Production of Nd and Nd–Fe Alloys from NdCl3 by Calciothermic Reduction
Abstract
1. Introduction
2. Materials and Methods
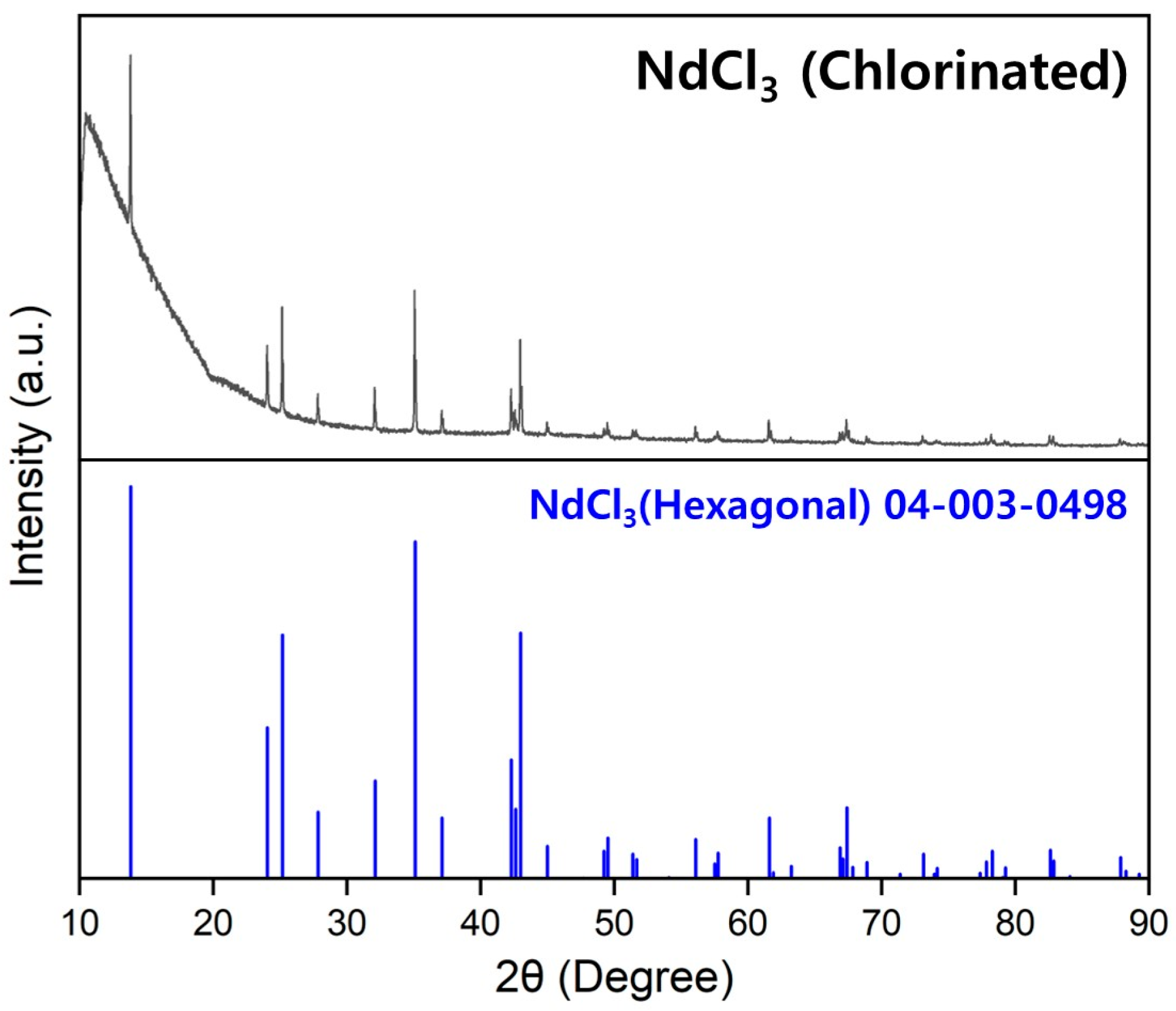
2.1. Materials
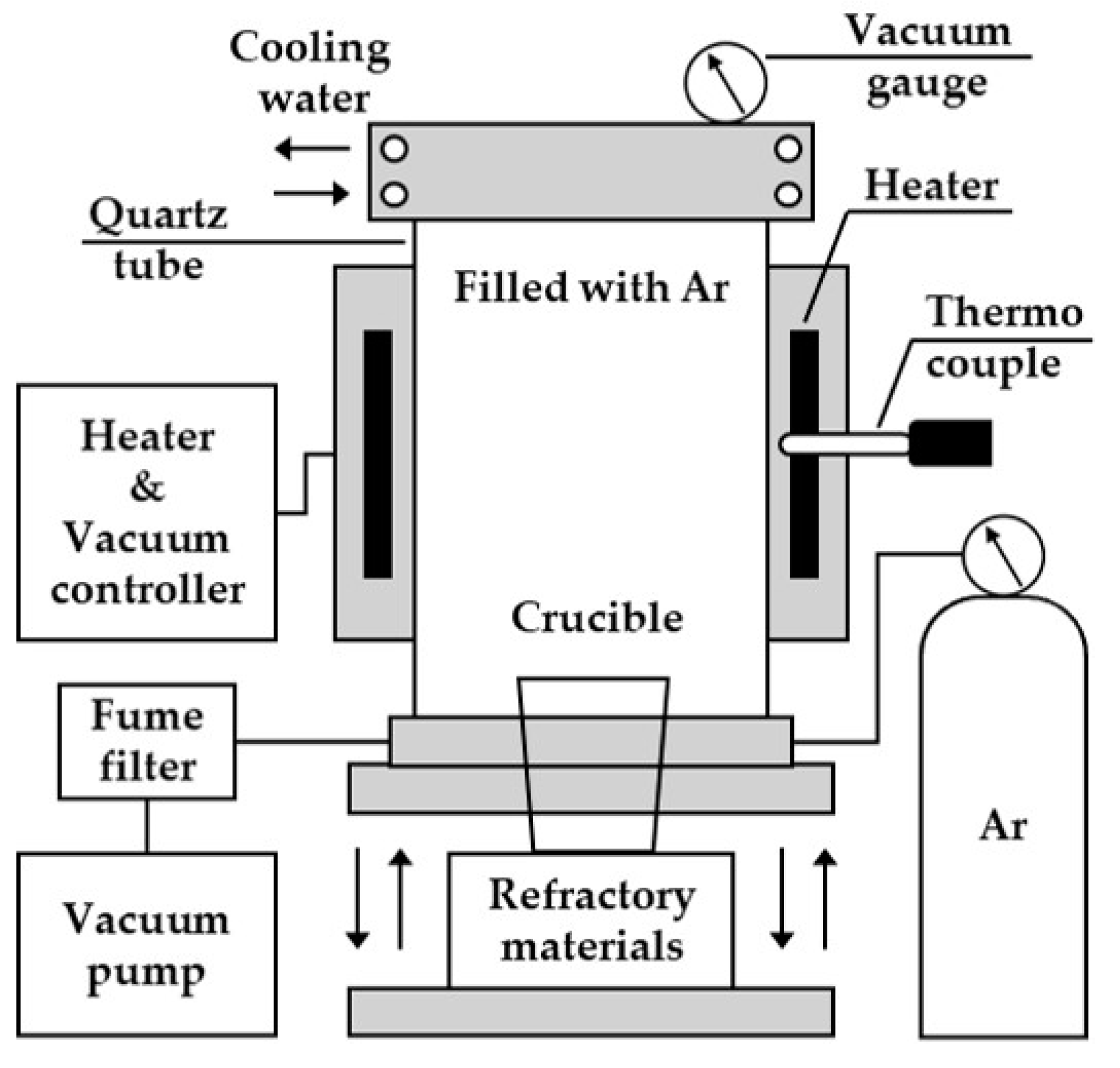
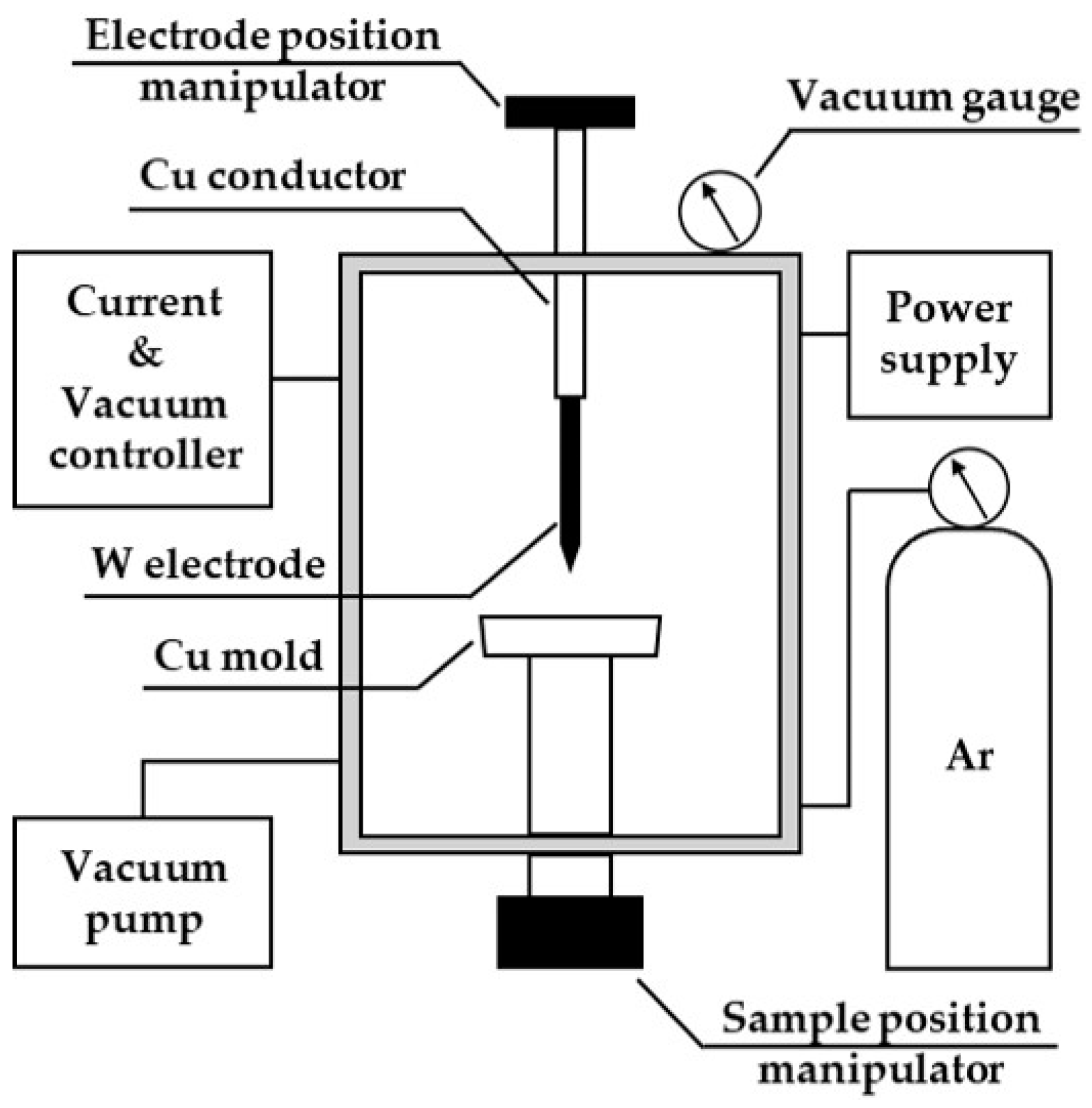
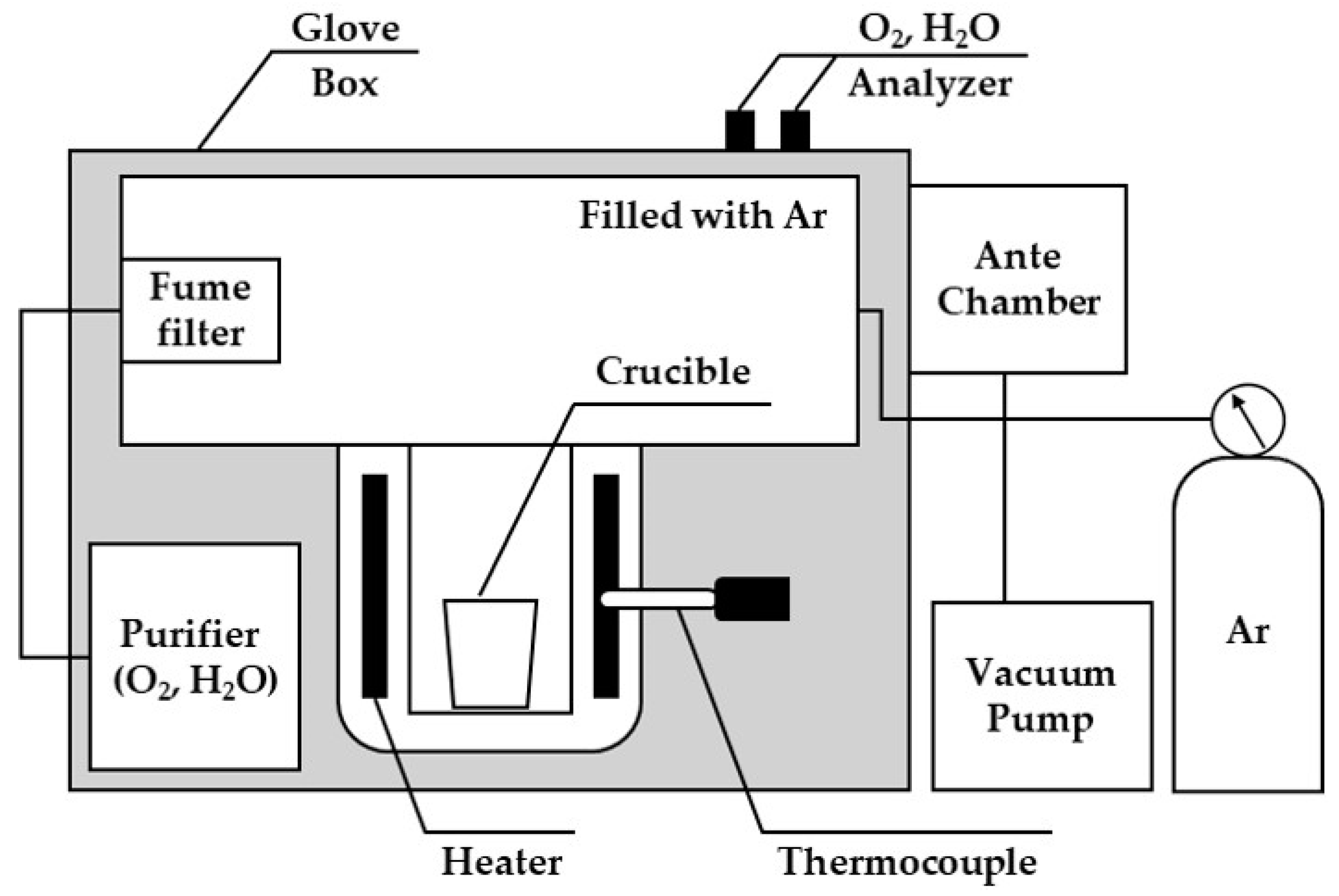
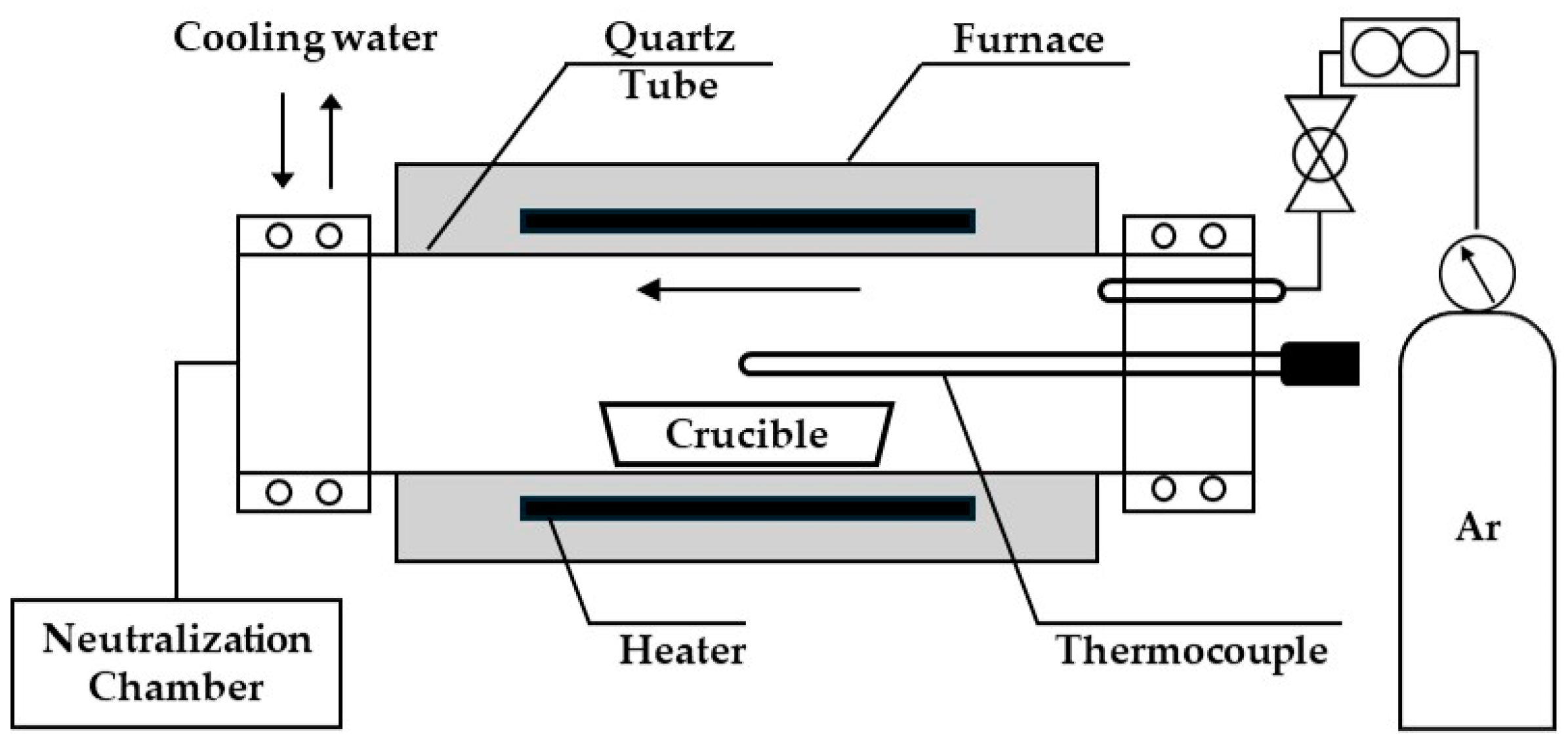
2.2. Experimental Apparatus
2.3. Experimental Analysis
2.4. Experimental Procedure
2.4.1. Drying of the Raw Materials
2.4.2. Vacuum Arc Melting
2.4.3. Metallothermic Reduction
- Manufacture of Nd: Approximately 100 g of arc-melted Nd was added to the bottom of a W crucible. After weighing the raw materials, mixed powders comprising NdCl3 and Ca and KCl and NaCl were sequentially added (Table 1). To minimize reactant evaporation during the reaction process, the upper part of the crucible was sealed with a W cover. The reactor was heated to 1050 °C at a rate of 5 °C/min and maintained for 4 h, with stirring performed at one-hour intervals. Following reaction completion, the liquid product in the crucible was poured into a mold. After natural air cooling, the metal and salt were separated using tools and a mortar, and metal particles of ˃45 μm were recovered following salt crushing. During analytical pre-treatment, cutting processing was conducted inside the glove box to avoid metal oxidation. The CaCl2 and CaCl2–KCl–NaCl crushed to ˂45 μm were sealed in an airtight holder during XRD analysis, considering the reaction with moisture. The W crucible and stirring rod were cleaned with HCl (35%) and reused in subsequent experiments;
- Manufacture of the Nd–Fe alloys: Before adding the reactants, homogenization of the Nd–Fe molten metal bath was performed. After adding Nd and Fe to the W crucible as shown in Table 1, it was heated above the melting point of Nd (to 1100 °C) at a heating rate of 5 °C/min and maintained for 20 min. Stirring was conducted before extracting the crucible, which was naturally air cooled inside a glove box. After adding the measured NdCl3, Ca, and Fe mixed powder to the top of the Nd–Fe alloy layer (Table 1), the temperature was increased to proceed with the reaction. Subsequent steps were performed in the same manner as the Nd manufacturing process.
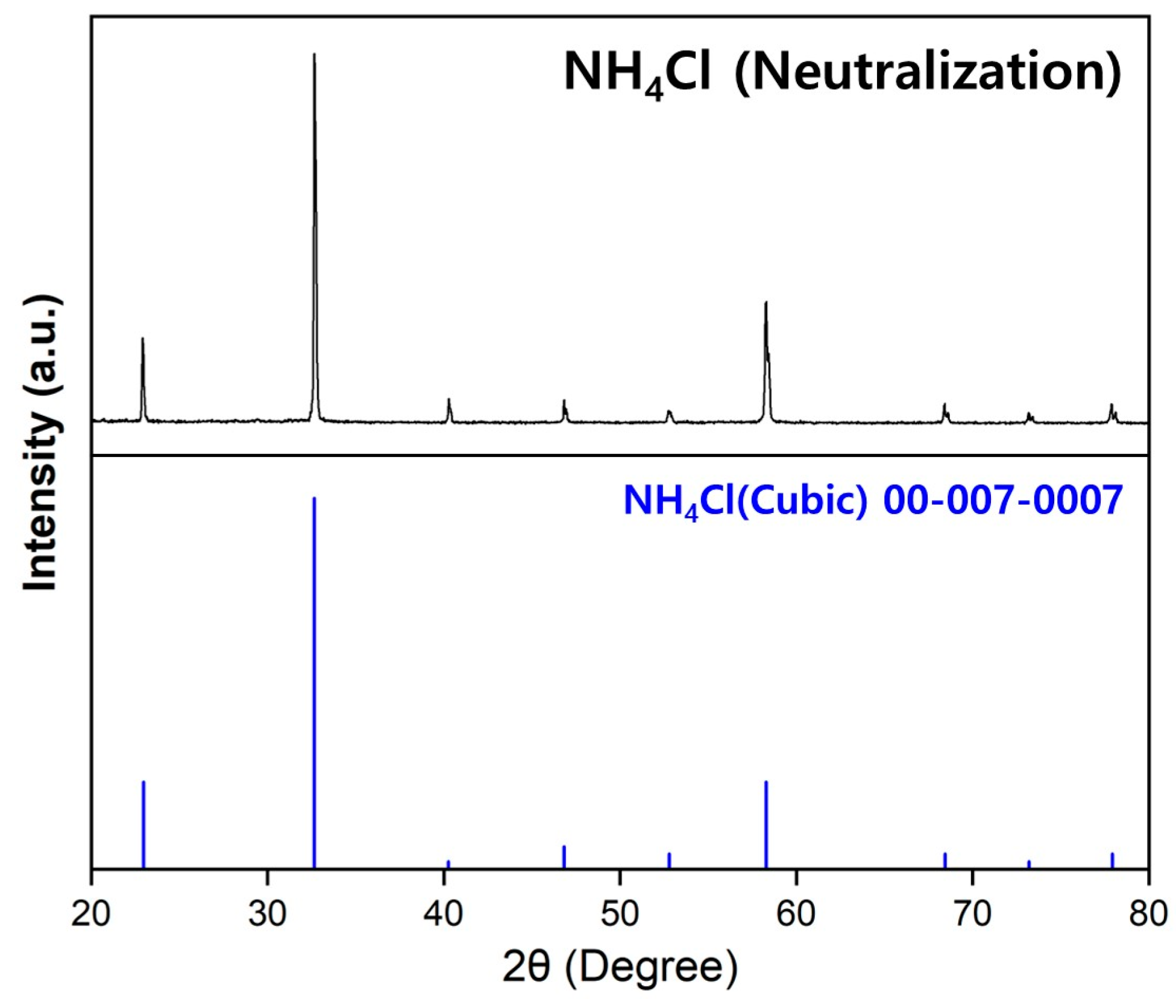
2.4.4. Capture of NH3 Gas
2.5. Variables of the Metallothermic Reduction Process
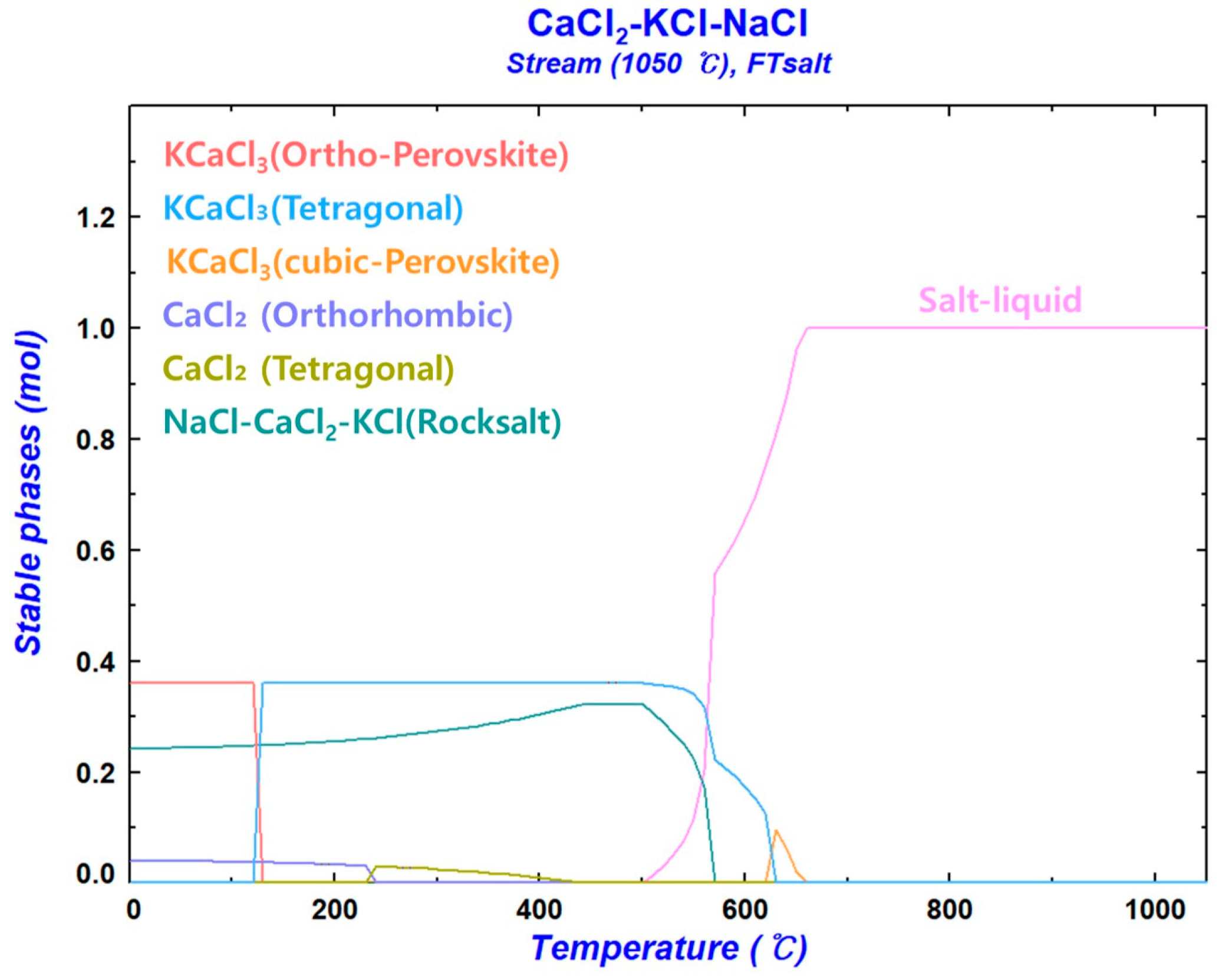
- Production of Nd: Considering the Gibbs free energy of the CaCl2–KCl–NaCl liquid solution derived from the thermodynamic analysis and the melting point of Nd, the reaction temperature was set at 1050 °C. The amounts of reactants were determined based on 100 g of NdCl3. The reductant Ca was used up to a maximum of 31.1859 g (×1.3), and the mass ratio (×1.n) was increased based on the stoichiometric ratio (23.9891 g) with NdCl3. The composition of KCl and NaCl in the CaCl2–KCl–NaCl molten salt was derived from the thermodynamic analysis using FactSage 8.3. The Nd metal used in the molten metal bath was close to 100 g;
- Production of the Nd–Fe Alloys: Considering the Gibbs free energy of the Nd–Fe liquid solution derived from the thermodynamic analysis and the melting point of the Nd–Fe alloy, the reaction temperature was set at 850–1050 °C. The composition of Nd (57.5584 g), which was theoretically producible from 100 g of NdCl3 and Fe, corresponded to the composition of Nd and Fe in the molten metal bath. Thus, the theoretical compositions of the Nd–Fe solution formed from the reactants, molten metal bath, and final product, the Nd–Fe alloy, were identical;
- Controlled Variables: The reaction time was fixed at 4 h under all conditions, and stirring was performed at one-hour intervals during the reaction. To minimize the effects of moisture and oxygen, an Ar atmosphere (O2: 1 ppm, H2O: 1 ppm) was maintained. The experimental conditions and equilibrium compositions are shown in Table 3 and Table 4, respectively.
3. Results and Discussion
3.1. Thermodynamic Considerations
3.1.1. Standard Gibbs Free Energy
3.1.2. CaCl2–KCl–NaCl Molten Salt System
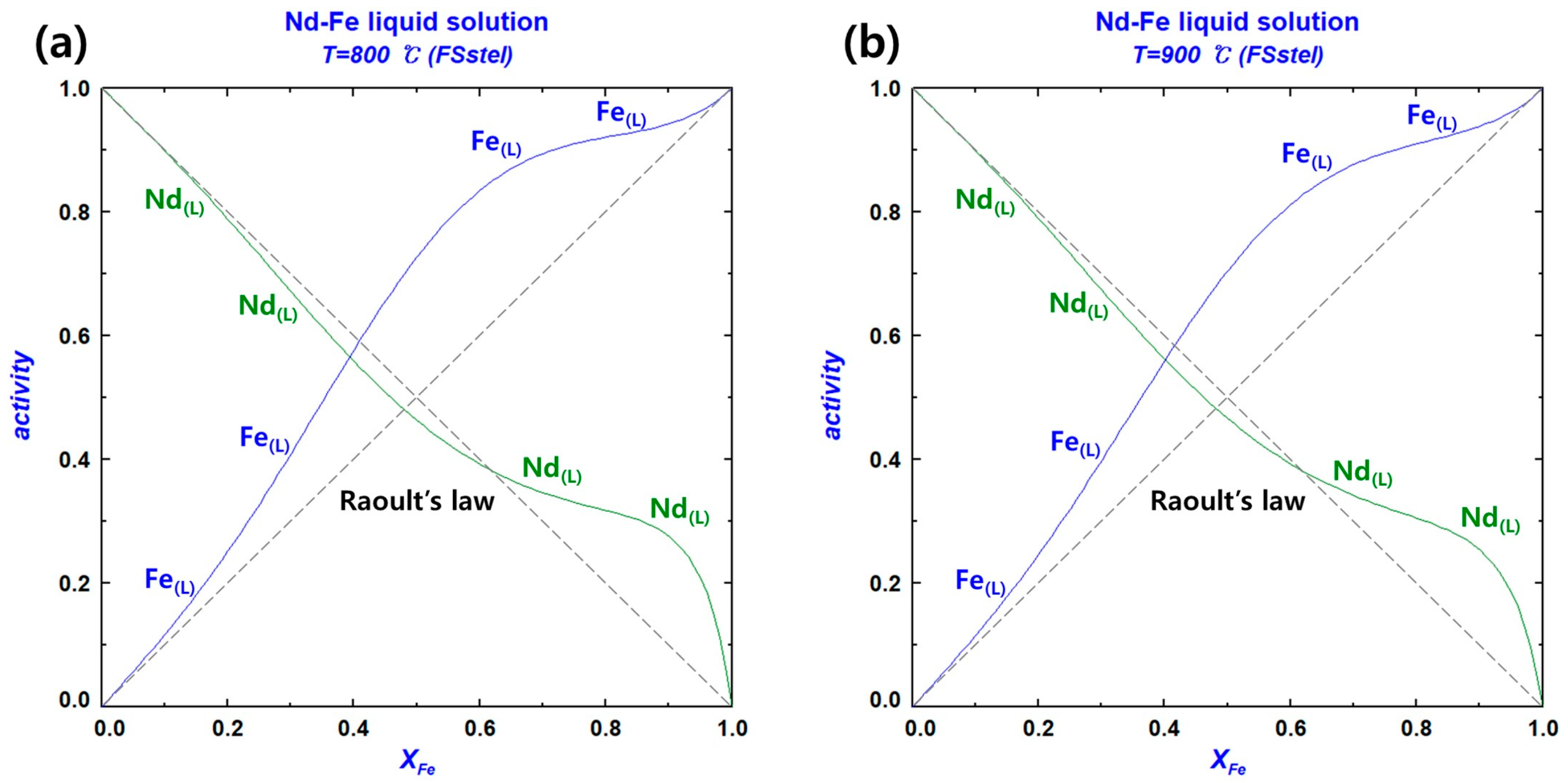
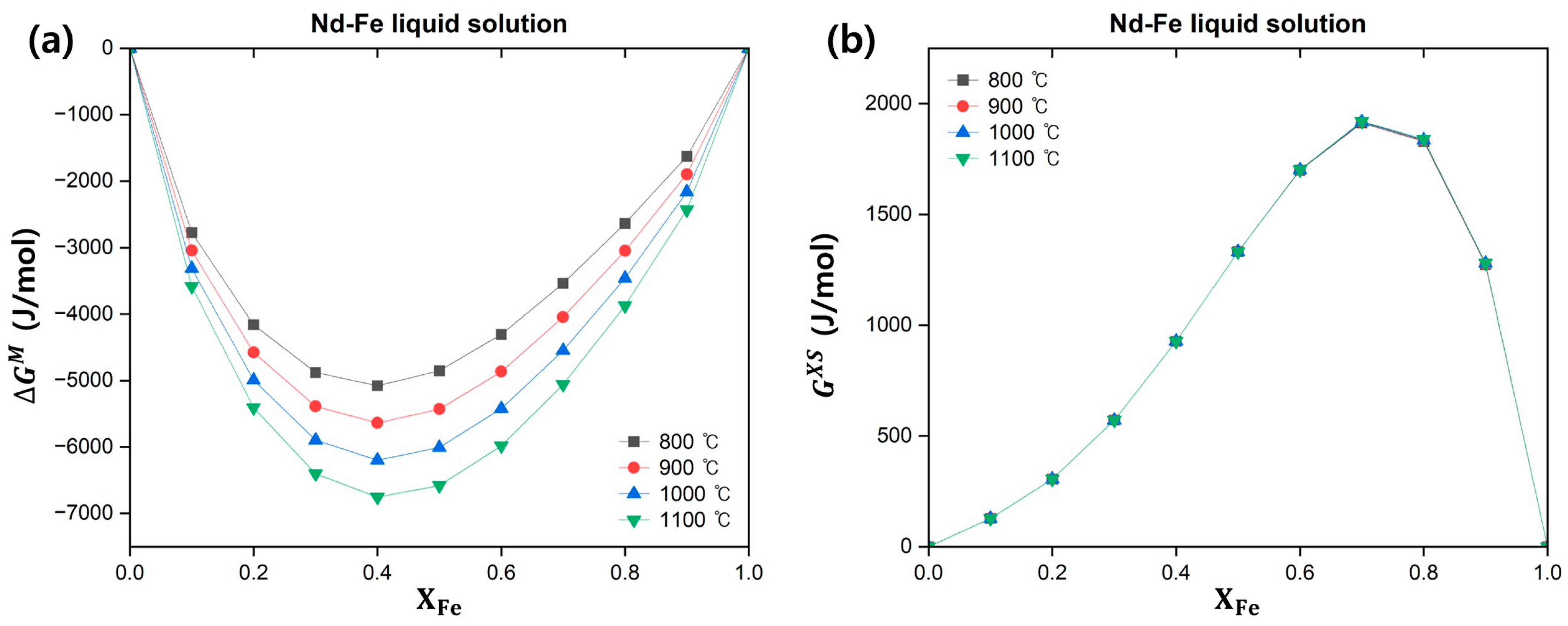
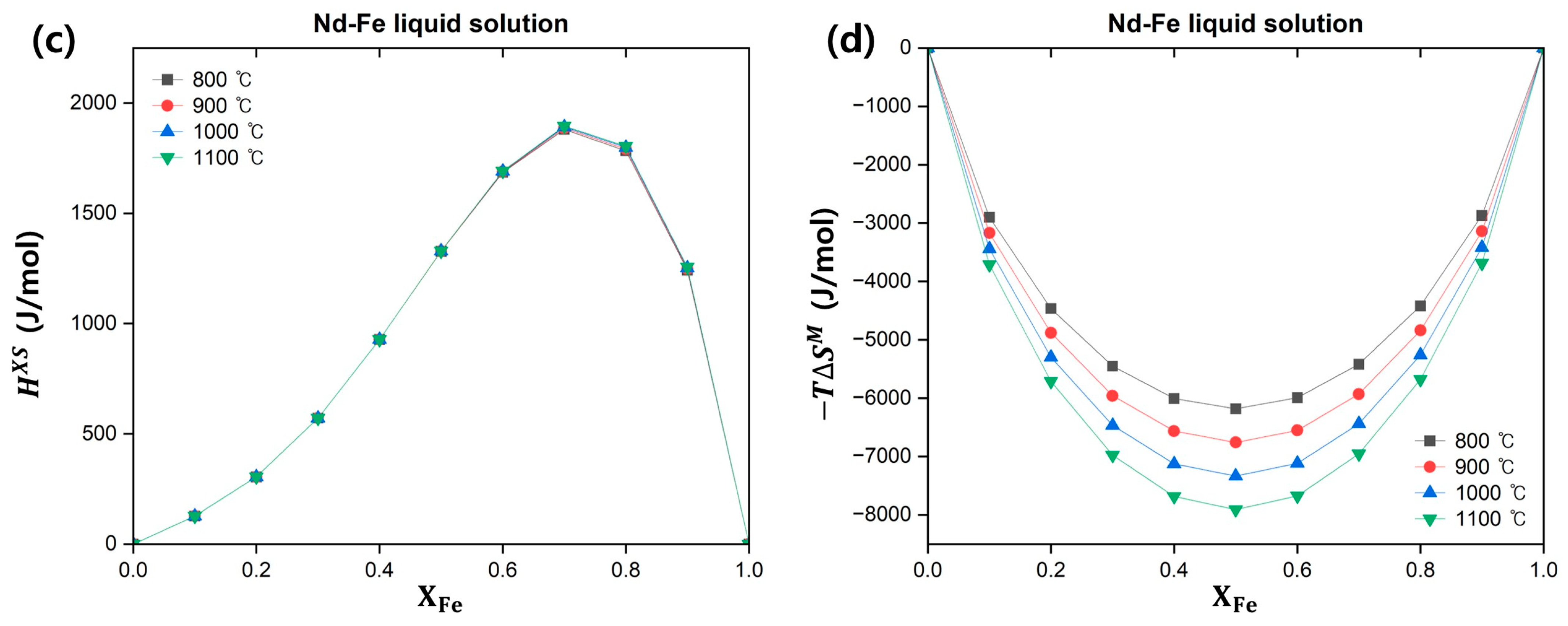
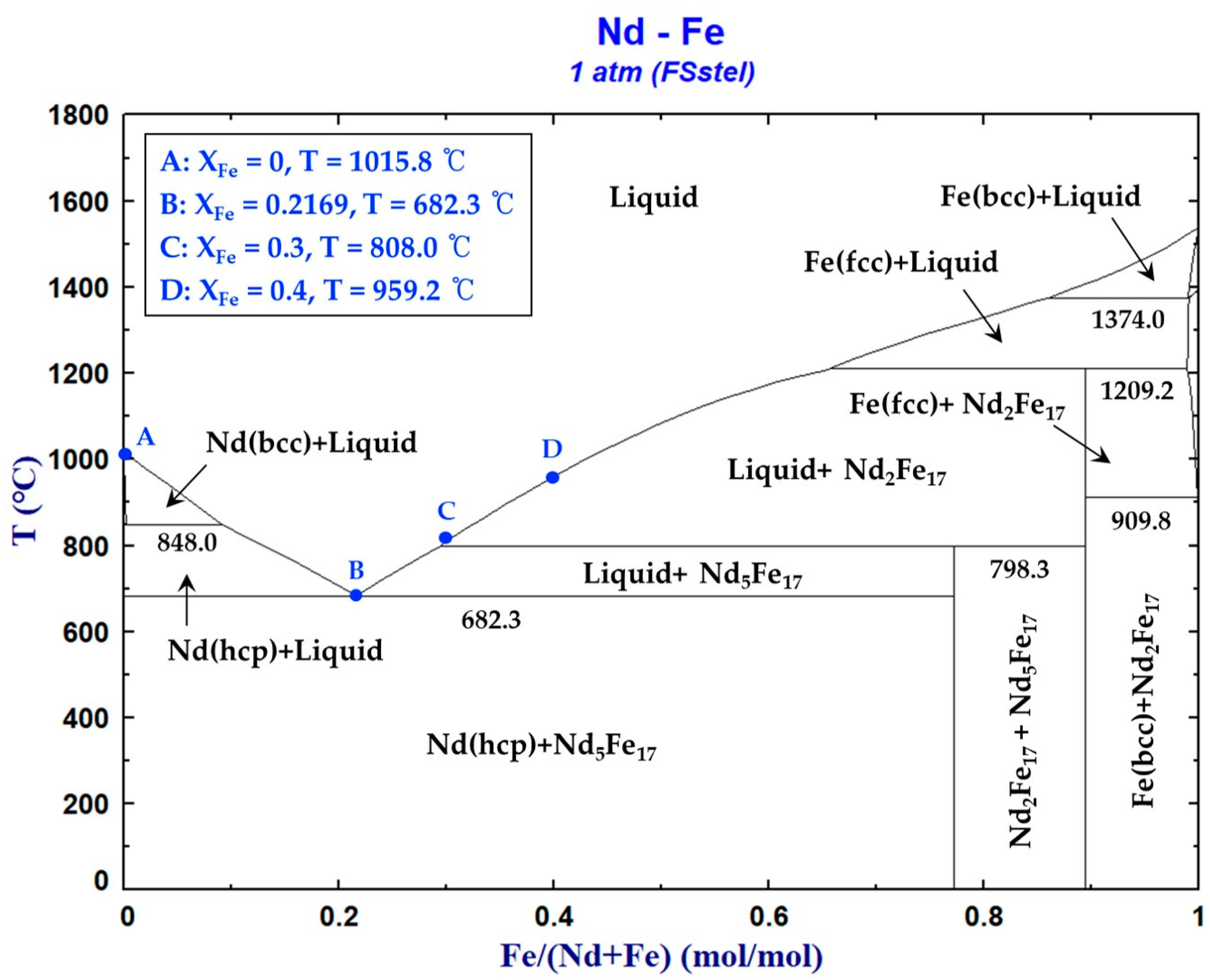
3.1.3. Nd–Fe Binary System
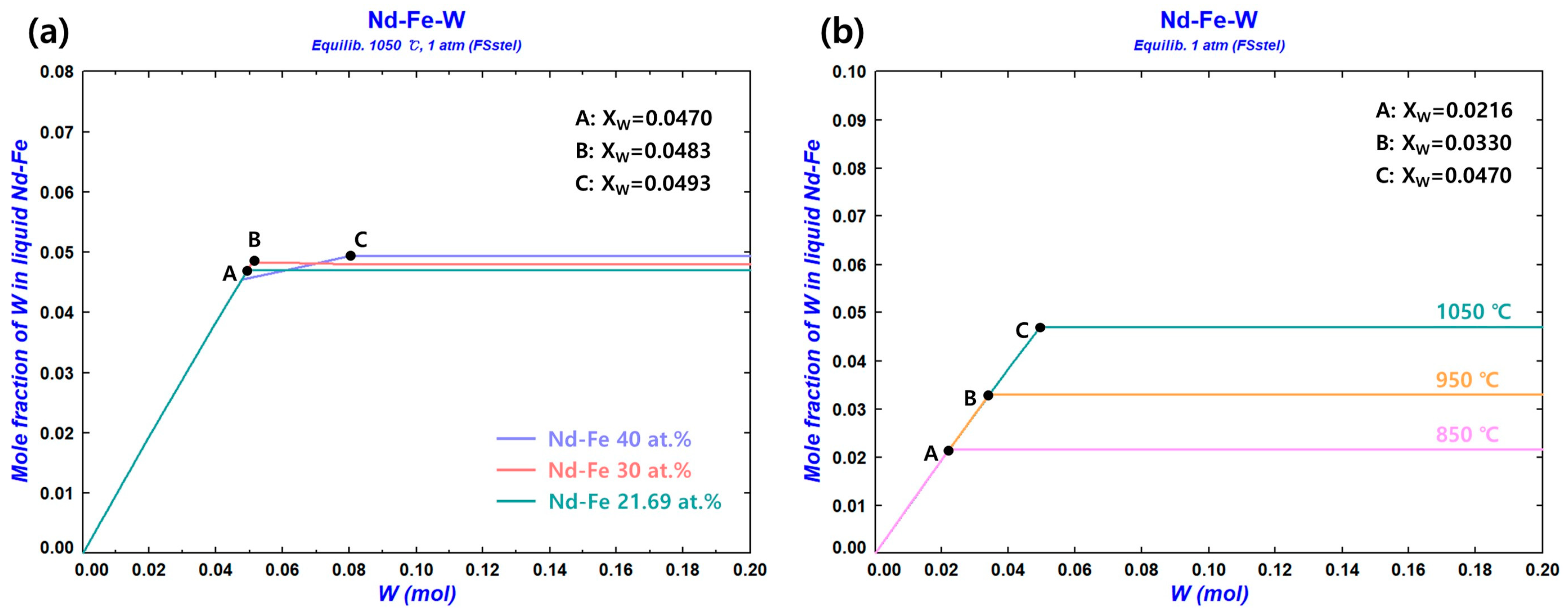
3.1.4. Solubility Limit of the W Crucible
3.2. Metallothermic Reduction Test
3.2.1. Recovery Rate of Nd
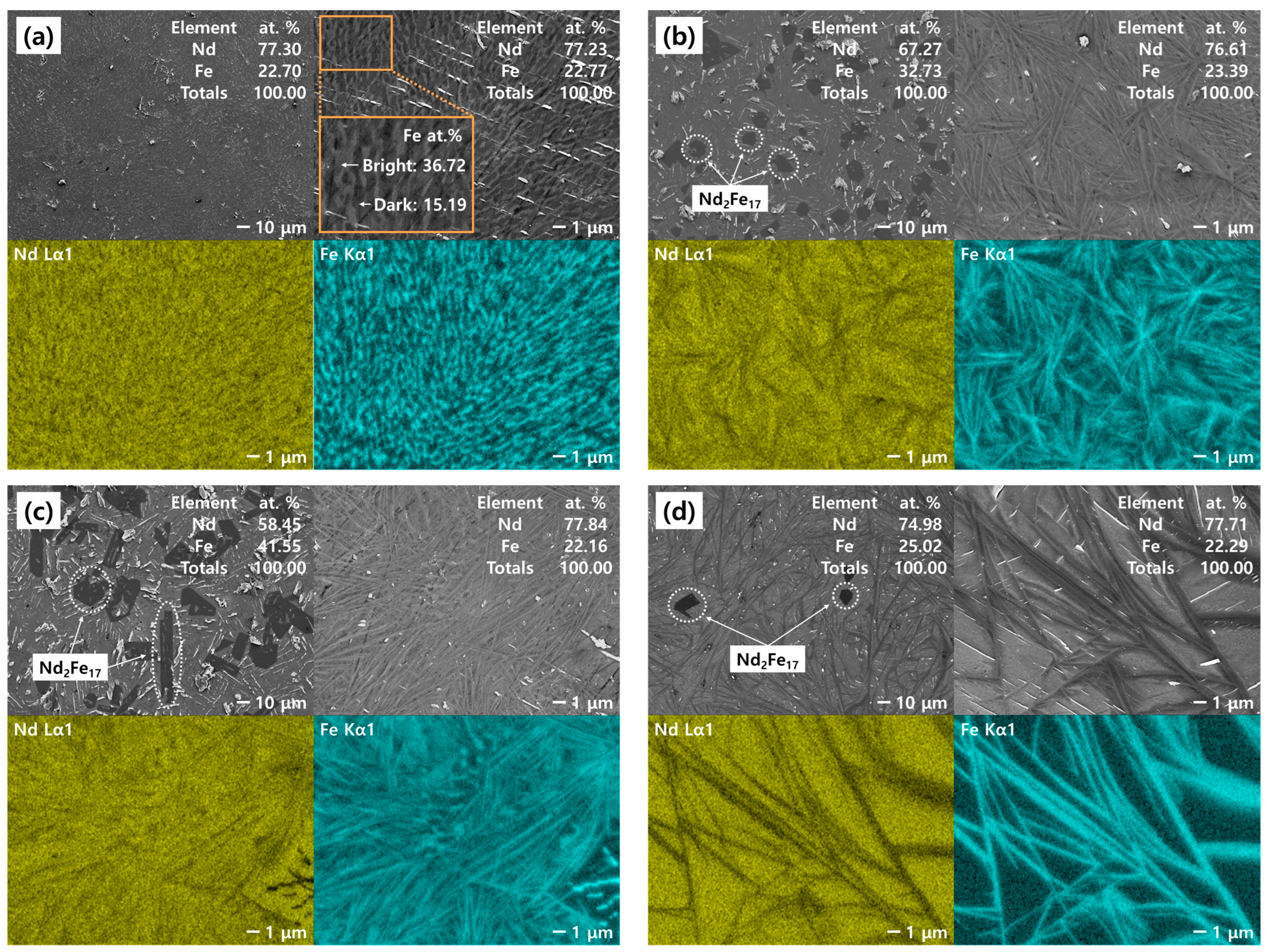
3.2.2. Microstructure of the Nd–Fe Alloys
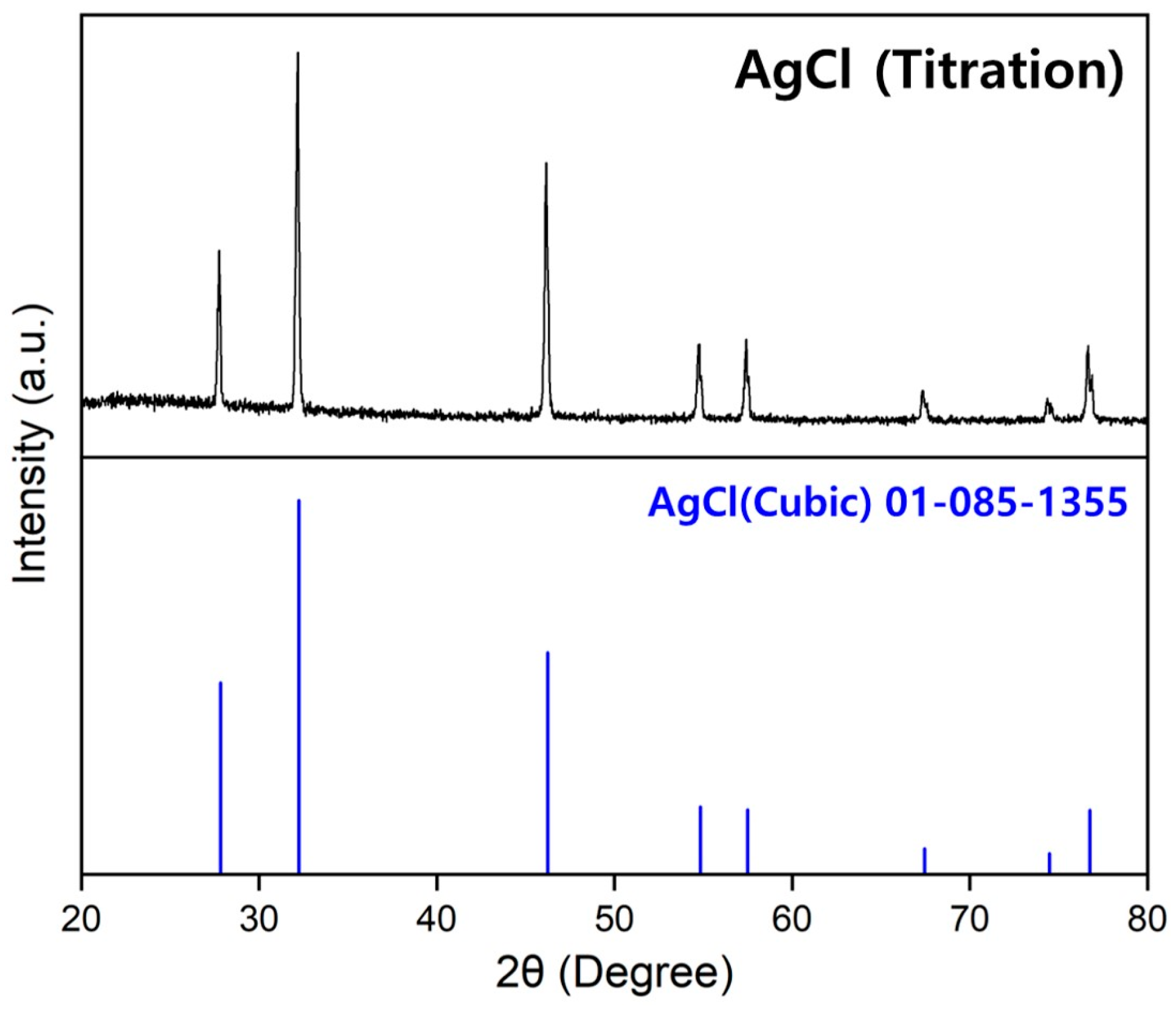
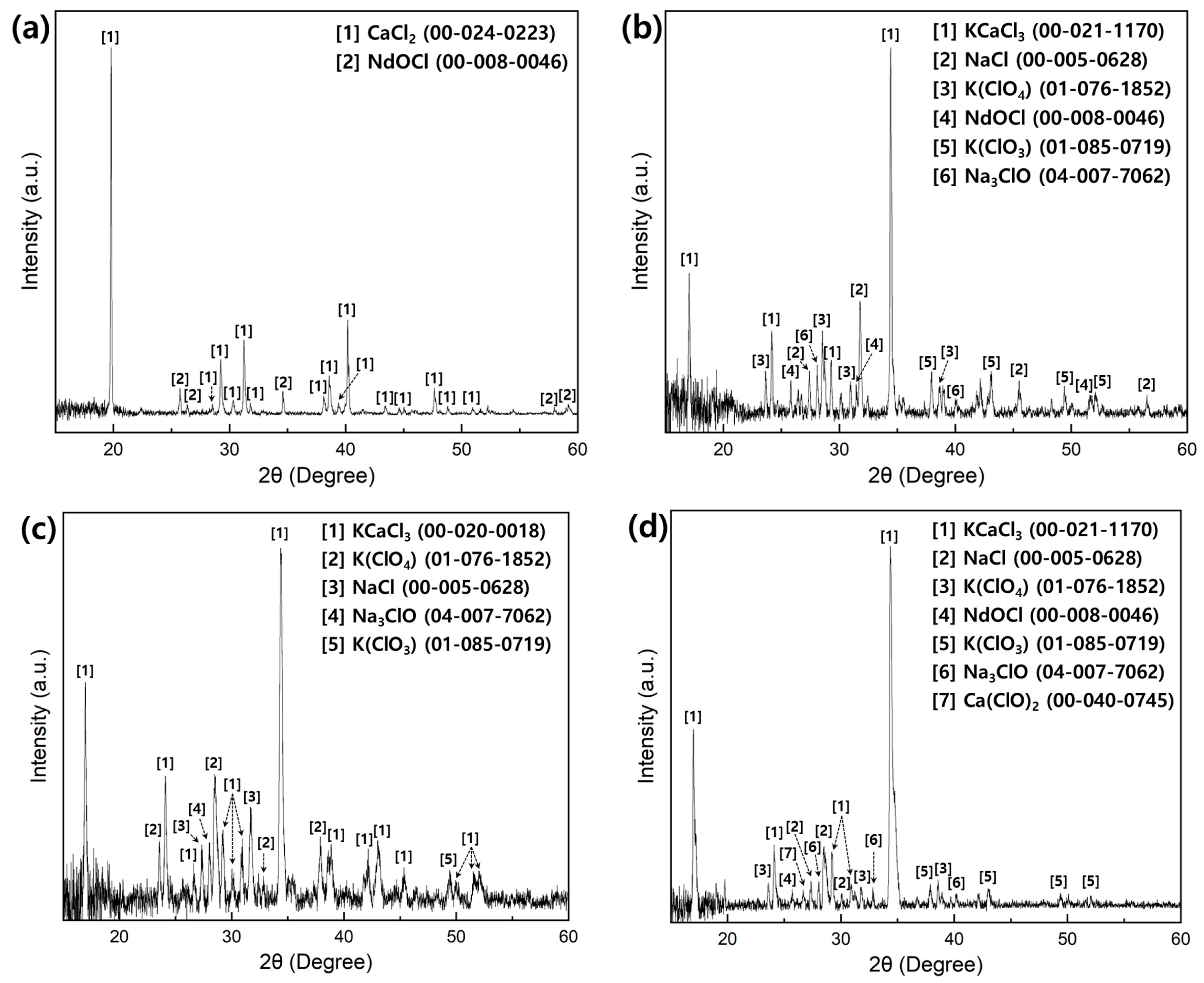
3.2.3. Salt Analysis
3.3. GHG Emissions
3.3.1. Production of NH4Cl and Ca
3.3.2. Recycling of NH3 Gas
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Irle, R. Global EV Sales for 2022. 2003. Available online: https://EV-Volumes.com/news/ev/global-ev-sales-for-2022/ (accessed on 1 July 2024).
- Rinawati, D.I.; Keeley, A.R.; Takeda, S.; Itsubo, N.; Managi, S. Potential for reducing CO2 emissions from passenger cars in Japan by 2030 to achieve carbon neutrality. IATSS Res. 2023, 47, 185–195. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, F.; Hao, H.; Liu, Z. Opportunities, challenges and strategies for developing electric vehicle energy storage systems under the carbon neutrality goal. World Electr. Veh. J. 2023, 14, 170. [Google Scholar] [CrossRef]
- Heim, J.W.; Vander Wal, R.L. NdFeB Permanent Magnet Uses, Projected Growth Rates and Nd Plus Dy Demands across End-Use Sectors through 2050: A Review. Minerals 2023, 13, 1274. [Google Scholar] [CrossRef]
- Pathapati, S.V.S.H.; Free, M.L.; Sarswat, P.K. A comparative study on recent developments for individual rare earth elements separation. Processes 2023, 11, 2070. [Google Scholar] [CrossRef]
- Stefanidaki, E.; Hasiotis, C.; Kontoyannis, C. Electrodeposition of neodymium from LiF–NdF3–Nd2O3 melts. Electrochim. Acta 2001, 46, 2665–2670. [Google Scholar] [CrossRef]
- Sarfo, P.; Das, A.; Young, C. Extraction and optimization of neodymium from molten fluoride electrolysis. Sep. Purif. Technol. 2021, 256, 117770. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Li, B.; Wang, L.; Yan, B.; Liu, M. Anode processes for Nd electrowinning from LiF-NdF3-Nd2O3 melt. Electrochim. Acta 2014, 147, 82–86. [Google Scholar] [CrossRef]
- Schreiber, A.; Marx, J.; Zapp, P.; Kuckshinrichs, W. Comparative life cycle assessment of neodymium oxide electrolysis in molten salt. Adv. Eng. Mater. 2020, 22, 1901206. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Chapter 2. [Google Scholar]
- Kim, J.; Thompson, R.; Park, H.; Bogle, S.; Mühle, J.; Park, M.-K.; Kim, Y.; Harth, C.M.; Salameh, P.K.; Schmidt, R.; et al. Emissions of tetrafluoromethane (CF4) and hexafluoroethane (C2F6) from East Asia: 2008 to 2019. J. Geophys. Res. Atmos. 2021, 126, e2021JD034888. [Google Scholar] [CrossRef] [PubMed]
- Say, D.; Manning, A.J.; Western, L.M.; Young, D.; Wisher, A.; Rigby, M.; Reimann, S.; Vollmer, M.K.; Maione, M.; Arduini, J. Global trends and European emissions of tetrafluoromethane (CF4), hexafluoroethane (C2F6) and octafluoropropane (C3F8). Atmos. Chem. Phys. Discuss. 2020, 21, 2149–2164. [Google Scholar] [CrossRef]
- Guan, X.; Pal, U.B.; Jiang, Y.; Su, S. Clean metals production by solid oxide membrane electrolysis process. J. Sustain. Metall. 2016, 2, 152–166. [Google Scholar] [CrossRef]
- Kvande, H.; Haupin, W. Inert anodes for AI smelters: Energy balances and environmental impact. JOM 2001, 53, 29–33. [Google Scholar] [CrossRef]
- Keniry, J. The economics of inert anodes and wettable cathodes for aluminum reduction cells. JOM 2001, 53, 43–47. [Google Scholar] [CrossRef]
- Krishnan, A.; Pal, U.B.; Lu, X.G. Solid oxide membrane process for magnesium production directly from magnesium oxide. Metall. Mater. Trans. B 2005, 36, 463–473. [Google Scholar] [CrossRef]
- Sharma, R.A. Neodymium production processes. JOM 1987, 39, 33–37. [Google Scholar] [CrossRef]
- Sharma, R.A.; Seefurth, R.N. Metallothermic Reduction of Nd2O3 with Ca in CaCl2-NaCl Melts. J. Electrochem. Soc. 1988, 135, 66. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Oxide reduction processes in the preparation of rare-earth metals. Min. Metall. Explor. 2013, 30, 38–44. [Google Scholar] [CrossRef]
- Rodliyah, I.; Septiarani, A.; Mamby, H.E.; Zulhan, Z.; Sitompul, C.A. Thermodynamic simulation and validation experiment of neodymium oxide reduction into metallic neodymium by metallothermic process. Indones. Min. J. 2018, 21, 21–34. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, F.; Liu, X.; Zhang, L. CaO solubility and activity coefficient in molten salts CaCl2–x (x = 0, NaCl, KCl, SrCl2, BaCl2 and LiCl). Thermochim. Acta 2008, 470, 105–107. [Google Scholar] [CrossRef]
- Novoselova, A.; Smolenski, V. Electrochemical behavior of neodymium compounds in molten chlorides. Electrochim. Acta 2023, 87, 657–662. [Google Scholar] [CrossRef]
- Im, S.; Smith, N.D.; Castro Baldivieso, S.C.; Gesualdi, J.; Liu, Z.-K.; Kim, H. Electrochemical recovery of Nd using liquid metals (Bi and Sn) in LiCl-KCl-NdCl3. Electrochim. Acta 2022, 425, 140655. [Google Scholar] [CrossRef]
- Akolkar, R. Perspective—Is sustainable electrowinning of neodymium metal achievable? J. Electrochem. Soc. 2022, 169, 043501. [Google Scholar] [CrossRef]
- Vasilenko, D.Y.; Shitov, A.V.; Vlasyuga, A.V.; Popov, A.G.; Kudrevatykh, N.V.; Pechischeva, N.V. Microstructure and properties of Nd–Fe–B alloys produced by strip casting and of permanent magnets fabricated from them. Met. Sci. Heat Treat. 2015, 56, 585–590. [Google Scholar] [CrossRef]
- Lei, M.; He, Y.; Ma, W.; Zhang, R.; Lei, Y. Novel approach for the simultaneous recovery of Nd from Nd2O3-Containing slag and the preparation of high-purity Si. ACS Sustain. Chem. Eng. 2021, 9, 15591–15602. [Google Scholar] [CrossRef]
- Yu, J.-W.; Wang, J.-P. A Study on the Production of Anhydrous Neodymium Chloride through the Chlorination Reaction of Neodymium Oxide and Ammonium Chloride. Minerals 2024, 14, 480. [Google Scholar] [CrossRef]
- Akpanyung, K.V.; Loto, R.T.; Fajobi, M.A. An overview of ammonium chloride (NH4Cl) corrosion in the refining unit. J. Phys. Conf. Ser. 2019, 1378, 022089. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and recent progress on ammonia synthesis catalysts for Haber–Bosch process. Adv. Energy Sustain. Res. 2021, 2, 2000043. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An electrochemical haber-bosch process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Firdaus, M.; Rhamdhani, M.A.; Durandet, Y.; Rankin, W.J.; Mcgregor, K. Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste. J. Sustain. Metall. 2016, 2, 276–295. [Google Scholar] [CrossRef]
- Barker, R. The reversibility of the reaction CaCO3⇄ CaO+ CO2. J. Appl. Chem. Biotechnol. 1973, 23, 733–742. [Google Scholar] [CrossRef]
- Jacob, K.T.; Srikanth, S. Physical chemistry of the reduction of calcium oxide with aluminum in vacuum. High Temp. Mater. Process. 1990, 9, 77–92. [Google Scholar] [CrossRef]




| GHG | Chemical Formula | Lifetime (Years) | GWP100 |
|---|---|---|---|
| Carbon dioxide | CO2 | - | 1 |
| Carbon tetrafluoride (PFC–14) | CF4 | 50,000 | 7390 |
| Hexafluoroethane (PFC–116) | C2F6 | 10,000 | 12,200 |
| GHG | Emissions | GWP100 | CO2e (kg) | |
|---|---|---|---|---|
| Individual | Totals | |||
| CO | 0.211 kg | - | - | 1.403 |
| CO2 | 0.055 kg | 1 | 0.387 | |
| CF4 | 0.118 g | 7390 | 0.872 | |
| C2F6 | 0.0118 g | 12,200 | 0.14396 | |
| No. | Temp. (°C) | Time (h) | Reactants (g) | Molten Metal Bath | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass (g) | Mole Fraction | ||||||||||
| NdCl3 | Ca | KCl | NaCl | Fe | Nd | Fe | |||||
| N1 | 1050 | 4 | 100 | 23.9891 | - | - | - | - | - | - | - |
| N2 | 1050 | 4 | 100 | 23.9891 | - | - | - | 100.6151 | - | 1 | 0 |
| N3 | 1050 | 4 | 100 | 23.9891 | 40.1612 | 20.9889 | 100.0425 | - | 1 | 0 | |
| N4 | 1050 | 4 | 100 | 26.3880 | 40.1612 | 20.9889 | 100.7577 | - | 1 | 0 | |
| N5 | 1050 | 4 | 100 | 28.7870 | 40.1612 | 20.9889 | 100.2423 | - | 1 | 0 | |
| N6 | 1050 | 4 | 100 | 31.1859 | 40.1612 | 20.9889 | 100.8409 | - | 1 | 0 | |
| NF1 | 1050 | 4 | 100 | 23.9891 | - | - | 6.1723 | 90.6053 | 9.7160 | 0.7831 | 0.2169 |
| NF2 | 1050 | 4 | 100 | 23.9891 | - | - | 9.5505 | 85.9442 | 14.2604 | 0.7 | 0.3 |
| NF3 | 1050 | 4 | 100 | 23.9891 | - | - | 14.8563 | 79.8113 | 20.5999 | 0.6 | 0.4 |
| NF4 | 950 | 4 | 100 | 23.9891 | - | - | 6.1723 | 90.6322 | 9.7189 | 0.7831 | 0.2169 |
| NF5 | 850 | 4 | 100 | 23.9891 | - | - | 6.1723 | 90.7136 | 9.7276 | 0.7831 | 0.2169 |
| No. | Nd/Nd–Fe Liquid Solution | Molten Salt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nd (g) | NdFe (g) | CaCl2 (g) | KCl (g) | NaCl (g) | ||||||
| N1 | 57.5584 | - | 1 | 0 | 66.4307 | - | - | 1 | 0 | 0 |
| N2 | 158.1735 | - | 1 | 0 | 66.4307 | - | - | 1 | 0 | 0 |
| N3 | 157.6009 | - | 1 | 0 | 66.4307 | 40.1612 | 20.9889 | 0.40 | 0.36 | 0.24 |
| N4 | 158.3161 | - | 1 | 0 | 66.4307 | 40.1612 | 20.9889 | 0.40 | 0.36 | 0.24 |
| N5 | 157.8007 | - | 1 | 0 | 66.4307 | 40.1612 | 20.9889 | 0.40 | 0.36 | 0.24 |
| N6 | 158.3993 | - | 1 | 0 | 66.4307 | 40.1612 | 20.9889 | 0.40 | 0.36 | 0.24 |
| NF1 | - | 164.0520 | 0.7831 | 0.2169 | 66.4307 | - | - | 1 | 0 | 0 |
| NF2 | - | 167.3135 | 0.7 | 0.3 | 66.4307 | - | - | 1 | 0 | 0 |
| NF3 | - | 172.8259 | 0.6 | 0.4 | 66.4307 | - | - | 1 | 0 | 0 |
| NF4 | - | 164.0818 | 0.7831 | 0.2169 | 66.4307 | - | - | 1 | 0 | 0 |
| NF5 | - | 164.1719 | 0.7831 | 0.2169 | 66.4307 | - | - | 1 | 0 | 0 |
| Reaction Formula | (J) | Temp. Range (K) |
|---|---|---|
| 2NdCl3(l) + 3Ca(s)→2Nd(s) + 3CaCl2(s) | 1,279,620 − 11,773.1 × T − 1.2 × 10−1 × T2 − 1.9 × 107 × T−1 −3.6 × 10−6 × T3 − 5.9 × 105 × lnT + 1.8 × 105 × T0.5 + 1232.3 × TlnT | 1032–1045 (CaCl2: Rutile→Liquid) |
| 2NdCl3(l) + 3Ca(s)→2Nd(s) + 3CaCl2(l) | −81,266 + 684.2 × T + 4.8 × 10−2×T2 − 6.7 × 106 × T−1 − 3.6 × 10−6 × T3 −5.1 × 104 × lnT − 91.7 × TlnT | 1045–1115 (Ca: β→Liquid) |
| 2NdCl3(l) + 3Ca(l)→2Nd(s) + 3CaCl2(l) | −341,380 − 300.5 × T − 8.4 × 10−3 × T2 − 3.6 × 10−6 × T3 + 44.8 × TlnT | 1115–1128 (Nd: HCP→BCC) |
| 2NdCl3(l) + 3Ca(l)→2Nd(s) + 3CaCl2(l) | −363,472 + 11.5 × T + 1.2 × TlnT | 1128–1289 (Nd: BCC→Liquid) |
| 2NdCl3(l) + 3Ca(l)→2Nd(l) + 3CaCl2(l) | −360,082 + 69.4 × T − 7.3 × TlnT | 1289–1774 (Ca: Liquid→Gas) |
| Temp. (°C) | (J/mol) | (J/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| 800 | 0.40 | 0.36 | 0.24 | 0.4990 | 0.5034 | 0.9025 | −14,514 | −4905 |
| 900 | 0.5393 | 0.5536 | 0.9098 | −15,211 | −4707 | |||
| 1000 | 0.5756 | 0.5991 | 0.9159 | −15,913 | −4514 | |||
| 1100 | 0.6086 | 0.6403 | 0.9210 | −16,619 | −4325 |
| 800 °C | 900 °C | 1000 °C | 1100 °C | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| 0.1 | 0.1172 | 0.8985 | 0.1156 | 0.8986 | 0.1143 | 0.8987 | 0.1132 | 0.8988 |
| 0.2 | 0.2517 | 0.7883 | 0.2469 | 0.7893 | 0.2428 | 0.7901 | 0.2394 | 0.7908 |
| 0.3 | 0.4094 | 0.6714 | 0.3987 | 0.6738 | 0.3899 | 0.6758 | 0.3825 | 0.6775 |
| 0.4 | 0.5772 | 0.5588 | 0.5594 | 0.5622 | 0.5449 | 0.5651 | 0.5328 | 0.5675 |
| 0.5 | 0.7275 | 0.4632 | 0.7048 | 0.4661 | 0.6862 | 0.4686 | 0.6706 | 0.4708 |
| 0.6 | 0.8350 | 0.3922 | 0.8123 | 0.3926 | 0.7936 | 0.3930 | 0.7780 | 0.3933 |
| 0.7 | 0.8943 | 0.3461 | 0.8765 | 0.3416 | 0.8617 | 0.3379 | 0.8492 | 0.3348 |
| 0.8 | 0.9209 | 0.3175 | 0.9104 | 0.3053 | 0.9015 | 0.2953 | 0.8939 | 0.2871 |
| 0.9 | 0.9424 | 0.2755 | 0.9387 | 0.2534 | 0.9356 | 0.2361 | 0.9330 | 0.2221 |
| 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| 800 °C | 900 °C | 1000 °C | 1100 °C | |||||
|---|---|---|---|---|---|---|---|---|
(J/mol) | (J/mol) | (J/mol) | (J/mol) | (J/mol) | (J/mol) | (J/mol) | (J/mol) | |
| 0.2169 | −4323 | 343 | −4758 | 343 | −5192 | 343 | −5627 | 343 |
| 0.3 | −4879 | 572 | −5387 | 572 | −5895 | 572 | −6403 | 572 |
| 0.4 | −5077 | 928 | −5637 | 928 | −6196 | 928 | −6756 | 928 |
| Sample | Ca | K | Na | W | Cl | C | O | Total (wt.) |
|---|---|---|---|---|---|---|---|---|
| Nd | 0.0739 | 0.0366 | 0.0344 | 0.0424 | 0.0415 | 0.9207 | 0.0452 | 1.1948 |
| Fe | 0.0888 | 0.0577 | 0.0038 | 0.0004 | N.D. | 0.0120 | 0.4074 | 0.5701 |
| Ca | - | 0.0124 | 0.0252 | 0.0017 | N.D. | 0.0212 | 2.3127 | 2.3732 |
| Sample | Ca | K | Na | W | Cl | C | O | Total (wt.%) |
|---|---|---|---|---|---|---|---|---|
| N1 | 0.1234 | 0.0024 | 0.0200 | 0.0165 | 0.0219 | 0.0420 | 0.0016 | 0.2278 |
| N2 | 0.1229 | 0.0116 | 0.0210 | 0.0220 | 0.0406 | 0.1403 | 0.0507 | 0.4092 |
| N3 | 0.1201 | 0.0135 | 0.0268 | 0.0268 | 0.0634 | 0.2007 | 0.0626 | 0.5140 |
| N4 | 0.2069 | 0.0327 | 0.0209 | 0.0225 | 0.0498 | 0.2056 | 0.1010 | 0.6394 |
| N5 | 0.6959 | 0.0921 | 0.0584 | 0.0283 | 0.0507 | 0.2029 | 0.1864 | 1.3148 |
| N6 | 1.4410 | 0.5074 | 0.1586 | 0.0333 | 0.0669 | 0.2748 | 0.2913 | 2.7734 |
| NF1 | 0.0985 | 0.0157 | 0.0169 | 0.0323 | 0.0506 | 0.1824 | 0.0466 | 0.4430 |
| NF2 | 0.1186 | 0.0108 | 0.0165 | 0.0467 | 0.0471 | 0.1909 | 0.0487 | 0.4793 |
| NF3 | 0.1517 | 0.0172 | 0.0151 | 0.0584 | 0.0543 | 0.1662 | 0.1157 | 0.5787 |
| NF4 | 0.1026 | 0.0138 | 0.0175 | 0.0286 | 0.0407 | 0.2069 | 0.0519 | 0.4621 |
| NF5 | 0.1234 | 0.0119 | 0.0180 | 0.0242 | 0.0335 | 0.1945 | 0.0291 | 0.4346 |
| No. | Nd/Nd–Fe Alloy (g) | Nd (Bath) (g) | Fe (Bath + Reactant) (g) | Nd Recovery | ||||
|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (C) | (D) | (E) | (F) | (g) | (%) | |
| N1 | 46.1052 | 46.0002 | - | - | - | - | 46.0002 | 79.9191 |
| N2 | 146.8214 | 146.2207 | 100.6151 | 99.4130 | - | - | 46.8077 | 81.3220 |
| N3 | 150.1836 | 149.4117 | 100.0425 | 98.8472 | - | - | 50.5645 | 87.8490 |
| N4 | 153.3236 | 152.3432 | 100.7577 | 99.5539 | - | - | 52.7893 | 91.7143 |
| N5 | 156.9405 | 154.8771 | 100.2423 | 99.0447 | - | - | 55.8324 | 97.0013 |
| N6 | 158.0924 | 153.7079 | 100.8409 | 99.6361 | - | - | 54.0718 | 93.9424 |
| NF1 | 161.4635 | 160.7482 | 90.6053 | 89.5228 | 15.8883 | 15.7977 | 55.4277 | 96.2981 |
| NF2 | 162.6488 | 161.8692 | 85.9442 | 84.9174 | 23.8109 | 23.6752 | 53.2767 | 92.5611 |
| NF3 | 166.9262 | 165.9603 | 79.8113 | 78.8578 | 35.4562 | 35.2541 | 51.8484 | 90.0796 |
| NF4 | 158.6776 | 157.9444 | 90.6322 | 89.5494 | 15.8912 | 15.8006 | 52.5944 | 91.3757 |
| NF5 | 157.8216 | 157.1357 | 90.7136 | 89.6298 | 15.8999 | 15.8093 | 51.6967 | 89.8160 |
| Components | 1.737 kg NdCl3 | 0.417 kg Ca | Total CO2 Emissions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NH4Cl | NH3 | H2 | CH4 | CO2 | CaO | CaCO3 | CO2 | ||
| (kg) | 1.113 | 0.354 | 0.031 | 0.125 | 0.343 | 0.778 | 1.388 | 0.610 | 0.953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.-W.; Chung, Y.-J.; Wang, J.-P. Production of Nd and Nd–Fe Alloys from NdCl3 by Calciothermic Reduction. Materials 2025, 18, 971. https://doi.org/10.3390/ma18050971
Yu J-W, Chung Y-J, Wang J-P. Production of Nd and Nd–Fe Alloys from NdCl3 by Calciothermic Reduction. Materials. 2025; 18(5):971. https://doi.org/10.3390/ma18050971
Chicago/Turabian StyleYu, Joo-Won, Yeon-Jun Chung, and Jei-Pil Wang. 2025. "Production of Nd and Nd–Fe Alloys from NdCl3 by Calciothermic Reduction" Materials 18, no. 5: 971. https://doi.org/10.3390/ma18050971
APA StyleYu, J.-W., Chung, Y.-J., & Wang, J.-P. (2025). Production of Nd and Nd–Fe Alloys from NdCl3 by Calciothermic Reduction. Materials, 18(5), 971. https://doi.org/10.3390/ma18050971







