Au Nanoclusters on Vanadium-Doped ZrO2 Nanoparticles for Propylene Oxidation: An Investigation into the Impact of V
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of ZrVx Supports
2.2. Deposition of Gold Nanoparticles on ZrVx Supports
2.3. Characterization
2.4. Propylene Oxidation
3. Results and Discussion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Zhang, X.; Bravo-Suárez, J.J.; Fujitani, T.; Oyama, S.T. Effect of composition and promoters in Au/TS-1 catalysts for direct propylene epoxidation using H2 and O2. Catal. Today 2009, 147, 186–195. [Google Scholar] [CrossRef]
- Huang, J.; Lima, E.; Akita, T.; Guzmán, A.; Qi, C.; Takei, T.; Haruta, M. Propene epoxidation with O2 and H2: Identification of the most active gold clusters. J. Catal. 2011, 278, 8–15. [Google Scholar] [CrossRef]
- Hayashi, T.; Tanaka, K.; Haruta, M. Selective vapor-phase epoxidation of propylene over Au/TiO2 catalysts in the presence of oxygen and hydrogen. J. Catal. 1998, 178, 566–575. [Google Scholar] [CrossRef]
- Qi, C. The production of propylene oxide over nanometer Au catalysts in the presence of H2 and O2. Gold Bull. 2008, 41, 224–234. [Google Scholar] [CrossRef]
- Haruta, M.; Uphade, B.S.; Tsubota, S.; Miyamoto, A. Selective oxidation of propylene over gold deposited on titanium-based oxides. Res. Chem. Interm. 1998, 24, 329–336. [Google Scholar] [CrossRef]
- Kalvachev, Y.A.; Hayashi, T.; Tsubota, S.; Haruta, M. Vapor-phase selective oxidation of aliphatic hydrocarbons over gold deposited on mesoporous titanium silicates in the co-presence of oxygen and hydrogen. J. Catal. 1999, 186, 228–233. [Google Scholar] [CrossRef]
- Nijhuis, T.A.; Huizinga, B.J.; Makkee, M.; Moulijn, J.A. Direct epoxidation of propene using gold dispersed on TS-1 and other titanium-containing supports. Ind. Eng. Chem. Res. 1999, 38, 884–891. [Google Scholar] [CrossRef]
- Uphade, B.S.; Okumura, M.; Yamada, N.; Tsubota, S.; Haruta, M. Vapor-phase epoxidation of propene using H2 and O2 over Au/Ti-MCM-48. J. Catal. 2002, 209, 331–340. [Google Scholar] [CrossRef]
- Uphade, B.S.; Okumura, M.; Tsubota, S.; Haruta, M. Effect of physical mixing of CsCl with Au/Ti-MCM-41 on the gas-phase epoxidation of propene using H2 and O2: Drastic depression of H2 consumption. Appl. Catal. A Gen. 2000, 190, 43–50. [Google Scholar] [CrossRef]
- Uphade, B.S.; Yamada, Y.; Akita, T.; Nakamura, T.; Haruta, M. Synthesis and characterization of Ti-MCM-41 and vapor-phase epoxidation of propylene using H2 and O2 over Au/Ti-MCM-41. Appl. Catal. A Gen. 2001, 215, 137–148. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Sun, B.; Zhu, H.; Shi, N.; Xu, W. TS-1 with Abundant Micropore Channel-Supported Au Catalysts toward Im-proved Performance in Gas-Phase Epoxidation of Propylene. ACS Sustain. Chem. Eng. 2023, 11, 7042–7052. [Google Scholar] [CrossRef]
- Hou, L.; Yan, S.; Liu, L.; Liu, J.; Xiong, G. Fabrication of gold nanoparticles supported on hollow microsphere of nanosized TS-1 for the epoxidation of propylene with H2 and O2. Chem. Eng. J. 2024, 481, 148676–148878. [Google Scholar] [CrossRef]
- Du, W.; Zhang, Z.; Wang, J.; Song, N.; Duan, X.; Zhou, X. Kinetic insights into reaction pathways of acrolein formation in propylene epoxidation by H2 and O2 over Au/TS-1 catalyst. Chem. Eng. J. 2024, 487, 150512–150523. [Google Scholar] [CrossRef]
- Das, I.; De, G. Zirconia based superhydrophobic coatings on cotton fabrics exhibiting excellent durability for versatile use. Sci. Rep. 2015, 5, 18503. [Google Scholar] [CrossRef] [PubMed]
- Pakharukova, V.P.; Moroz, E.M.; Zyuzin, D.A.; Ishchenko, A.V.; Dolgikh, L.Y.; Strizhak, P.E. Structure of copper oxide species supported on monoclinic zirconia. J. Phys. Chem. C 2015, 119, 28828–28835. [Google Scholar] [CrossRef]
- Wyrwalski, F.; Lamonier, J.F.; Siffert, S.; Aboukaïs, A. Additional effects of cobalt precursor and zirconia support modifications for the design of efficient VOC oxidation catalysts. Appl. Catal. B Environ. 2007, 70, 393–399. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Xu, B.Q. Remarkable nanosize effect of zirconia in Au/ZrO2 catalyst for CO oxidation. J. Phys. Chem. B 2005, 109, 9678–9683. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Lin, M.; Ge, M.; Zhao, Y.; Wang, F. Interface effect of mixed phase Pt/ZrO2 catalysts for HCHO oxidation at ambient temperature. J. Mater. Chem. A 2017, 5, 13799–13806. [Google Scholar] [CrossRef]
- Jin, G.; Lu, G.; Guo, Y.; Guo, Y.; Wang, J.; Liu, X. Effect of additive in feedstock on performance of Ag-MoO3/ZrO2 catalyst for propylene epoxidation by molecular oxygen. React. Kinet. Catal. Lett. 2006, 89, 253–260. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, J.; Seo, Y.J.; Lee, J.W.; Ro, Y.; Yi, J.; Song, I.K. Direct epoxidation of propylene to propylene oxide with molecular oxygen over Ag-Mo-W/ZrO2 catalysts. Catal. Commun. 2017, 89, 156–160. [Google Scholar] [CrossRef]
- Zheng, Y.; Okumura, M.; Hua, X.; Sonour, A.; Su, H.; Nobutou, H.; Sun, X.; Sun, L.; Xiao, F.; Qi, C. Partial oxidation of propylene with H2 and O2 over Au supported on ZrO2 with different structural and surface properties. J. Catal. 2021, 401, 188–199. [Google Scholar] [CrossRef]
- Chen, S.; Ma, F.; Xu, A.; Wang, L.; Chen, F.; Lu, W. Study on the structure, acidic properties of V-Zr nanocrystal catalysts in oxidative dehydrogenation of propane. Appl. Surf. Sci. 2014, 289, 316–325. [Google Scholar] [CrossRef]
- Blin, J.L.; Flamant, R.; Su, B.L. Synthesis of nanostructured mesoporous zirconia using CTMABr-ZrOCl2·8H2O systems: A kinetic study of synthesis mechanism. Intern. J. Inorg. Mater. 2001, 3, 959–972. [Google Scholar] [CrossRef]
- Peng, S.; Sun, X.; Sun, L.; Zhang, M.; Zheng, Y.; Su, H.; Qi, C. Selective hydrogenation of acetylene over gold nanoparticles supported on CeO2 pretreated under different atmospheres. Catal. Lett. 2019, 149, 465–472. [Google Scholar] [CrossRef]
- Gazzoli, D.; De Rossi, S.; Ferraris, G.; Mattei, G.; Spinicci, R.; Valigi, M. Bulk and surface structures of V2O5/ZrO2 catalysts for n-butane oxidative dehydrogenation. J. Mol. Catal. A Chem. 2009, 310, 17–23. [Google Scholar] [CrossRef]
- Khodakov, A.; Yang, J.; Su, S.; Iglesia, E.; Bell, A.T. Structure and properties of vanadium oxide-zirconia catalysts for propane oxidative dehydrogenation. J. Catal. 1998, 177, 343–351. [Google Scholar] [CrossRef]
- Barberis, P.; Merle-Méjean, T.; Quintard, P. On Raman spectroscopy of zirconium oxide films. J. Nucl. Mater. 1997, 246, 232–243. [Google Scholar] [CrossRef]
- Štefanić, G.; Musić, S.; Popović, S.; Sekulić, A. FT-IR and laser Raman spectroscopic investigation of the formation and stability of low temperature t-ZrO2. J. Mol. Struct. 1997, 408, 391–394. [Google Scholar] [CrossRef]
- Wang, G.; Meng, F.; Ding, C.; Chu, P.K.; Liu, X. Microstructure, bioactivity and osteoblast behavior of monoclinic zirconia coating with nanostructured surface. Acta Biomater. 2010, 6, 990–1000. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, J.; Hu, Z.; Yu, W.; Li, Q.; Sun, J.; Xu, N.; Wu, J.; Ying, Z. Infrared and Raman spectroscopic studies of optically transparent zirconia (ZrO2) films deposited by plasma-assisted reactive pulsed laser deposition. Appl. Spectrosc. 2011, 65, 522–527. [Google Scholar] [CrossRef]
- Zhao, X.; Vanderbilt, D. Phonons and lattice dielectric properties of zirconia. Phys. Rev. B 2002, 65, 075105. [Google Scholar] [CrossRef]
- Khodakov, A.; Olthof, B.; Bell, A.T.; Iglesia, E. Structure and catalytic properties of supported vanadium oxides: Support effects on oxidative dehydrogenation reactions. J. Catal. 1999, 181, 205–216. [Google Scholar] [CrossRef]
- Burcham, L.J.; Deo, G.; Gao, X.; Wachs, I.E. In situ IR, Raman, and UV-Vis DRS spectroscopy of supported vanadium oxide catalysts during methanol oxidation. Top. Catal. 2000, 11, 85–100. [Google Scholar] [CrossRef]
- Gao, X.; Jehng, J.M.; Wachs, I.E. In situ UV-vis-NIR diffuse reflectance and Raman spectroscopic studies of propane oxidation over ZrO2-supported vanadium oxide catalysts. J. Catal. 2002, 209, 43–50. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, L.L.; Lu, S.L.; Duan, H.H.; Li, Z.X.; Zhu, Y.X.; Yan, C.H. Facile synthesis of Zr-based functional materials with highly ordered mesoporous structures. J. Phy. Chem. C 2009, 113, 4117–4124. [Google Scholar] [CrossRef]
- Ebiad, M.A.; Abd El-Hafiz, D.R.; Elsalamony, R.A.; Mohamed, L.S. Ni supported high surface area CeO2-ZrO2 catalysts for hydrogen production from ethanol steam reforming. Rsc. Adv. 2012, 2, 8145–8156. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, Y.; Chen, C.; Cao, Y.; Lin, X.; Zheng, Q. Highly efficient Au/ZrO2 catalysts for low-temperature water-gas shift reaction: Effect of precalcination temperature of ZrO2. Int. J. Hydrog. Energ. 2012, 37, 12292–12300. [Google Scholar] [CrossRef]
- Mendialdua, J.; Casanova, R.; Barbaux, Y. XPS studies of V2O5, V6O13, VO2 and V2O3. J. Electron Spectros. Relat. Phenom. 1995, 71, 249–261. [Google Scholar] [CrossRef]
- Sinha, A.K.; Seelan, S.; Tsubota, S.; Haruta, M. Catalysis by gold nanoparticles: Epoxidation of propene. Top. Catal. 2004, 29, 95–102. [Google Scholar] [CrossRef]
- Hern’andez, J.A.; G’omez, S.A.; Zepeda, T.A.; Fierro-Gonz’alez, J.C.; Fuentes, G.A. Insight into the Deactivation of Au/CeO2 Catalysts Studied by In Situ Spectroscopy during the CO-PROX Reaction. ACS Catal. 2015, 5, 4003–4012. [Google Scholar] [CrossRef]
- Qi, C.; Cheng, Y.; Yang, Z.; Ishida, T.; Su, H.; Zhang, J.; Sun, X.; Sun, L.; Zhao, L.; Murayama, T. Efficient formation of propylene oxide under low hydrogen concentration in propylene epoxidation over Au nanoparticles supported on V-doped TS-1. J. Catal. 2024, 436, 115608–115708. [Google Scholar] [CrossRef]

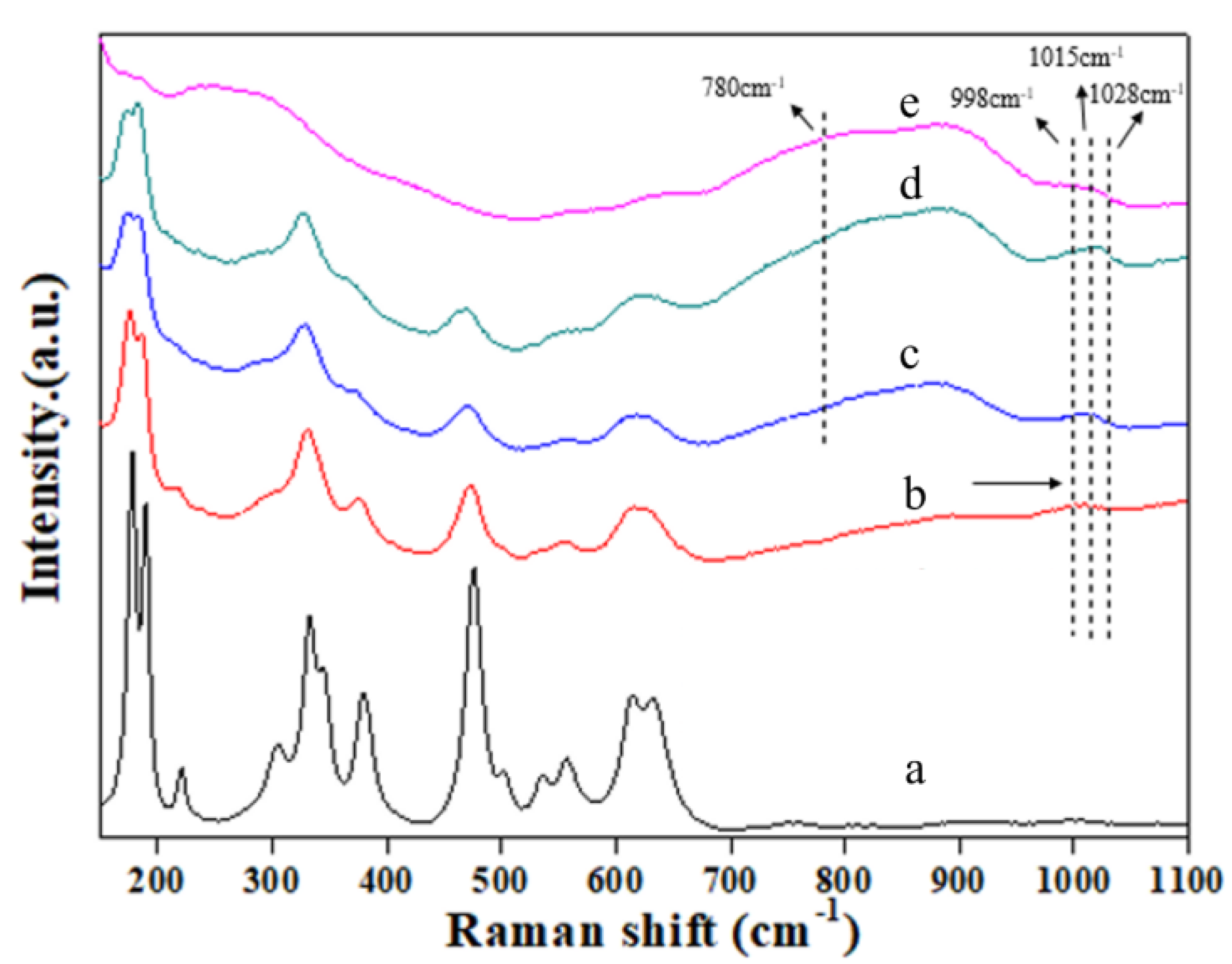

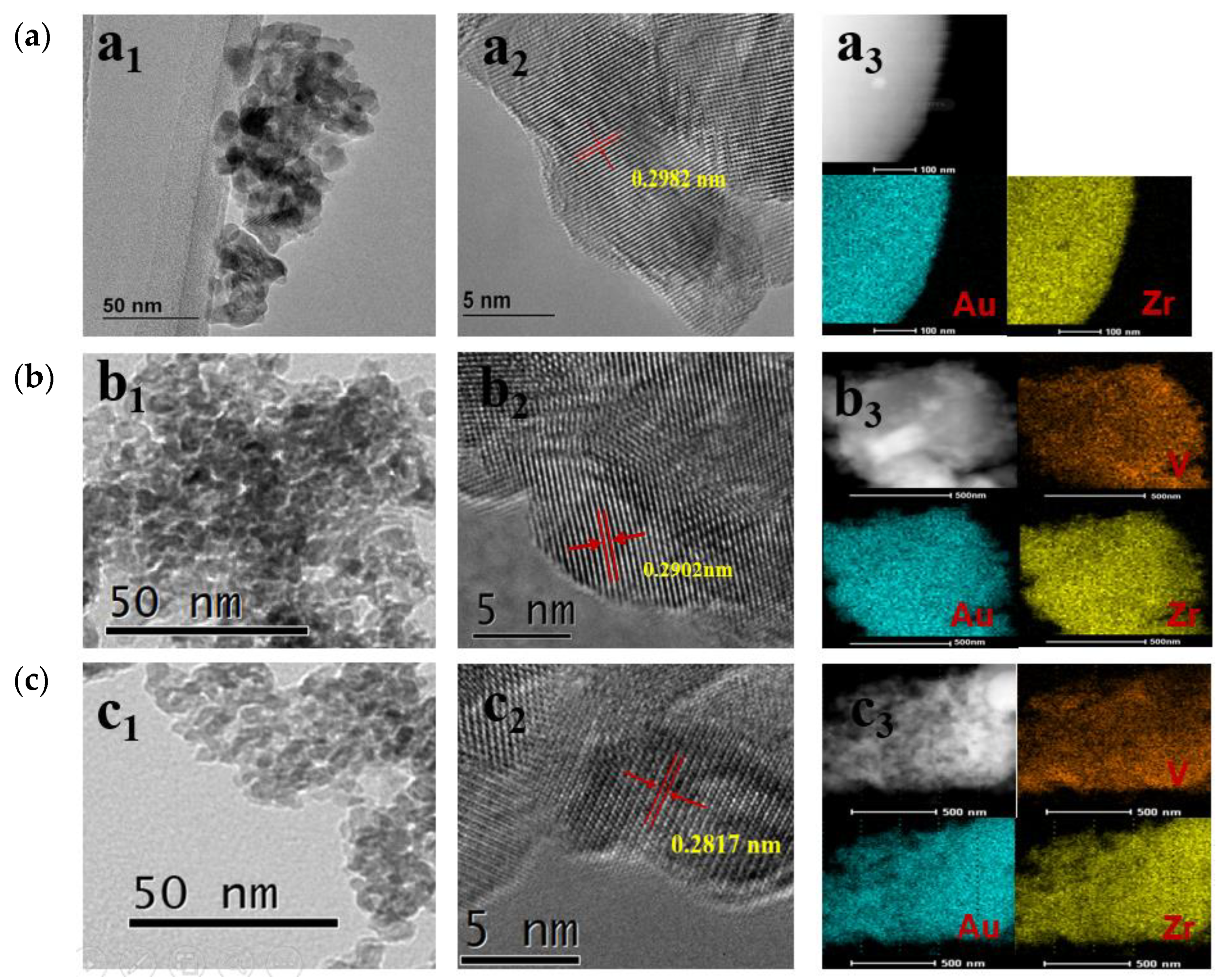
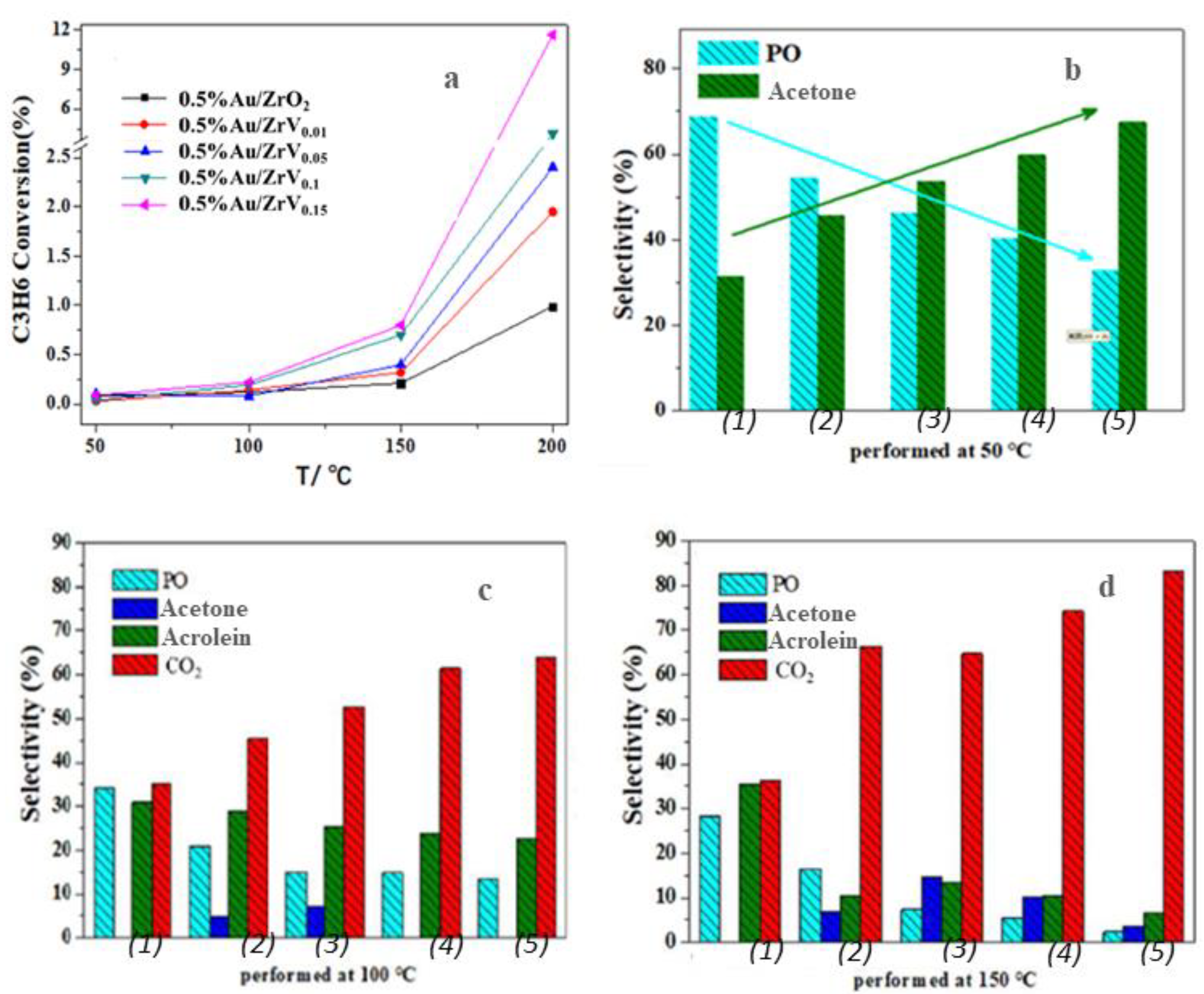
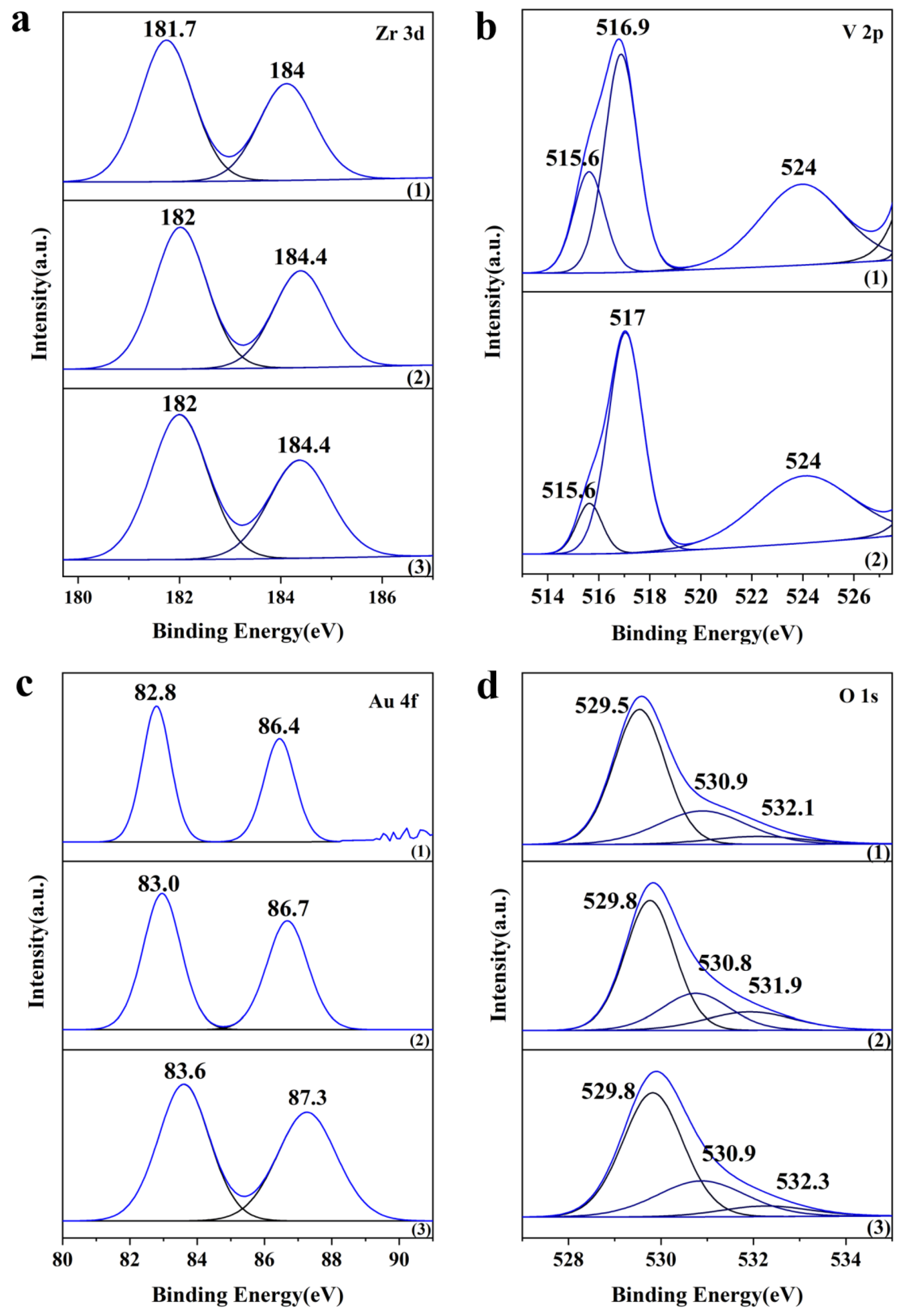
| Catalysts | SBET (m2 g−1) | Average Particle Size (nm) a | Crystal Size (nm) b | Pore Vol. (cm3 g−1) c | Pore Size (nm) d | Au Loading (wt%) | Acid Amount (mmol g−1) e |
|---|---|---|---|---|---|---|---|
| 0.5 wt% Au/ZrO2 | 85 | 14.8 | 15.5 | 0.32 | 15.2 | 0.33 | 0.116 |
| 0.5 wt% Au/ZrV0.01 | 101 | - | 10.3 | 0.17 | 4.6 | 0.27 | 0.166 |
| 0.5 wt% Au/ZrV0.05 | 197 | 9.4 | 9.2 | 0.18 | 3.7 | 0.23 | 0.292 |
| 0.5 wt% Au/ZrV0.1 | 193 | - | 8.3 | 0.15 | 3.1 | 0.27 | 0.362 |
| 0.5 wt% Au/ZrV0.15 | 249 | 6.9 | 7.8 | 0.19 | 3.1 | 0.22 | 0.452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, C.; Zhang, J.; Sun, X.; Sun, L.; Su, H.; Murayama, T. Au Nanoclusters on Vanadium-Doped ZrO2 Nanoparticles for Propylene Oxidation: An Investigation into the Impact of V. Materials 2025, 18, 1118. https://doi.org/10.3390/ma18051118
Qi C, Zhang J, Sun X, Sun L, Su H, Murayama T. Au Nanoclusters on Vanadium-Doped ZrO2 Nanoparticles for Propylene Oxidation: An Investigation into the Impact of V. Materials. 2025; 18(5):1118. https://doi.org/10.3390/ma18051118
Chicago/Turabian StyleQi, Caixia, Jingzhou Zhang, Xun Sun, Libo Sun, Huijuan Su, and Toru Murayama. 2025. "Au Nanoclusters on Vanadium-Doped ZrO2 Nanoparticles for Propylene Oxidation: An Investigation into the Impact of V" Materials 18, no. 5: 1118. https://doi.org/10.3390/ma18051118
APA StyleQi, C., Zhang, J., Sun, X., Sun, L., Su, H., & Murayama, T. (2025). Au Nanoclusters on Vanadium-Doped ZrO2 Nanoparticles for Propylene Oxidation: An Investigation into the Impact of V. Materials, 18(5), 1118. https://doi.org/10.3390/ma18051118





