New Three Dimensional-Printed Polyethylene Terephthalate Glycol Liners for Hip Joint Endoprostheses: A Bioactive Platform for Bone Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Scaffold Fabrication and the Synthesis of Biocomposite Scaffold Materials
2.2.2. Physicochemical Characterization Techniques
2.2.3. Assessment of Biocompatibility Using In Vitro Testing
MTT Assay
Live/Dead Test
LDH Assay
Griess Test
3. Results
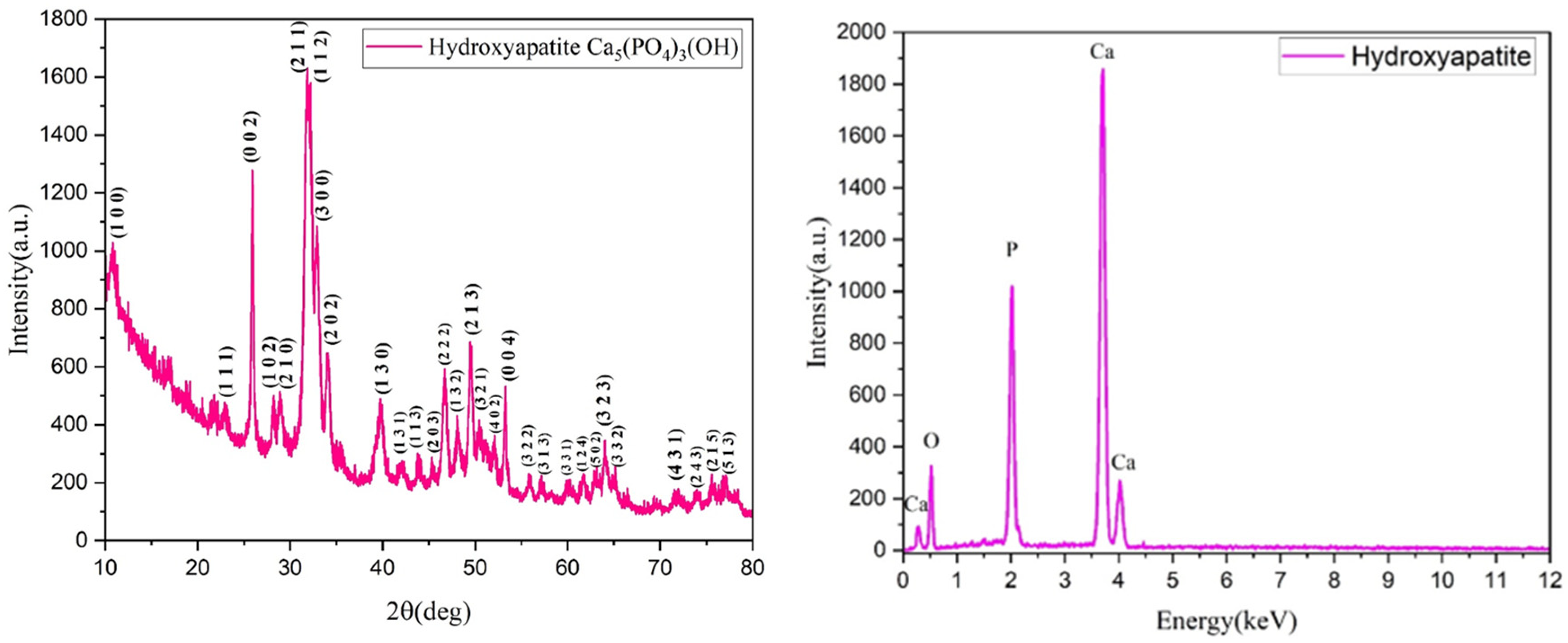
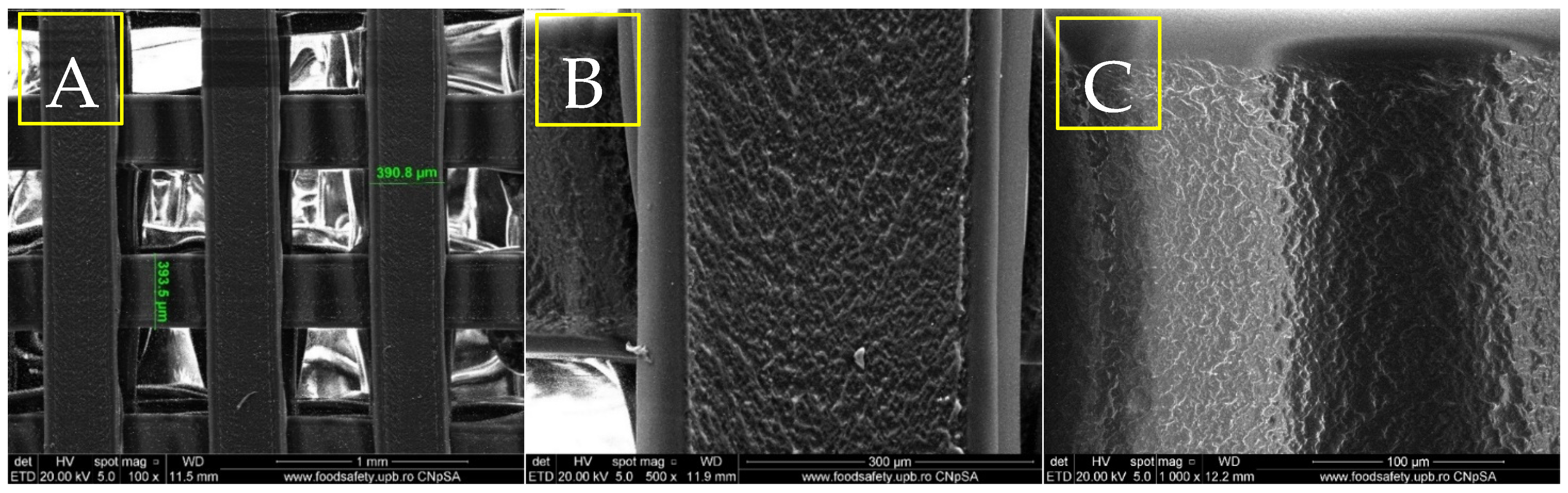
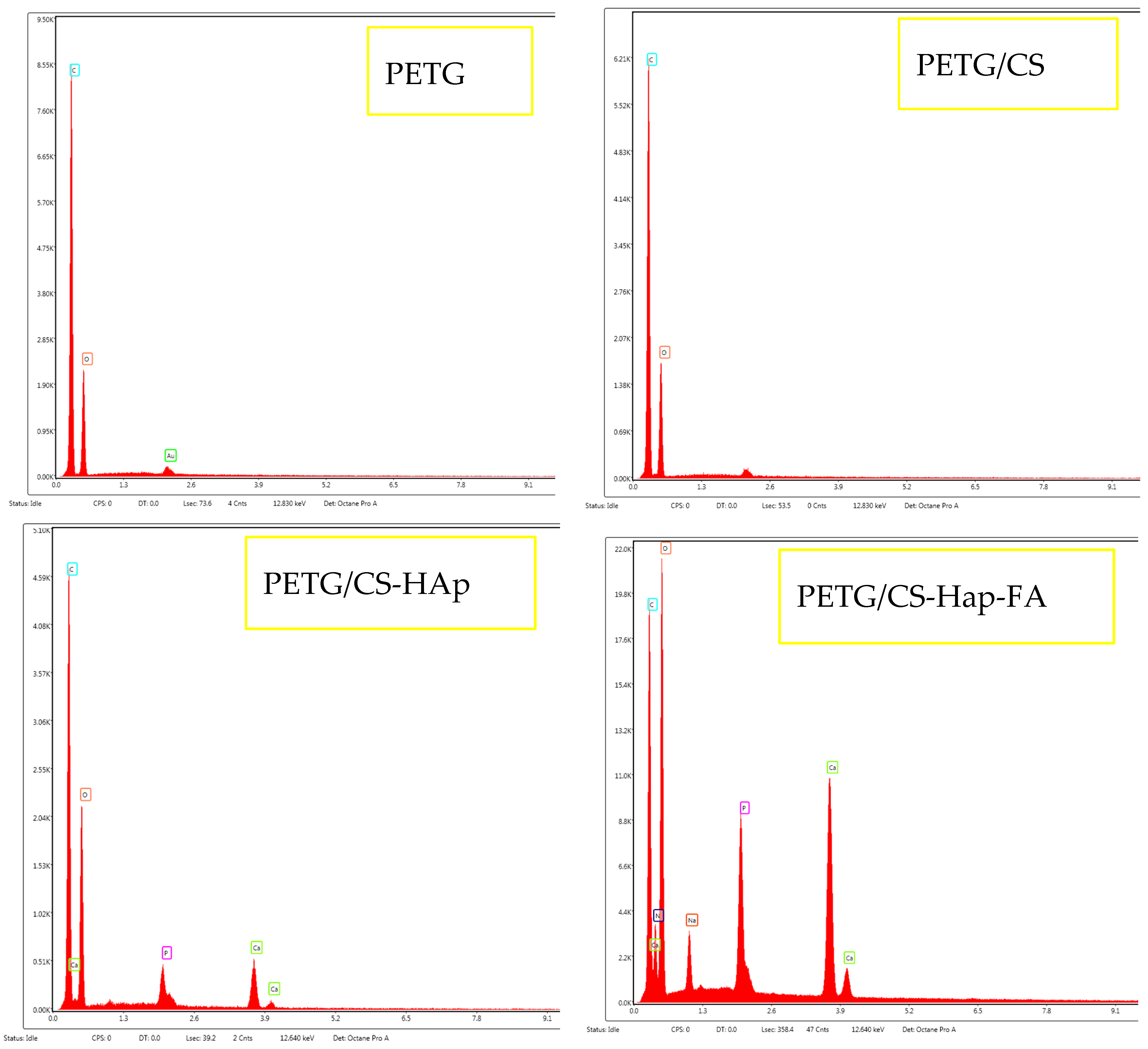
3.1. Phisicochemical and Morphological Characteristics of the HAp Powder and of the Fabricated PETG-Based Scaffolds
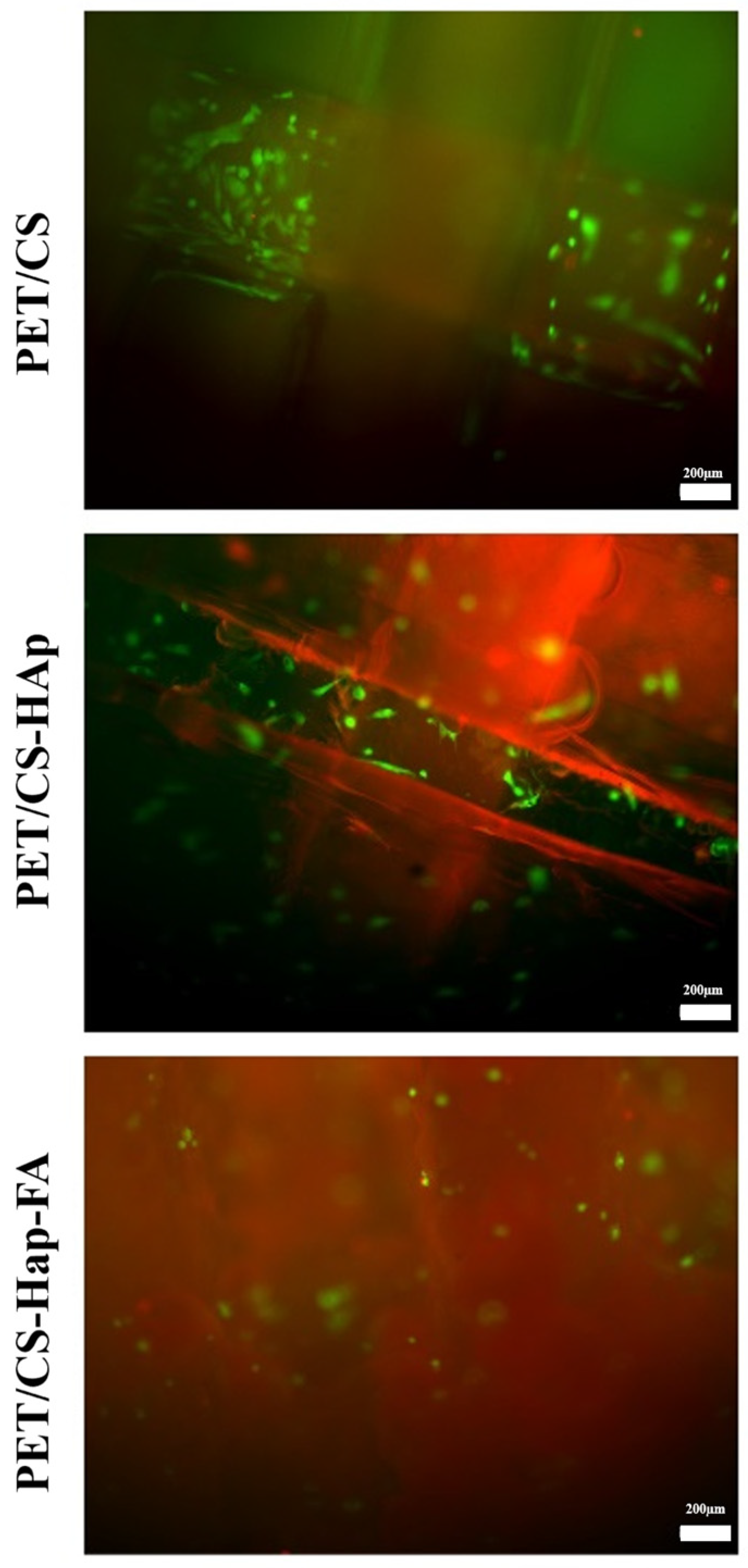
3.2. Biological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue engineering: Current strategies and future directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A.; Zhang, Q. 3D printing method for bone tissue engineering scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef]
- Zarei, M.; Shabani Dargah, M.; Hasanzadeh Azar, M.; Alizadeh, R.; Mahdavi, F.S.; Sayedain, S.S.; Kaviani, A.; Asadollahi, M.; Azami, M.; Beheshtizadeh, N. Enhanced bone tissue regeneration using a 3D-printed poly(lactic acid)/Ti6Al4V composite scaffold with plasma treatment modification. Sci. Rep. 2023, 13, 3139. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Francipane, M.G.; Lagasse, E. Toward Organs on Demand: Breakthroughs and Challenges in Models of Organogenesis. Curr. Pathobiol. Rep. 2016, 4, 77–85. [Google Scholar] [CrossRef][Green Version]
- Yuan, X.; Zhu, W.; Yang, Z.; He, N.; Chen, F.; Han, X.; Zhou, K. Recent Advances in 3D Printing of Smart Scaffolds for Bone Tissue Engineering and Regeneration. Adv. Mater. 2024, 36, 2403641. [Google Scholar] [CrossRef]
- Seol, Y.J.; Park, D.Y.; Park, J.Y.; Kim, S.W.; Park, S.J.; Cho, D.W. A new method of fabricating robust freeform 3D ceramic scaffolds for bone tissue regeneration. Biotechnol. Bioeng. 2013, 110, 1444–1455. [Google Scholar] [CrossRef]
- Buradagunta, R.S.; Madiga, J.; Dumpala, R. Producing Calcium Deficient Nano-hydroxyapatite from Silver pomfret Fish Bones for Biomedical Applications. Lett. Appl. NanoBioScience 2024, 13, 130. [Google Scholar] [CrossRef]
- Hutmacher, D.; Woodfield, T.; Dalton, P.; Lewis, J. Scaffold design and fabrication. In Tissue Engineering; van Blitterswijk, C.A., Thomsen, P., Lindahl, A., Hubbell, J., Williams, D.F., Cancedda, R., Eds.; Chapter 14; Academic Press: Burlington, NJ, USA, 2008; pp. 403–454. [Google Scholar]
- Aslam Khan, M.U.; Mehboob, H.; Abd Razak, S.I.; Yahya, M.Y.; Mohd Yusof, A.H.; Ramlee, M.H.; Sahaya Anand, T.J.; Hassan, R.; Aziz, A.; Amin, R. Development of Polymeric Nanocomposite (Xyloglucan-co-Methacrylic Acid/Hydroxyapatite/SiO2) Scaffold for Bone Tissue Engineering Applications—In-Vitro Antibacterial, Cytotoxicity and Cell Culture Evaluation. Polymers 2020, 12, 1238. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Kocak-Oztug, N.A.; Sheikh, F.A.; Han, P.; Anwar, S.; Fournier, B.P.J.; Ivanovski, S. Fabrication of 3D bioactive melt electrowriting composite scaffold with high osteogenic potential. Colloids Surf. B Biointerfaces 2025, 245, 114270. [Google Scholar] [CrossRef]
- Aijaz, M.; Ahmad, M.; Ansari, M.A.; Ahmad, S.; Kumar, A. Tools and Techniques Used for the Development of Scaffold for Bone Tissue Regeneration: A Detailed Review. Biointerface Res. Appl. Chem. 2024, 14, 123. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. New Perspectives of Hydrogels in Chronic Wound Management. Molecules 2025, 30, 686. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef] [PubMed]
- Blokhuis, T.J.; Arts, J.J.C. Bioactive and osteoinductive bone graft substitutes: Definitions, facts and myths. Injury 2011, 42, S26–S29. [Google Scholar] [CrossRef] [PubMed]
- Brie, I.-C.; Soritau, O.; Dirzu, N.; Berce, C.; Vulpoi, A.; Popa, C.; Todea, M.; Simon, S.; Perde-Schrepler, M.; Virag, P.; et al. Comparative in vitro study regarding the biocompatibility of titanium-base composites infiltrated with hydroxyapatite or silicatitanate. J. Biol. Eng. 2014, 8, 14. [Google Scholar] [CrossRef]
- Daculsi, G.; Fellah, B.H.; Miramond, T.; Durand, M. Osteoconduction, Osteogenicity, Osteoinduction, what are the fundamental properties for a smart bone substitutes. IRBM 2013, 34, 346–348. [Google Scholar] [CrossRef]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef]
- Es-Saddik, M.; Laasri, S.; Bensemlali, M.; Hariti, N.; Laghzizil, A.; Taha, M. Experimental and Finite Element Study of the Mechanical Behavior of Hydroxyapatite/Tricalcium Phosphate/Alumina Biocomposite. Biointerface Res. Appl. Chem. 2024, 14, 37. [Google Scholar] [CrossRef]
- Egan, P.F.; Ferguson, S.J.; Shea, K. Design of Hierarchical Three-Dimensional Printed Scaffolds Considering Mechanical and Biological Factors for Bone Tissue Engineering. J. Mech. Des. 2017, 139, 061401. [Google Scholar] [CrossRef]
- Soleymani, S.; Naghib, S.M. 3D and 4D printing hydroxyapatite-based scaffolds for bone tissue engineering and regeneration. Heliyon 2023, 9, e19363. [Google Scholar] [CrossRef]
- Podgorbunsky, A.B.; Imshinetskiy, I.M.; Mashtalyar, D.V.; Sidorova, M.V.; Gnedenkov, A.S.; Sinebryukhov, S.L.; Gnedenkov, S.V. Bioresorbable composites based on magnesium and hydroxyapatite for use in bone tissue engineering: Focus on controlling and minimizing corrosion activity. Ceram. Int. 2025, 51, 423–436. [Google Scholar] [CrossRef]
- Chopra, V.; Fuentes-Velasco, V.; Nacif-Lopez, S.R.; Melendez-Malpicca, J.; Mendez-Hernandez, A.S.; Ramos-Mendez-Iris, L.F.; Arroyo-Jimenez, D.A.; Reyes-Segura, D.G.; Gonzalez-Y-Mendoza, P.; Sanchez-Hernandez, K.A.; et al. Advancements in 3D-4D printing of hydroxyapatite composites for bone tissue engineering. Ceram. Int. 2024, 50, 38819–38840. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, M.; Jiang, Y.; Zhang, X.; Li, J.; Zhu, Y.; Yao, Q. Calcium-based biomaterials: Unveiling features and expanding applications in osteosarcoma treatment. Bioact. Mater. 2024, 32, 385–399. [Google Scholar] [CrossRef]
- Hess, U.; Shahabi, S.; Treccani, L.; Streckbein, P.; Heiss, C.; Rezwan, K. Co-delivery of cisplatin and doxorubicin from calcium phosphate beads/matrix scaffolds for osteosarcoma therapy. Mater. Sci. Eng. C 2017, 77, 427–435. [Google Scholar] [CrossRef]
- Zhou, Z.-F.; Sun, T.-W.; Chen, F.; Zuo, D.-Q.; Wang, H.-S.; Hua, Y.-Q.; Cai, Z.-D.; Tan, J. Calcium phosphate-phosphorylated adenosine hybrid microspheres for anti-osteosarcoma drug delivery and osteogenic differentiation. Biomaterials 2017, 121, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Grămadă, A.M.; Niculescu, A.-G.; Bîrcă, A.C.; Holban, A.M.; Ciceu, A.; Balta, C.; Herman, H.; Hermenean, A.; Stoica, A.-E.; Ardelean, S.; et al. In Vitro and In Vivo Evaluation of rPET/Cu-Alg Nanofibers for Anti-Infective Therapy. Polymers 2025, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Grămadă, A.M.; Stoica, A.-E.; Niculescu, A.-G.; Bîrcă, A.C.; Vasile, B.Ș.; Holban, A.M.; Mihaiescu, T.; Șerban, A.I.; Ciceu, A.; Balta, C.; et al. Zinc Oxide-Loaded Recycled PET Nanofibers for Applications in Healthcare and Biomedical Devices. Polymers 2025, 17, 45. [Google Scholar] [CrossRef]
- Cai, J.; Ai, C.; Chen, J.; Chen, S. Biomineralizaion of hydroxyapatite on polyethylene terephthalate artificial ligaments promotes graft-bone healing after anterior cruciate ligament reconstruction: An in vitro and in vivo study. J. Biomater. Appl. 2020, 35, 193–204. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Q.; Chen, J.; Jiang, J.; Mo, X.; He, C.; Zhao, J. Electrodeposition of calcium phosphate onto polyethylene terephthalate artificial ligament enhances graft-bone integration after anterior cruciate ligament reconstruction. Bioact. Mater. 2021, 6, 783–793. [Google Scholar] [CrossRef]
- Tai, C.C.; Huang, C.C.; Chou, B.H.; Chen, C.Y.; Chen, S.Y.; Huang, Y.H.; Sun, J.S.; Chao, Y.H. Profiled polyethylene terephthalate filaments that incorporate collagen and calcium phosphate enhance ligamentisation and bone formation. Eur. Cell Mater. 2022, 43, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Quispe-López, N.; Gómez-Polo, C.; Zubizarreta-Macho, Á.; Montero, J. How do the dimensions of peri-implant mucosa affect marginal bone loss in equicrestal and subcrestal position of implants? A 1-year clinical trial. Clin. Implant Dent. Relat. Res. 2024, 26, 442–456. [Google Scholar] [CrossRef]
- Meyer, D.C.; Bachmann, E.; Darwiche, S.; Moehl, A.; von Rechenberg, B.; Gerber, C.; Snedeker, J.G. Rotator Cuff Repair and Overlay Augmentation by Direct Interlocking of a Nonwoven Polyethylene Terephthalate Patch Into the Tendon: Evaluation in an Ovine Model. Am. J. Sports Med. 2023, 51, 3235–3242. [Google Scholar] [CrossRef]
- Cowling, P.; Hackney, R.; Dube, B.; Grainger, A.J.; Biglands, J.D.; Stanley, M.; Song, D.; Conaghan, P.G.; Kingsbury, S.R. The use of a synthetic shoulder patch for large and massive rotator cuff tears—A feasibility study. BMC Musculoskelet. Disord. 2020, 21, 213. [Google Scholar] [CrossRef] [PubMed]
- Smolen, D.; Haffner, N.; Mittermayr, R.; Hess, F.; Sternberg, C.; Leuzinger, J. Application of a new polyester patch in arthroscopic massive rotator cuff repair—A prospective cohort study. J. Shoulder Elb. Surg. 2020, 29, e11–e21. [Google Scholar] [CrossRef]
- Prasad, S.G.; Lal, C.; Sahu, K.R.; De, U. Modification of Optical Bandgap and Formation of Carbonaceous Clusters Due to 1.75 MeV N5+ Ion Irradiation in PET Polymers and Search for Chemical Reaction Mechanisms. Biointerface Res. Appl. Chem. 2023, 13, 35. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chuang, Y.-H.; Chen, C.-M.; Wang, J.-Y.; Wang, J. Development of hybrid scaffolds with biodegradable polymer composites and bioactive hydrogels for bone tissue engineering. Biomater. Adv. 2023, 153, 213562. [Google Scholar] [CrossRef] [PubMed]
- El-Bahrawy, N.R.; Elgharbawy, H.; Elmekawy, A.; Salem, M.; Morsy, R. Development of porous hydroxyapatite/PVA/gelatin/alginate hybrid flexible scaffolds with improved mechanical properties for bone tissue engineering. Mater. Chem. Phys. 2024, 319, 129332. [Google Scholar] [CrossRef]
- Khodabandeh, A.; Yousefi, A.A.; Jafarzadeh-Holagh, S.; Vasheghani-Farahani, E. Fabrication of 3D microfibrous composite polycaprolactone/hydroxyapatite scaffolds loaded with piezoelectric poly (lactic acid) nanofibers by sequential near-field and conventional electrospinning for bone tissue engineering. Biomater. Adv. 2025, 166, 214053. [Google Scholar] [CrossRef]
- Nnamani, P.; Kenechukwu, F.; Okoye, C.; Attama, A. Surface-engineered Oral Sub-micron Particles and Topical Microgels Matrixed with Transcutol-HP/Capra hircus Composite for Amplification of Piroxicam Delivery and Anti-inflammatory Activity. Lett. Appl. NanoBioScience 2024, 13, 15. [Google Scholar] [CrossRef]
- Soetaredjo, F.E.; Ismadji, S.; Santoso, S.P.; Putro, J.N.; Foe, K.; Waworuntu, G.L. pH-Sensitive CNC-Chitosan Hydrogel for Drug Delivery. Lett. Appl. NanoBioScience 2024, 13, 168. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.-I.; Niculescu, A.-G.; Grumezescu, A.M. Advancements in Wound Dressing Materials: Highlighting Recent Progress in Hydrogels, Foams, and Antimicrobial Dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Koyyada, A.; Orsu, P. Recent Advancements and Associated Challenges of Scaffold Fabrication Techniques in Tissue Engineering Applications. Regen. Eng. Transl. Med. 2021, 7, 147–159. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Smith, J.A.; Mele, E. Electrospinning and Additive Manufacturing: Adding Three-Dimensionality to Electrospun Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 674738. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ghadikolaei, M.; Vasheghani-Farahani, E.; Bagheri, F.; Khorrami Moghaddam, A.; Mellati, A.; Karimizade, A. Fabrication of 3D chitosan/polyvinyl alcohol/brushite nanofibrous scaffold for bone tissue engineering by electrospinning using a novel falling film collector. Int. J. Biol. Macromol. 2024, 272, 132874. [Google Scholar] [CrossRef]
- Autissier, A.; Visage, C.L.; Pouzet, C.; Chaubet, F.; Letourneur, D. Fabrication of porous polysaccharide-based scaffolds using a combined freeze-drying/cross-linking process. Acta Biomater. 2010, 6, 3640–3648. [Google Scholar] [CrossRef]
- Vigani, B.; Ianev, D.; Adami, M.; Valentino, C.; Ruggeri, M.; Boselli, C.; Icaro Cornaglia, A.; Sandri, G.; Rossi, S. Porous Functionally Graded Scaffold prepared by a single-step freeze-drying process. A bioinspired approach for wound care. Int. J. Pharm. 2024, 656, 124119. [Google Scholar] [CrossRef]
- Schantz, J.T.; Brandwood, A.; Hutmacher, D.W.; Khor, H.L.; Bittner, K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J. Mater. Sci. Mater. Med. 2005, 16, 807–819. [Google Scholar] [CrossRef]
- Xu, N.; Ye, X.; Wei, D.; Zhong, J.; Chen, Y.; Xu, G.; He, D. 3D Artificial Bones for Bone Repair Prepared by Computed Tomography-Guided Fused Deposition Modeling for Bone Repair. ACS Appl. Mater. Interfaces 2014, 6, 14952–14963. [Google Scholar] [CrossRef]
- Chen, C.-H.; Shyu, V.B.-H.; Chen, J.-P.; Lee, M.-Y. Selective laser sintered poly-ε-caprolactone scaffold hybridized with collagen hydrogel for cartilage tissue engineering. Biofabrication 2014, 6, 015004. [Google Scholar] [CrossRef]
- Roskies, M.G.; Fang, D.; Abdallah, M.-N.; Charbonneau, A.M.; Cohen, N.; Jordan, J.O.; Hier, M.P.; Mlynarek, A.; Tamimi, F.; Tran, S.D. Three-dimensionally printed polyetherketoneketone scaffolds with mesenchymal stem cells for the reconstruction of critical-sized mandibular defects. Laryngoscope 2017, 127, E392–E398. [Google Scholar] [CrossRef] [PubMed]
- Wanibuchi, M.; Noshiro, S.; Sugino, T.; Akiyama, Y.; Mikami, T.; Iihoshi, S.; Miyata, K.; Komatsu, K.; Mikuni, N. Training for Skull Base Surgery with a Colored Temporal Bone Model Created by Three-Dimensional Printing Technology. World Neurosurg. 2016, 91, 66–72. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Chu, H.; Yang, W.; Sun, L.; Cai, S.; Yang, R.; Liang, W.; Yu, H.; Liu, L. 4D Printing: A Review on Recent Progresses. Micromachines 2020, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Salari, P.; Abdollahi, M.; Heshmat, R.; Meybodi, H.A.; Razi, F. Effect of folic acid on bone metabolism: A randomized double blind clinical trial in postmenopausal osteoporotic women. DARU J. Pharm. Sci. 2014, 22, 62. [Google Scholar] [CrossRef] [PubMed]
- Mariangela, R.; Alice, T.; Federica, F.; Viviana, V.; Milena Anna, F.; Maurizio, N.; Simone, P.; Mara, N.; Gabriella, P. Adequate Intake and Supplementation of B Vitamins, in Particular Folic Acid, can Play a Protective Role in Bone Health. Curr. Aging Sci. 2022, 15, 110–120. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, L.; Wang, X.; Zhang, Y. Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: A systematic review and meta-analysis. Arch. Osteoporos. 2021, 16, 4. [Google Scholar] [CrossRef]
- Mrozikiewicz, A.; Bogacz, A.; Barlik, M.; Górska, A.; Wolek, M.; Kalak, M. The role of folate receptor and reduced folate carrier polymorphisms in osteoporosis development. Herba Pol. 2019, 65, 30–36. [Google Scholar] [CrossRef]
- Burdusel, A.-C.; Neacsu, I.A.; Birca, A.C.; Chircov, C.; Grumezescu, A.-M.; Holban, A.M.; Curutiu, C.; Ditu, L.M.; Stan, M.; Andronescu, E. Microwave-Assisted Hydrothermal Treatment of Multifunctional Substituted Hydroxyapatite with Prospective Applications in Bone Regeneration. J. Funct. Biomater. 2023, 14, 378. [Google Scholar] [CrossRef]
- Ibrahim, I.; Ashour, A.G.; Zeiada, W.; Salem, N.; Abdallah, M. A Systematic Review on the Technical Performance and Sustainability of 3D Printing Filaments Using Recycled Plastic. Sustainability 2024, 16, 8247. [Google Scholar] [CrossRef]
- Loskot, J.; Jezbera, D.; Loskot, R.; Bušovský, D.; Barylski, A.; Glowka, K.; Duda, P.; Aniołek, K.; Voglová, K.; Zubko, M. Influence of print speed on the microstructure, morphology, and mechanical properties of 3D-printed PETG products. Polym. Test. 2023, 123, 108055. [Google Scholar] [CrossRef]
- Michailidis, N.; Petousis, M.; Saltas, V.; Papadakis, V.; Spiridaki, M.; Mountakis, N.; Argyros, A.; Valsamos, J.; Nasikas, N.K.; Vidakis, N. Investigation of the Effectiveness of Silicon Nitride as a Reinforcement Agent for Polyethylene Terephthalate Glycol in Material Extrusion 3D Printing. Polymers 2024, 16, 1043. [Google Scholar] [CrossRef]
- Van’T Hof, R.J.; Ralston, S.H. Nitric oxide and bone. Immunology 2001, 103, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Rezaiian, F.; Saadati, S.; Naseri, K.; Ashtary-Larky, D.; Yousefi, M.; Golalipour, E.; Clark, C.C.T.; Rastgoo, S.; Asbaghi, O. The effects of folic acid supplementation on endothelial function in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Nutr. J. 2023, 22, 12. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, W.; Zhang, W.; Hong, N.; Liao, Y.; Yao, Y.; Cai, L.; Xiong, C.; Yao, L. A joint experimental and theoretical investigation of the binding mechanism between zein and folic acid in an ethanol–water solution. Int. J. Food Prop. 2024, 27, 729–749. [Google Scholar] [CrossRef]
- Liu, H.; Rosen, C.J. Nitric oxide and bone: The phoenix rises again. J. Clin. Investig. 2021, 131, e147072. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, T.; Zhang, Y.; Zhao, Y.; Shi, X.; Zhang, Q. Biomimetic mineralized hierarchical hybrid scaffolds based on in situ synthesis of nano-hydroxyapatite/chitosan/chondroitin sulfate/hyaluronic acid for bone tissue engineering. Colloids Surf. B Biointerfaces 2017, 157, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.R.; El-Turki, A.; Ramakrishna, S.; Su, B.; Lim, C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials 2008, 29, 4314–4322. [Google Scholar] [CrossRef]
- Wang, H.; Sun, R.; Huang, S.; Wu, H.; Zhang, D. Fabrication and properties of hydroxyapatite/chitosan composite scaffolds loaded with periostin for bone regeneration. Heliyon 2024, 10, e25832. [Google Scholar] [CrossRef]
- Nazeer, M.A.; Yilgör, E.; Yilgör, I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr. Polym. 2017, 175, 38–46. [Google Scholar] [CrossRef]
- Santos, C.; Gomes, P.; Duarte, J.A.; Almeida, M.M.; Costa, M.E.V.; Fernandes, M.H. Development of hydroxyapatite nanoparticles loaded with folic acid to induce osteoblastic differentiation. Int. J. Pharm. 2017, 516, 185–195. [Google Scholar] [CrossRef]
- Shi, W.; Hatori, S.; Noda, D.; Yamada, I.; Tagaya, M. Direct Immobilization of Folic Acid Molecules on Hydroxyapatite Nanoparticles with Substitution and Coordination Phenomena. ACS Biomater. Sci. Eng. 2024, 10, 6615–6624. [Google Scholar] [CrossRef]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Hou, Y.; Huang, B.; Strashnov, I.; Grieve, B.D.; Bártolo, P. The Potential of Polyethylene Terephthalate Glycol as Biomaterial for Bone Tissue Engineering. Polymers 2020, 12, 3045. [Google Scholar] [CrossRef] [PubMed]
- Çaykara, T.; Sande, M.G.; Azoia, N.; Rodrigues, L.R.; Silva, C.J. Exploring the potential of polyethylene terephthalate in the design of antibacterial surfaces. Med. Microbiol. Immunol. 2020, 209, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Sughanthy, S.A.P.; Ansari, M.N.M.; Atiqah, A. Dynamic mechanical analysis of polyethylene terephthalate/hydroxyapatite biocomposites for tissue engineering applications. J. Mater. Res. Technol. 2020, 9, 2350–2356. [Google Scholar] [CrossRef]
- Dupaix, R.B.; Boyce, M.C. Finite strain behavior of poly(ethylene terephthalate) (PET) and poly(ethylene terephthalate)-glycol (PETG). Polymer 2005, 46, 4827–4838. [Google Scholar] [CrossRef]
- Yan, C.; Kleiner, C.; Tabigue, A.; Shah, V.; Sacks, G.; Shah, D.; DeStefano, V. PETG: Applications in Modern Medicine. Eng. Regen. 2024, 5, 45–55. [Google Scholar] [CrossRef]
- Martins, R.F.; Branco, R.; Martins, M.; Macek, W.; Marciniak, Z.; Silva, R.; Trindade, D.; Moura, C.; Franco, M.; Malça, C. Mechanical Properties of Additively Manufactured Polymeric Materials—PLA and PETG—For Biomechanical Applications. Polymers 2024, 16, 1868. [Google Scholar] [CrossRef]
- Soleyman, E.; Aberoumand, M.; Rahmatabadi, D.; Soltanmohammadi, K.; Ghasemi, I.; Baniassadi, M.; Abrinia, K.; Baghani, M. Assessment of controllable shape transformation, potential applications, and tensile shape memory properties of 3D printed PETG. J. Mater. Res. Technol. 2022, 18, 4201–4215. [Google Scholar] [CrossRef]
- Jiang, J.; Hao, W.; Li, Y.-z.; Chen, J.; Yao, J.-r.; Shao, Z.-z.; Li, H.; Yang, J.-j.; Chen, S.-y. Biocompatibility evaluation of polyethylene terephthalate artificial ligament coating hydroxyapatite by fibroblasts cells in vitro. J. Shanghai Jiaotong Univ. Sci. 2012, 17, 717–722. [Google Scholar] [CrossRef]
- Li, H.; Ge, Y.; Wu, Y.; Jiang, J.; Gao, K.; Zhang, P.; Wu, L.; Chen, S. Hydroxyapatite coating enhances polyethylene terephthalate artificial ligament graft osseointegration in the bone tunnel. Int. Orthop. 2011, 35, 1561–1567. [Google Scholar] [CrossRef]
- Sreeja, S.; Muraleedharan, C.V.; Varma, P.R.H.; Sailaja, G.S. Surface-transformed osteoinductive polyethylene terephthalate scaffold as a dual system for bone tissue regeneration with localized antibiotic delivery. Mater. Sci. Eng. C 2020, 109, 110491. [Google Scholar] [CrossRef]
- Strähle, U.T.; Pütz, N.; Hannig, M. A coating machine for coating filaments with bioactive nanomaterials for extrusion 3D printing. Heliyon 2024, 10, e33223. [Google Scholar] [CrossRef] [PubMed]
- Durgashyam, K.; Indra Reddy, M.; Balakrishna, A.; Satyanarayana, K. Experimental investigation on mechanical properties of PETG material processed by fused deposition modeling method. Mater. Today Proc. 2019, 18, 2052–2059. [Google Scholar] [CrossRef]
- Srinivasan, R.; Ruban, W.; Deepanraj, A.; Bhuvanesh, R.; Bhuvanesh, T. Effect on infill density on mechanical properties of PETG part fabricated by fused deposition modelling. Mater. Today Proc. 2020, 27, 1838–1842. [Google Scholar] [CrossRef]
- Lakshman Sri, S.V.; Kartchick, A.; Dinesch, C. Evaluation of mechanical properties of 3D printed PETG and Polyamide (6) polymers. Chem. Phys. Impact 2024, 8, 100491. [Google Scholar] [CrossRef]
- Ronca, A.; Abbate, V.; Redaelli, D.F.; Storm, F.A.; Cesaro, G.; De Capitani, C.; Sorrentino, A.; Colombo, G.; Fraschini, P.; Ambrosio, L. A Comparative Study for Material Selection in 3D Printing of Scoliosis Back Brace. Materials 2022, 15, 5724. [Google Scholar] [CrossRef]
- Cagnacci, A.; Bagni, B.; Zini, A.; Cannoletta, M.; Generali, M.; Volpe, A. Relation of folates, vitamin B12 and homocysteine to vertebral bone mineral density change in postmenopausal women. A five-year longitudinal evaluation. Bone 2008, 42, 314–320. [Google Scholar] [CrossRef]




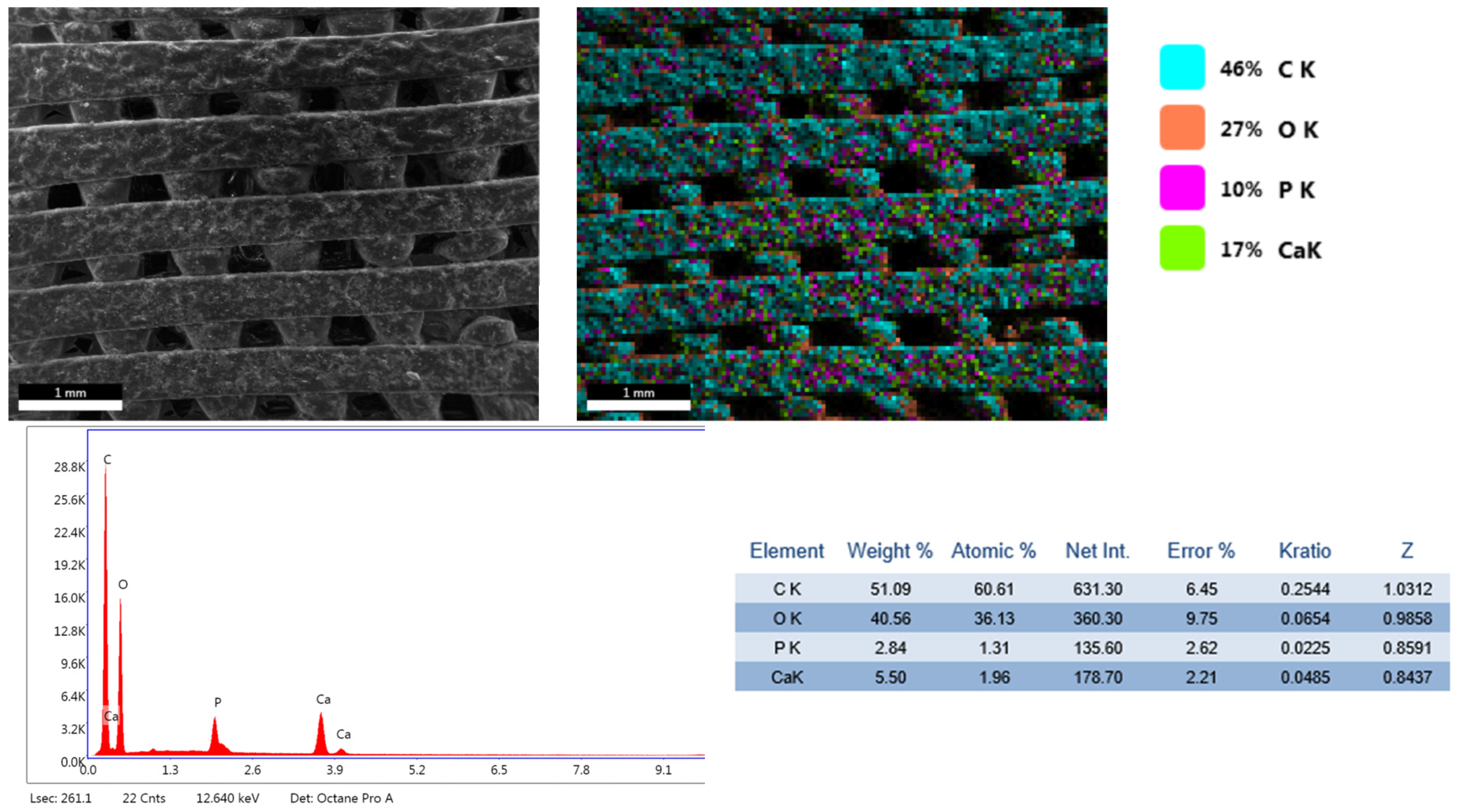
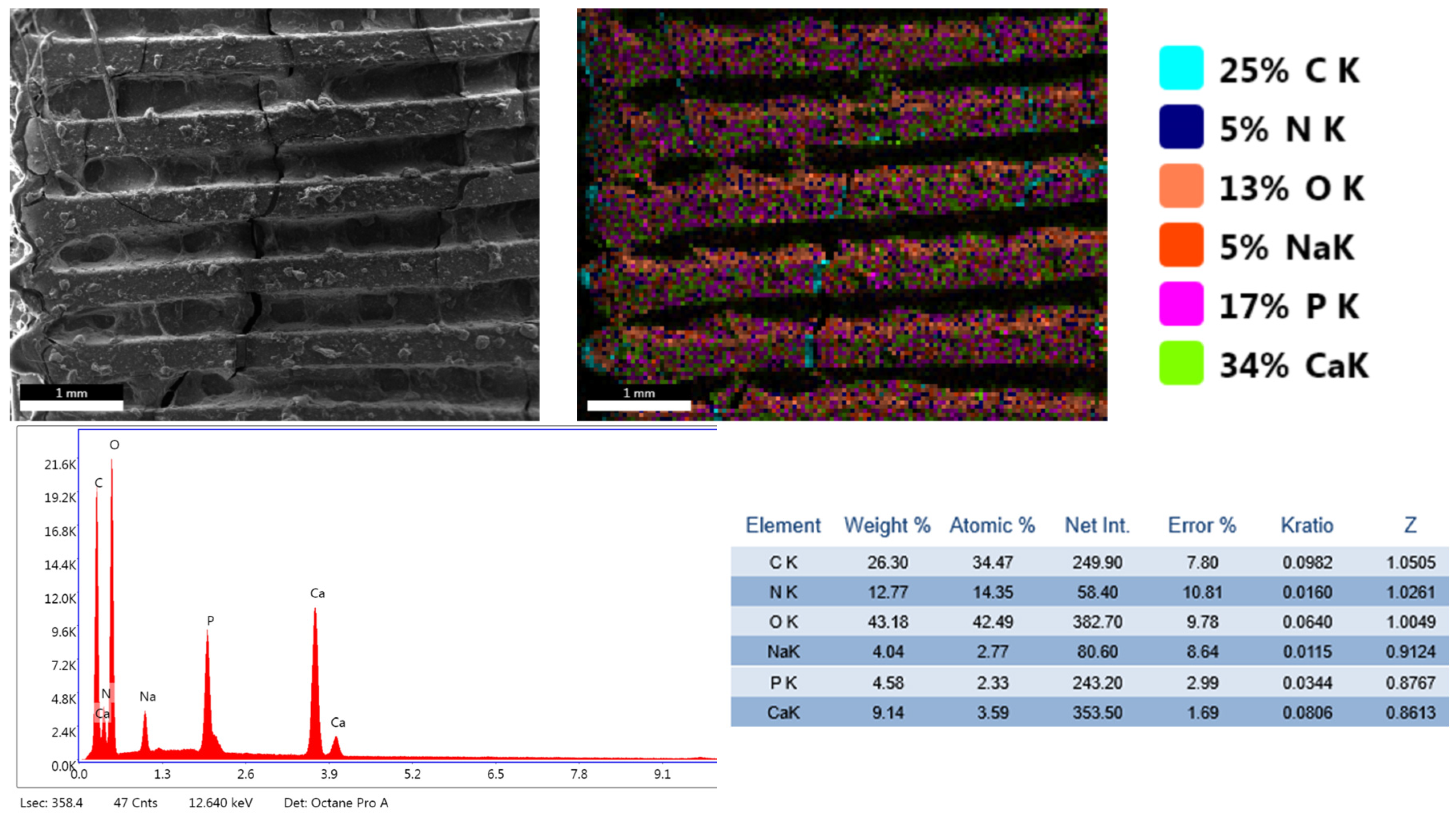
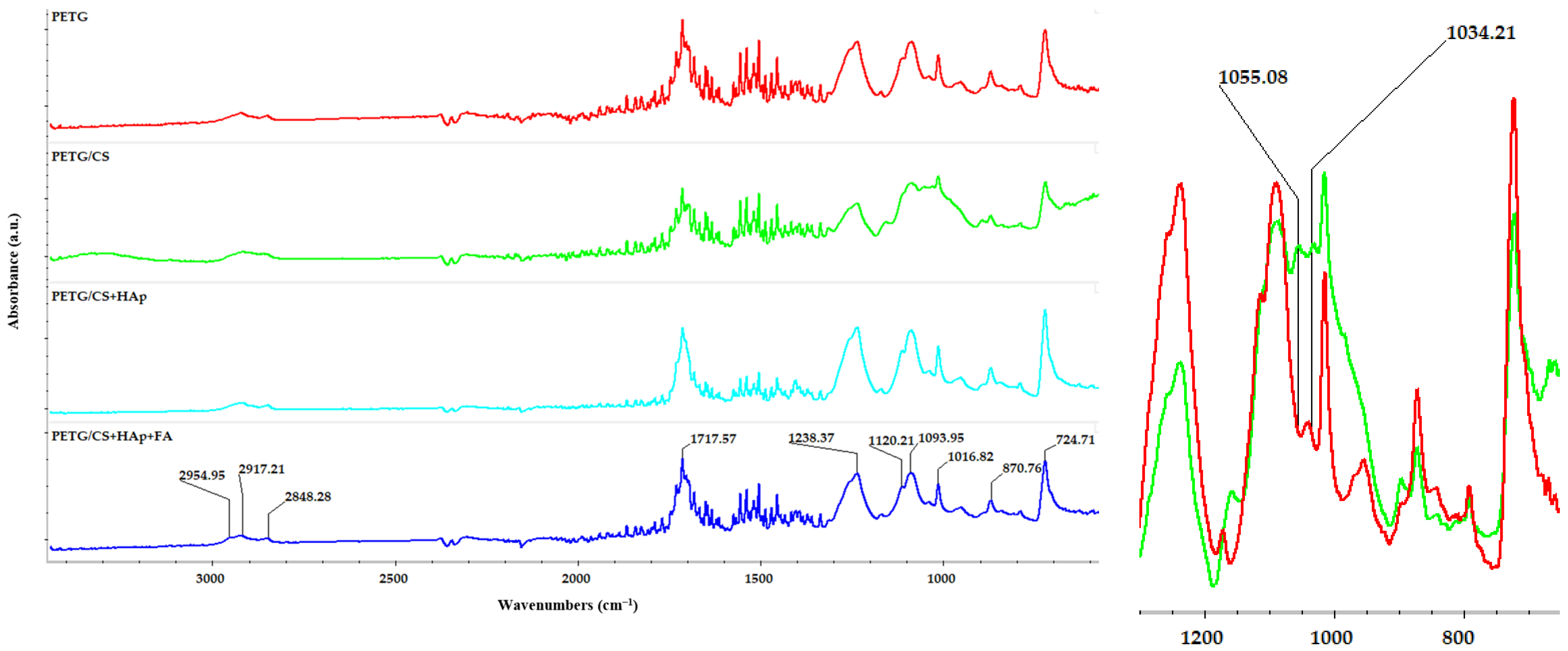
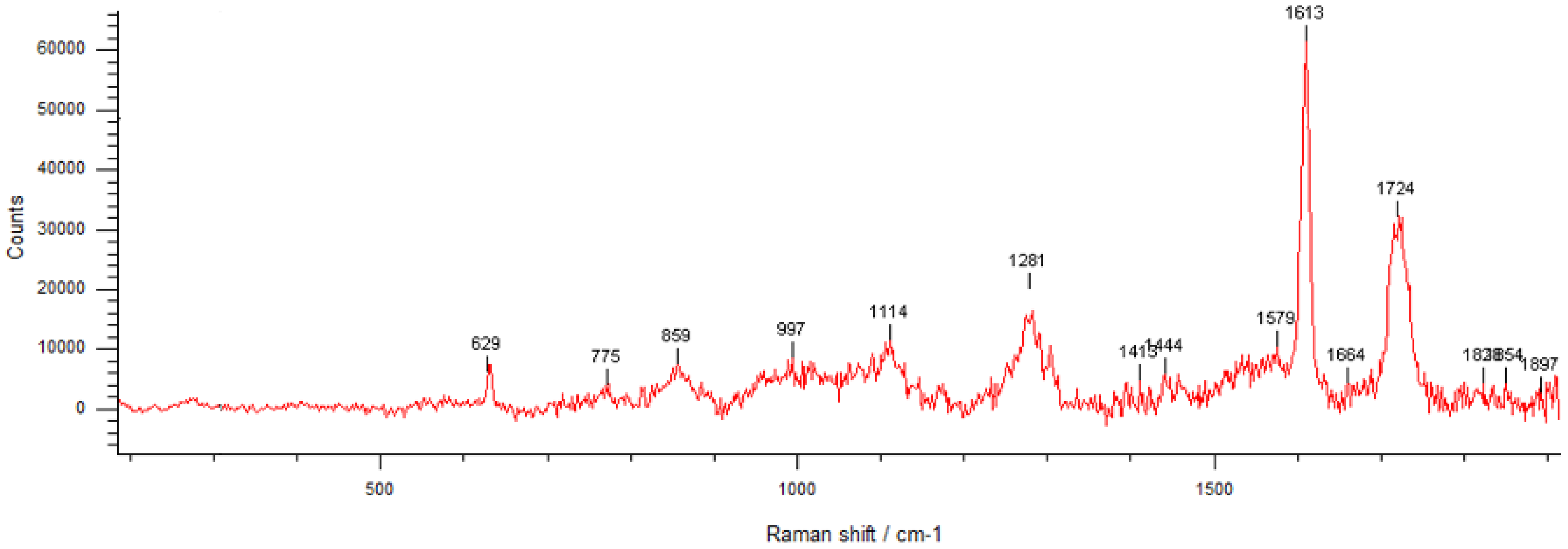



| Printing Parameter | Improved Effect on Tensile Strength | Improved Effect on Elastic Modulus | Improved Effect on Compressive Strength | Improved Effect on Impact Strength |
|---|---|---|---|---|
| Layer Height (mm) | ↓ | ↓ | ↓ | ↓ |
| Infill Density (%) | ↑ | ↑ | ↑ | ↑ |
| Printing Speed (mm/s) | ↓ | ↓ | ↓ | ↓ |
| Bed Temperature (°C) | ↑ | ↑ | ↑ | ↑ |
| Nozzle Temperature (°C) | ↑ | ↑ | ↑ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iosub, G.; Lungescu, I.-A.; Bîrcă, A.C.; Niculescu, A.-G.; Balaure, P.C.; Constantinescu, S.; Mihaiescu, B.; Rădulescu, D.M.; Grumezescu, A.M.; Hudiță, A.; et al. New Three Dimensional-Printed Polyethylene Terephthalate Glycol Liners for Hip Joint Endoprostheses: A Bioactive Platform for Bone Regeneration. Materials 2025, 18, 1206. https://doi.org/10.3390/ma18061206
Iosub G, Lungescu I-A, Bîrcă AC, Niculescu A-G, Balaure PC, Constantinescu S, Mihaiescu B, Rădulescu DM, Grumezescu AM, Hudiță A, et al. New Three Dimensional-Printed Polyethylene Terephthalate Glycol Liners for Hip Joint Endoprostheses: A Bioactive Platform for Bone Regeneration. Materials. 2025; 18(6):1206. https://doi.org/10.3390/ma18061206
Chicago/Turabian StyleIosub, Gheorghe, Ioana-Alexandra Lungescu, Alexandra Cătălina Bîrcă, Adelina-Gabriela Niculescu, Paul Catalin Balaure, Sorin Constantinescu, Bogdan Mihaiescu, Dragoș Mihai Rădulescu, Alexandru Mihai Grumezescu, Ariana Hudiță, and et al. 2025. "New Three Dimensional-Printed Polyethylene Terephthalate Glycol Liners for Hip Joint Endoprostheses: A Bioactive Platform for Bone Regeneration" Materials 18, no. 6: 1206. https://doi.org/10.3390/ma18061206
APA StyleIosub, G., Lungescu, I.-A., Bîrcă, A. C., Niculescu, A.-G., Balaure, P. C., Constantinescu, S., Mihaiescu, B., Rădulescu, D. M., Grumezescu, A. M., Hudiță, A., Neacșu, I. A., & Rădulescu, A. R. (2025). New Three Dimensional-Printed Polyethylene Terephthalate Glycol Liners for Hip Joint Endoprostheses: A Bioactive Platform for Bone Regeneration. Materials, 18(6), 1206. https://doi.org/10.3390/ma18061206











