Effect of SiC Concentration on the Microstructure and Anti-Wear Performance of Electrodeposited Ni-SiC Composite Coatings Constructed for Piston Ring Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
3. Results and Discussion
3.1. Surface Morphology Observation
3.2. XRD Pattern Analysis
3.3. Microhardness Value Measurement
3.4. Anti-Wear Performance Investigation
4. Conclusions
- (1)
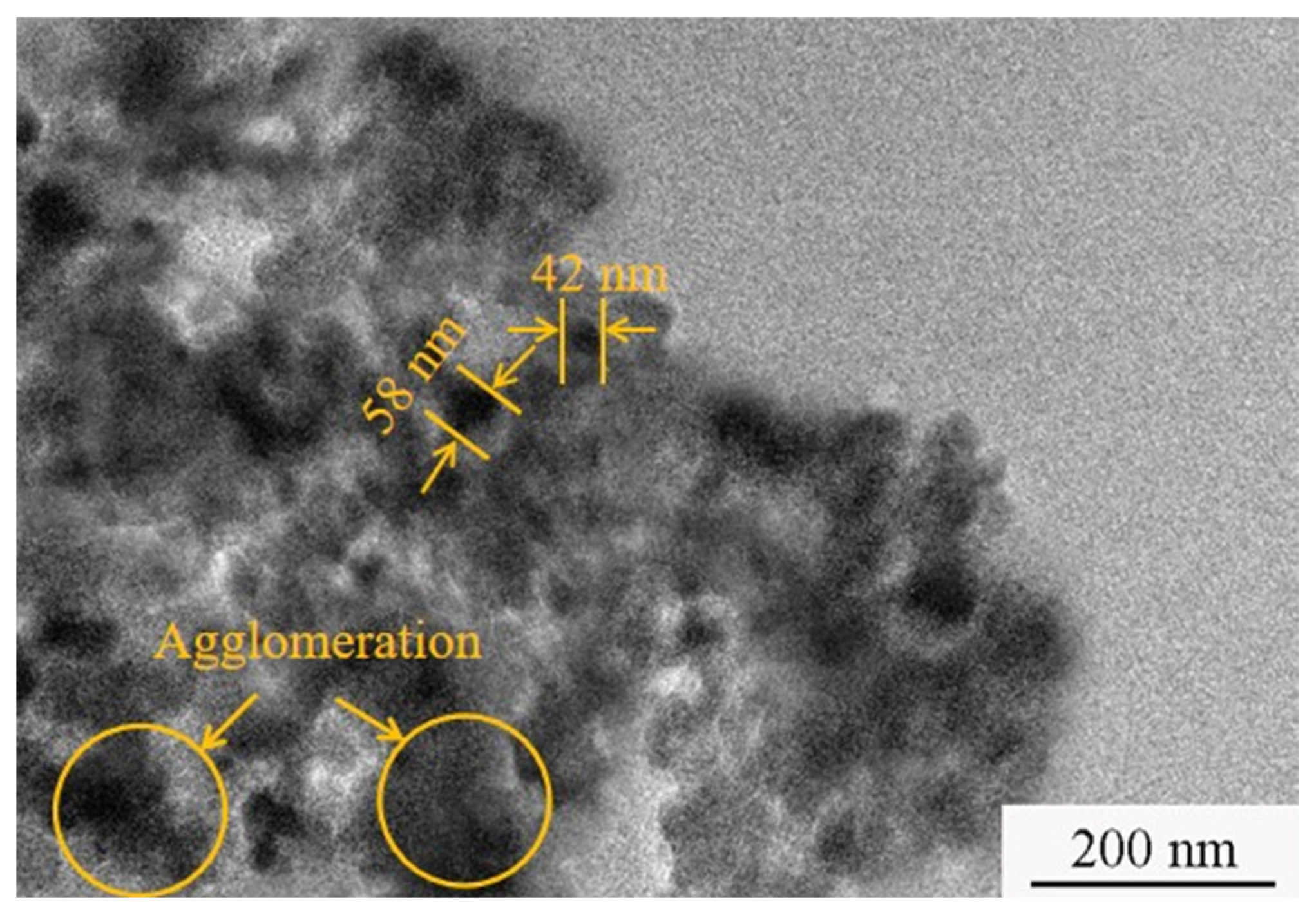
- The SEM images show that a dense, flat microstructure with a fine grain size appeared on the surface of the NSc-9, while the NSc-9 and NSc-15 coatings had a cauliflower-like morphology. Meanwhile, the grain sizes of the NSc-3, NSc-9, and NSc-15 coatings were 874 nm, 429 nm, and 635 nm, respectively.
- (2)
- In comparison to the NSc-3 and NSc-15 coatings, the NSc-9 coatings possessed a maximum microhardness of 672 Hv and a minimum indentation depth of 13.7 μm, demonstrating an excellent anti-wear performance.
- (3)
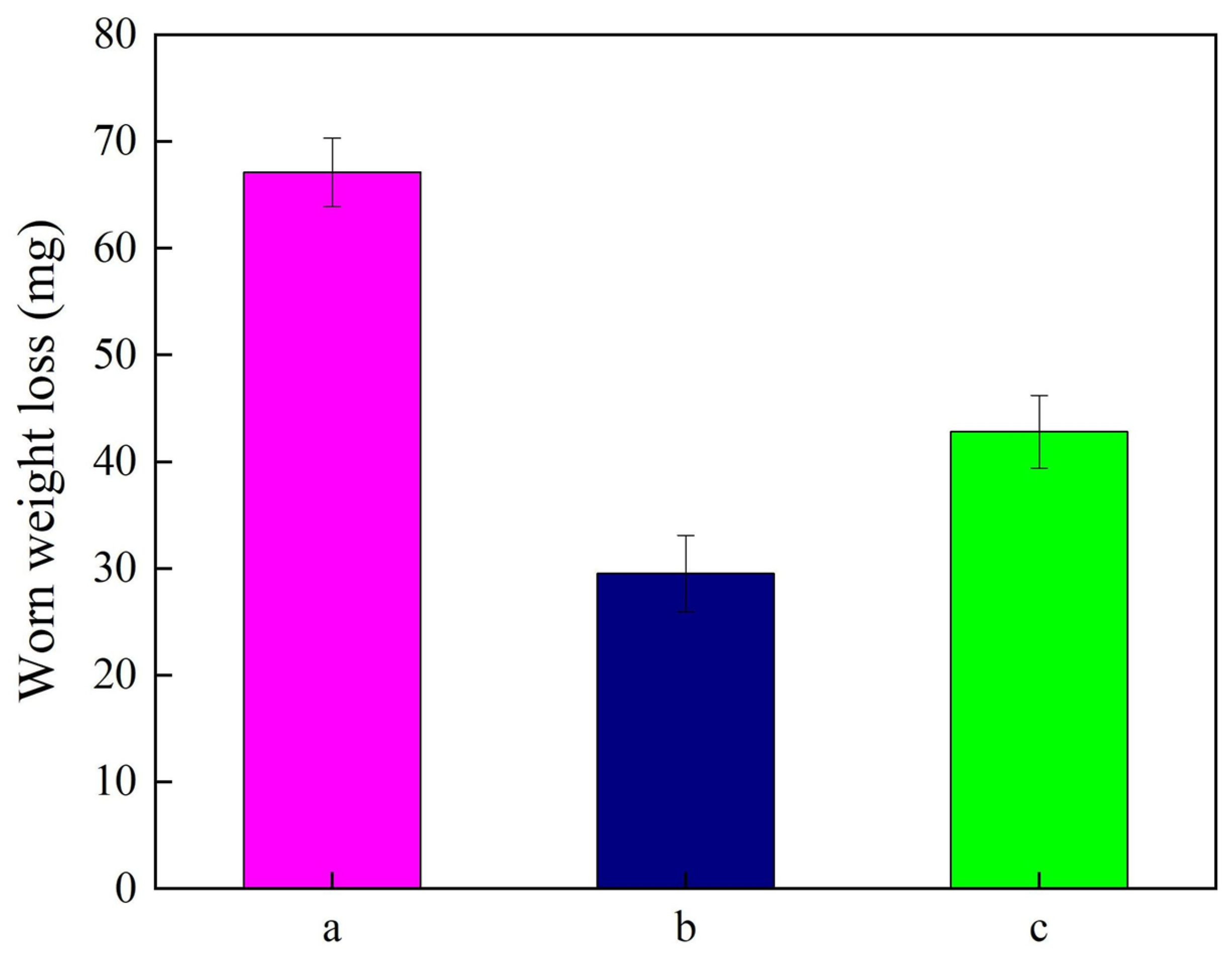
- Furthermore, the NSc-9 coatings had the lowest friction coefficient of 0.46 and the smallest worn weight loss of 29.5 mg, indicating their outstanding anti-wear performance and their expanded application in piston rings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vural, E.; Ozzel, S.; Ozer, S. Coating of diesel engine with new generation ceramic material to improve combustion and performance. Therm. Sci. 2021, 25, S101–S110. [Google Scholar] [CrossRef]
- Gu, W.S.; Yang, S.; Zhang, S.Y.; Pei, Z.L.; Xue, W.H.; Duan, D.L.; Gong, J.; Sun, C. High-Speed Rubbing Behavior of Abrasive Coating Coated on Titanium Alloy Blade Tips. ACTA Metall. Sin.-Engl. Lett. 2024, 37, 749–762. [Google Scholar] [CrossRef]
- Yilmaz, A.C.; Esen, M. Improving Tribological Performance of Piston Ring Steel Substrates by DLC/Nano-crystalline Diamond Coating. Arab. J. Sci. Eng. 2022, 47, 15441–15453. [Google Scholar] [CrossRef]
- Zhong, Z.Y.; Zhu, Y.S.; Zhang, Y.Y. Experiments on effects of dust particles on the wear of cylinder liner in internal combustion engine. Ind. Lubr. Tribol. 2012, 64, 321–330. [Google Scholar] [CrossRef]
- Huang, P.C.; Chou, C.C.; Hsu, L.S. Preparation and tribological research of the electrodeposited Ni-W alloy coatings for piston ring application. Surf. Coat. Technol. 2021, 411, 126980. [Google Scholar] [CrossRef]
- EI-Nemr, M.A.; Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; EI Nemr, A.; Ragab, S.; Osibote, O.A.; Hassaan, M.A. Adsorption of Cr6+ ion using activated Pisum sativum peels-triethylenetetramine. Evironment Sci. Pollut. Res. 2022, 29, 91036–91060. [Google Scholar] [CrossRef]
- Priyadarshi, P.; Katiyar, P.K.; Maurya, R. A review on mechanical, tribological and electrochemical performance of ceramic particle-reinforced Ni-based electrodeposited composite coatings. J. Mater. Sci. 2022, 57, 19179–19211. [Google Scholar] [CrossRef]
- Kucharska, B.; Brojanowska, A.; Poplawski, K.; Sobiecki, J.R. Corrosion resistance of Ni-Al2O3 nanocomposite coatings. Mater. Sci.-Medzg. 2016, 22, 31–35. [Google Scholar] [CrossRef][Green Version]
- Ali, K.; Narayana, S.; Shakkoor, R.A.; Okonkkwo, P.C.; Yusuf, M.M.; Alashraf, A.; Kahraman, R. Synthesis and Performance evaluation of pulse electrodeposited Ni-AlN nanocomposite coatings. Scanning 2018, 7187024. [Google Scholar] [CrossRef]
- Yang, Z.G.; Yi, S.J.; Cao, L.K.; Tang, S.H.; Li, Q. Process simulation and abrasion behavior of jet electrodeposited Ni-TiN nanocoatings. Coatings 2022, 12, 86. [Google Scholar] [CrossRef]
- Ji, R.J.; Han, K.; Jin, H.; Li, X.P.; Liu, Y.H.; Liu, S.G.; Dong, T.C.; Cai, B.P.; Cheng, W.H. Preparation of Ni-SiC nano-composite coating by rotating magnetic field-assisted electrodeposition. J. Manuf. Process 2020, 57, 787–797. [Google Scholar] [CrossRef]
- Loganathan, A.; Kamdi, Z.; Ainuddin, A.R.; Husin, R.; Ibrahim, A.; Salleh, M.A.A.M. Microstructure characterisation, hardness, and corrosion behaviour of nickel-silicon carbide electrodeposition coating with the influence of ultrasonic action. Arch. Metall. Mater. 2024, 69, 859–863. [Google Scholar] [CrossRef]
- Islam, M.; Azhar, M.R.; Khalid, Y.; Khan, R.; Abdo, H.S.; Dar, M.A.; Oloyede, O.R.; Burleigh, T.D. Electroless Ni-P/SiC nanocomposite coatings with small amounts of SiC nanoparticles for superior corrosion resistance and hardness. J. Mater. Eng. Perform. 2015, 24, 4835–4843. [Google Scholar] [CrossRef]
- Ying, L.X.; Liu, Y.; Liu, G.N.; Li, Z.H.; Wang, G.X. Preparation and Properties of Electroless Plating Wear-resistant and Antifriction Composite Coatings Ni-P-SiC-WS2. Rare Met. Mater. Eng. 2015, 44, 28–31. [Google Scholar]
- Farzaneh, A.; Ehteshamzadeh, M.; Can, M.; Mermer, O.; Okur, S. Effect of SiC particles size on electrochemical of SiC particles size on the electrochemical properties of electroless Ni-P-SiC nanocomposite coatings. Protecction Met. Phys. Chem. Surf. 2016, 52, 632–636. [Google Scholar] [CrossRef]
- Gyawali, G.; Hamal, K.; Joshi, B.; Rajbhandari, A.; Lee, S.W. Microstructural and electrochemical analysis of Ni-SiC composite coatings prepared in presence of additives. Mater. Lett. 2014, 126, 228–231. [Google Scholar] [CrossRef]
- Cui, L.F.; Li, X.; Li, C.Y.; Zhu, L.J.; Zhang, Q.G.; Li, Z.; Liu, H.Y. Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application. Materials 2024, 17, 4616. [Google Scholar] [CrossRef]
- Yan, Y.T.; Lu, L.F.; Huo, Y.Q.; Zhao, Y. Characterization and Wear Behaviors of Electrodeposited Ni-MoS2/SiC Composite Coating. Coatings 2022, 12, 1223. [Google Scholar] [CrossRef]
- Dogan, F.; Uysal, M.; Algül, H.; Duru, E.; Akbulut, H.; Aslan, S. Pulsed electrodeposition of Ni-B/TiN composites: Effect of current density on the structure, mechanical, tribological, and corrosion properties. J. Asian Ceram. Soc. 2020, 8, 1271–1284. [Google Scholar] [CrossRef]
- Xian, J.Y.; Shen, Z.Y.; Zhang, Z.W.; Wu, H.B.; Jin, M.F.; Jiang, M.J. Effect of current density on the wear resistance of Ni-P alloy coating prepared through immersion-assisted jet-electrodeposition. Coatings 2021, 11, 527. [Google Scholar] [CrossRef]
- Wang, C.; Shen, L.D.; Qiu, M.B.; Tian, Z.J.; Jiang, W. Characterizations of Ni-CeO2 nanocomposite coating by interlaced jet electrodeposition. J. Alloys Compd. 2017, 727, 269–277. [Google Scholar] [CrossRef]
- Dai, P.Q.; Zhong, Y.H.; Zhou, X. Corrosion characteristic of pulsed electrodeposition Ni-Co/SiC nanocomposite coating. Surf. Eng. 2011, 27, 71–76. [Google Scholar] [CrossRef]
- Ben Temam, H.; Zeroual, L.; Chala, A.; Rahmane, S.; Nouveau, C. Microhardness and Corrosion Behavior of Ni-SiC Electrodeposited Coatings. Plasma Process. Polym. 2007, 4, S618–S621. [Google Scholar] [CrossRef]
- Kan, H.M.; Yang, Z.Y.; Zhang, N. Preparation of Ni-SiC Composite Coating by Electrochemical Deposition. Rare Met. Mater. Eng. 2015, 44, 2960–2964. [Google Scholar]
- Zheng, L.; Li, Y.F.; Liu, M.Y.; Wang, Y.X.; Sun, B.; Zhang, C.M.; Leng, H.Y. Fabrication and Characterization of Al2O3 Particles Reinforced Ni-P-Polytetrafluoroethylene Nanocomposite Coating by Jet Electrodeposition. J. Mater. Eng. Perform. 2023, 32, 8503–8515. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, H.G.; Zhu, J.C.; Fang, C.; Li, Z.Q.; Jiang, W. Fabrication of Ni-TiN nanocomposite coatings by ultrasonic assisted jet electrodeposition. Proc. Inst. Mech. Eng. Part B-J. Eng. Manuf. 2024, 238, 1507–1518. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.D.; Qiu, M.B.; Xu, M.Y.; Tian, Z.J. Microhardness, wear, and corrosion resistance of Ni-SiC composite coating with magnetic-field-assisted jet electrodeposition. Mater. Res. Express 2018, 5, 96407. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Yao, L.M.; Li, H.X.; Jia, X. Investigation of the mechanical properties and corrosion behaviors of Ni-BN-TiC layers constructed via laser cladding technique. Ceram. Int. 2023, 49, 6671–6677. [Google Scholar] [CrossRef]
- Lamovec, J.; Jovic, V.; Vorkapic, M.; Popovic, B.; Radojevic, V.; Aleksic, R. Microhardness analysis of thin metallic multilayer composite films on copper substrates. J. Min. Metall. Sect. B-Metall. 2011, 47, 53–61. [Google Scholar] [CrossRef]
- Ma, C.Y.; Zhao, D.Q.; Liu, W.Q.; Xia, F.F.; Jin, P.; Sun, C.F. Magnetic assisted pulse electrodeposition and characterization of Ni-TiC nanocomposites. Ceram. Int. 2020, 46, 17631–79639. [Google Scholar] [CrossRef]
- Liu, W.Q.; Jiang, K.D.; Li, Q.; Ma, C.Y.; Xia, F.F. Jet Pulse Electrodeposited Ni-SiC Thin Coatings by Using Experimental System Designed for Potential Industrial Application. Int. J. Electrochem. Sci. 2021, 16, 21044. [Google Scholar] [CrossRef]
- Xia, F.F.; Li, C.Y.; Ma, C.Y.; Li, Q.; Xing, H.Y. Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Kozik, A.; Nowak, M.; Gawlik, M.; Bigaj, M.; Karas, M. The effecT of dIspersIon phases of sic and AL2O3 on The properTIes of galvanIc nIckel coaTIngs. Arch. Metall. Mater. 2016, 61, 375–380. [Google Scholar] [CrossRef][Green Version]
- Balaji, R.; Pushpavanam, M.; Kumar, K.Y.; Subramanian, K. Electrodeposition of bronze-PTFE composite coatings and study on their tribological characteristics. Surf. Coat. Technol. 2006, 201, 3205–3211. [Google Scholar] [CrossRef]
- Wang, S.Q.; Xie, F.Q.; Wu, X.Q. Effect of SiC contents on wear resistance performance of electro-codeposited Ni-SiC composite Coatings. Coatings 2024, 14, 1224. [Google Scholar] [CrossRef]









| Chemical Reagent | Specific |
|---|---|
| NiSO4·6H2O (g/L) | 240 |
| NiCl2·6H2O (g/L) | 35 |
| CTAB (mg/L) | 50 |
| H3BO3 (g/L) | 30 |
| SiC concentration (g/L) | 3, 9, and 15 |
| Operation Parameters | Specific |
|---|---|
| Current density (A/dm2) | 4.5 |
| Electrolyte temperature (°C) | 55 |
| Electrodeposition time (min) | 40 |
| pH | 4.1 |
| Duty cycle (%) | 30 |
| Agitation speed (rmp) | 600 |
| SiC Concentration | 3 g/L | 9 g/L | 15 g/L |
| Si Content of Composite Coatings | 1.9 wt.% | 3.2 wt.% | 4.5 wt.% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, Q.; Shen, H.; Bai, C.; Li, C.; Tian, D.; Wang, B. Effect of SiC Concentration on the Microstructure and Anti-Wear Performance of Electrodeposited Ni-SiC Composite Coatings Constructed for Piston Ring Application. Materials 2025, 18, 1117. https://doi.org/10.3390/ma18051117
Zhang F, Wang Q, Shen H, Bai C, Li C, Tian D, Wang B. Effect of SiC Concentration on the Microstructure and Anti-Wear Performance of Electrodeposited Ni-SiC Composite Coatings Constructed for Piston Ring Application. Materials. 2025; 18(5):1117. https://doi.org/10.3390/ma18051117
Chicago/Turabian StyleZhang, Fengwu, Qiuhua Wang, Huajie Shen, Caixia Bai, Chaoyu Li, Dehao Tian, and Baojin Wang. 2025. "Effect of SiC Concentration on the Microstructure and Anti-Wear Performance of Electrodeposited Ni-SiC Composite Coatings Constructed for Piston Ring Application" Materials 18, no. 5: 1117. https://doi.org/10.3390/ma18051117
APA StyleZhang, F., Wang, Q., Shen, H., Bai, C., Li, C., Tian, D., & Wang, B. (2025). Effect of SiC Concentration on the Microstructure and Anti-Wear Performance of Electrodeposited Ni-SiC Composite Coatings Constructed for Piston Ring Application. Materials, 18(5), 1117. https://doi.org/10.3390/ma18051117






