natural binder brings significantly better environmental benefits than EPS and cement, which can cause great damage to the environment due to their high energy consumption and carbon emissions during the production process.
- (4)
- The microstructure revealed that the SS supported more robust growth of hyphae and resulted in stronger MBCs than the bagasse. Adding SS improved the thermal stability and thermal degradation resistance of the MBCs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MBCs | mycelium-based composites |
| SS | sewage sludge |
| LBMs | lightweight backfill materials |
| EPS | expanded polystyrene |
References
- Camilleri, E.; Narayan, S.; Lingam, D.; Blundell, R. Mycelium-based composites: An updated comprehensive overview. Biotechnol. Adv. 2025, 79, 108517. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–162. [Google Scholar] [CrossRef]
- Soh, E.; Saeidi, N.; Javadian, A.; Hebel, D.E.; Le Ferrand, H. Effect of common foods as supplements for the mycelium growth of Ganoderma lucidum and Pleurotus ostreatus on solid substrates. PLoS ONE 2021, 16, e0260170. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered mycelium composite construction materials from fungal biorefineries: A critical review. Mater. Des. 2019, 187, 108397. [Google Scholar] [CrossRef]
- Appels, F.V.; Camere, S.; Montalti, M.; Karana, E.; Jansen, K.M.; Dijksterhuis, J.; Krijgsheld, P.; Wösten, H.A. Fabrication factors influencing mechanical, moisture- and water-related properties of mycelium-based composites. Mater. Des. 2019, 161, 64–71. [Google Scholar] [CrossRef]
- Appels, F.V.W.; Dijksterhuis, J.; Lukasiewicz, C.E.; Jansen, K.M.B.; Wösten, H.A.B.; Krijgsheld, P. Hydrophobin gene deletion and environmental growth conditions impact mechanical properties of mycelium by affecting the density of the material. Sci. Rep. 2018, 8, 4703. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, F.; Still, B.; White, M.; Amstislavski, P. Physical and mechanical properties of fungal mycelium-based biofoam. J. Mater. Civ. Eng. 2017, 29, 04017030. [Google Scholar] [CrossRef]
- Bruscato, C.; Malvessi, E.; Brandalise, R.N.; Camassola, M. High performance of macrofungi in the production of mycelium-based biofoams using sawdust—Sustainable technology for waste reduction. J. Clean. Prod. 2019, 234, 225–232. [Google Scholar] [CrossRef]
- Canda, K.; Ducut, E.; Laxamana, J.C.; Manganti, J.M.; Mercado, J.P.; Velasco, B.J.; Culala, J.; Malonzo, A. Mycelium-Based Composite: An Experimental Study on the Utilization of Sawdust and Glass Fines as Substrates for Potential Fungi-Based Bio-Formed Insulation Board. Int. J. Progress. Res. Sci. Eng. 2023, 4, 103–112. [Google Scholar]
- Holt, G.A.; Mcintyre, G.; Flagg, D.; Bayer, E.; Wanjura, J.D.; Pelletier, M.G. Fungal mycelium and cotton plant materials in the manufacture of biodegradable molded packaging material: Evaluation study of select blends of cotton byproducts. J. Biobased Mater. Bioenergy 2012, 6, 431–439. [Google Scholar] [CrossRef]
- Womer, S.; Huynh, T.; John, S. Hybridizations and reinforcements in mycelium composites: A review. Bioresour. Technol. Rep. 2023, 22, 101456. [Google Scholar] [CrossRef]
- Alemu, D.; Tafesse, M.; Deressa, Y.G. Production of Mycoblock from the Mycelium of the Fungus pleurotus ostreatus for Use as Sustainable Construction Materials. Adv. Mater. Sci. Eng. 2022, 2022, 2876643. [Google Scholar] [CrossRef]
- Cai, J.; Han, J.; Ge, F.; Lin, Y.; Pan, J.; Ren, A. Development of impact-resistant mycelium-based composites (MBCs) with agricultural waste straws. Constr. Build. Mater. 2023, 389, 131730. [Google Scholar] [CrossRef]
- Attias, N.; Danai, O.; Abitbol, T.; Tarazi, E.; Ezov, N.; Pereman, I.; Grobman, Y.J. Mycelium bio-composites in industrial design and architecture: Comparative review and experimental analysis. J. Clean. Prod. 2019, 246, 119037. [Google Scholar] [CrossRef]
- César, E.; Castillo-Campohermoso, M.; Ledezma-Pérez, A.; Villarreal-Cárdenas, L.; Montoya, L.; Bandala, V.; Rodríguez-Hernández, A. Guayule bagasse to make mycelium composites: An alternative to enhance the profitability of a sustainable guayule crop. Biocatal. Agric. Biotechnol. 2023, 47, 102602. [Google Scholar] [CrossRef]
- Sisti, L.; Gioia, C.; Totaro, G.; Verstichel, S.; Cartabia, M.; Camere, S.; Celli, A. Valorization of wheat bran agro-industrial byproduct as an upgrading filler for mycelium-based composite materials. Ind. Crops Prod. 2021, 170, 113742. [Google Scholar] [CrossRef]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; McIntyre, G.; Gardner, D.J. Fully Bio-Based Hybrid Composites Made of Wood, Fungal Mycelium and Cellulose Nanofibrils. Sci. Rep. 2019, 9, 3766. [Google Scholar] [CrossRef]
- Haneef, M.; Ceseracciu, L.; Canale, C.; Bayer, I.S.; Heredia-Guerrero, J.A.; Athanassiou, A. Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Sci. Rep. 2017, 7, 41292. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.; Le Ferrand, H. Woodpile structural designs to increase the stiffness of mycelium-bound composites. Mater. Des. 2022, 225, 111530. [Google Scholar] [CrossRef]
- Liu, T.; Hou, T.-S.; Liu, H.-Y. Active Earth Pressure Characteristics of Light Weight Soil with EPS Particles Behind Rigid Retaining Wall. Int. J. Geosynth. Ground Eng. 2024, 10, 21. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Jiang, B.; Ma, Z.; Wen, S.; Wang, F. Preparation and experimental study of saponified slag fly ash foam lightweight soil. Constr. Build. Mater. 2024, 431, 136504. [Google Scholar] [CrossRef]
- Abdrabbo, F.M.; Gaaver, K.E.; Elwakil, A.Z.; Khalifa, S.A. Towards construction of embankment on soft soil: A comparative study of lightweight materials and deep replacement techniques. Sci. Rep. 2024, 14, 29628. [Google Scholar] [CrossRef] [PubMed]
- Layachi, S.; Izemmouren, O.; Dakhia, A.; Taallah, B.; Atiki, E.; Almeasar, K.S.; Layachi, M.; Guettala, A. Effect of incorporating Expanded polystyrene beads on Thermophysical, mechanical properties and life cycle analysis of lightweight earth blocks. Constr. Build. Mater. 2023, 375, 130948. [Google Scholar] [CrossRef]
- Gou, L.; Li, S.; Yin, J.; Li, T.; Liu, X. Morphological and physico-mechanical properties of mycelium biocomposites with natural reinforcement particles. Constr. Build. Mater. 2021, 304, 124656. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; Mckay, G.; Al-Ansari, T. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Alokika; Anu; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Rigobello, A.; Ayres, P. Compressive behaviour of anisotropic mycelium-based composites. Sci. Rep. 2022, 12, 6846. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Salifu, A.A.; Osafo, S.; Eluu, S.C.; Obayemi, J.D.; Soboyejo, W.O. Fracture and Toughening of Mycelium-based Biocomposites. Mater. Des. 2024, 237, 112592. [Google Scholar] [CrossRef]
- Tacer-Caba, Z.; Varis, J.J.; Lankinen, P.; Mikkonen, K.S. Comparison of novel fungal mycelia strains and sustainable growth substrates to produce humidity-resistant biocomposites. Mater. Des. 2020, 192, 108728. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Huynh, T.; Kandare, E.; Yuen, R.; Wang, C.H.; John, S. Waste-derived low-cost mycelium composite construction materials with improved fire safety. Fire Mater. 2018, 42, 816–825. [Google Scholar] [CrossRef]
- Peng, L.; Yi, J.; Yang, X.; Xie, J.; Chen, C. Development and characterization of mycelium bio-composites by utilization of different agricultural residual byproducts. J. Bioresour. Bioprod. 2022, 8, 78–89. [Google Scholar] [CrossRef]
- ISO 22007-2: 2022; Plastics-Determination of Thermal Conductivity and Thermal Diffusivity, Part 2: Transient Plane Heat Source (Hot Disc) Method. International Organization for Standardization: Geneva, Switzerland, 2022. Available online: https://www.iso.org/standard/81836.html (accessed on 1 June 2022).
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium composites: A review of engineering characteristics and growth kinetics. J. Bionanosci. 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Kung’u, J. The Phases of Fungal Growth in Indoor Environment. 2016. Available online: http://www.moldbacteria.com/mold/the-phases-of-fungal-growth-in-indoor-environment.html (accessed on 8 June 2024).
- Nawawi, W.; Lee, K.-Y.; Kontturi, E.; Murphy, R.; Bismarck, A. Chitin nanopaper from mushroom extract: Natural composite of nanofibres and glucan from a single bio-based source. ACS Sustain. Chem. Eng. 2019, 7, 6492–6496. [Google Scholar]
- Lelivelt, R.J.J. The Mechanical Possibilities of Mycelium Materials. Master’s Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2015. [Google Scholar]
- Schritt, H.; Vidi, S.; Pleissner, D. Spent mushroom substrate and sawdust to produce mycelium-based thermal insulation composites. J. Clean. Prod. 2021, 313, 127910. [Google Scholar] [CrossRef]
- Abdelhady, O.; Spyridonos, E.; Dahy, H. Bio-Modules: Mycelium-Based Composites Forming a Modular Interlocking System through a Computational Design towards Sustainable Architecture. Designs 2023, 7, 20. [Google Scholar] [CrossRef]
- Elsacker, E.; De Laet, L.; Peeters, E. Functional Grading of Mycelium Materials with Inorganic Particles: The Effect of Nanoclay on the Biological, Chemical and Mechanical Properties. Biomimetics 2022, 7, 57. [Google Scholar] [CrossRef]
- Yang, L.; Qin, Z. Mycelium-based wood composites for light weight and high strength by experiment and machine learning. Cell Rep. Phys. Sci. 2023, 4, 101424. [Google Scholar] [CrossRef]
- Ziegler, A.R.; Bajwa, S.G.; Holt, G.A.; McIntyre, G.; Bajwa, D.S. Evaluation of physico-mechanical properties of mycelium reinforced green biocomposites made from cellulosic fibers. Appl. Eng. Agric. 2016, 32, 931–938. [Google Scholar] [CrossRef]
- Nava, J.A.L.; González, J.M.; Chacón, X.R.; Luna, J.A.N. Assessment of Edible Fungi and Films Bio-Based Material Simulating Expanded Polystyrene. Mater. Manuf. Process. 2016, 31, 1085–1090. [Google Scholar] [CrossRef]
- Pelletier, M.; Holt, G.; Wanjura, J.; Greetham, L.; McIntyre, G.; Bayer, E.; Kaplan-Bie, J. Acoustic evaluation of mycological biopolymer, an all-natural closed cell foam alternative. Ind. Crop. Prod. 2019, 139, 111533. [Google Scholar] [CrossRef]
- Etinosa, P.O.; Salifu, A.A.; Azeko, S.T.; Obayemi, J.D.; Onche, E.O.; Aina, T.; Soboyejo, W.O. Self-organized mycelium biocomposites: Effects of geometry and laterite composition on compressive behavior. J. Mech. Behav. Biomed. Mater. 2023, 142, 105831. [Google Scholar] [CrossRef] [PubMed]
- Lingam, D.; Narayan, S.; Mamun, K.; Charan, D. Engineered mycelium-based composite materials: Comprehensive study of various properties and applications. Constr. Build. Mater. 2023, 391, 131841. [Google Scholar] [CrossRef]
- T/CHTS 10165-2024; Technical Guidelines for Application of Foamed Lightweight Soil in Highway. China Highway and Transportation Society: Beijing, China, 2024. Available online: https://www.chts.cn/bz/BZTZ/art/2023/art_85cb191255db457ea202e0c3013111a6.html (accessed on 1 July 2023).
- Zhang, M.; Zhang, Z.; Zhang, R.; Peng, Y.; Wang, M.; Cao, J. Lightweight, thermal insulation, hydrophobic mycelium composites with hierarchical porous structure: Design, manufacture and applications. Compos. Part B Eng. 2023, 266, 111003. [Google Scholar] [CrossRef]
- Girometta, C.; Picco, A.M.; Baiguera, R.M.; Dondi, D.; Babbini, S.; Cartabia, M.; Pellegrini, M.; Savino, E. Physico-mechanical and thermodynamic properties of mycelium-based biocomposites: A review. Sustainability 2019, 11, 281. [Google Scholar] [CrossRef]
- Răut, I.; Călin, M.; Vuluga, Z.; Oancea, F.; Paceagiu, J.; Radu, N.; Doni, M.; Alexandrescu, E.; Purcar, V.; Gurban, A.-M.; et al. Fungal based biopolymer composites for construction materials. Materials 2021, 14, 2906. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Bridgwater, A.V. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. J. Anal. Appl. Pyrol. 2010, 87, 199–206. [Google Scholar] [CrossRef]
- Weng, S.; Xu, Y. Fourier Transform Infrared Spectroscopy; Chemical Industry Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Shao, G.; Xu, D.; Xu, Z.; Jin, Y.; Wu, F.; Yang, N.; Xu, X.M. Green and sustainable biomaterials: Edible bio-plastic films from mushroom mycelium. Food Hydrocoll. 2024, 146, 109289. [Google Scholar] [CrossRef]
- Liu, R.; Long, L.; Sheng, Y.; Xu, J.; Qiu, H.; Li, X.; Wang, Y.; Wu, H. Preparation of a kind of novel sustainable mycelium/cotton stalk composites and effects of pressing temperature on the properties. Ind. Crops Prod. 2019, 141, 111732. [Google Scholar] [CrossRef]






| Types | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Bagasse | 21.3 | 36.1 | 41.1 |
| SS | 64.4 | 2.7 | 30.4 |
| Types | N (%) | C (%) | H (%) | S (%) | C/N | Dry Density (kg/m3) | Wet Density (kg/m3) | Moisture Content (wt%) |
|---|---|---|---|---|---|---|---|---|
| Bagasse | 0.32 | 44.96 | 6.89 | 0.03 | 138.33 | 86.65 ± 7.09 | 317.12 ± 15.08 | 87.56 ± 1.74 |
| SS | 2.94 | 15.03 | 4.90 | 3.18 | 5.11 | 670.68 ± 19.29 | 547.90 ± 23.23 | 68.91 ± 0.15 |
| Types (%) | SiO2 | Al2O3 | Fe2O3 | CaO | Loss |
|---|---|---|---|---|---|
| Bagasse | 6.33 | 0.32 | 0.12 | 0.36 | 92.41 |
| SS | 16.69 | 10.92 | 0.93 | 23.16 | 43.38 |
| Group | The Protocols of Proportion of MBCs | Ratio (Mass) | Label |
|---|---|---|---|
| Group 1 | Bagasse–ready-made mycelium | 1:2 | B1M2 |
| 1:1 | B1M1 | ||
| 2:1 | B2M1 | ||
| Group 2 | Bagasse–SS–ready-made mycelium | 0.5:0.5:1 | B0.5S0.5M1 |
| 1:1:1 | B1S1M1 | ||
| Group 3 | SS–ready-made mycelium | 1:2 | S1M2 |
| 1:1 | S1M1 | ||
| 2:1 | S2M1 |
| Label | Temperature/°C | Thermal Conductivity (Wm−1K−1) |
|---|---|---|
| B1M2 | 25 | 0.12 ± 2.52 × 10−4 |
| S2M1 | 25 | 0.13 ± 1.15 × 10−4 |
| IR Shift (cm−1) | Peak Detected (cm−1) | Assignment | Main Contribution |
|---|---|---|---|
| 3600–3300 | 3410 | O-H s | Cellulose and hemicellulose |
| 3050–2800 | 2975, 2925, 2848 | CH s, as | Lipids, waxes, and oils |
| 1700–1600 | 1650 | C=O (Amide I), -NH2 | Proteins |
| 1500–1400 | 1490 | C=O s | Proteins |
| 1400–1200 | 1325 | Amide III | Lipids and lignin |
| 1200–1000 | 1090, 1043 | C-C s | Proteins, lignin, and polysaccharides |
| 1000–800 | 880 | C-H b | Cellulose |
| Below 800 | 619, 605, 570, 470 | C-H b | Polysaccharides |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Cao, X. Experimental Assessment of Multiple Properties of Mycelium-Based Composites with Sewage Sludge and Bagasse. Materials 2025, 18, 1225. https://doi.org/10.3390/ma18061225
Hu M, Cao X. Experimental Assessment of Multiple Properties of Mycelium-Based Composites with Sewage Sludge and Bagasse. Materials. 2025; 18(6):1225. https://doi.org/10.3390/ma18061225
Chicago/Turabian StyleHu, Min, and Xuejuan Cao. 2025. "Experimental Assessment of Multiple Properties of Mycelium-Based Composites with Sewage Sludge and Bagasse" Materials 18, no. 6: 1225. https://doi.org/10.3390/ma18061225
APA StyleHu, M., & Cao, X. (2025). Experimental Assessment of Multiple Properties of Mycelium-Based Composites with Sewage Sludge and Bagasse. Materials, 18(6), 1225. https://doi.org/10.3390/ma18061225






