Basic Research on the Preparation of Electrolytic Manganese Residue–Red Mud–Ground Granulated Blast Furnace Slag–Calcium Hydroxide Composite Cementitious Material and Its Mechanical Properties
Abstract
1. Introduction
2. Reaction Mechanism of Geopolymers
3. Experimental Design
3.1. Raw Materials
3.2. Mix Proportions and Preparation
3.3. Testing Items and Methods
3.3.1. Physical and Mechanical Properties
3.3.2. Microstructure Characterization
3.3.3. Solidification Time Test
4. Results and Discussion
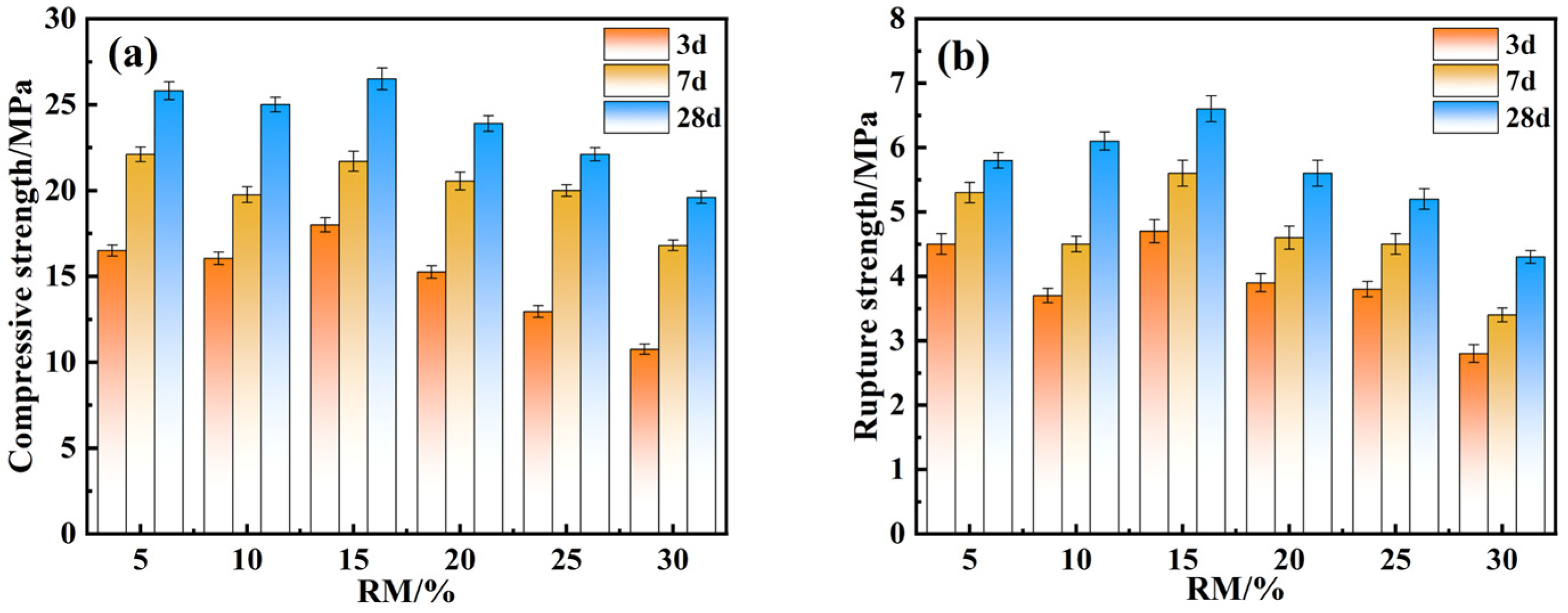
4.1. Effect of Various RM Dosages on Mechanical Strength
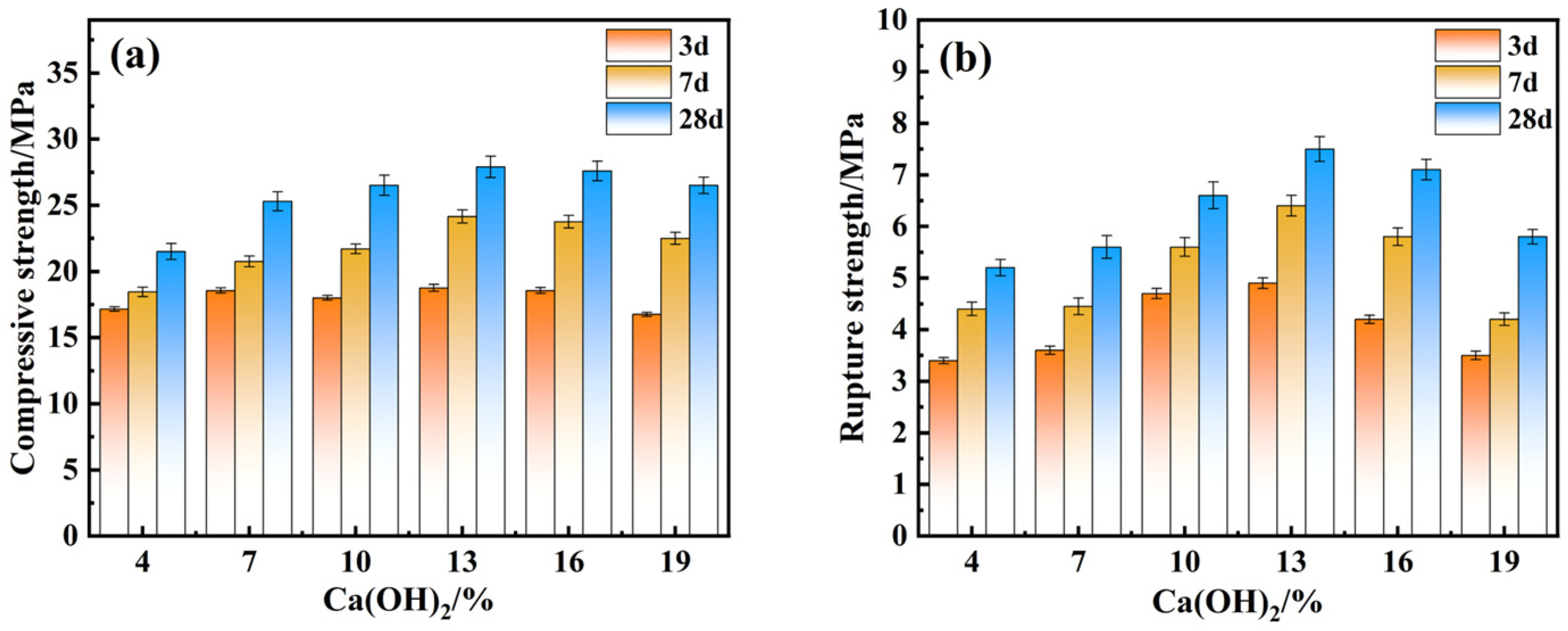
4.2. Effect of Alkali Exciter Dosage on Mechanical Properties
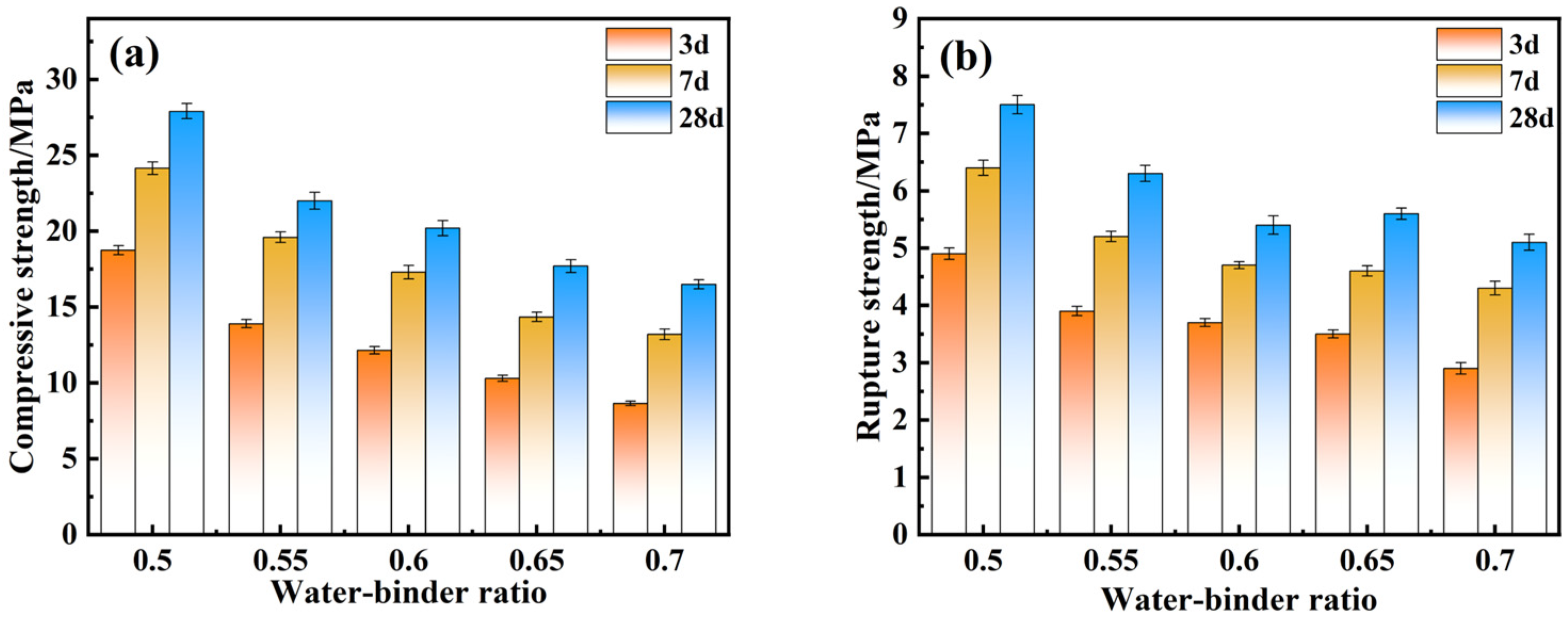
4.3. Effect of Mechanical Properties of Water-Binder Ratio
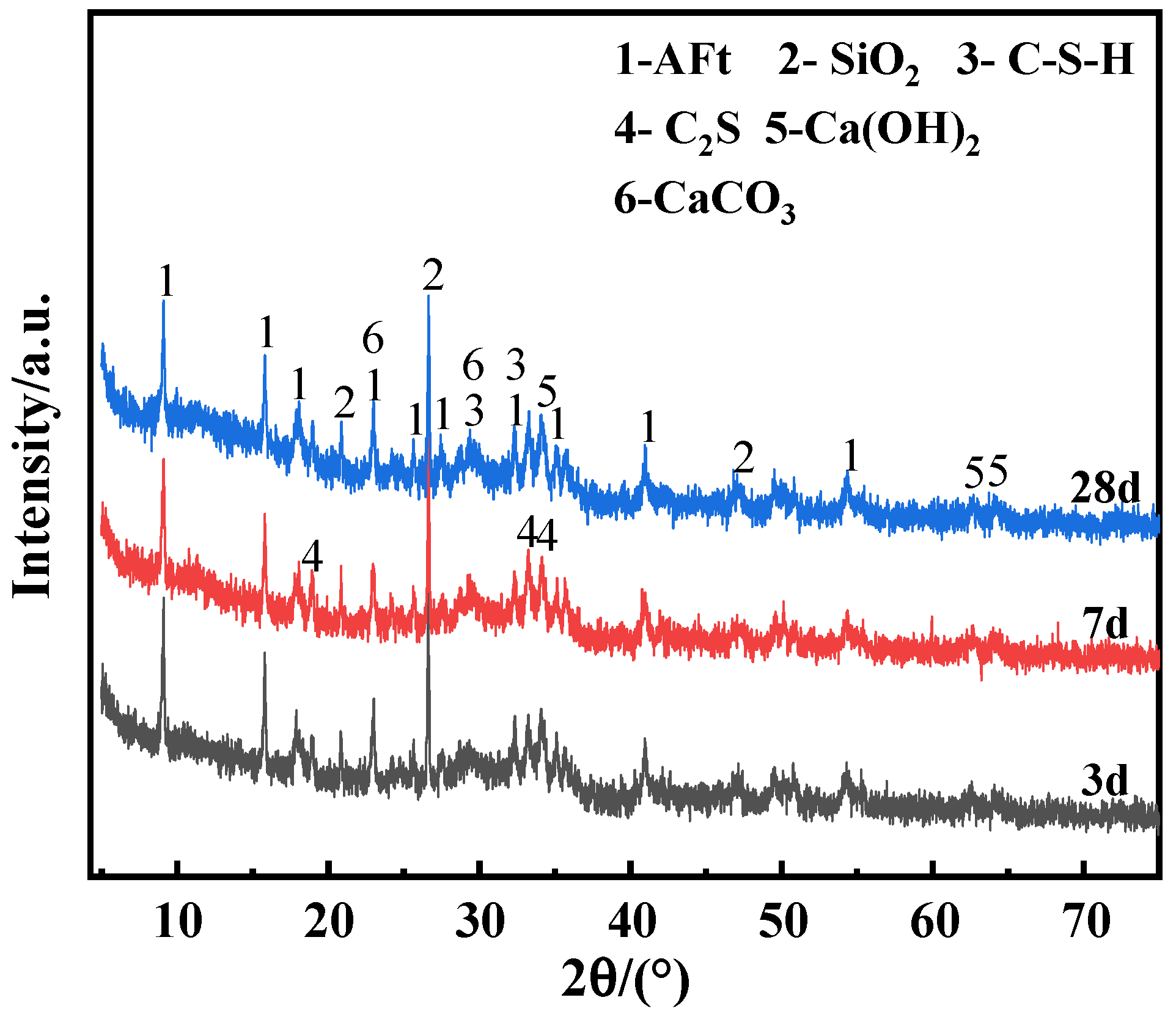
4.4. Physical Analysis of Hydration Products
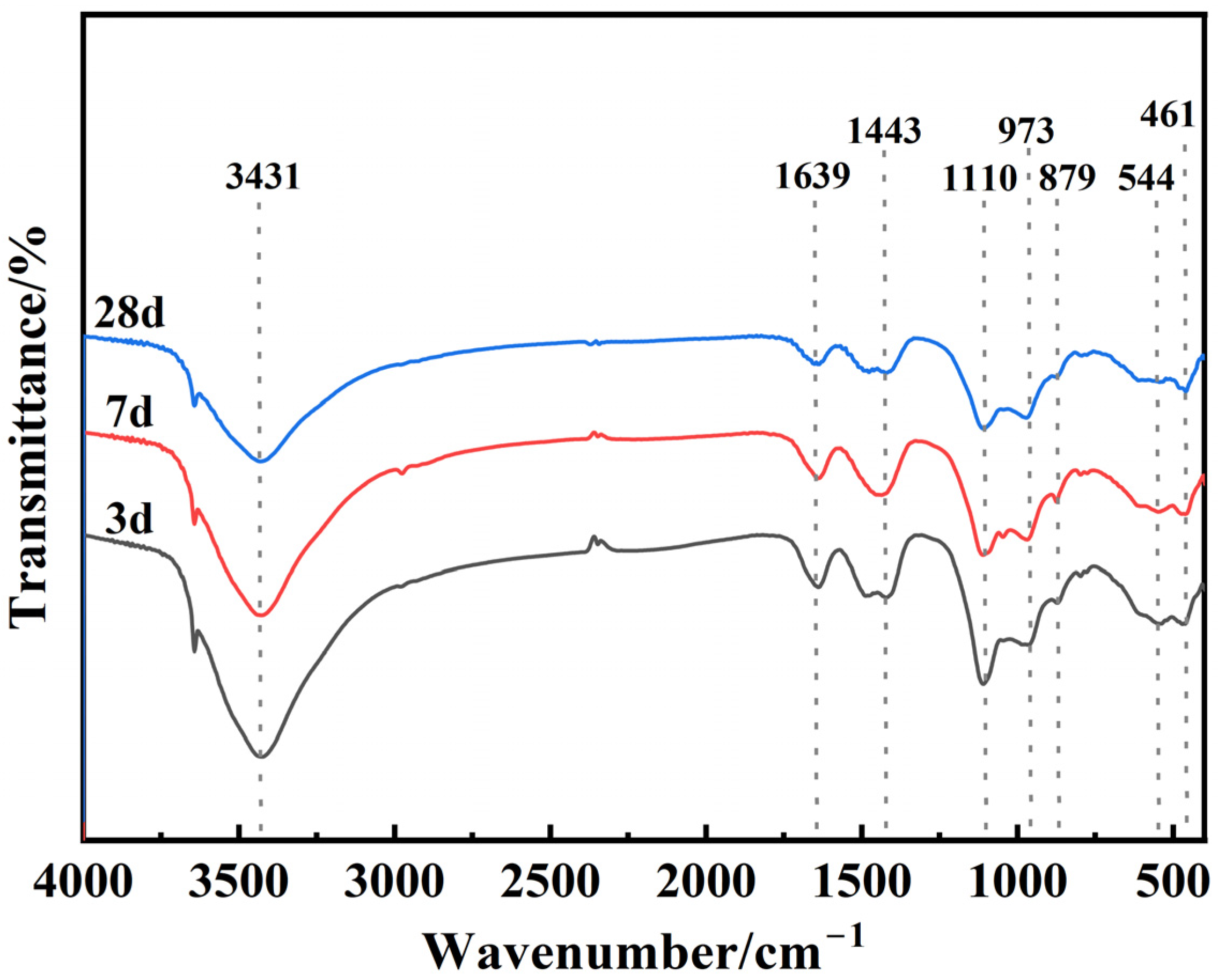
4.5. Chemical Bonding Analysis of Hydration Products
4.6. TG-DTG Analysis of Hydration Products
4.7. Microscopic Morphology of Cementitious Materials
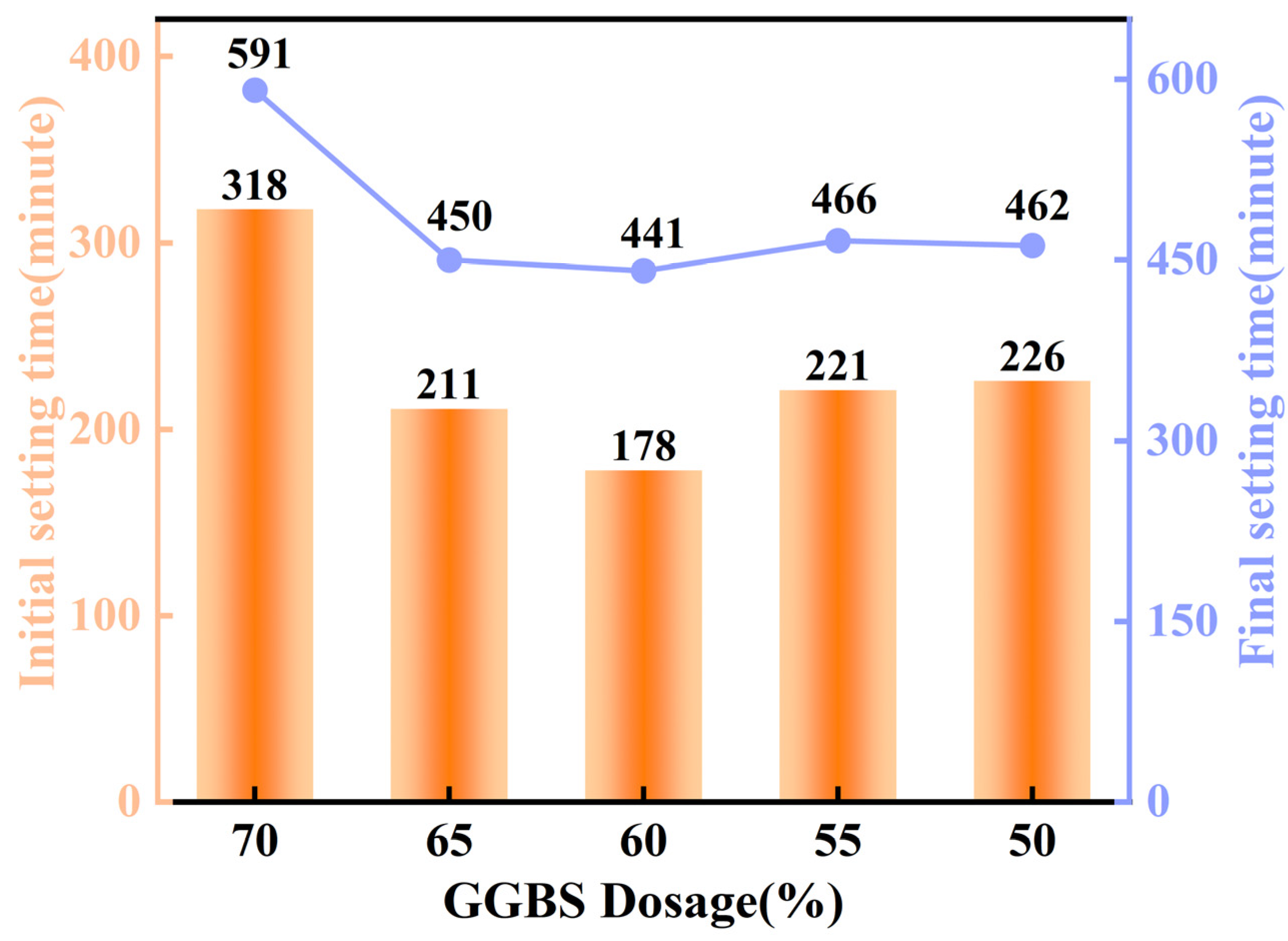
4.8. Setting Time Analysis
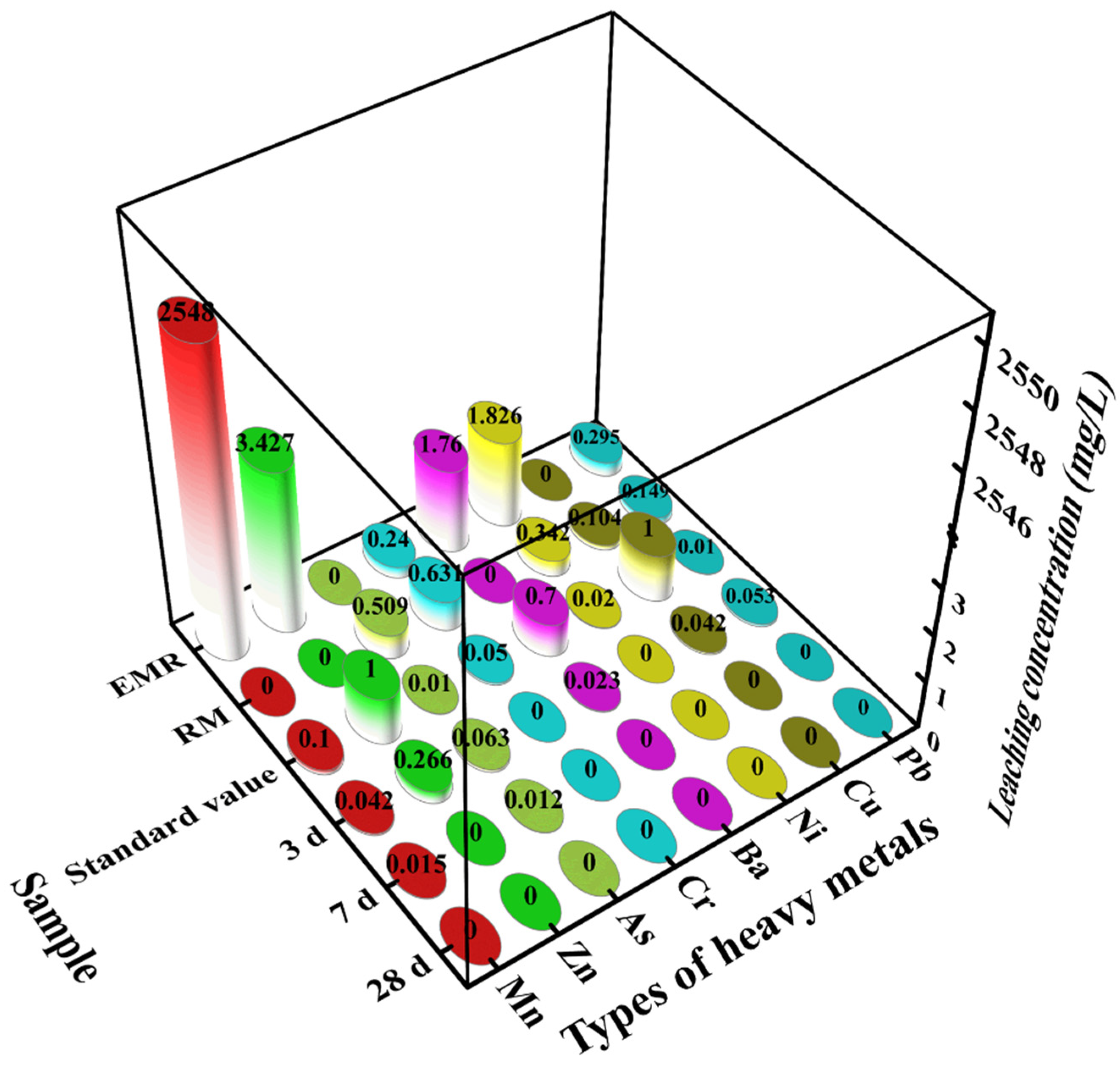
4.9. Toxicity Leaching Test
5. Conclusions
- (1)
- Through optimization of the preparation process of composite cementitious materials, the best preparation process conditions were obtained: the optimal ratio of electrolytic manganese slag was 20%, that of red mud was 15%, that of ground blast furnace slag was 52%, that of calcium hydroxide was 13%, and the optimal water–cementitious ratio was 0.5. Under these conditions, the compressive and flexural strengths of the specimens after 28 days of curing were 27.9 MPa and 7.5 MPa, respectively.
- (2)
- The hydration products in the composite cementitious material are mainly AFt and C-S-H gel, and the RM and EMR mixed in suitable proportions effectively stimulated the activity of GGBS. The combined effect of alkali in red mud and SO42− in EMR, as well as alkali excitation, further promoted the participation of active substances such as SiO2 and Al2O3 in the composite gelling system in the hydration reaction to generate more AFt and C-S-H gels.
- (3)
- By conducting toxic leaching experiments on the composite cementitious material, we found that the composite cementitious material had a significant effect on the curing of heavy metals. The concentrations of all the heavy metals in the leachate of the samples at the age of 28 d did not exceed the standard limit values of the Hygienic Standard for Drinking Water (GB 5749-2022). The results show that the composite gelling system can effectively solidify and stabilize the harmful substances in electrolytic manganese slag and red mud, reducing the harm to the environment, and is thus an environmentally friendly cementitious material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, S.J.; Caldeira, K.; Matth, H.D. Future CO2 Emissions and Climate Change from Existing Energy Infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef]
- Lan, J.; Zhang, S.; Mei, T.; Dong, Y.; Hou, H. Mechanochemical modification of electrolytic manganese residue: Ammonium nitrogen recycling, heavy metal solidification, and baking-free brick preparation. J. Clean. Prod. 2021, 329, 129727. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Y.; Zhang, Z.; Liu, X.; Wang, Y. Harmless treatment of electrolytic manganese residue: Ammonia nitrogen recovery, preparation of struvite and nonsintered bricks. Chem. Eng. J. 2022, 455, 140739. [Google Scholar] [CrossRef]
- Liu, R.; Poon, C.S. Utilization of red mud derived from bauxite in self-compacting concrete. J. Clean. Prod. 2016, 112, 384–391. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Liu, H.; Peng, B. Clean strengthening reduction of lead and zinc from smelting waste slag by iron oxide. J. Clean. Prod. 2017, 143, 311–318. [Google Scholar] [CrossRef]
- Tanvar, H.; Mishra, B. Comprehensive utilization of bauxite residue for simultaneous recovery of base metals and critical elements. Sustain. Mater. Technol. 2022, 33, 466. [Google Scholar] [CrossRef]
- Vilarinho, I.S.; Dias, A.C.; Carneiro, J.; Pinto, C.; Labrincha, J.A.; Seabra, M.P. Red mud valorization in stoneware pastes: Technical and environmental assessment. Sustain. Mater. Technol. 2023, 38, 762. [Google Scholar] [CrossRef]
- Wei, M.; Wang, B.; Wu, P.; Zhang, X.; Chen, M.; Wang, S. Electrolytic manganese residue-biochar composite for simultaneous removal of antimony and arsenic from water: Adsorption performance and mechanisms. J. Clean. Prod. 2024, 437, 140623. [Google Scholar] [CrossRef]
- Wang, K.; Dou, Z.; Liu, Y.; Li, X.; Lv, G.; Zhang, T.A. Summary of research progress on separation and extraction of valuable metals from Bayer red mud. Environ. Sci. Pollut. Res. 2022, 29, 89834–89852. [Google Scholar] [CrossRef]
- Georgiades, M.; Shah, I.H.; Steubing, B.; Cheeseman, C.; Myers, R.J. Prospective life cycle assessment of European cement production. Resour. Conserv. Recycl. 2023, 194, 106998. [Google Scholar] [CrossRef]
- Cheng, X.; Long, D.; Zhang, C.; Gao, X.; Yu, Y.; Mei, K.; Zhang, C.; Guo, X.; Chen, Z. Utilization of red mud, slag and waste drilling fluid for the synthesis of slag-red mud cementitious material. J. Clean. Prod. 2019, 238, 117902. [Google Scholar] [CrossRef]
- Ozden, B.; Brennan, C.; Landsberger, S. Investigation of bauxite residue (red mud) in terms of its environmental risk. J. Radioanal. Nucl. Chem. 2019, 319, 339–346. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.; Wen, Q.; Meegoda, J.N. Mechanical Properties of Geopolymers Synthesized from Fly Ash and Red Mud under Ambient Conditions. Crystals 2019, 9, 572. [Google Scholar] [CrossRef]
- Wu, P.; Liu, X.; Zhang, Z.; Wei, C.; Wang, J.; Gu, J. The harmless and value-added utilization of red mud: Recovering iron from red mud by pyrometallurgy and preparing cementitious materials with its tailings. J. Ind. Eng. Chem. 2024, 132, 50–65. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Zhou, H.; Zhong, X.; Zhou, Z.; Li, J.; Hou, H. Preparation of environmental-friendly cementitious material from red mud and waste glass sludge by mechanical activation. Constr. Build. Mater. 2024, 423, 135861. [Google Scholar] [CrossRef]
- Cui, W.; Liu, J.; Duan, W.; Xie, M.; Li, X.; Dong, X. Study on the synergistic effects and eco-friendly performance of red mud-based quaternary cementitious materials. Constr. Build. Mater. 2024, 428, 136352. [Google Scholar] [CrossRef]
- Wang, J.; Peng, B.; Chai, L.; Zhang, Q.; Liu, Q. Preparation of electrolytic manganese residue–ground granulated blastfurnace slag cement. Powder Technol. 2013, 241, 12–18. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Pu, S.; Cai, G.; Duan, W.; Song, H.; Zeng, C.; Yang, Y. Synergistic preparation of geopolymer using electrolytic manganese residue, coal slag and granulated blast furnace slag. J. Build. Eng. 2024, 91, 109609. [Google Scholar] [CrossRef]
- Li, J.; Sun, P.; Li, J.; Lv, Y.; Ye, H.; Shao, L.; Du, D. Synthesis of electrolytic manganese residue-fly ash based geopolymers with high compressive strength. Constr. Build. Mater. 2020, 248, 118489. [Google Scholar] [CrossRef]
- He, D.; Chen, M.; Liu, H.; Wang, J. Preparation of activated electrolytic manganese residue-slag-cement ternary blended cementitious material: Hydration characteristics and carbon reduction potential. Constr. Build. Mater. 2024, 425, 135990. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Lingyu, T.; Dongpo, H.; Jianing, Z.; Hongguang, W. Durability of geopolymers and geopolymer concretes: A review. Rev. Adv. Mater. Sci. 2021, 60, 1–14. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Liu, X. Hydration mechanism and leaching behavior of bauxite-calcination-method red mud-coal gangue based cementitious materials. J. Hazard. Mater. 2016, 314, 172–180. [Google Scholar] [CrossRef]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Chen, L.; Lin, Z.; Liu, Z.; Zhang, W.; Guo, R. Microstructure and Key Properties of Phosphogypsum-Red Mud-Slag Composite Cementitious Materials. Materials 2022, 15, 6096. [Google Scholar] [CrossRef] [PubMed]
- GB/T 17671-2021; Test Methods for Cement Mortar Strength (ISO Method). Standardization Administration of China: Beijing, China, 2021.
- Li, Y.; Min, X.; Ke, Y.; Liu, D.; Tang, C. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhao, Y.X.; Chen, B. Efflorescence mitigation in fly ash/slag-based geopolymers: The role of precursor composition and proportions, and admixtures. Constr. Build. Mater. 2024, 438, 137216. [Google Scholar] [CrossRef]
- Nie, Q.; Hu, W.; Ai, T.; Huang, B.; Shu, X.; He, Q. Strength properties of geopolymers derived from original and desulfurized red mud cured at ambient temperature. Constr. Build. Mater. 2016, 125, 905–911. [Google Scholar] [CrossRef]
- Choudhary, H.K.; Anupama, A.V.; Kumar, R.; Panzi, M.E.; Matteppanavar, S.; Sherikar, B.N.; Sahoo, B. Observation of phase transformations in cement during hydration. Constr. Build. Mater. 2015, 101, 122–129. [Google Scholar] [CrossRef]
- Wei, X.; Ni, W.; Zhang, S.; Wang, X.; Li, J.; Du, H. Influence of the key factors on the performance of steel slag-desulphurisation gypsum-based hydration-carbonation materials. J. Build. Eng. 2022, 45, 103591. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Qiao, C.; Suraneni, P.; Ying, T.N.W.; Choudhary, A.; Weiss, J. Chloride binding of cement pastes with fly ash exposed to CaCl2 solutions at 5 and 23 °C. Cem. Concr. Compos. 2019, 97, 43–53. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Cao, X.; Liu, Q.; Zang, H. Influence of the combination of calcium oxide and sodium carbonate on the hydration reactivity of alkali-activated slag binders. J. Clean. Prod. 2018, 171, 622–629. [Google Scholar] [CrossRef]
- Kuri, J.C.; Nuruzzaman, M.; Sarker, P.K. Sodium sulphate resistance of geopolymer mortar produced using ground ferronickel slag with fly ash. Ceram. Int. 2023, 49, 2765–2773. [Google Scholar] [CrossRef]
- Zheng, C.; Mao, Z.; Chen, L.; Qian, H.; Wang, J. Development of a novel rapid repairing agent for concrete based on GFRP waste powder/GGBS geopolymer mortars. J. Build. Eng. 2023, 71, 106542. [Google Scholar] [CrossRef]
- Hou, D.; Wu, D.; Wang, X.; Gao, S.; Yu, R.; Li, M.; Wang, P.; Wang, Y. Sustainable use of red mud in ultra-high performance concrete (UHPC): Design and performance evaluation. Cem. Concr. Compos. 2021, 115, 103862. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y.; Qi, Y. Properties of red mud blended with magnesium phosphate cement paste: Feasibility of grouting material preparation. Constr. Build. Mater. 2020, 260, 119704. [Google Scholar] [CrossRef]
- HJ/T 299-2007; Solid Waste—Extraction Procedure for Leaching Toxicity—Sulphuric Acid and Nitric Acid Method. Standardization Administration of China: Beijing, China, 2007.
- GB 5749-2022; Standards for Drinking Water Quality. Standardization Administration of China: Beijing, China, 2022.
- Fan, J.; Yan, J.; Zhou, M.; Xu, Y.; Lu, Y.; Duan, P.; Zhu, Y.; Zhang, Z.; Li, W.; Wang, A.; et al. Heavy metals immobilization of ternary geopolymer based on nickel slag, lithium slag and metakaolin. J. Hazard. Mater. 2023, 453, 131380. [Google Scholar] [CrossRef]
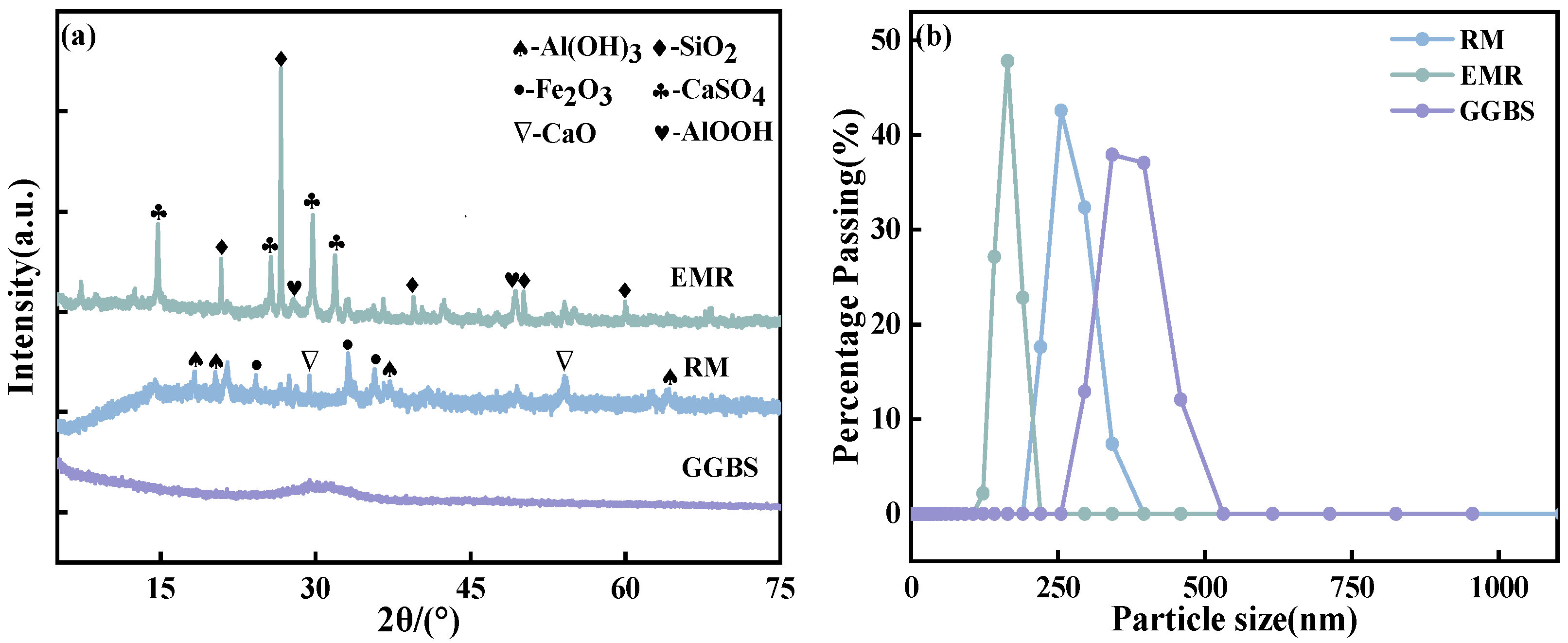
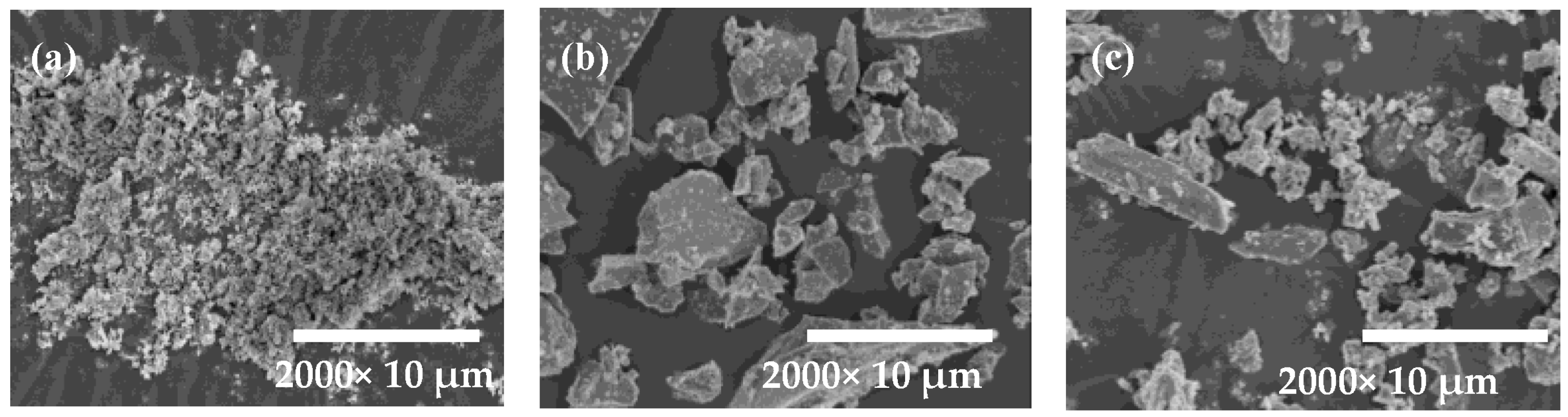
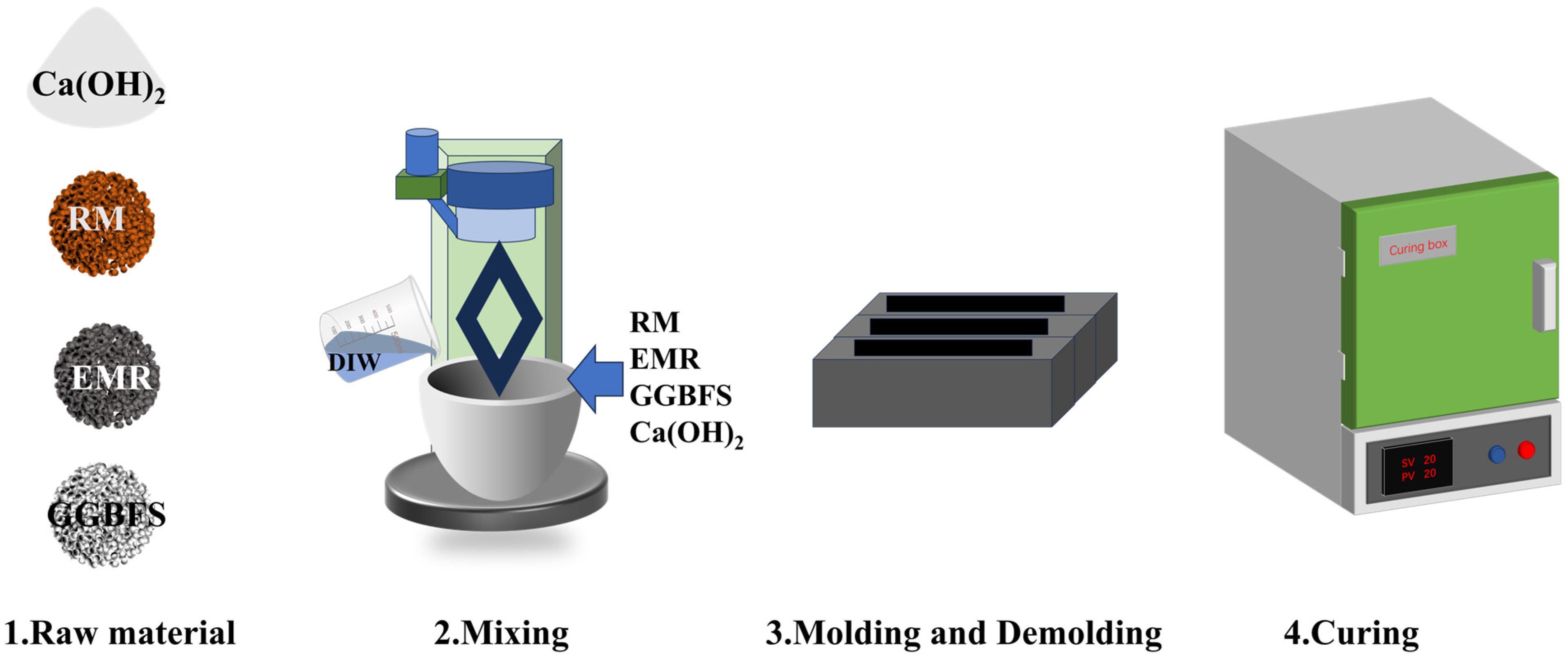


| Raw Material | CaO | SiO2 | SO3 | Al2O3 | Fe2O3 |
|---|---|---|---|---|---|
| GGBS | 49.32 | 30.84 | - | 16.32 | 0.487 |
| RM | 2.64 | 4.17 | - | 22.08 | 63.74 |
| EMR | 17.07 | 22.96 | 36.00 | 1.77 | 13.08 |
| Specimen ID | Mass/% | Water-Binder Ratio | |||
|---|---|---|---|---|---|
| EMR | RM | GGBS | Ca(OH)2 | ||
| 1 | 20 | 5 | 65 | 10 | 0.5 |
| 2 | 20 | 10 | 60 | 10 | 0.5 |
| 3 | 20 | 15 | 55 | 10 | 0.5 |
| 4 | 20 | 20 | 50 | 10 | 0.5 |
| 5 | 20 | 25 | 45 | 10 | 0.5 |
| 6 | 20 | 15 | 61 | 4 | 0.5 |
| 7 | 20 | 15 | 58 | 7 | 0.5 |
| 9 | 20 | 15 | 52 | 13 | 0.5 |
| 10 | 20 | 15 | 49 | 16 | 0.5 |
| 11 | 20 | 15 | 46 | 19 | 0.5 |
| 12 | 20 | 15 | 52 | 13 | 0.55 |
| 13 | 20 | 15 | 52 | 13 | 0.6 |
| 14 | 20 | 15 | 52 | 13 | 0.65 |
| 15 | 20 | 15 | 52 | 13 | 0.7 |
| Area | C | O | Na | Mg | Al | Si | S | K | Ca | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.25 | 60.83 | 0.56 | 4.03 | 5.69 | 8.00 | 1.97 | 0.15 | 12.88 | 0.14 | 0.52 |
| 2 | 13.00 | 61.85 | 1.07 | 1.64 | 3.51 | 4.26 | 2.24 | 0.16 | 9.90 | 0.19 | 2.18 |
| 3 | 9.80 | 68.19 | 0.64 | 2.79 | 3.53 | 3.50 | 1.38 | 0.13 | 7.66 | 0.26 | 2.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Wang, L.; Li, Z.; Xu, Y.; Zhang, W.; Li, Y. Basic Research on the Preparation of Electrolytic Manganese Residue–Red Mud–Ground Granulated Blast Furnace Slag–Calcium Hydroxide Composite Cementitious Material and Its Mechanical Properties. Materials 2025, 18, 1218. https://doi.org/10.3390/ma18061218
Peng B, Wang L, Li Z, Xu Y, Zhang W, Li Y. Basic Research on the Preparation of Electrolytic Manganese Residue–Red Mud–Ground Granulated Blast Furnace Slag–Calcium Hydroxide Composite Cementitious Material and Its Mechanical Properties. Materials. 2025; 18(6):1218. https://doi.org/10.3390/ma18061218
Chicago/Turabian StylePeng, Biao, Lusen Wang, Zhonglin Li, Ye Xu, Weiguang Zhang, and Yibing Li. 2025. "Basic Research on the Preparation of Electrolytic Manganese Residue–Red Mud–Ground Granulated Blast Furnace Slag–Calcium Hydroxide Composite Cementitious Material and Its Mechanical Properties" Materials 18, no. 6: 1218. https://doi.org/10.3390/ma18061218
APA StylePeng, B., Wang, L., Li, Z., Xu, Y., Zhang, W., & Li, Y. (2025). Basic Research on the Preparation of Electrolytic Manganese Residue–Red Mud–Ground Granulated Blast Furnace Slag–Calcium Hydroxide Composite Cementitious Material and Its Mechanical Properties. Materials, 18(6), 1218. https://doi.org/10.3390/ma18061218






