Impacts of Hydrogen Adsorption on Carbon Nanotube–Metal Schottky Contacts
Abstract
1. Introduction
2. Materials and Methods
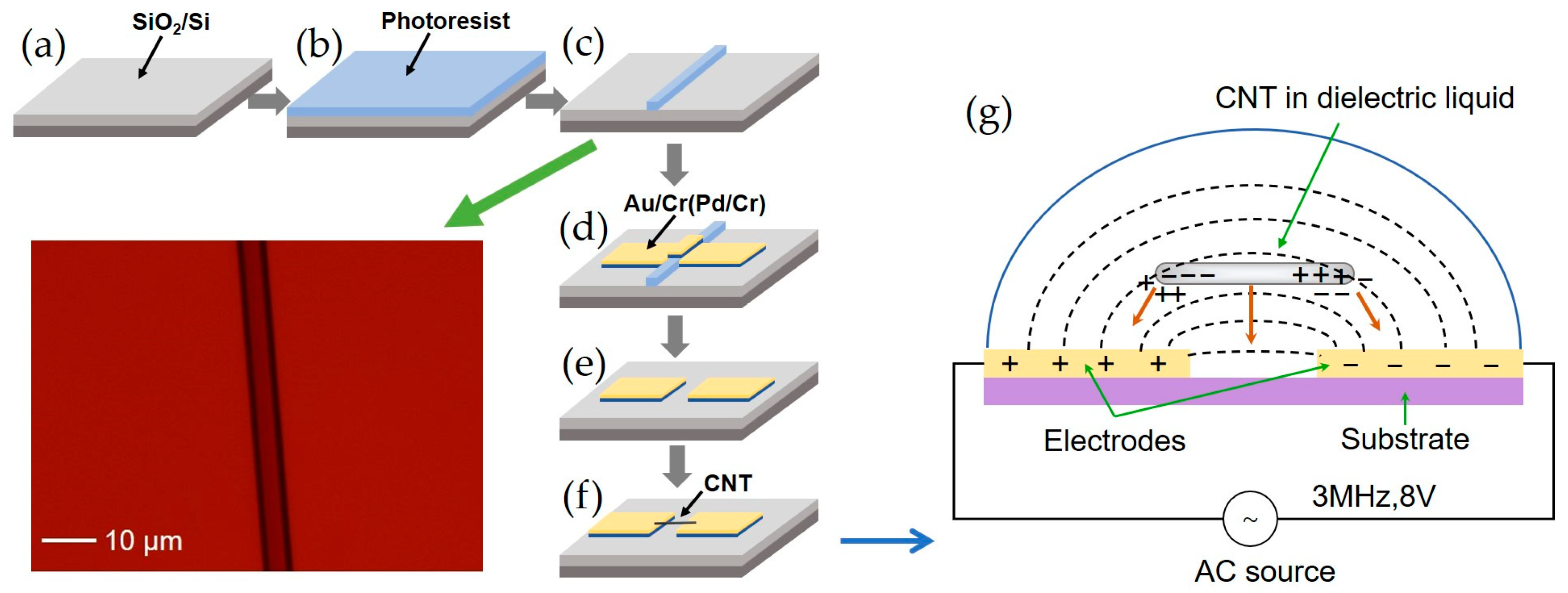
2.1. Device Preparation
2.2. Characterization and Performance Tests
2.3. The First-Principles Simulation Study
3. Results and Discussion
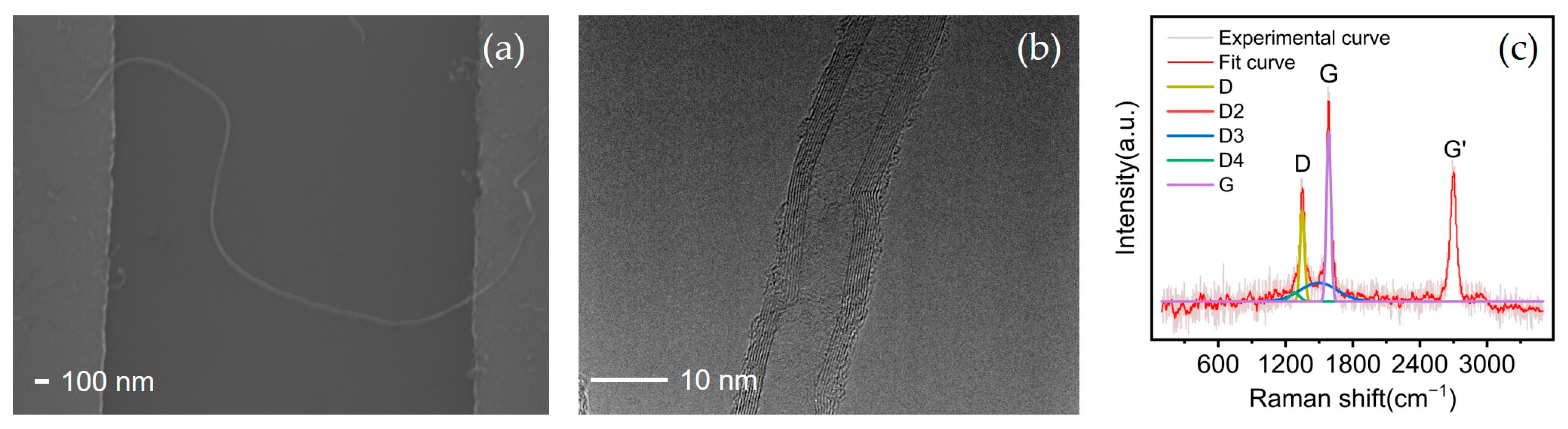
3.1. Carbon Nanotubes and Schottky Contact
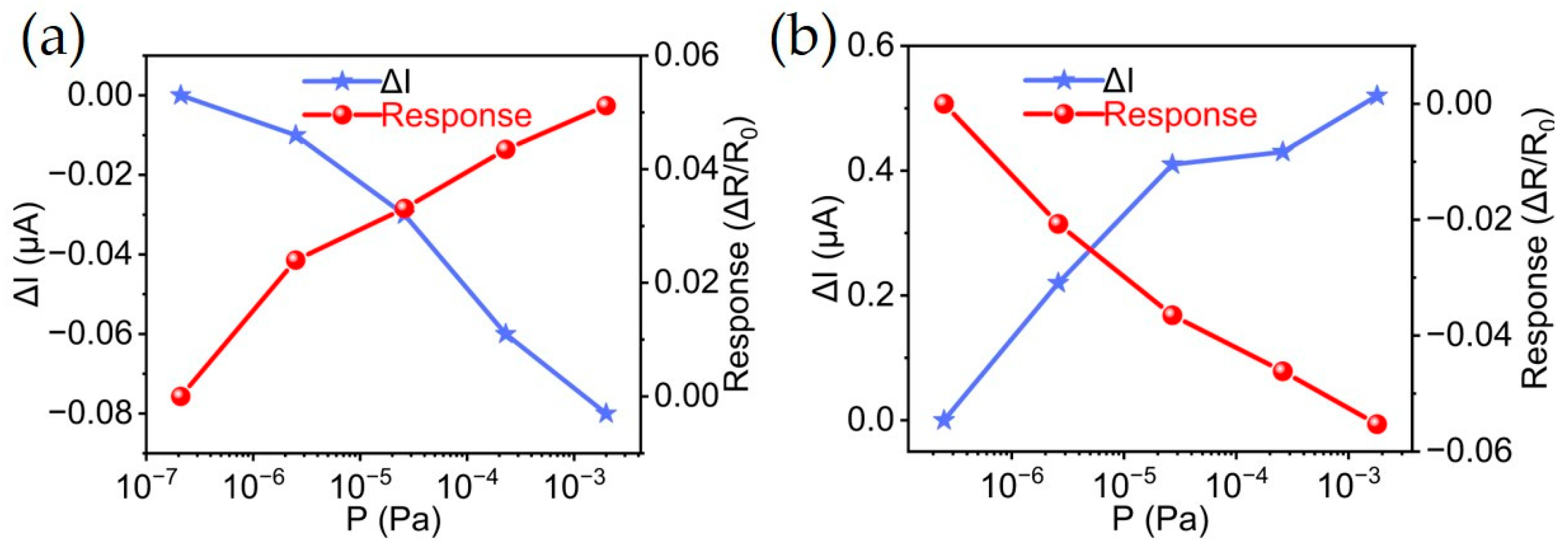
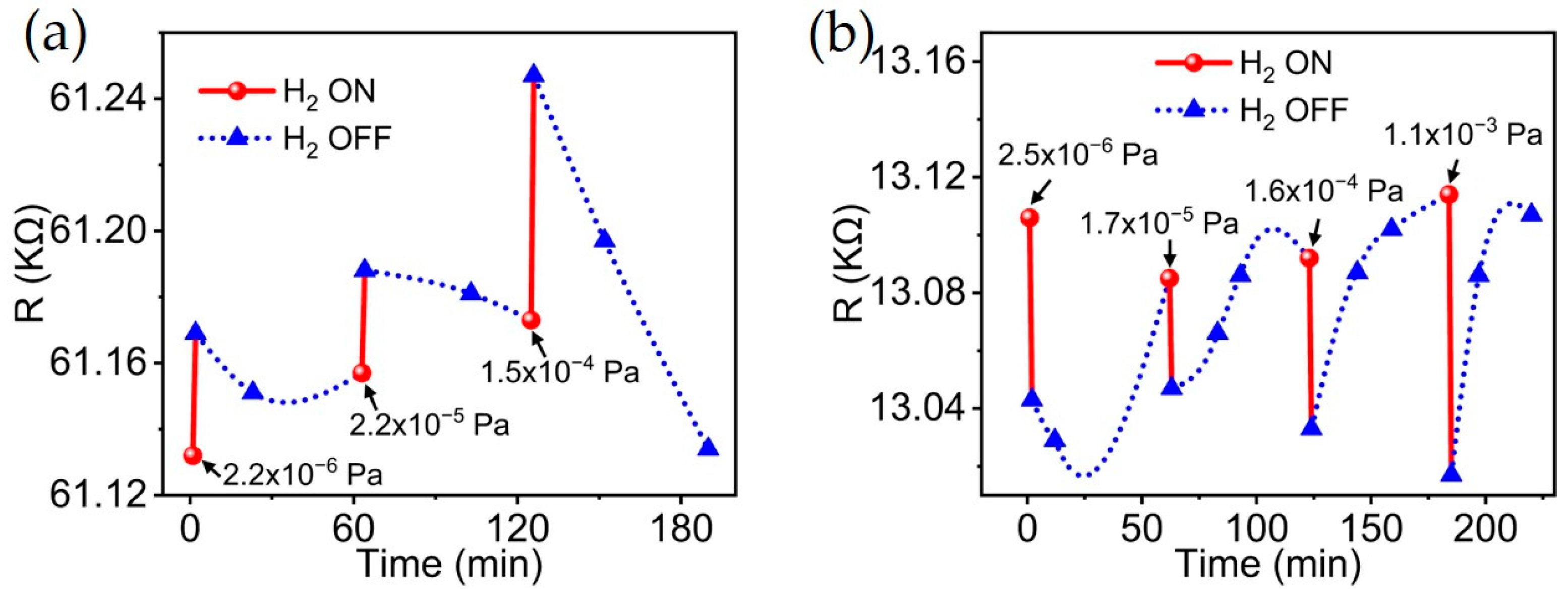
3.2. Hydrogen Sensing Performances
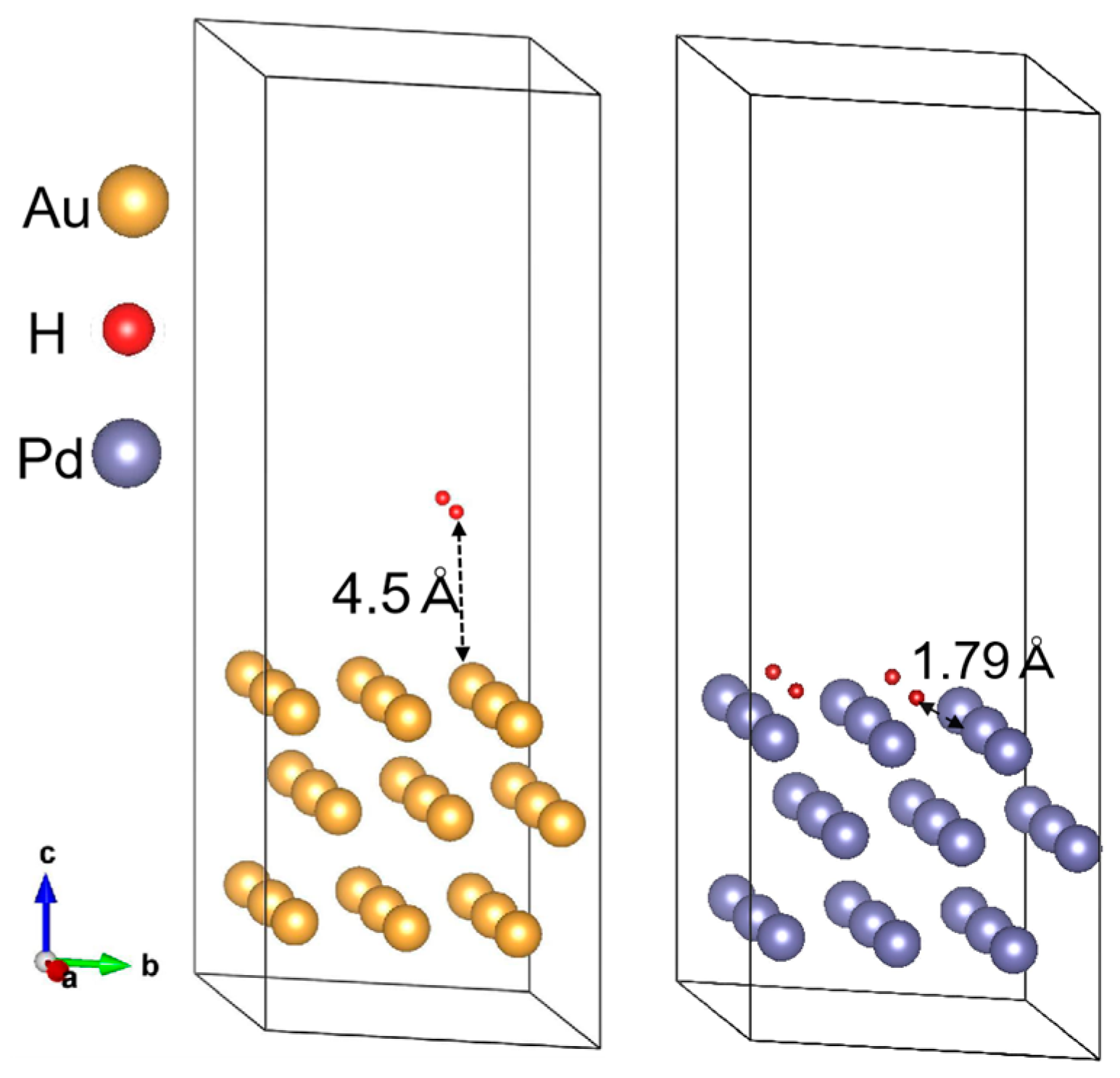
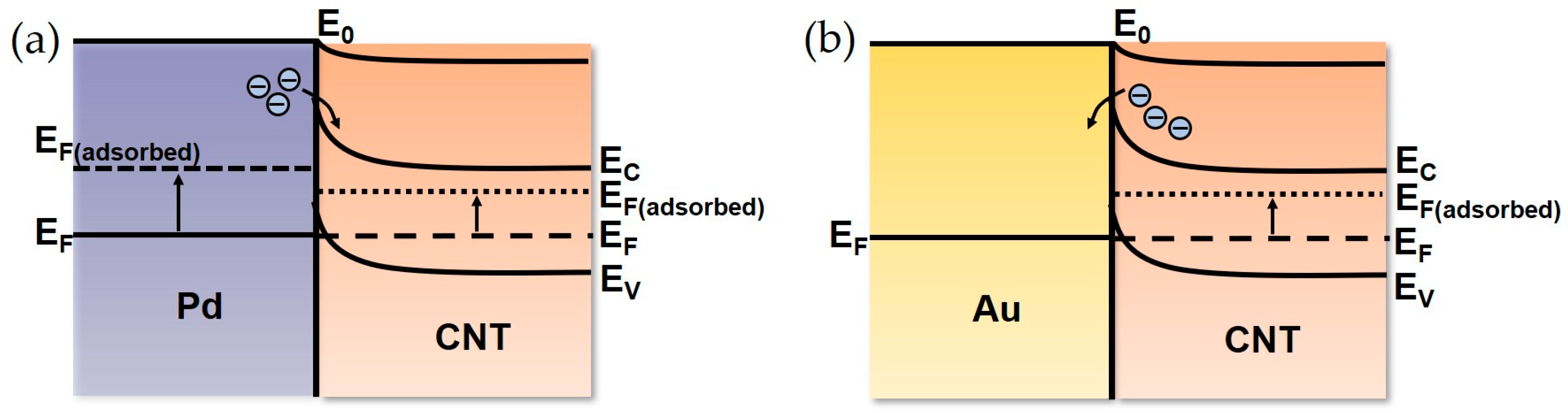
3.3. Mechanism of Hydrogen Adsorption
3.4. Recovery After Hydrogen Adsorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, B.X.; Niu, J.S.; Jian, J.J.; Liu, W.C. Hydrogen sensing characteristics of an Al-doped SnO2 thin film incorporated with palladium nanoparticles. Int. J. Hydrogen Energy 2024, 55, 1234–1241. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Liu, Y.N.; Lei, Y.S.; Mao, X.B.; Qian, H.Y.; Wen, H.M.; Xia, S.J.; Xiang, Y.; Chen, Q.L.; Xie, B.; Hu, J. Wide-Concentration-range hydrogen sensing using palladium-loaded SnO2 nanoparticle films and understanding of hydrogen concentration-dependent sensing mechanism. Int. J. Hydrogen Energy 2024, 62, 783–793. [Google Scholar] [CrossRef]
- Choudhary, M.; Shrivastav, A.; Sinha, A.K.; Chawla, A.K.; Avasthi, D.K.; Saravanan, K.; Chandra, R.; Wadhwa, S. Emerging nanomaterials for hydrogen sensing: Mechanisms and prospects. Int. J. Hydrogen Energy 2024, 77, 557–574. [Google Scholar] [CrossRef]
- Wischmeyer, T.; Stetter, J.R.; Buttner, W.J.; Patel, V.; Peaslee, D. Characterization of a selective, zero power sensor for distributed sensing of hydrogen in energy applications. Int. J. Hydrogen Energy 2021, 46, 31489–31500. [Google Scholar] [CrossRef]
- Kumar, M.K.; Ramaprabhu, S. Palladium dispersed multiwalled carbon nanotube based hydrogen sensor for fuel cell applications. Int. J. Hydrogen Energy 2007, 32, 2518–2526. [Google Scholar]
- Ho, R.; Lau, C.; Hills, G.; Shulaker, M.M. Carbon Nanotube CMOS Analog Circuitry. IEEE Trans. Nanotechnol. 2019, 18, 845–848. [Google Scholar] [CrossRef]
- Sacco, L.; Forel, S.; Florea, I.; Cojocaru, C.S. Ultra-sensitive NO gas sensors based on single-wall carbon nanotube field effect transistors: Monitoring from ppm to ppb level. Carbon 2020, 157, 631–639. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-nanotube-based thermoelectric materials and devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef]
- Hills, G.; Lau, C.; Wright, A.; Fuller, S.; Bishop, M.D.; Srimani, T.; Kanhaiya, P.; Ho, R.; Amer, A.; Stein, Y.; et al. Modern microprocessor built from complementary carbon nanotube transistors. Nature 2019, 572, 595–602. [Google Scholar] [CrossRef]
- Takakura, A.; Beppu, K.; Nishihara, T.; Fukui, A.; Kozeki, T.; Namazu, T.; Miyauchi, Y.H.; Itami, K. Strength of carbon nanotubes depends on their chemical structures. Nat. Commun. 2019, 10, 3040. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.M.; Zhang, Z.Y.; Qiu, C.G. Carbon nanotube digital electronics. Nat. Electron. 2019, 2, 499–505. [Google Scholar] [CrossRef]
- Kumar, S.; Pavelyev, V.; Tripathi, N.; Platonov, V.; Sharma, P.; Ahmad, R.; Mishra, P.; Khosla, A. Review-Recent Advances in the Development of Carbon Nanotubes Based Flexible Sensors. J. Electrochem. Soc. 2020, 167, 047506. [Google Scholar] [CrossRef]
- Javey, A.; Guo, J.; Wang, Q.; Lundstrom, M.; Dai, H. Ballistic carbon nanotube field-effect transistors. Nature 2003, 424, 654–657. [Google Scholar] [CrossRef]
- Skucha, K.; Fan, Z.Y.; Jeon, K.; Javey, A.; Boser, B. Palladium/Silicon nanowire Schottky barrier-based hydrogen sensors. Sens. Actuators B Chem. 2010, 145, 232–238. [Google Scholar] [CrossRef]
- Lin, Y.X.; Liang, S.B.; Xu, L.; Liu, L.J.; Hu, Q.L.; Fan, C.W.; Liu, Y.F.; Han, J.; Zhang, Z.Y.; Peng, L.M. Enhancement-mode field-effect transistors and high-speed integrated circuits based on aligned carbon nanotube films. Adv. Funct. Mater. 2022, 32, 2104539. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Lee, G.S. Fast-Response Room Temperature Hydrogen Gas Sensors Using Platinum-Coated Spin-Capable Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 3050–3057. [Google Scholar] [CrossRef]
- Kim, J.H.; Jean, J.G.; Ovalle-Robles, R.; Kang, T.J. Aerogel sheet of carbon nanotubes decorated with palladium nanoparticles for hydrogen gas sensing. Int. J. Hydrogen Energy 2018, 43, 6456–6461. [Google Scholar] [CrossRef]
- Young, S.J.; Liu, Y.H.; Lin, Z.D.; Ahmed, K.; Shiblee, M.N.I.; Romanuik, S.; Sekhar, K.P.; Thundat, T.; Nagahara, L.; Arya, S.; et al. Multi-Walled Carbon Nanotubes Decorated with Silver Nanoparticles for Acetone Gas Sensing at Room Temperature. J. Electrochem. Soc. 2020, 167, 167519. [Google Scholar] [CrossRef]
- Xiao, M.M.; Liang, S.B.; Han, J.; Zhong, D.L.; Liu, J.X.; Zhang, Z.Y.; Peng, L.M. Batch fabrication of ultrasensitive carbon nanotube hydrogen sensors with sub-ppm detection limit. ACS Sens. 2018, 3, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.Y.; Toku, Y.; Ju, Y. Highly sensitive hydrogen sensor based on a new suspended structure of cross-stacked multiwall carbon nanotube sheet. Int. J. Hydrogen Energy 2019, 44, 6344–6352. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Singh, R.C.; Sharma, S. Hydrothermally synthesized MoS-multi-walled carbon nanotube composite as a novel room-temperature ammonia sensing platform. Appl. Surf. Sci. 2020, 532, 147373. [Google Scholar] [CrossRef]
- Dong, C.K.; Luo, H.J.; Cai, J.Q.; Wang, F.Q.; Zhao, Y.Y.; Li, D.T. Hydrogen sensing characteristics from carbon nanotube field emissions. Nanoscale 2016, 8, 5599–5604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Cai, J.Q.; Luo, H.J.; Kang, S.; Qian, W.J.; Dong, C.K. Low pressure hydrogen sensing based on carbon nanotube field emission: Mechanism of atomic adsorption induced work function effects. Carbon 2017, 124, 669–674. [Google Scholar] [CrossRef]
- Meyer, D.J.; Downey, B.P.; Katzer, D.S.; Nepal, N.; Wheeler, V.D.; Hardy, M.T.; Anderson, T.J.; Storm, D.F. Epitaxial Lift-Off and Transfer of III-N Materials and Devices from SiC Substrates. IEEE Trans. Semicond. Manuf. 2016, 29, 384–389. [Google Scholar] [CrossRef]
- Rabbani, M.T.; Sonker, M.; Ros, A. Carbon nanotube dielectrophoresis: Theory and applications. Electrophoresis 2020, 41, 1893–1914. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Elike, J.; Reynolds, A.; Moten, R.; Zhao, X. The fabrication of carbon nanotube electronic circuits with dielectrophoresis. Microelectron. Eng. 2016, 164, 123–127. [Google Scholar] [CrossRef]
- Abdulhameed, A.; Halim, M.M.; Halin, I.A. Dielectrophoretic alignment of carbon nanotubes: Theory, applications, and future. Nanotechnology 2023, 34, 242001. [Google Scholar] [CrossRef]
- Kareer, S.; Park, J. Metallic CNT Tolerant Field Effect Transistor Using Dielectrophoresis. IEEE Open J. Nanotechnol. 2023, 4, 95–101. [Google Scholar] [CrossRef]
- Vedaei, S.S.; Nadimi, E. Gas sensing properties of CNT-BNNT-CNT nanostructures: A first principles study. Appl. Surf. Sci. 2019, 470, 933–942. [Google Scholar] [CrossRef]
- Bets, K.V.; Penev, E.S.; Yakobson, B.I. Janus segregation at the carbon nanotube–catalyst interface. ACS Nano 2019, 13, 8836–8841. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhu, Y.; Yang, W.; Sun, J.; Li, H.; Yuan, F.; Xiu, Z. Effect of defects and oxidation on CNT–Copper interface: First-principles calculation and experiment. Materials 2023, 16, 6845. [Google Scholar] [CrossRef]
- Radovanović, M.P.; Zdolšek, N.; Brković, S.; Dučić, M.J.; Anićijević, D.V.; Častvan, I.J.; Pavićević, V.; Janković, B. Production and characterization of biochar and modified biochars by carbonization process of bearberry (Arctostaphylos uva-ursi. L.): Adsorption capacities and kinetic studies of Pb2+, Cd2+ and rhodamine B removal from aqueous solutions. Diam. Relat. Mater. 2025, 151, 111794. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Chen, X.N.; Wang, X.H.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Qian, W.J.; Li, M.J.; Chen, L.H.; Zhang, J.H.; Dong, C.K. Improving thermo-electrochemical cell performance by constructing Ag–MgO–CNTs nanocomposite electrodes. RSC Adv. 2015, 5, 97982–97987. [Google Scholar] [CrossRef]
- Sivkov, D.V.; Petrova, O.V.; Nekipelov, S.V.; Vinogradov, A.S.; Skandakov, R.N.; Bakina, K.A.; Isaenko, S.I.; Ob’edkov, A.M.; Kaverin, B.S.; Vilkov, I.V.; et al. Quantitative Characterization of Oxygen-Containing Groups on the Surface of Carbon Materials: XPS and NEXAFS Study. Appl. Sci. 2022, 12, 7744. [Google Scholar] [CrossRef]
- Xu, T.; Yang, J.H.; Liu, J.W.; Fu, Q. Surface modification of multi-walled carbon nanotubes by O2 plasma. Appl. Surf. Sci. 2007, 253, 8945–8951. [Google Scholar] [CrossRef]
- Suehiro, J.; Hidaka, S.I.; Yamane, S.; Imasaka, K. Fabrication of interfaces between carbon nanotubes and catalytic palladium using dielectrophoresis and its application to hydrogen gas sensor. Sens. Actuators B Chem. 2007, 127, 505–511. [Google Scholar] [CrossRef]
- Mandelis, A.; Christofides, C. Physics, Chemistry and Technology of Solid State Gas Sensor Devices; John Wiley & Sons: New York, NY, USA, 1993; pp. 8–13. [Google Scholar]
- Halas, S.; Durakiewicz, T.; Mackiewicz, P. Temperature-dependent work function shifts of hydrogenated/deuteriated palladium: A new theoretical explanation. Surf. Sci. 2004, 555, 43–50. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Barrio, L.; Liu, P.; Rodríguez, J.A.; Campos-Martín, J.M.; Fierro, J.L.G. A density functional theory study of the dissociation of H on gold clusters: Importance of fluxionality and ensemble effects. J. Chem. Phys. 2006, 125, 16. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Pradhan, B.K.; EKlund, P.C.; Kostov, M.K.; Cole, M.W. Raman spectroscopic investigation of H2, HD, and D2 physisorption on ropes of single-walled, carbon nanotubes. Phys. Rev. Lett. 2002, 88, 165502–165505. [Google Scholar] [CrossRef] [PubMed]
- Arellano, J.S.; Molina, L.M.; Rubio, A.; Lopez, M.J.; Alonso, J.A. Interaction of molecular and atomic hydrogen with (5, 5) and (6, 6) single-wall carbon nanotubes. J. Chem. Phys. 2002, 117, 2281–2288. [Google Scholar] [CrossRef]
- Lu, Y.H.; Yuan, P.F. Adsorptions of hydrogen on graphene and other forms of carbon structures: First principle calculations. Nanoscale 2011, 3, 2444–2453. [Google Scholar] [CrossRef]
- Zhu, W.; Qian, W.J.; Liu, R.Z.; Huang, W.J.; Luo, H.J.; Dong, C.K. Field emission energy distribution studies of single multi-walled carbon nanotube emitter with gas adsorptions. Vacuum 2022, 199, 110933. [Google Scholar] [CrossRef]
- Iordache, S.M.; Ionete, E.I.; Iordache, A.M.; Tanasa, E.; Stamatin, I.; Eugenia, C.; Grigorescu, A. Pd-Decorated CNT as sensitive material for applications in hydrogen isotopes sensing-Application as gas sensor. Int. J. Hydrogen Energy 2021, 46, 11015–11024. [Google Scholar] [CrossRef]
- Collins, P.G.; Bradley, K.; Ishigami, M.; Zettl, A. Extreme oxygen sensitivity of electronic properties of carbon nanotubes. Science 2000, 287, 1801–1804. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Ward, T.L.; Dao, T. Model of hydrogen permeation behavior in palladium membranes. J. Membr. Sci. 1999, 153, 211–231. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Ye, N.; Luo, H.; Shao, H.; Qian, W.; Fang, C.; Dong, C. Impacts of Hydrogen Adsorption on Carbon Nanotube–Metal Schottky Contacts. Materials 2025, 18, 1202. https://doi.org/10.3390/ma18061202
Huang C, Ye N, Luo H, Shao H, Qian W, Fang C, Dong C. Impacts of Hydrogen Adsorption on Carbon Nanotube–Metal Schottky Contacts. Materials. 2025; 18(6):1202. https://doi.org/10.3390/ma18061202
Chicago/Turabian StyleHuang, Chuntian, Nini Ye, Haijun Luo, Hezhu Shao, Weijin Qian, Chaolong Fang, and Changkun Dong. 2025. "Impacts of Hydrogen Adsorption on Carbon Nanotube–Metal Schottky Contacts" Materials 18, no. 6: 1202. https://doi.org/10.3390/ma18061202
APA StyleHuang, C., Ye, N., Luo, H., Shao, H., Qian, W., Fang, C., & Dong, C. (2025). Impacts of Hydrogen Adsorption on Carbon Nanotube–Metal Schottky Contacts. Materials, 18(6), 1202. https://doi.org/10.3390/ma18061202





