Adhesion Characteristics of an Asphalt Binder–Aggregate Interface Based on Molecular Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Asphalt Binder and Aggregates
2.2. Contact Angle Test
2.3. Molecular Dynamics Simulation
3. Results
3.1. Results of Contact Angle Test
3.2. Results of Surface Free Energy
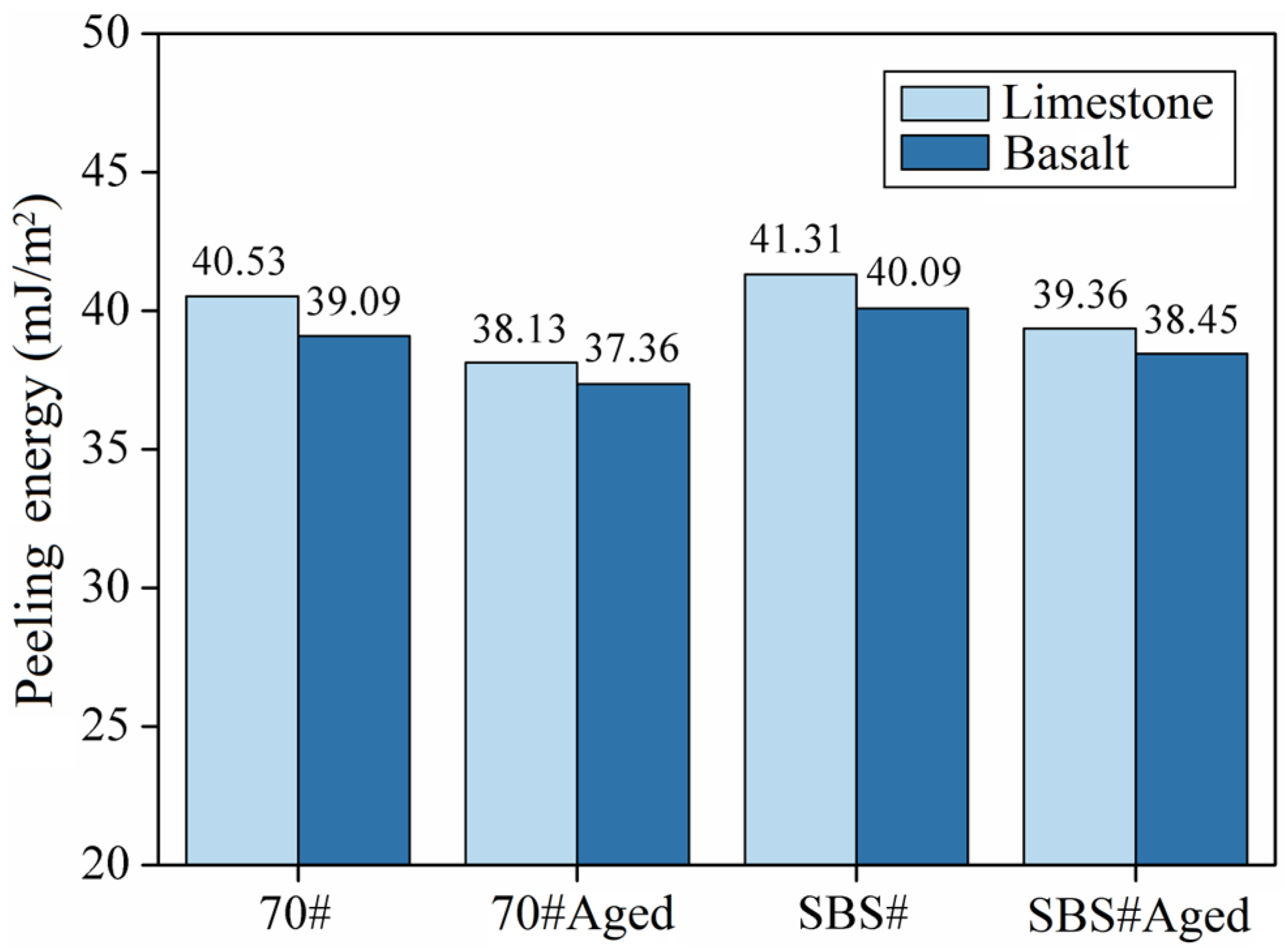
3.3. Results of Adhesion and Peeling
3.4. Results of Model Verification
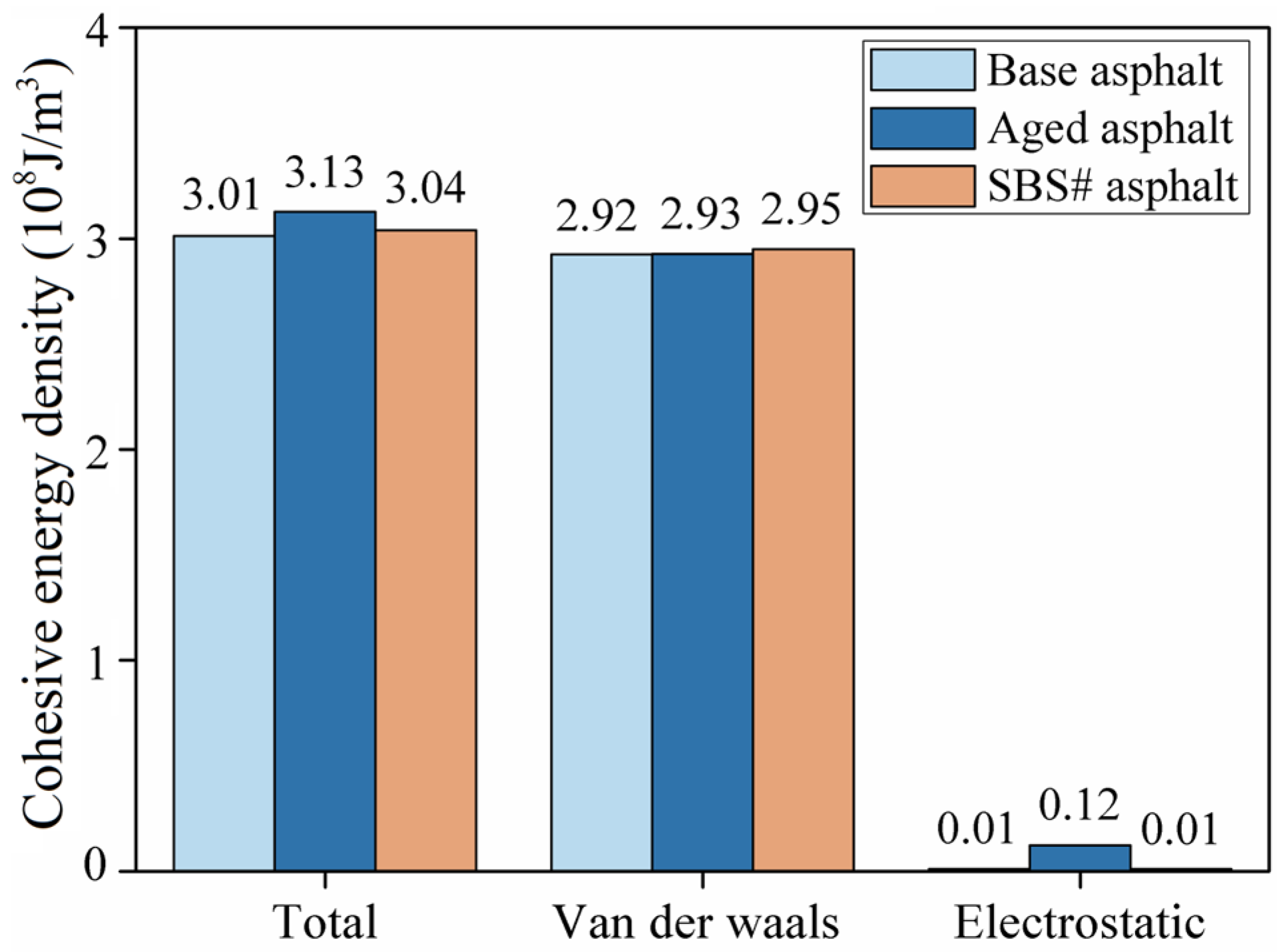
3.5. Results of Interface Energy
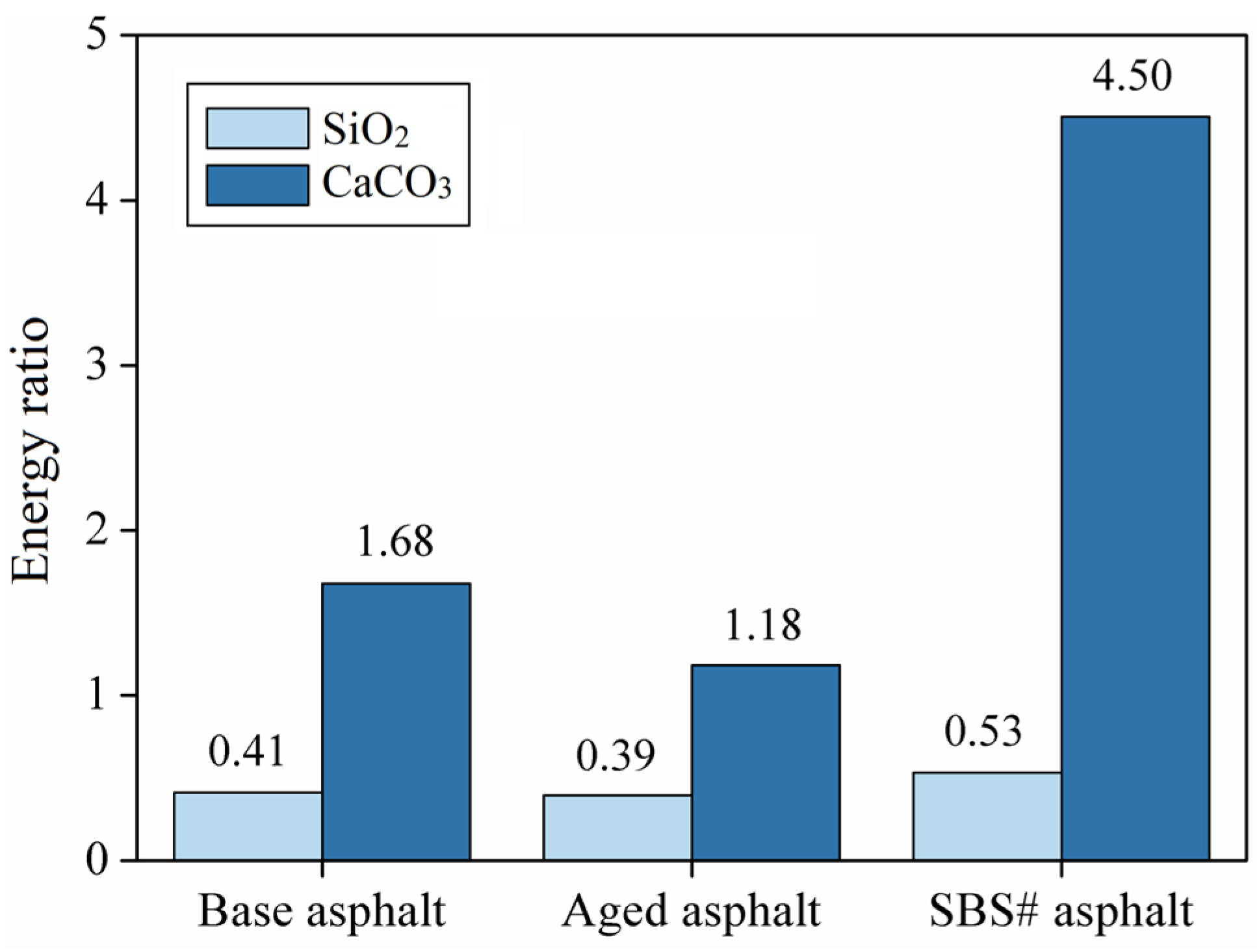
3.6. Results of Energy Ratio
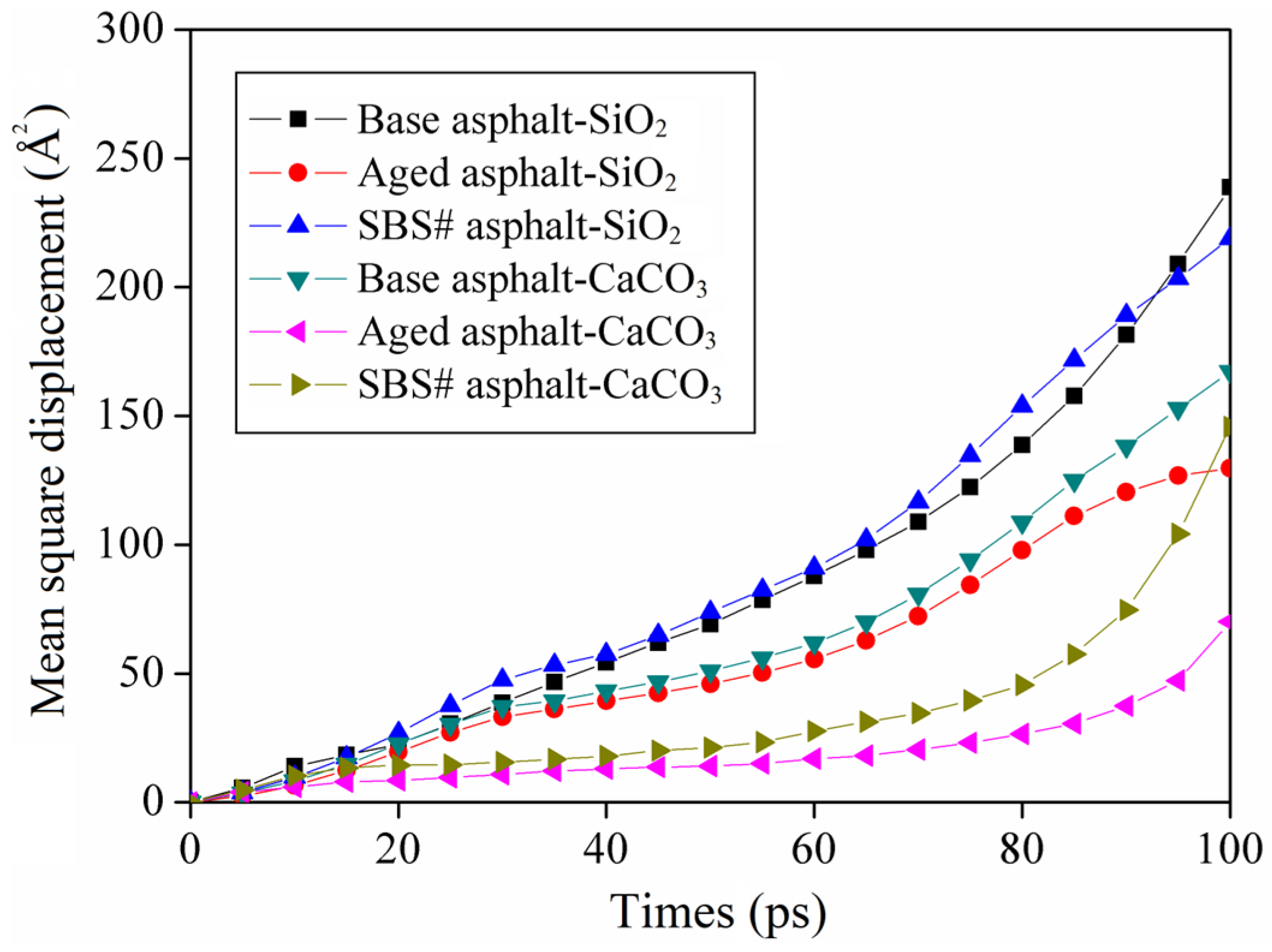
3.7. Results of Mean Square Displacement
4. Conclusions
- (1)
- The indoor test results were consistent with the conclusions of the MD numerical simulations. The adhesion of limestone to asphalt was significantly better than that of basalt. The alkaline aggregate forms a strong interface with asphalt through electrostatic forces, whereas the acidic aggregate mainly relies on van der Waals forces, resulting in a weak adhesion performance. In addition, the SBS modifier can improve the adhesion between asphalt and aggregates; however, the improvement in resistance to water damage is limited;
- (2)
- The calculation results of the interfacial energy and energy ratio show that SBS-modified asphalt has the best adhesion with aggregates and a strong resistance to water damage. In contrast, both the energy ratio and interfacial energy of the aged asphalt decreased significantly, indicating that aging significantly reduced the adhesion of asphalt to aggregates and its resistance to water damage;
- (3)
- Thermal oxidative aging caused the diffusion coefficient of asphalt at the interface of the CaCO3 aggregate to decrease significantly, with a decrease of more than 70%, whereas the diffusion coefficient at the interface of the SiO2 aggregate was higher, indicating that the different behaviors of asphalt on the surfaces of different types of aggregates affect the final physical properties and significantly impact durability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, G.; Chen, Z.; Wang, S.; Hu, D.; Zhang, J.; Pei, J. Investigation of fracture failure and water damage behavior of asphalt mixtures and their correlation with asphalt-aggregate bonding performance. Constr. Build. Mater. 2024, 449, 138352. [Google Scholar] [CrossRef]
- Zhu, S.; Kong, L.; Peng, Y.; Chen, Y.; Zhao, T.; Jian, O.; Zhao, P.; Sheng, X.; Li, Z. Mechanisms of interface electrostatic potential induced asphalt-aggregate adhesion. Constr. Build. Mater. 2024, 438, 137255. [Google Scholar] [CrossRef]
- Yu, H.; Ge, J.; Qian, G.; Shi, C.; Zhang, C.; Dai, W.; Xie, T.; Nian, T. Evaluation of the interface adhesion mechanism between SBS asphalt and aggregates under UV aging through molecular dynamics. Constr. Build. Mater. 2023, 409, 133995. [Google Scholar] [CrossRef]
- Su, A.; Yin, H.; Bao, S.; Zhao, J.; Li, J.; Li, S. A multiscale study of the influence of aggregate type on adhesion at the asphalt–aggregate interface. AIP Adv. 2024, 14, 055207. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, J.; Wang, Z.; Guo, F.; Li, Y.; Wang, L.; He, Y.; Xu, Z.; Huang, X. Evaluation of asphalt-aggregate adhesive property and its correlation with the interaction behavior. Constr. Build. Mater. 2023, 374, 130909. [Google Scholar] [CrossRef]
- Cala, A.; Caro, S. Predictive quantitative model for assessing the asphalt-aggregate adhesion quality based on aggregate chemistry. Road Mater. Pavement Des. 2022, 23, 1523–1543. [Google Scholar] [CrossRef]
- Wang, H.; Chen, G.; Kang, H.; Zhang, J.; Rui, L.; Lyu, L.; Pei, J. Asphalt-aggregates interface interaction: Correlating oxide composition and morphology with adhesion. Constr. Build. Mater. 2024, 457, 139317. [Google Scholar] [CrossRef]
- Ge, J.; Yu, H.; Qian, G.; Dai, W.; Zhang, C.; Shi, C.; Zhou, H.; Nian, T.; Zhong, T. Enhancement mechanism of aggregate surface roughness structure on interfacial properties of asphalt mixtures. Constr. Build. Mater. 2024, 449, 138388. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhou, X.; Zhang, Z.; Chen, X. Mechanical properties of the interfacial bond between asphalt-binder and aggregates under different aging conditions. Materials 2021, 14, 1221. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.; Hasan, M.; Xin, T.; Sougui, O.; Khan, D. Effects of bonding enhancers on shear stress and bonding strength of modified asphalt binders under different aging and moisture conditions. Constr. Build. Mater. 2024, 453, 139020. [Google Scholar] [CrossRef]
- Guo, M.; Yin, X.; Du, X.; Tan, Y. Effect of aging, testing temperature and relative humidity on adhesion between asphalt binder and mineral aggregate. Constr. Build. Mater. 2023, 363, 129775. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Ma, F.; Zhao, P.; Hou, Y.; Jiang, X.; Peng, C. Effect of water molecular behavior on adhesion properties of asphalt-aggregate interface. Constr. Build. Mater. 2023, 402, 133028. [Google Scholar] [CrossRef]
- Mihandoust, M.; Ghabchi, R. Mechanical, thermodynamical, and microstructural characterization of adhesion evolution in asphalt binder-aggregate interface subjected to calcium chloride deicer. Constr. Build. Mater. 2023, 408, 133663. [Google Scholar] [CrossRef]
- Meng, Y.; Tan, S.; Mo, S.; Rong, H.; Lai, J.; Yao, Q.; Chen, J.; Meng, F. Study on the effect of acid rain erosion on the adhesion properties of asphalt-aggregate interface. Int. J. Pavement Eng. 2024, 25, 2408623. [Google Scholar] [CrossRef]
- Pstrowska, K.; Gunka, V.; Sidun, I.; Demchuk, Y.; Vytrykush, N.; Kułażyński, M.; Bratychak, M. Adhesion in bitumen/aggregate system: Adhesion mechanism and test methods. Coatings 2022, 12, 1934. [Google Scholar] [CrossRef]
- Koc, M.; Bulut, R. Assessment of a sessile drop device and a new testing approach measuring contact angles on aggregates and asphalt binders. J. Mater. Civ. Eng. 2014, 26, 391–398. [Google Scholar] [CrossRef]
- Valentin, J.; Trejbal, J.; Nežerka, V.; Valentová, T.; Vacková, P.; Tichá, P. A comprehensive study on adhesion between modified bituminous binders and mineral aggregates. Constr. Build. Mater. 2021, 305, 124686. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, J.; Yan, E. Intrinsic temperature and moisture sensitive adhesion characters of asphalt-aggregate interface based on molecular dynamics simulations. Constr. Build. Mater. 2021, 292, 123462. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Raos, G.; Ma, F.; Zhao, P.; Hou, Y. Molecular dynamics simulation of adhesion at the asphalt-aggregate interface: A review. Surf. Interfaces 2024, 44, 103706. [Google Scholar] [CrossRef]
- Xu, J.; Ma, B.; Mao, W.; Si, W.; Wang, X. Review of interfacial adhesion between asphalt and aggregate based on molecular dynamics. Constr. Build. Mater. 2023, 362, 129642. [Google Scholar] [CrossRef]
- Cui, B.; Gu, X.; Wang, H.; Hu, D. Numerical and experimental evaluation of adhesion properties of asphalt-aggregate interfaces using molecular dynamics simulation and atomic force microscopy. Road Mater. Pavement Des. 2022, 23, 1564–1584. [Google Scholar] [CrossRef]
- Guo, F.; Pei, J.; Zhang, J.; Liu, P.; Wang, D. Study on adhesion property and moisture effect between SBS modified asphalt binder and aggregate using molecular dynamics simulation. Materials 2022, 15, 6912. [Google Scholar] [CrossRef]
- Li, N.; Wang, J.; Si, W.; Hu, D. Quantitative analysis of adhesion characteristics between crumb rubber modified asphalt and aggregate using surface free energy theory. Materials 2022, 15, 5735. [Google Scholar] [CrossRef]
- Zhao, K.; Song, S.; Wei, Y.; Li, G.; Guo, F. Adhesion properties of recycled high-viscosity asphalt–aggregate interface under dynamic water erosion. Materials 2023, 16, 6203. [Google Scholar] [CrossRef]
- Xiang, H.; He, Z.; Tang, H.; Hu, M. Healing behavior of thermo-oxygen aged asphalt based on molecular dynamics simulations. Constr. Build. Mater. 2022, 349, 128740. [Google Scholar] [CrossRef]
- Han, J.; Li, B.; Ji, H.; Guo, F.; Wei, D.; Cao, S.; Zhang, W.; Chen, X. Interfacial adhesion between recycled asphalt binder and aggregates considering aggregate surface anisotropy: A molecular dynamics simulation. Constr. Build. Mater. 2024, 438, 137176. [Google Scholar] [CrossRef]
- Peng, C.; Yang, H.; You, Z.; Ma, H.; Xu, F.; You, L.; Aboelkasim, D.; Lu, L.; Hu, Y.; Liu, Y.; et al. Investigation of Adhesion Performance of Wax Based Warm Mix Asphalt with Molecular Dynamics Simulation. Materials 2022, 15, 5930. [Google Scholar] [CrossRef]
- Liu, S.; Wang, H.; Yang, J.; Luo, S.; Liu, Y.; Huang, W.; Hu, J.; Xu, G.; Min, Z. Force field benchmark of asphalt materials: Density, viscosity, glass transition temperature, diffusion coefficient, cohesive energy density and molecular structures. J. Mol. Liq. 2024, 398, 124166. [Google Scholar] [CrossRef]
- Yan, S.; Dong, Q.; Chen, X.; Wang, X.; Shi, B.; Yao, K. Study on inherent characteristics of asphalt regenerating agents: Insights from density functional theory calculations and molecular dynamics simulations. Constr. Build. Mater. 2024, 411, 134390. [Google Scholar] [CrossRef]
- Tu, C.; Xi, L.; Luo, R.; Huang, T.; Luo, J. Effect of water vapor concentration on adhesion between asphalt and aggregate. Mater. Struct. 2022, 55, 237. [Google Scholar] [CrossRef]
- Dong, F.; Wang, S.; Yu, X.; Guo, Y.; Jin, Y.; Zhu, H.; Yang, J.; Lu, J. Investigation on the diffusion behavior of dry modified sbs at the asphalt-aggregate interface: Molecular simulation and experiments. J. Mater. Civ. Eng. 2024, 36, 04023564. [Google Scholar] [CrossRef]
- Luo, L.; Chu, L.; Fwa, T. Molecular dynamics analysis of oxidative aging effects on thermodynamic and interfacial bonding properties of asphalt mixtures. Constr. Build. Mater. 2021, 269, 121299. [Google Scholar] [CrossRef]
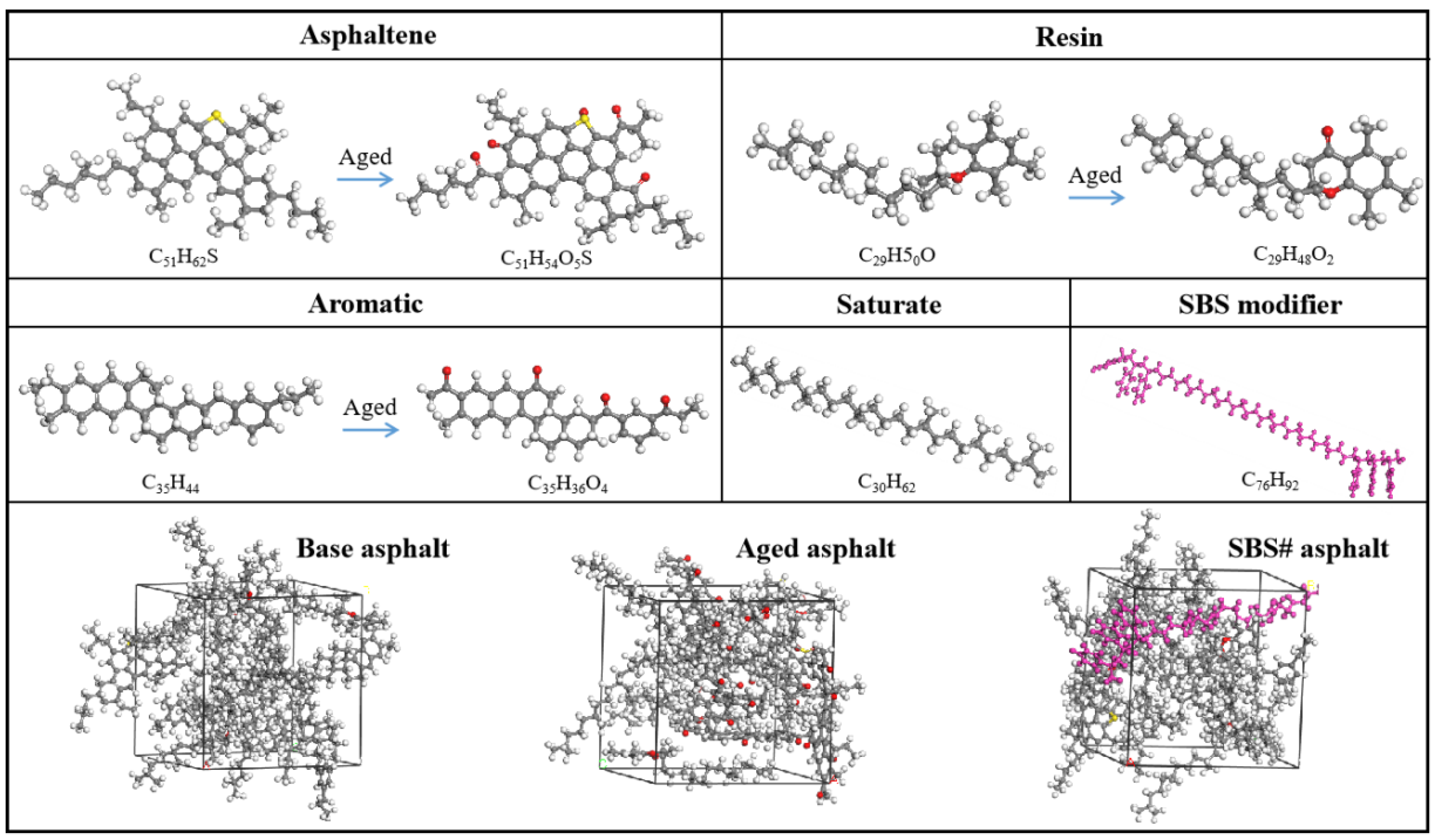
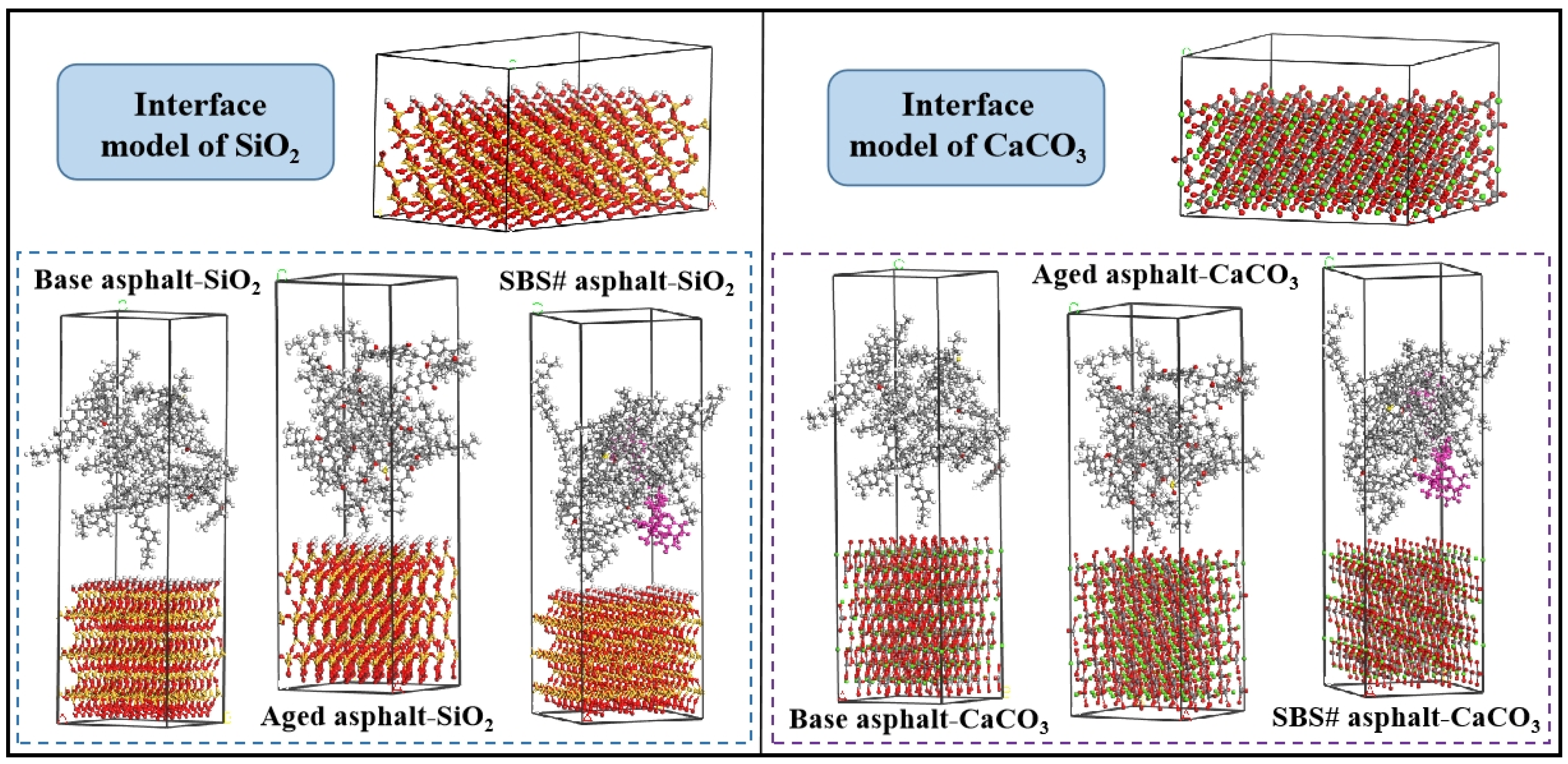
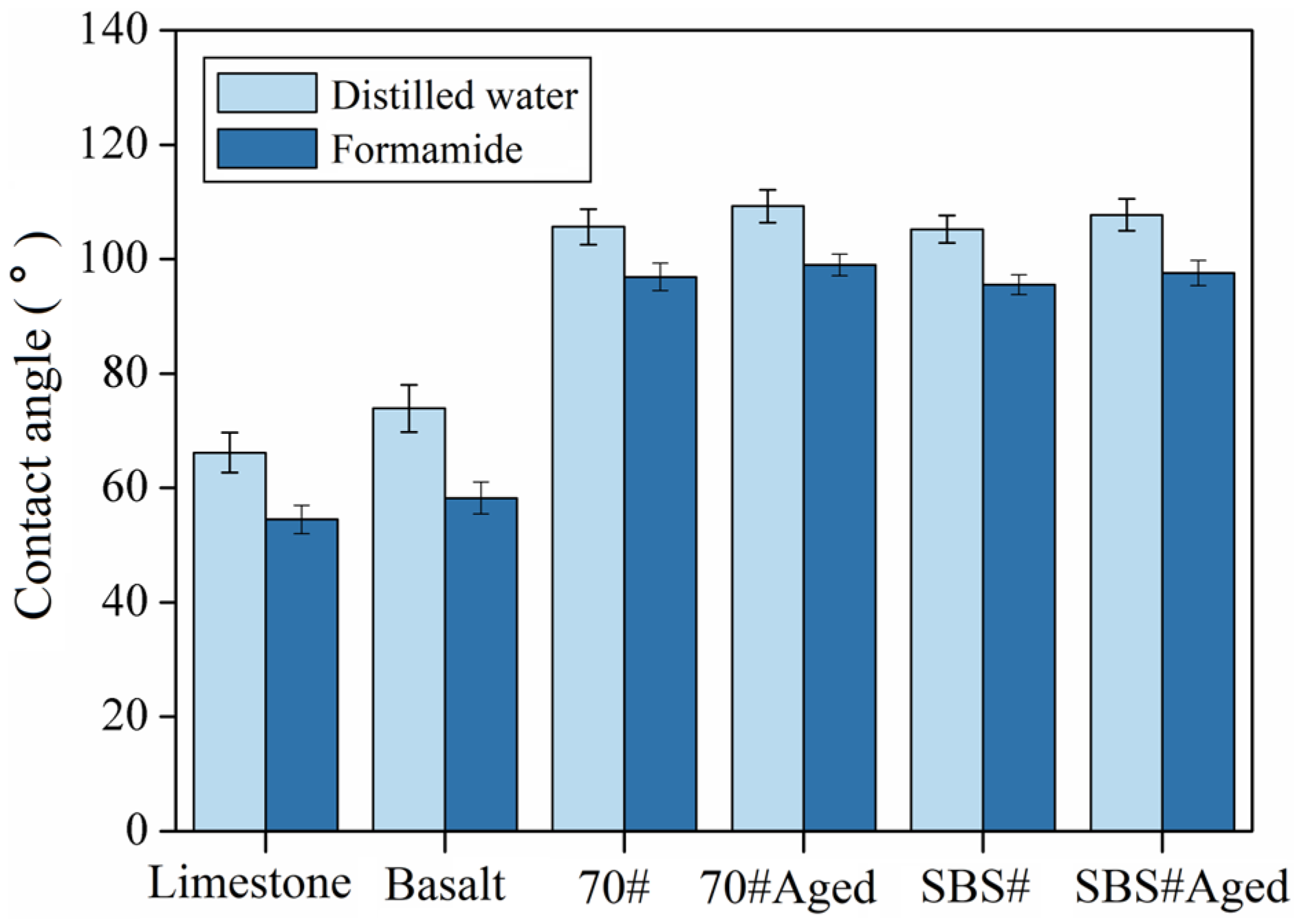

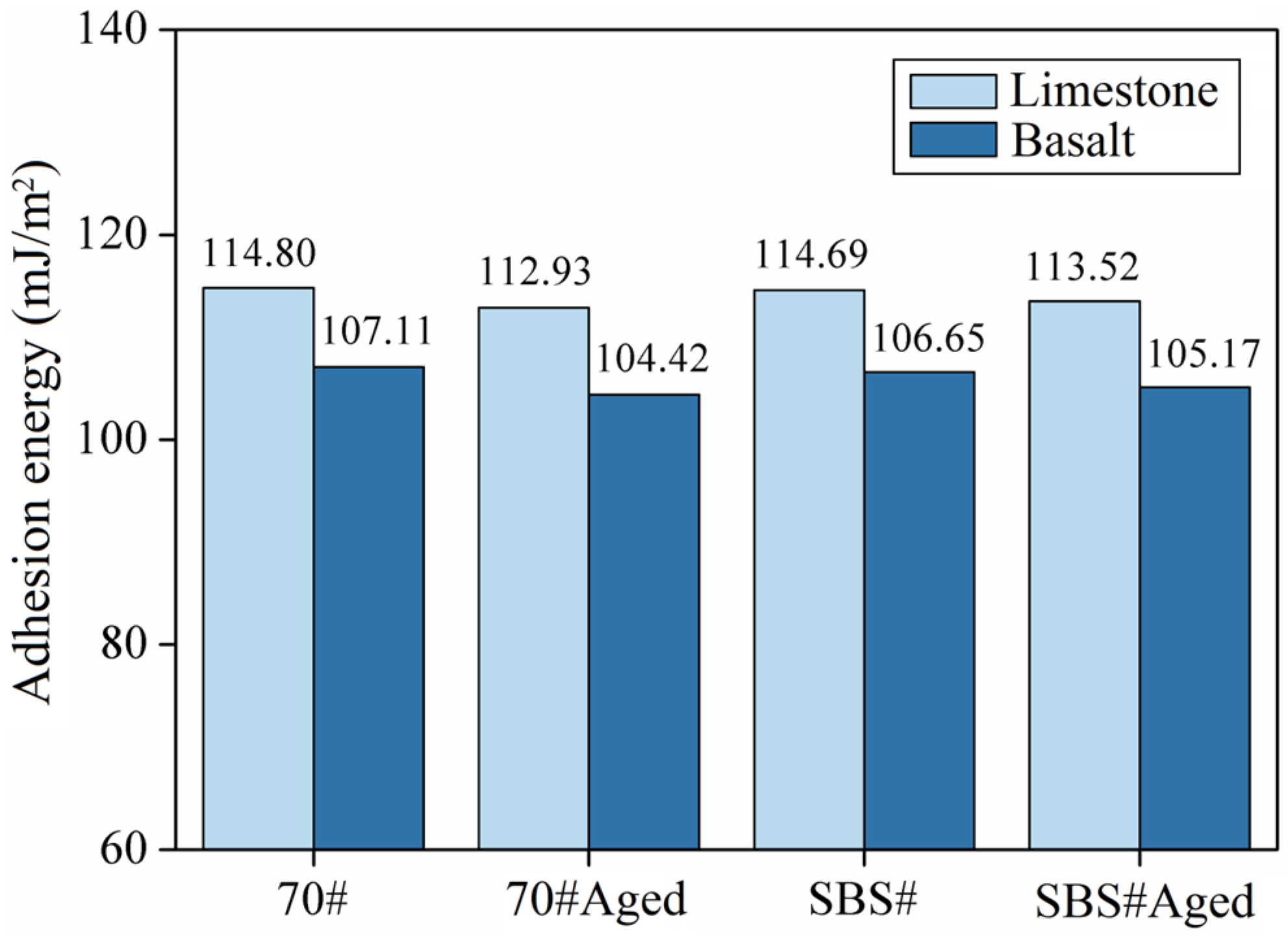


| Type | Apparent Density (g/cm3) | Water Absorption (%) | Crushing Value (%) | Los Angeles Abrasion Loss (%) |
|---|---|---|---|---|
| Limestone | 2.715 | 0.67 | 17.1 | 14.8 |
| Basalt | 2.873 | 0.52 | 13.2 | 12.7 |
| Type | Total Surface Free Energy (mJ/m2) | Dispersive Component (mJ/m2) | Polar Component (mJ/m2) |
|---|---|---|---|
| Distilled Water | 72.8 | 21.8 | 51.0 |
| Formamide | 58.2 | 39.5 | 18.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Wang, Z.; Deng, M.; Tan, S.; Liang, H. Adhesion Characteristics of an Asphalt Binder–Aggregate Interface Based on Molecular Dynamics. Materials 2025, 18, 981. https://doi.org/10.3390/ma18050981
Xiang H, Wang Z, Deng M, Tan S, Liang H. Adhesion Characteristics of an Asphalt Binder–Aggregate Interface Based on Molecular Dynamics. Materials. 2025; 18(5):981. https://doi.org/10.3390/ma18050981
Chicago/Turabian StyleXiang, Hao, Zhengxing Wang, Mingyang Deng, Silu Tan, and Haoning Liang. 2025. "Adhesion Characteristics of an Asphalt Binder–Aggregate Interface Based on Molecular Dynamics" Materials 18, no. 5: 981. https://doi.org/10.3390/ma18050981
APA StyleXiang, H., Wang, Z., Deng, M., Tan, S., & Liang, H. (2025). Adhesion Characteristics of an Asphalt Binder–Aggregate Interface Based on Molecular Dynamics. Materials, 18(5), 981. https://doi.org/10.3390/ma18050981





