Polysulfones Prepared by Radical Ring-Opening Polymerization of Cyclic Sulfolane Derivatives: Density Functional Theory Calculations, Synthesis, Structure, and Polymer Reactions
Abstract
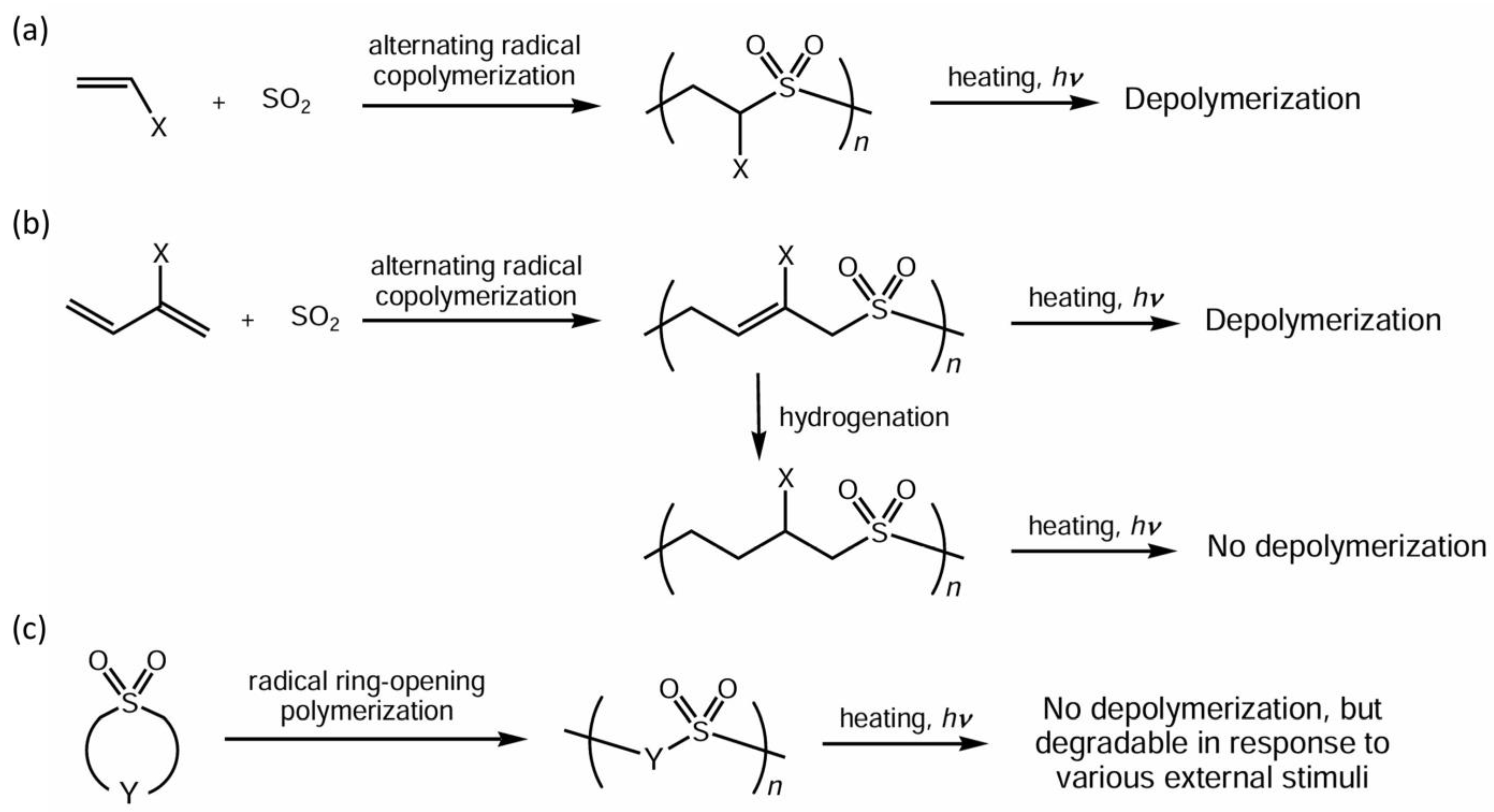
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of 2.5-Dimethyl-3-Sulfolene (DMS)
- DMS: solid; 1H NMR (300 MHz, CDCl3) δ5.94 (s, CH=CH, 2H), 3.80 (q, J = 6.9 Hz, CH3CH, 1H), 1.42 (d, CH3CH, J = 6.9 Hz, 3H);13C NMR (75 MHz, CDCl3) δ129.72 (CH=CH), 59.73 (CH), 13.65 (CH3).
2.2.2. Synthesis of 2-Vinylsulfolane (2VS)
Synthesis of Allyl-3-bromopropylsulfide [51]
- Allyl-3-bromopropylsulfide: liquid; 1H NMR (300 MHz, CDCl3) δ5.83–5.72 (m, CH=CH2, 1H), 5.15–5.08 (m, CH=CH2, 2H), 3.51 (t, J = 6.3 Hz, CH2Br, 2H), 3.16–3.12 (m, CH2CH=CH2, 2H), 2.61 (t, J = 6.9 Hz, CH2CH2S, 7 2H), 2.10 (tt, J = 6.9 Hz and 6.3 Hz, CH2CH2CH2, 2H); 13C NMR (75 MHz, CDCl3) δ134.32 (CH=CH2), 117.32 (CH=CH2), 34.84 (CH2), 32.29 (CH2), 32.15 (CH2), 28.88 (CH2).
Synthesis of 2-Vinylthiolane [43]
- 2-Vinylthiolane: liquid; 1H NMR (300 MHz, CDCl3) δ5.86–5.74 (m, CH=CH2, 1H), 5.12 (d, J = 16.8 Hz, CH=CH2 (trans), 1H), 4.95 (d, J = 9.6 Hz, CH=CH2 (cis), 1H), 3.92 (dt, J = 9.6 and 5.7 Hz, SCH, 1H), 3.01–2.81 (m, CH2S, 2H), 2.22–1.60 (m, CH2CH2, 4H); 13C NMR (75 MHz, CDCl3) δ140.15 (CH=CH2), 114.58 (CH=CH2), 51.59 (SCH), 37.96 (CH2CH), 33.22 (CH2S), 31.00 (CH2CH2CH2).
Synthesis of 2VS [43]
- 2VS: liquid; 1H NMR (300 MHz, CDCl3) δ5.86–5.73 (m, CH=CH2, 1H), 5.48–5.39 (m, CH=CH2, 2H), 3.62–3.56 (m, SO2CH, 1H), 3.19–2.98 (m, CH2SO2, 2H), 2.41–2.02 (m, CH2CH2, 4H); 13C NMR (75 MHz, CDCl3) δ128.62 (CH=CH2), 122.67 (CH=CH2), 65.26 (SO2CH), 50.80 (CH2SO2), 29.23 (CH2CH), 20.22 (CH2CH2CH2).
2.3. Polymerization
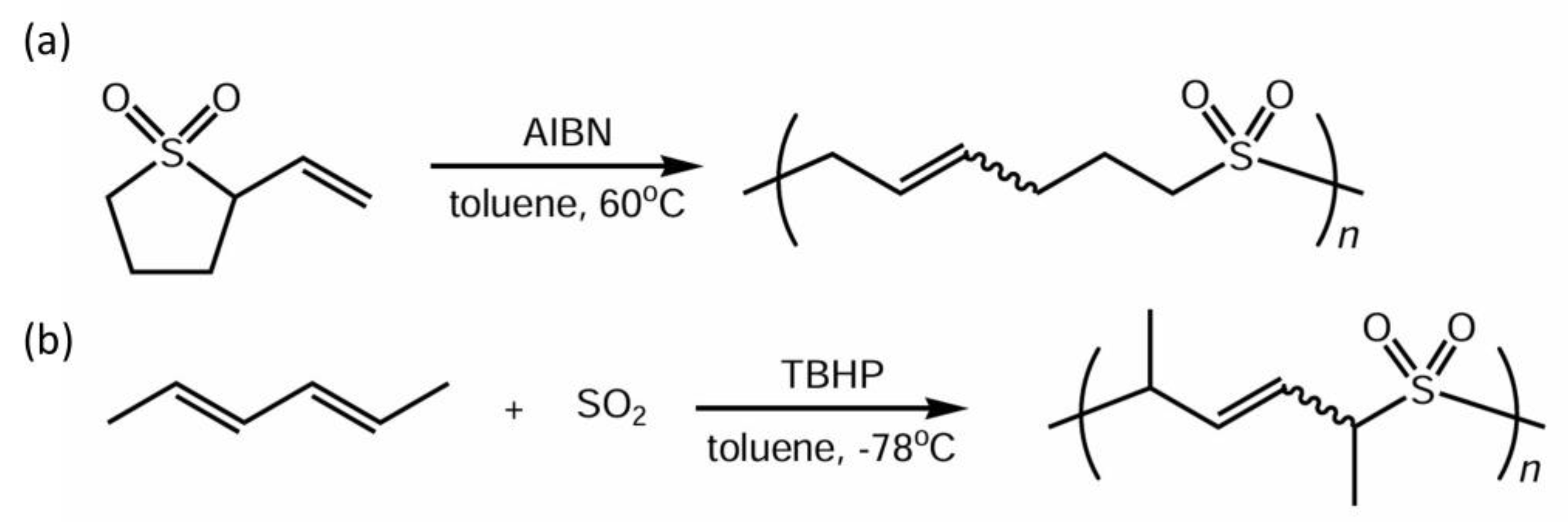
2.3.1. Ring-Opening Polymerization of Cyclic Sulfones
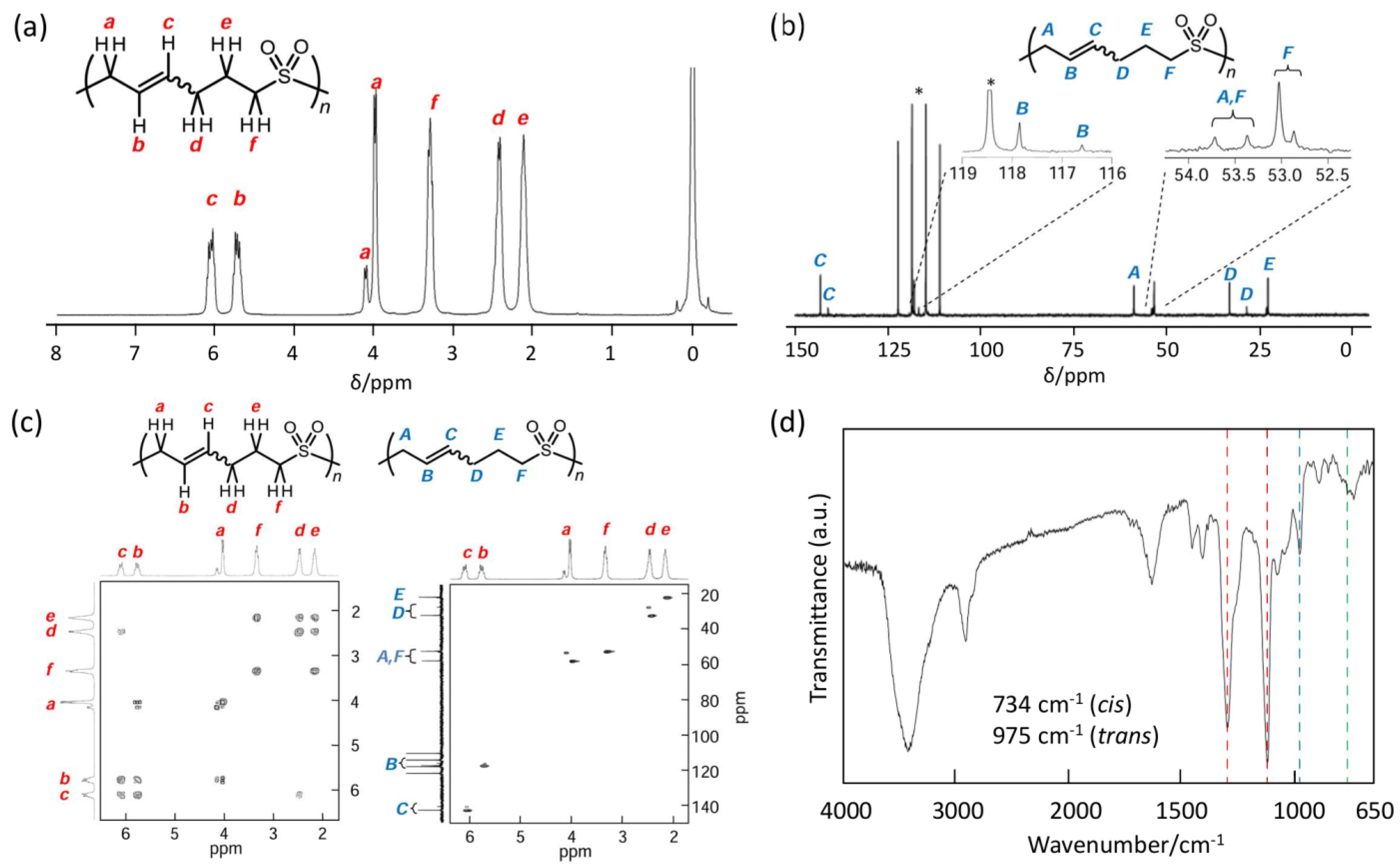
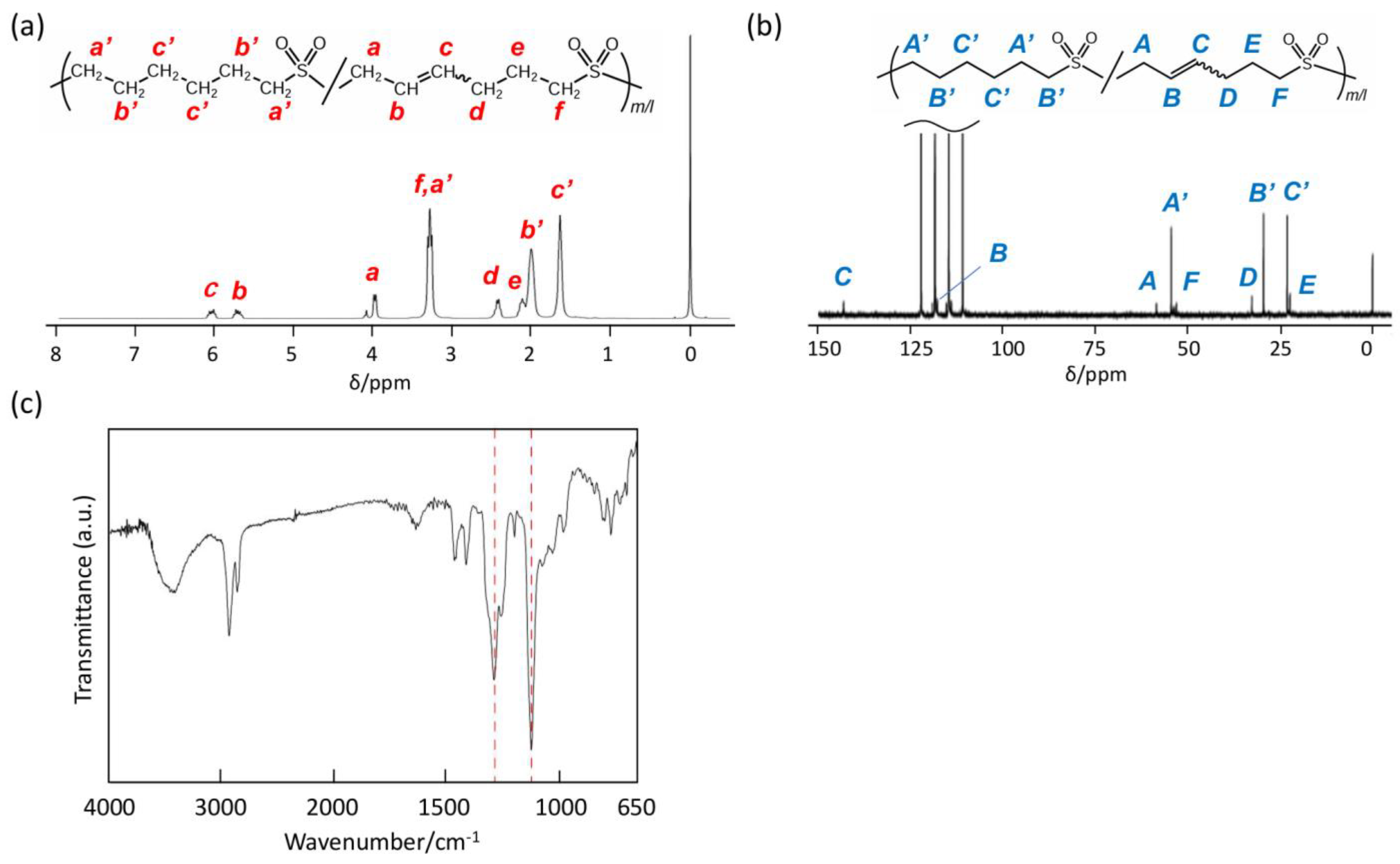
- P2VS: solid; 1H NMR (300 MHz, TFA-d1) δ6.16–5.96 (SO2CH2CH=CH, 1H), 5.84–5.64 (SO2CH2CH=CH, 1H), 4.19–3.81 (SO2CH2CH, 2H), 3.48–3.09 (SO2CH2CH2, 2H), 2.60–2.28 (CHCH2CH2, 2H), 2.24–1.92 (CH2CH2CH2, 2H); 13C NMR (75 MHz, TFA-d1) δ143.21, 141.13 (SO2CH2CH=CH), 117.85, 116.60 (SO2CH2CH=CH), 58.45, 53.70 (SO2CH2CH), 53.00 (SO2CH2CH2), 32.63, 27.92 (CHCH2CH2), 22.24 (CH2CH2CH2). IR (KBr) 3438, 2925, 1632, 1453, 1407, 1296, 1119, 975, 888, 734 cm−1.
2.3.2. Radical Copolymerization of HD and Sulfur Dioxide
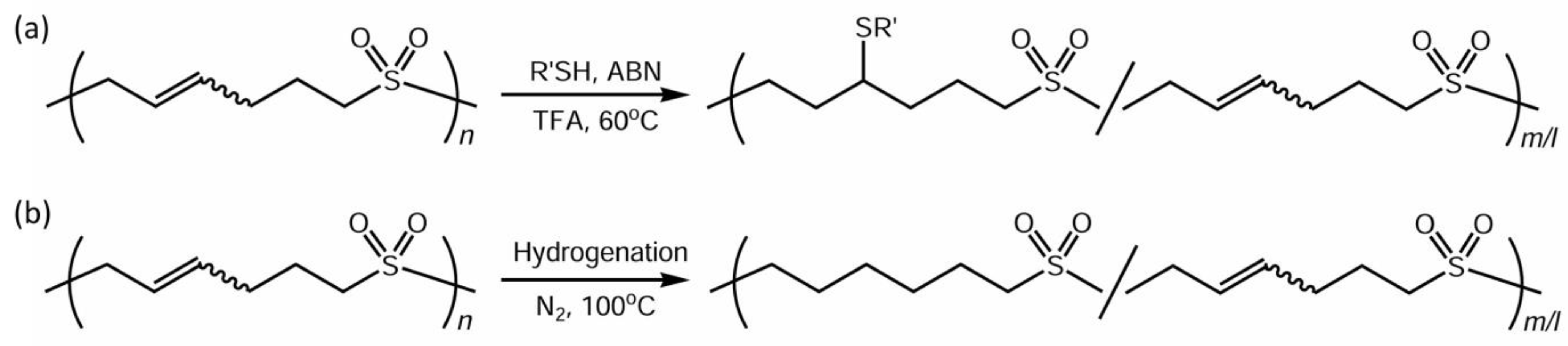
2.4. Polymer Reaction of Polysulfones
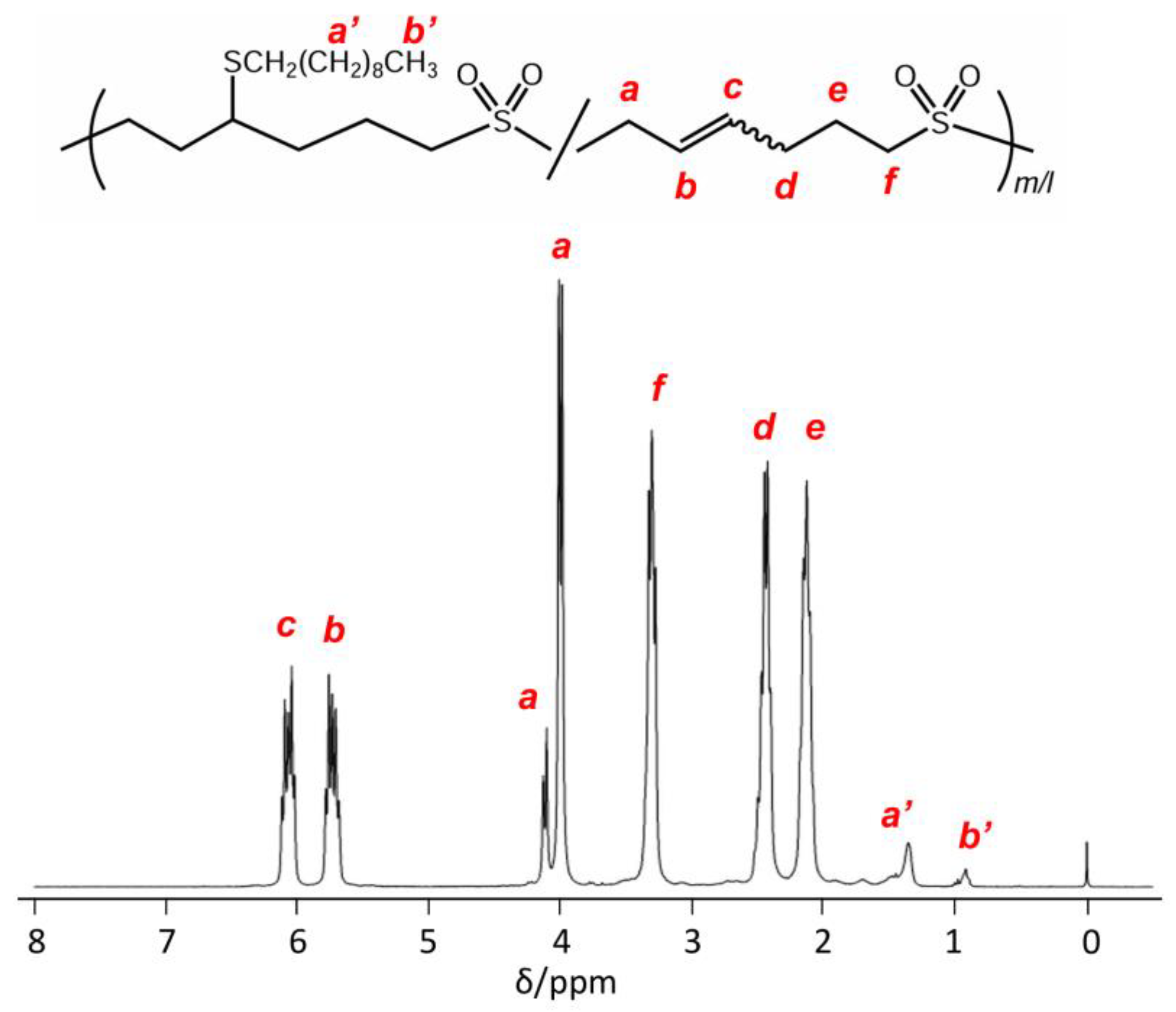
2.4.1. Thiol Addition
2.4.2. Hydrogenation
2.5. Measurements
3. Results and Discussion
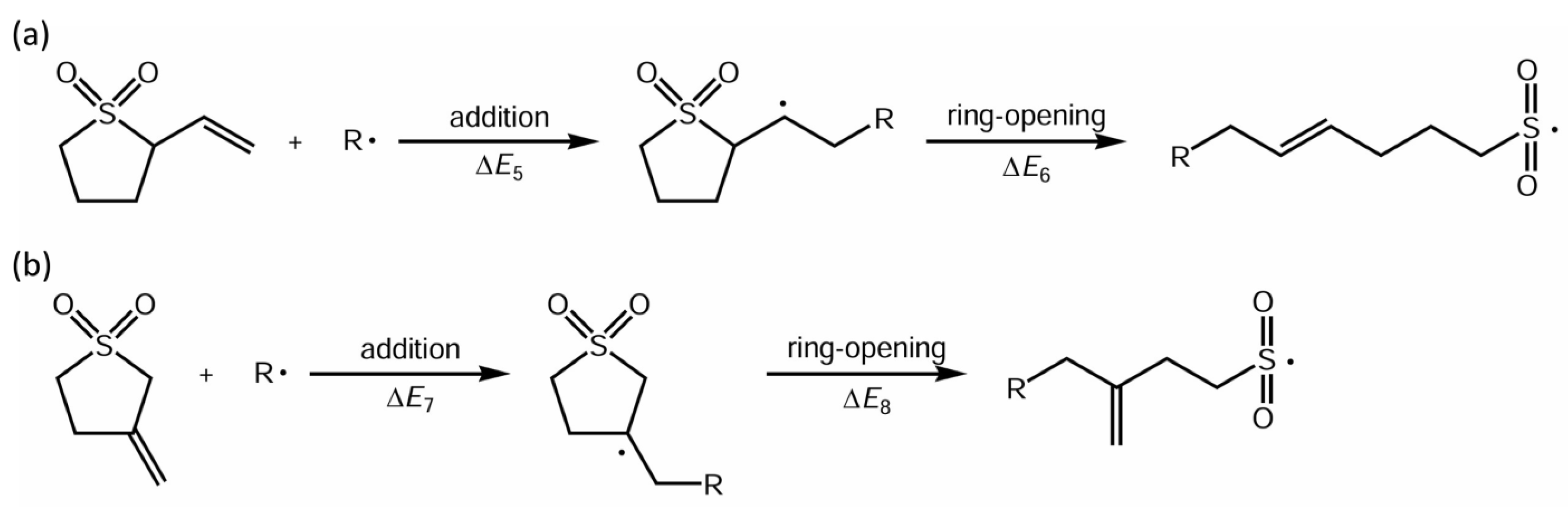
3.1. Ring-Opening Reactivity of Sulfolene Derivatives
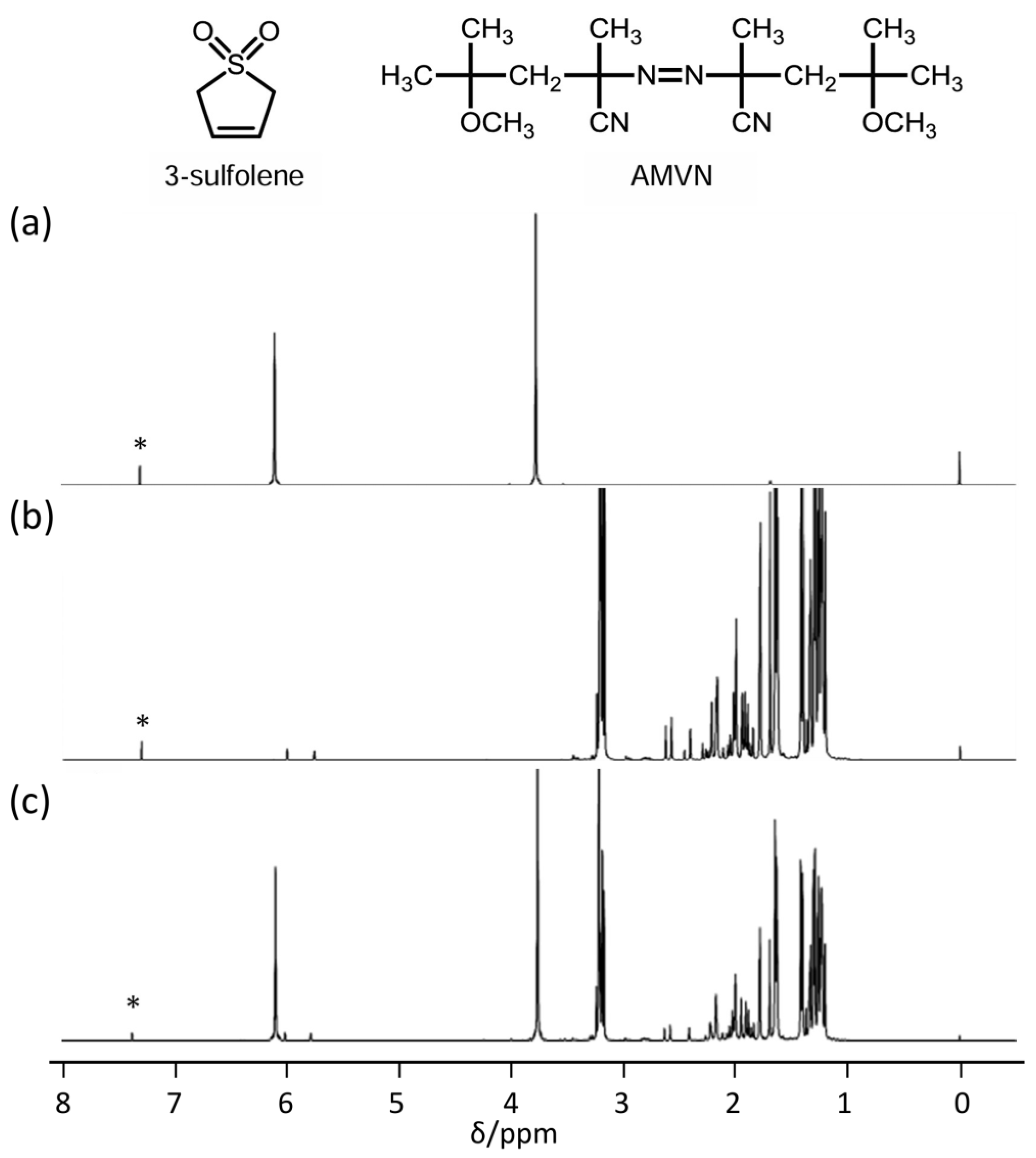
3.2. Polymerization of 2VS
3.3. Polymer Reaction of P2VS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Odian, G. Principles of Polymerization, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 1991. [Google Scholar]
- El-Hibri, M.J.; Weinberg, S.A. Encyclopedia of Polymer Science and Technology, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2003; Volume 4, pp. 1–25. [Google Scholar]
- Staudinger, H.; Ritzenthaler, B. Highly polymerized compounds. CIV. The addition of sulfur dioxide to ethylene derivatives. Chem. Ber. 1935, 68, 455–471. [Google Scholar] [CrossRef]
- Snow, R.D.; Frey, F.E. Reaction of sulfur dioxide with olefins. Ind. Eng. Chem. 1938, 30, 176–182. [Google Scholar] [CrossRef]
- Dainton, F.S.; Ivin, K.J. The kinetics of polysulfone formation. II. The formation of 1-butene polysulfone in the region of the ceiling temperature. Proc. R. Soc. 1952, 212, 207–220. [Google Scholar]
- Ivin, K.J. Thermodynamics of addition polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2137–2146. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Lu, X.-Y.; Qi, H.; Wang, Y.; Wu, G.-P. Sulfur-containing polymers derived from SO2: Synthesis, properties, and applications. Polym. Chem. 2022, 13, 5282–5299. [Google Scholar] [CrossRef]
- Kitamura, T.; Tanaka, N.; Mihashi, A.; Matsumoto, A. Soluble and thermally stable polysulfones prepared by the regiospecific and alternating radical copolymerization of 2,4-hexadiene with sulfur dioxide. Macromolecules 2010, 43, 1800–1806. [Google Scholar] [CrossRef]
- Tanaka, N.; Sato, E.; Matsumoto, A. Thermally Stable Polysulfones Obtained by Regiospecific Radical Copolymerization of Various Acyclic and Cyclic 1,3-Diene Monomers with Sulfur Dioxide and Subsequent Hydrogenation. Macromolecules 2011, 44, 9125–9137. [Google Scholar] [CrossRef]
- Tanaka, N.; Sato, E.; Matsumoto, A. Highly-controlled regiospecific free-radical copolymerization of 1,3-diene monomers with sulfur dioxide. Org. Biomol. Chem. 2011, 9, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Lee, S.; Okamura, H. Molecular design of diene monomers containing an ester functional group for the synthesis of poly(diene sulfone)s by radical alternating copolymerization with sulfur dioxide. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1000–1009. [Google Scholar] [CrossRef]
- Sasaki, T.; Kondo, T.; Noro, M.; Saida, K.; Yaguchi, H.; Naka, Y. Photoinduced depolymerization in poly(olefin sulfone) films comprised of volatile monomers doped with a photobase generator. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1462–1468. [Google Scholar] [CrossRef]
- Sasaki, T.; Yoneyama, T.; Hashimoto, S.; Takemura, S.; Naka, Y. Photoinduced depolymerization of poly(olefin sulfone)s possessing photobase generator side-chains: Effect of spacer-chain length. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3873–3880. [Google Scholar] [CrossRef]
- Sasaki, T.; Hashimoto, S.; Nogami, N.; Sugiyama, Y.; Mori, M.; Naka, Y.; Le, K.V. Dismantlable Thermosetting Adhesives Composed of a Cross-Linkable Poly(olefin sulfone) with a Photobase Generator. ACS Appl. Mater. Interfaces 2016, 8, 5580–5585. [Google Scholar] [CrossRef]
- Kumar, K.; Goodwin, A.P. Alternating Sulfone Copolymers Depolymerize in Response to Both Chemical and Mechanical Stimuli. ACS Macro Lett. 2015, 4, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Castano, E.J.; Weidner, A.R.; Yildirim, A.; Goodwin, A.P. Depolymerizable Poly(O-vinyl carbamate-alt-sulfones) as Customizable Macromolecular Scaffolds for Mucosal Drug Delivery. ACS Macro Lett. 2016, 5, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.P.; Lopez Hernandez, H.; Moore, J.S. Tunable Thermal Degradation of Poly(vinyl butyl carbonate sulfone)s via Side-Chain Branching. ACS Macro Lett. 2015, 4, 665–668. [Google Scholar] [CrossRef]
- Possanza Casey, C.M.; Moore, J.S. Base-Triggered Degradation of Poly(vinyl ester sulfone)s with Tunable Sensitivity. ACS Macro Lett. 2016, 5, 1257–1260. [Google Scholar] [CrossRef]
- Sanda, F.; Endo, T. Radical ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2000, 39, 265–276. [Google Scholar] [CrossRef]
- Endo, T.; Sudo, A. Ring-Opening Polymerization. ACS Symp. Ser. 2015, 1187, 19–38. [Google Scholar] [CrossRef]
- Tardy, A.; Nicolas, J.; Gigmes, D.; Lefay, C.; Guillaneuf, Y. Radical Ring-Opening Polymerization: Scope, Limitations, and Application to (Bio)Degradable Materials. Chem. Rev. 2017, 117, 1319–1406. [Google Scholar] [CrossRef] [PubMed]
- Pesenti, T.; Nicolas, J. Degradable Polymers from Radical Ring-Opening Polymerization: Latest Advances, New Directions, and Ongoing Challenges. ACS Macro Lett. 2020, 9, 1812–1835. [Google Scholar] [CrossRef] [PubMed]
- Sbordone, F.; Frisch, H. Plenty of Space in the Backbone: Radical Ring-Opening Polymerization. Chem. Eur. J. 2024, 30, e202401547. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.R.; Kubo, T.; Goodrich, S.L.; Figg, C.A.; Sumerlin, B.S. Alternating Radical Ring-Opening Polymerization of Cyclic Ketene Acetals: Access to Tunable and Functional Polyester Copolymers. Macromolecules 2018, 51, 5079–5084. [Google Scholar] [CrossRef]
- Folini, J.; Murad, W.; Mehner, F.; Meier, W.; Gaitzsch, J. Updating radical ring-opening polymerisation of cyclic ketene acetals from synthesis to degradation. Eur. Polym. J. 2020, 134, 109851. [Google Scholar] [CrossRef]
- Mothe, S.R.; Zhao, W.; van Herk, A.M.; Thoniyot, P. Backbone-Degradable Acrylate Latex: Toward Overcoming Hydrolysis Limitations of Cyclic Ketene Acetal Monomers. Macromolecules 2024, 57, 2937–2949. [Google Scholar] [CrossRef]
- Volz, H.K.-H.; Agarwal, S. High Tg Poly(ester-co-vinyl)s with Compositional Homogeneity by Radical Ring-Opening Polymerization. ACS Appl. Polym. Mater. 2023, 5, 8754–8763. [Google Scholar] [CrossRef]
- Hardy, C.; Levere, M.E.; Kociok-Köhn, G.; Buchard, A. Radical Ring Opening Polymerization of Cyclic Ketene Acetals Derived From D-Glucal. ACS Macro Lett. 2023, 12, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.-C.; Zhou, Z.; Niu, J. Quantitative, Regiospecific, and Stereoselective Radical Ring-Opening Polymerization of Monosaccharide Cyclic Ketene Acetals. J. Am. Chem. Soc. 2024, 146, 5056–5062. [Google Scholar] [CrossRef]
- Ding, J.; Han, Y.; Wu, J.; Chen, D.; Xiong, C.; Huang, D.; Xiong, Z. Degradable Underwater Adhesive Utilizing Free Radical Ring-Opening Polymerization. ACS Appl. Polym. Mater. 2024, 6, 5980–5987. [Google Scholar] [CrossRef]
- Gardoni, G.; Zanoni, A.; de San Román, E.G.; Moscatelli, D.; Gabirondo, E.; Leiza, J.R.; Barquero, A.; Sardon, H. Enhancing the incorporation of 2-methylene-1,3-dioxepane (MDO) into industrial monomers by the addition of crotonate comonomers. Polymer 2024, 307, 127298. [Google Scholar] [CrossRef]
- Thoniyot, J.-B.L.P.; Ramalingam, B.; van Herk, A.M.; Rusli, W. Insertion of ester bonds in three terpolymerization systems. Eur. Polym. J. 2022, 181, 111627. [Google Scholar] [CrossRef]
- Deng, Y.; Schäfer, S.; Kronstein, D.; Atabay, A.; Susewind, M.; Krieg, E.; Seiffert, S.; Gaitzsch, J. Amphiphilic Block Copolymers PEG-b-PMTCs: Synthesis, Self-assembly, Degradation Properties and Biocompatibility. Biomacromolecules 2024, 25, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Spick, M.P.; Bingham, N.M.; Li, Y.; de Jesus, J.; Costa, C.; Bailey, M.J.; Roth, P.J. Fully Degradable Thioester-Functional Homo- and Alternating Copolymers Prepared through Thiocarbonyl Addition-Ring-Opening RAFT Radical Polymerization. Macromolecules 2020, 53, 539–547. [Google Scholar] [CrossRef]
- Gil, N.; Caron, B.; Siri, D.; Roche, J.; Hadiouch, S.; Khedaioui, D.; Ranque, S.; Cassagne, C.; Montarnal, D.; Gigmes, D.; et al. Degradable Polystyrene via the Cleavable Comonomer Approach. Macromolecules 2022, 55, 6680–6694. [Google Scholar] [CrossRef]
- Luzel, B.; Gil, N.; Désirée, P.; Monot, J.; Bourissou, D.; Siri, D.; Gigmes, D.; Martin-Vaca, B.; Lefay, C.; Guillaneuf, Y. Development of an Efficient Thionolactone for Radical Ring-Opening Polymerization by a Combined Theoretical/Experimental Approach. J. Am. Chem. Soc. 2023, 145, 27437–27449. [Google Scholar] [CrossRef] [PubMed]
- Lages, M.; Gil, N.; Galanopoulo, P.; Mougin, J.; Lefay, C.; Guillaneuf, Y.; Lansalot, M.; D’Agosto, F.; Nicolas, J. Degradable Latexes by Nitroxide-mediated Aqueous Seeded Emulsion Copolymerization Using a Thionolactone. Macromolecules 2023, 56, 7973–7983. [Google Scholar] [CrossRef]
- Lages, M.; Pesenti, T.; Zhu, C.; Le, D.; Mougin, J.; Guillaneuf, Y.; Nicolas, J. Degradable polyisoprene by radical ring-opening polymerization and application to polymer prodrug nanoparticles. Chem. Sci. 2023, 14, 3311–3325. [Google Scholar] [CrossRef] [PubMed]
- Dawson, F.; Kazmi, T.; Roth, P.J.; Kopeć, M. Strands vs. crosslinks: Topology-dependent degradation and regelation of polyacrylate networks synthesised by RAFT polymerization. Polym. Chem. 2023, 14, 5166–5177. [Google Scholar] [CrossRef]
- Gil, N.; Thomas, C.; Mhanna, R.; Mauriello, J.; Maury, R.; Leuschel, B.; Malval, J.-P.; Clément, J.-L.; Gigmes, D.; Lefay, C.; et al. Thionolactone as a Resin Additive to Prepare (Bio)degradable 3D Objects via VAT Photopolymerization. Angew. Chem. Int. Ed. 2022, 61, e202117700. [Google Scholar] [CrossRef] [PubMed]
- Kamiki, R.; Kubo, T.; Satoh, K. Addition–Fragmentation Ring-Opening Polymerization of Bio-Based Thiocarbonyl l-Lactide for Dual Degradable Vinyl Copolymers. Macromol. Rapid Commun. 2023, 44, 2200537. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, K. Polysulfone Synthesis Using Radical Ring-Opening Reactions. Diploma Thesis, Osaka City University, Osaka, Japan, 2012. [Google Scholar]
- Cho, I.; Kim, S.K.; Lee, M.H. Exploratory ring-opening polymerization. XIIL Ring-opening polymerization of 2-vinyl cyclic sulfones. J. Polym. Sci. Polym. Symp. 1986, 74, 219–226. [Google Scholar] [CrossRef]
- Cho, I.; Lee, M.H. Exploratory ring-opening polymerization. XIV. Synthesis of 2-vinylthiepane-1,1-dioxide and its ring-opening polymerization. J. Polym. Sci. Part C Polym. Lett. 1987, 25, 309–311. [Google Scholar] [CrossRef]
- Cho, I.; Choi, S.Y. Radical ring-opening polymerization of 2-methyl-2-vinyl-1,3-dithiane-1,1,3,3-tetroxide. Makromol. Chem. Rapid Commun. 1991, 12, 399–402. [Google Scholar] [CrossRef]
- Tanaka, S.; Furusho, Y.; Endo, T. Radical ring-opening polymerization of five-membered cyclic vinyl sulfone using p-toluenesulfonyl halides. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 222–227. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Costa, M.F.; Mouneyrac, C. (Eds.) Handbook of Microplastics in the Environment; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Delplace, V.; Nicolas, J. Degradable vinyl polymers for biomedical applications. Nat. Chem. 2015, 7, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Lefay, C.; Guillaneuf, Y. Recyclable/degradable materials via the insertion of labile/cleavable bonds using a comonomer approach. Prog. Polym. Sci. 2023, 147, 101764. [Google Scholar] [CrossRef]
- Zheng, J.; Png, Z.M.; Quek, X.C.N.; Loh, J.; Li, Z. Stimuli-cleavable moiety enabled vinyl polymer degradation and emerging applications. Green Chem. 2023, 25, 8903–8934. [Google Scholar] [CrossRef]
- Vedejs, E.; Hagen, J.P.; Roach, B.L.; Spear, K.L. Ring expansion by [2,3] sigmatropic shift: Conversion of five-membered into eight-membered heterocycles. J. Org. Chem. 1978, 43, 1185–1190. [Google Scholar] [CrossRef]
- Fujii, Y.; Takasu, M.; Matsumoto, A. Synthesis of polysulfones using radical ring-opening polymerization. In Proceedings of the 98th Spring Annual Meeting of the Chemical Society of Japan, Funabashi, Japan, 20–23 March 2018. Presentation Number 1B2-10. [Google Scholar]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol–ene “click” reactions and recent applications in polymer and materials synthesis: A first update. Polym. Chem. 2014, 5, 4820–4870. [Google Scholar] [CrossRef]
- Theato, P.; Klok, H.-A. (Eds.) Functional Polymers by Post-Polymerization Modification; Wiley-VCH: Hoboken, NJ, USA, 2013. [Google Scholar]


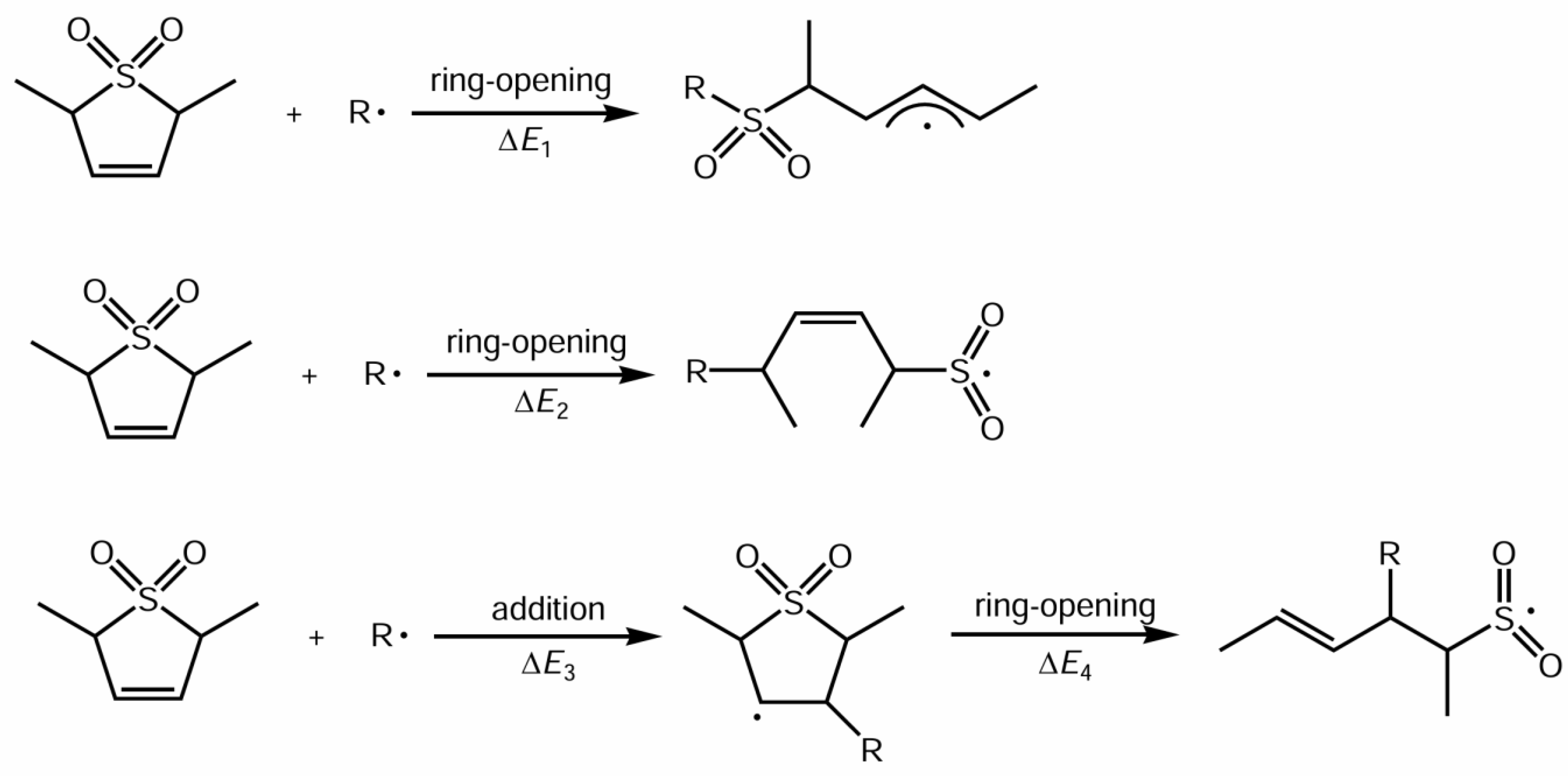


| Cyclic Monomer | Radical | Addition (kcal/mol) | Ring Opening (kcal/mol) |
|---|---|---|---|
| DMS | ·CH3 | - | ΔE1 = −26.5 |
| ·SO2CH3 | - | Not calculated | |
| ·CH3 | - | ΔE2 = −36.9 | |
| ·SO2CH3 | - | ΔE2 = −8.6 | |
| ·CH3 | ΔE3 = −26.4 | ΔE4 = −10.0 | |
| ·SO2CH3 | ΔE3 = +4.5 | ΔE4 = −9.6 | |
| 2VS | ·CH3 | ΔE5 = −30.1 | ΔE6 = −8.6 |
| ·SO2CH3 | ΔE5 = −3.6 | ΔE6 = −9.2 | |
| 3EMS | ·CH3 | ΔE7 = −29.9 | ΔE8 = −7.0 |
| ·SO2CH3 | ΔE7 = −2.4 | ΔE8 = −5.7 |
| Polymer | Thiol | Solvent | Yield (%) | Conv. a (%) |
|---|---|---|---|---|
| P2VS | 1-Decanethiol | TFA | 51 | 2.1 |
| P(HD-alt-SO2) | 1-Butanethiol | 1,2-Dichloroethane | 0 | Not determined |
| Polymer | Time (h) | Conv. a (%) | Td5 (°C) | Tmax (°C) | Tg b (°C) |
|---|---|---|---|---|---|
| P2VS | 0 | 0 | 187 | 353 | c |
| 12 | 80.8 | 221 | 385 | c | |
| P(HD-alt-SO2) | 0 | 0 | 135 | 235 | c |
| 12 | 98.2 | 278 | 346 | 90.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanishi, K.; Sato, E.; Matsumoto, A. Polysulfones Prepared by Radical Ring-Opening Polymerization of Cyclic Sulfolane Derivatives: Density Functional Theory Calculations, Synthesis, Structure, and Polymer Reactions. Materials 2025, 18, 928. https://doi.org/10.3390/ma18050928
Yamanishi K, Sato E, Matsumoto A. Polysulfones Prepared by Radical Ring-Opening Polymerization of Cyclic Sulfolane Derivatives: Density Functional Theory Calculations, Synthesis, Structure, and Polymer Reactions. Materials. 2025; 18(5):928. https://doi.org/10.3390/ma18050928
Chicago/Turabian StyleYamanishi, Keisuke, Eriko Sato, and Akikazu Matsumoto. 2025. "Polysulfones Prepared by Radical Ring-Opening Polymerization of Cyclic Sulfolane Derivatives: Density Functional Theory Calculations, Synthesis, Structure, and Polymer Reactions" Materials 18, no. 5: 928. https://doi.org/10.3390/ma18050928
APA StyleYamanishi, K., Sato, E., & Matsumoto, A. (2025). Polysulfones Prepared by Radical Ring-Opening Polymerization of Cyclic Sulfolane Derivatives: Density Functional Theory Calculations, Synthesis, Structure, and Polymer Reactions. Materials, 18(5), 928. https://doi.org/10.3390/ma18050928






