Microstructure and Mechanical Properties of AlSi10MnMg Alloy with Increased Content of Recycled Scrap
Abstract
1. Introduction
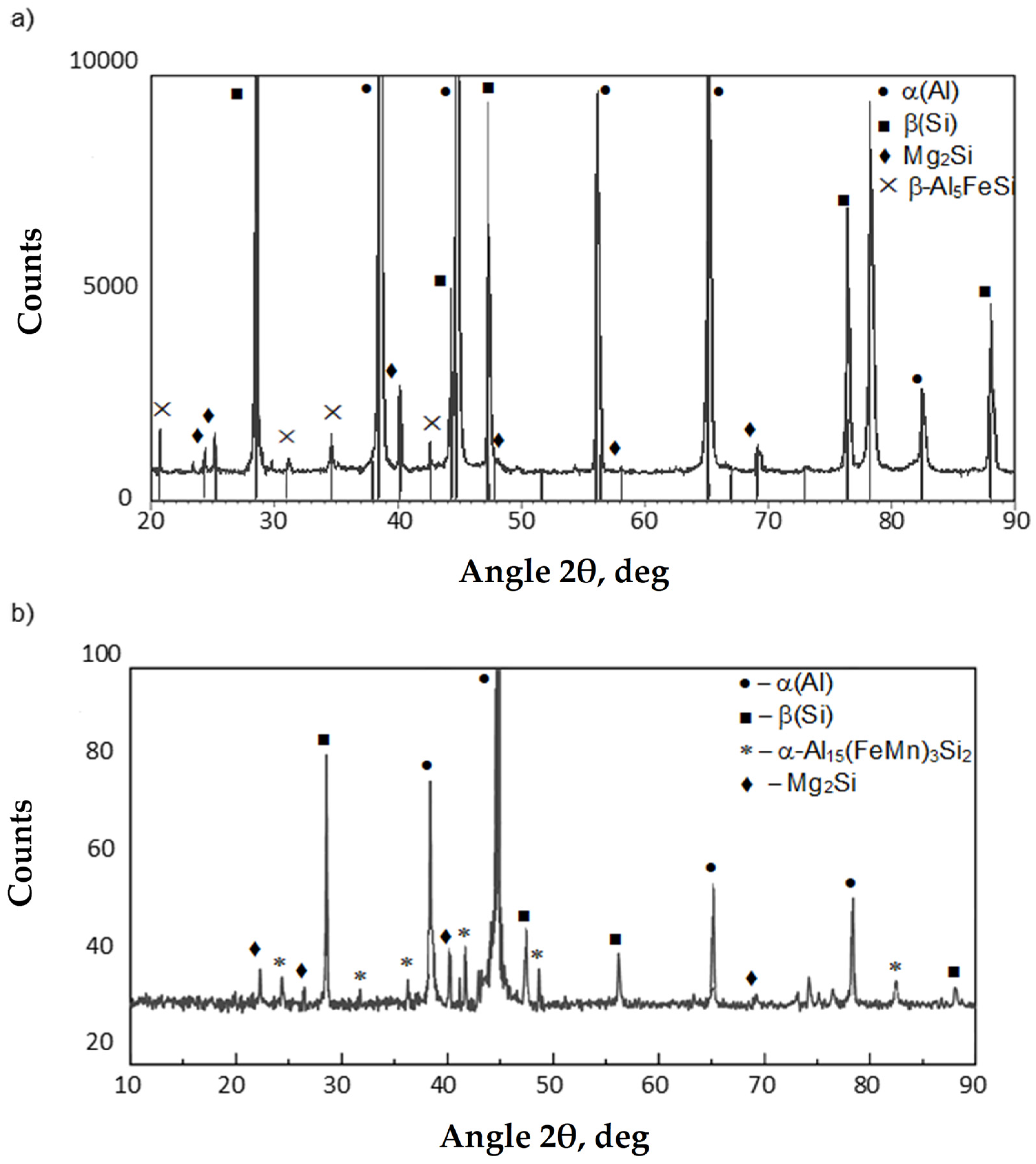
- β-Al5FeSi, also referred to as Al9Fe2Si in Group 6XX.X (Al-Mg-Si) alloys [22];
- α-Al8Fe2Si (possibly α-Al12Fe3Si2), which has a hexagonal structure under thermodynamic equilibrium conditions and only stable in Al-Si-Fe alloys with a very high purity of charge components [23];
- δ-Al4FeSi2 present in Al-Si alloys with a more than 18 wt.% Si [24];
- γ-Al3FeSi occurring at more than 4% wt. content Fe and more than 16 wt.% Si [25].
- Shrinkage of the metal during cooling of the casting, which causes a vacuum of the liquid phase in the dendrite growth zone of the solid solution α(Al)—Darcy’s law [39];
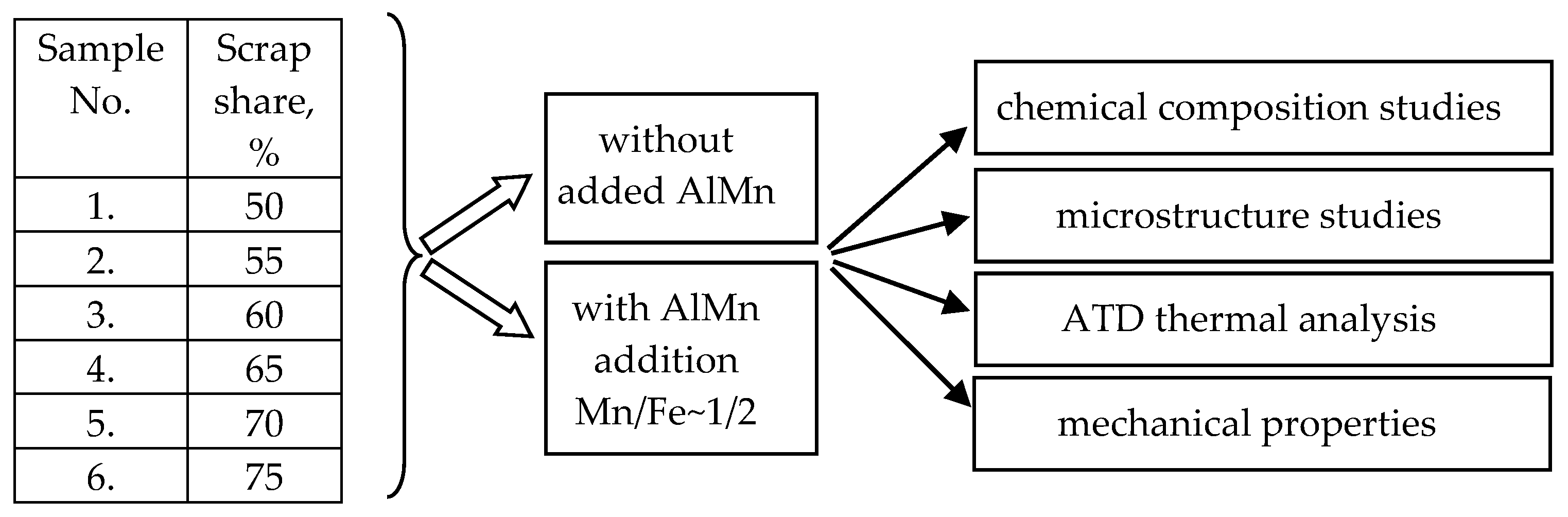
- Perform smelting of AlSi10MnMg alloy with an increasing proportion of circulating scrap according to the experimental plan (Figure 1);
- Investigate the chemical composition of AlSi10MnMg alloy with different iron and manganese contents;
- Analyze the crystallization process by the ATD method for AlSi10MnMg alloy with different iron and manganese contents;
- Test the mechanical properties, i.e., hardness (HB), tensile strength (UTS), conventional yield strength (YS), and elongation (A), based on the static tensile test;
- Characterize the microstructure (including porosity) of the tested alloys.
2. Materials and Methods
2.1. Method of Preparing Research Material
2.2. Method of Performing ATD and Chemical Composition Tests
2.3. Methodology for Testing Mechanical Properties
2.4. Method of Conducting Microstructure Tests
3. Results and Discussion
3.1. Chemical Composition Test Results
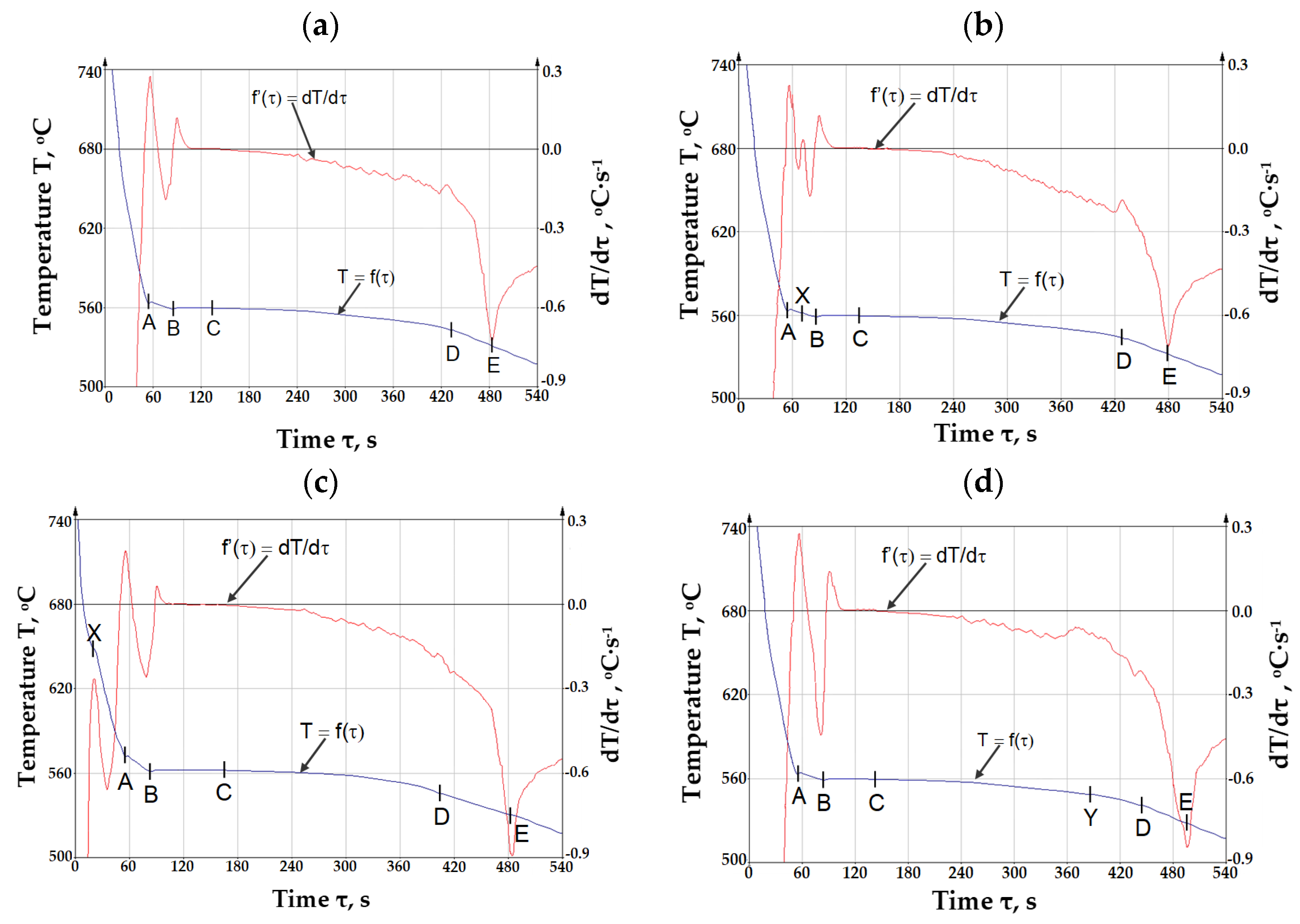
3.2. ATD Thermal Derivative Analysis Test Results
- T = f(τ)—the curve of temperature T change at time τ—the so-called TA (temperature analysis) curve;
- dT/dτ = f’(τ)—the first derivative of the temperature change over time—the so-called ATD (thermal derivative analysis) curve;
- Point A—temperature of crystallization of dendrites of solid solution α(Al)—Tα(Al) (Tliq.), °C;
- A–B—range of crystallization of dendrites of solid solution α(Al), °C;
- Point B—minimum temperature of crystallization of eutectic α(Al) + β(Si)—TEmin(α+β), °C;
- Point C—average crystallization temperature of the α(Al) + β(Si)—TE(α+β), °C;
- Point X—crystallization temperature of iron intermetallic phases—TE(Fe), °C;
- Point Y—crystallization temperature of iron intermetallic phases—TE(AlFeMn), °C;
- Point D—crystallization temperature of magnesium intermetallic phases—TE(Mg), °C;
- Point E—temperature of the end of crystallization of AlSi10MnMg alloy (Tsol.), °C.
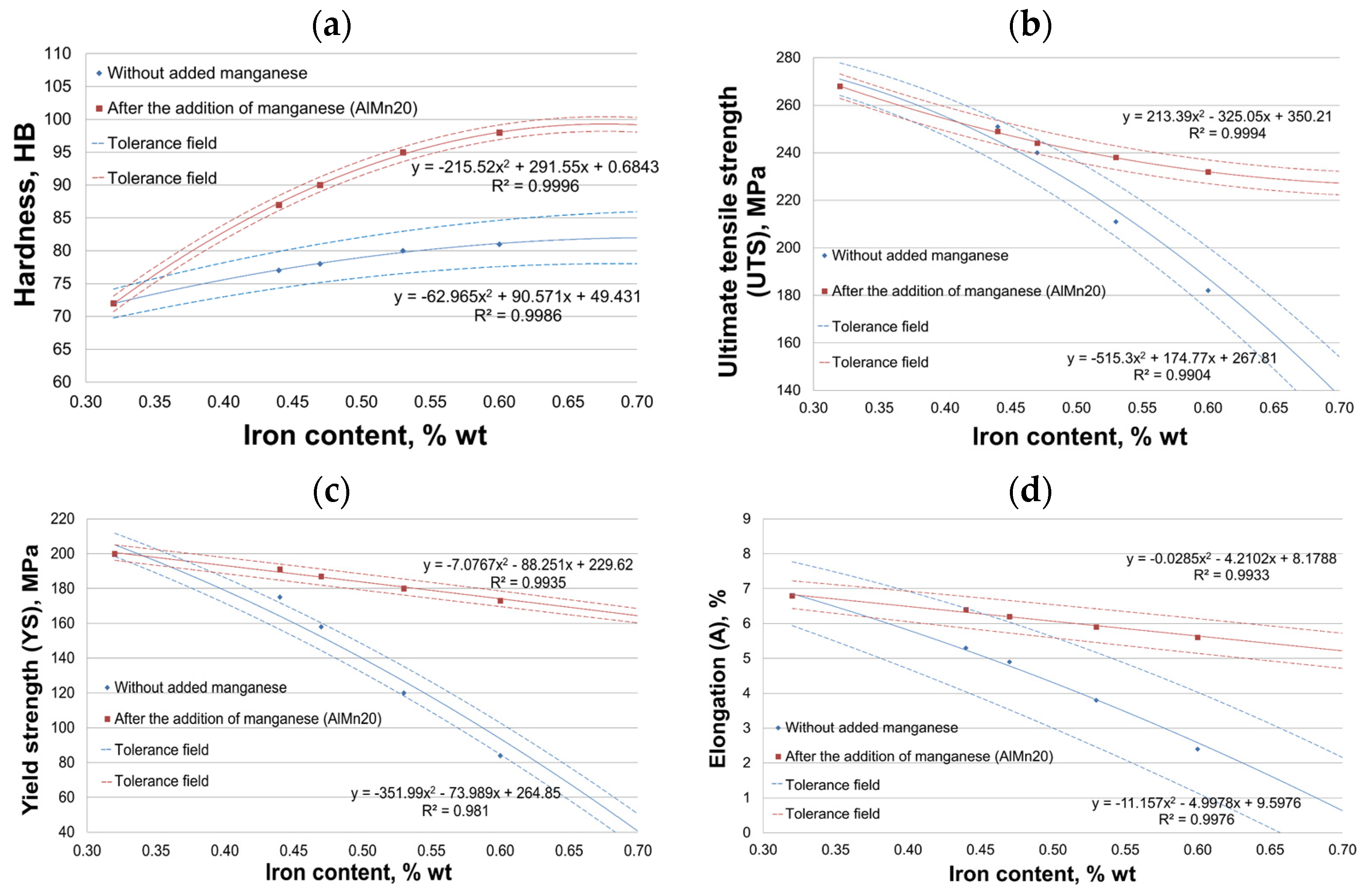
3.3. Mechanical Properties Test Results
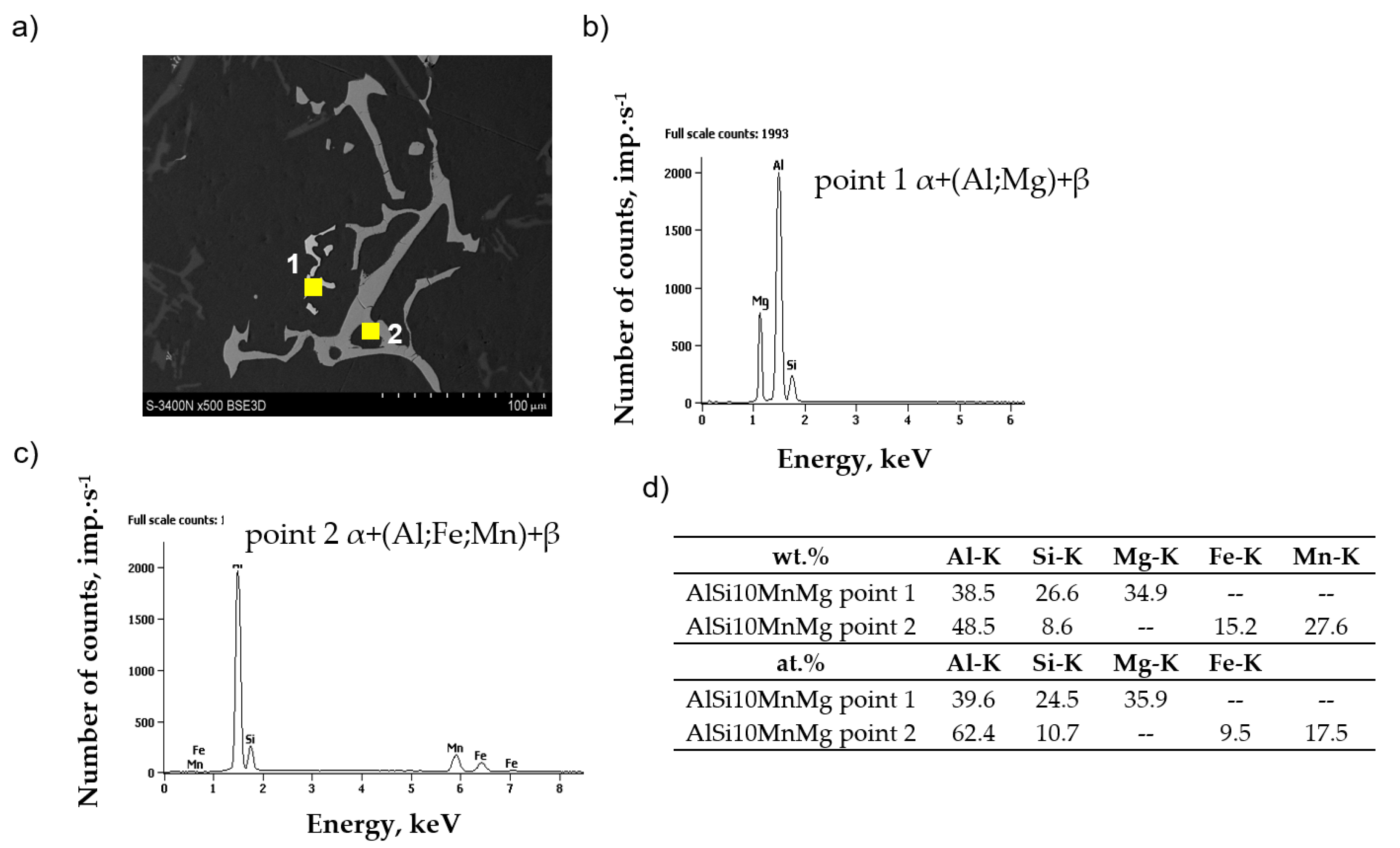
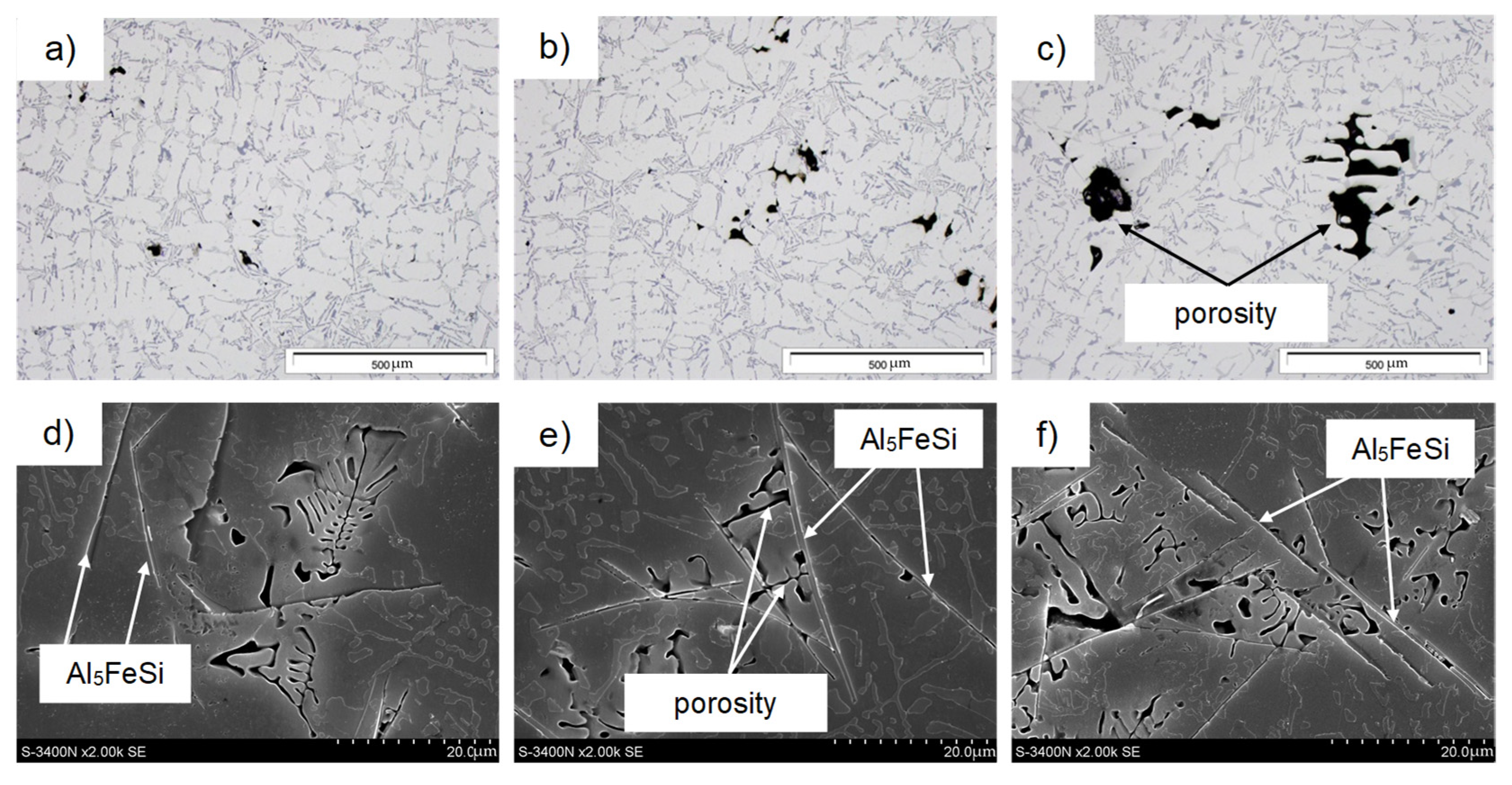
3.4. Microstructure Test Results
4. Summary and Conclusions
- To a content of about 0.45 wt.% Fe:Liq. → α(Al) + α(Al) + β(Si) + α(Al) + (Al;Si;Fe) + β(Si) + α(Al) + (Mg2Si) + β(Si)(these eutectics can crystallize simultaneously);
- With a content of about 0.45 to 0.7 wt.% Fe:Liq. → α(Al) + Al5FeSi + α(Al) + β(Si) + α(Al) + (Mg2Si) + β(Si)(crystallization of eutectics as above);
- With a content of more than 0.7 wt.% Fe:Liq. → Al5FeSi + α(Al) + α(Al) + β(Si) + α(Al) + (Mg2Si) + β(Si)(crystallization of eutectics as above).
- Improvement in HB hardness by about 37%;
- Reduction in UTS tensile strength by about 16%;
- Reduction in YS yield strength by about 16%;
- Slight reduction in elongation of about 18% compared to an alloy with 50% scrap (0.32 wt.% Fe content).
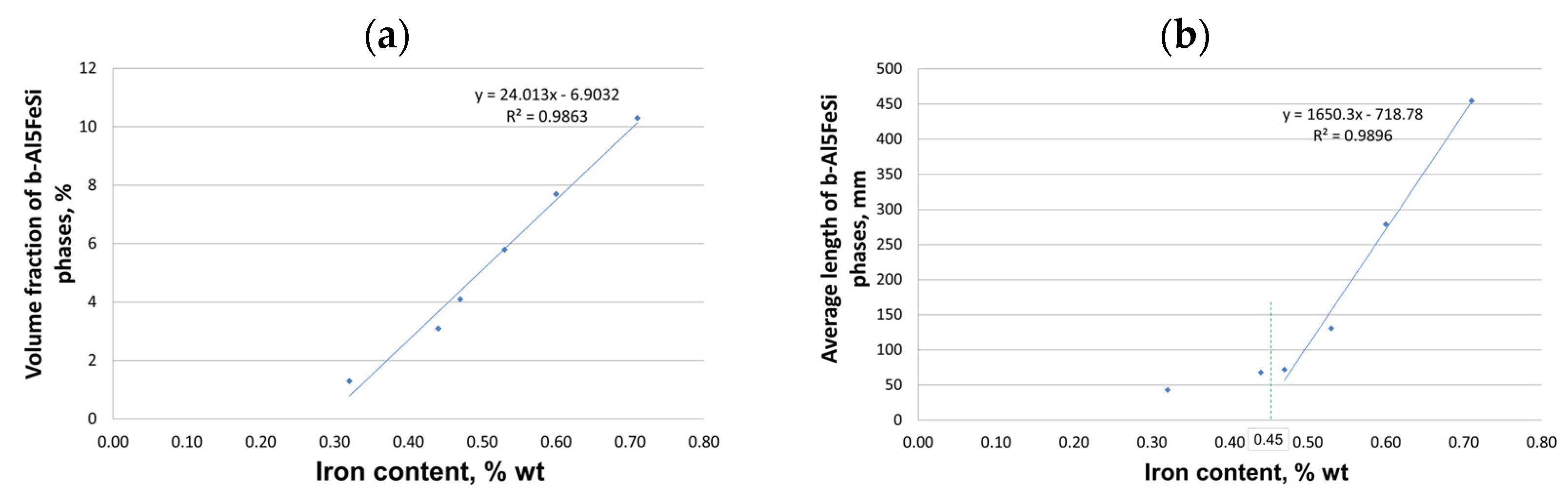
- The alloy’s crystallization (under gravitational conditions) with a content of up to 0.45 wt.% Fe proceeds traditionally, as for silumin, which has a slightly sub-eutectic composition with manganese and magnesium content. At contents of about 0.45 to 0.7 wt.% Fe, a change in the order of crystallization takes place—the Al5FeSi phase separates pre-eutectically if the iron content exceeds about 0.7 wt.%—the Al5FeSi phase crystallizes originally. This phase does not affect the crystallization of the other components, i.e., dendrites α(Al), eutectics α(Al) + β(Si), and eutectics containing the Mg2Si phase.
- After adding AlMn20 master alloy, the Al15(Fe,Mn)3Si2 phase was identified, whose crystallization occurs in the temperature range of 540–555 °C and increases slightly with increasing manganese addition.
- When introducing manganese into the alloy, the quotient Mn/Fe of 1/2 must be observed. A higher manganese content results in the formation of sludge particles, which are deposited on the surface of the liquid alloy and can adversely affect its mechanical properties.
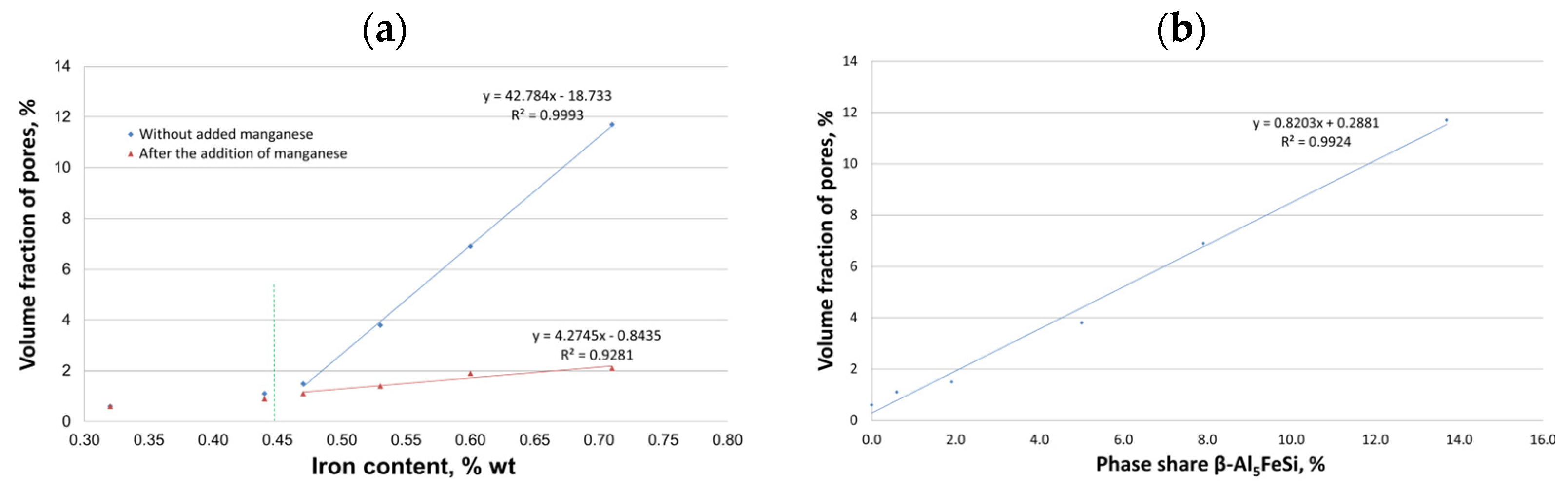
- Increasing the scrap content of the AlSi10MnMg alloy decreases UTS, YS, and elongation values, hence the need for manganese addition. This transformation transforms the morphologically unfavorable β-Al5FeSi phase into α-Al15(Fe, Mn)3Si2 with dendritic morphology.
- Without the addition of manganese, the results of mechanical properties are characterized by a large scatter (measured by standard deviation). In contrast, after the introduction of manganese, the scatter is much smaller, indicating a more homogeneous alloy structure.
- Up to a content of about 0.45 wt.% Fe, there was no effect of iron on shrinkage porosity. Only after this value is exceeded is there a correlation between the amount of Al5FeSi phase precipitates and porosity.
- The effect of casting process parameters (e.g., mold temperature, pouring speed) on the morphology and distribution of the β-Al5FeSi phase in castings with an iron content of more than 0.7 wt.% will be examined. It is known that in die-cast Al-Si-Mn(Fe) alloys, the allowable iron content is much higher (from about 1.0 to 2.0 wt.%) compared to gravity castings (from 0.4 to about 0.7 wt.%).
- Heat flow and solidification, pore formation, and phase transformation processes will be simulated to ensure the best possible metallurgical quality of the alloys studied.
- The effect of heat treatment on the properties of alloys with different manganese content will be investigated, especially in terms of aging enhancement and grain refinement of casting alloys.
- Long-term performance of AlSiMnMg alloys will be evaluated under extreme conditions (e.g., for different operating temperatures; the influence of iron and manganese on corrosion; and the possibility of carrying out thermo-chemical treatment of the surface of castings made of Al-Si-Me (Me=Cu; Mg; Mn; Ni) alloys with an increased proportion of scrap).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalai, B. Material Characterization of Aluminum Alloys for Automotive and Aerospace Applications; Luleå University of Technology: Luleå, Sweden, 2024; ISBN 978-91-8048-644-6. [Google Scholar]
- Czerwinski, F. Current Trends in Automotive Lightweighting Strategies and Materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef] [PubMed]
- Bakedano, A.; Niklas, A.; Fernández-Calvo, A.I.; Plata, G.; Lozares, J.; Berlanga-Labari, C. Comparative Study of the Metallurgical Quality of Primary and Secondary AlSi10MnMg Aluminium Alloys. Metals 2021, 11, 1147. [Google Scholar] [CrossRef]
- Soares, G.; Neto, R.; Madureira, R.; Soares, R.; Silva, J.; Silva, R.P.; Araújo, L. Characterization of Al Alloys Injected through Vacuum-Assisted HPDC and Influence of T6 Heat Treatment. Metals 2023, 13, 389. [Google Scholar] [CrossRef]
- Jiao, X.Y.; Zhang, Y.F.; Wang, J.; Nishat, H.; Liu, Y.X.; Liu, W.N.; Chen, H.X.; Xiong, S.M. Characterization of Externally Solidified Crystals in a High-Pressure Die-Cast AlSi10MnMg Alloy and Their Effect on Porosities and Mechanical Properties. J. Mater. Process. Technol. 2021, 298, 117299. [Google Scholar] [CrossRef]
- Niklas, A.; Orden, S.; Bakedano, A.; Da Silva, M.; Nogués, E.; Fernández-Calvo, A.I. Effect of Solution Heat Treatment on Gas Porosity and Mechanical Properties in a Die Cast Step Test Part Manufactured with a New AlSi10MnMg(Fe) Secondary Alloy. Mater. Sci. Eng. A 2016, 667, 376–382. [Google Scholar] [CrossRef]
- Primary Aluminium Casting Alloys. Available online: https://www.foundry-planet.com/fileadmin/redakteur/Material/08-03-10-Leporello_engl.pdf (accessed on 26 February 2025).
- Winkler, M.; Gawert, C.; Bähr, R.; Jüttner, S.; Trommer, F. Investigation of the Friction Weldability of an AlSi10MnMg-Alloy Reinforced with 30 Vol.-% Silicon Carbide Particles with the Adequate Monolithic Material. J. Adv. Join. Process. 2022, 5, 100101. [Google Scholar] [CrossRef]
- Krochmal, M.; Rajan, A.N.R.; Moeini, G.; Sajadifar, S.V.; Wegener, T.; Niendorf, T. Microstructural and Mechanical Properties of AlSi10Mg: Hybrid Welding of Additively Manufactured and Cast Parts. J. Mater. Res. 2023, 38, 297–311. [Google Scholar] [CrossRef]
- Schlesinger, M.E. Aluminum Recycling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-10127-4. [Google Scholar]
- Gaustad, G.; Olivetti, E.; Kirchain, R. Improving Aluminum Recycling: A Survey of Sorting and Impurity Removal Technologies. Resour. Conserv. Recycl. 2012, 58, 79–87. [Google Scholar] [CrossRef]
- Kuchariková, L.; Tillová, E.; Bokůvka, O. Recycling and Properties of Recycled Aluminium Alloys Used in the Transportation Industry. Transp. Probl. 2017, 11, 117–122. [Google Scholar] [CrossRef]
- Gutowski, T.G.; Sahni, S.; Allwood, J.M.; Ashby, M.F.; Worrell, E. The Energy Required to Produce Materials: Constraints on Energy-Intensity Improvements, Parameters of Demand. Philos. Trans. R. Soc. A 2013, 371, 20120003. [Google Scholar] [CrossRef]
- Das, S.K.; Green, J.A.S.; Kaufman, J.G.; Emadi, D.; Mahfoud, M. Aluminum Recycling—An Integrated, Industrywide Approach. JOM 2010, 62, 23–26. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. CO2 and Greenhouse Gas Emissions; Our World in Data: Oxford, UK, 2023. [Google Scholar]
- Bertram, M.; Ramkumar, S.; Rechberger, H.; Rombach, G.; Bayliss, C.; Martchek, K.J.; Müller, D.B.; Liu, G. A Regionally-Linked, Dynamic Material Flow Modelling Tool for Rolled, Extruded and Cast Aluminium Products. Resour. Conserv. Recycl. 2017, 125, 48–69. [Google Scholar] [CrossRef]
- Stotz, P.M.; Niero, M.; Bey, N.; Paraskevas, D. Environmental Screening of Novel Technologies to Increase Material Circularity: A Case Study on Aluminium Cans. Resour. Conserv. Recycl. 2017, 127, 96–106. [Google Scholar] [CrossRef]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Deschamps, A.; Hutchinson, C.R. Precipitation Kinetics in Metallic Alloys: Experiments and Modeling. Acta Mater. 2021, 220, 117338. [Google Scholar] [CrossRef]
- Ebenberger, P.; Uggowitzer, P.J.; Gerold, B.; Pogatscher, S. Effect of Compositional and Processing Variations in New 5182-Type AlMgMn Alloys on Mechanical Properties and Deformation Surface Quality. Materials 2019, 12, 1645. [Google Scholar] [CrossRef] [PubMed]
- Sukiman, N.L.; Zhou, X.; Birbilis, N.; Hughes, A.E.; Mol, J.M.C.; Garcia, S.J.; Zhou, X.; Thompson, G.E. Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments. In Aluminium Alloys—New Trends in Fabrication and Applications; Ahmad, Z., Ed.; InTech: Houston, TX, USA, 2012; ISBN 978-953-51-0861-0. [Google Scholar]
- Cao, X.; Campbell, J. Morphology of β-Al5FeSi Phase in Al-Si Cast Alloys. Mater. Trans. 2006, 47, 1303–1312. [Google Scholar] [CrossRef]
- Boulouma, A.; Drici, A.; Benaldjia, A.; Guerioune, M.; Vrel, D. The Formation of (Al8Fe2Si, Al13Fe4) Phases from Al-Fe-Si System by TE Mode; AIP Publishing LLC: Fethiye, Turkey, 2015; p. 0023. [Google Scholar]
- Khalifa, W. Role of Inclusions in the Nucleation of A-Aluminum and Fe-Intermetallics in the Aluminum-Rich Corner of the Al-Si-Fe Ternary System; Université du Québec à Chicoutimi: Chicoutimi, QC, Canada, 2003; ISBN 978-1-4123-1106-9. [Google Scholar]
- Belov, N.A.; Aksenov, A.A.; Eskin, D.G. Iron in Aluminium Alloys: Impurity and Alloying Element, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-429-18990-6. [Google Scholar]
- Moustafa, M.A. Effect of Iron Content on the Formation of β-Al5FeSi and Porosity in Al–Si Eutectic Alloys. J. Mater. Process. Technol. 2009, 209, 605–610. [Google Scholar] [CrossRef]
- Niklas, A.; Baquedano, A.; Orden, S.; Noguès, E.; Da Silva, M.; Fernández-Calvo, A.I. Microstructure and Mechanical Properties of a New Secondary AlSi10MnMg(Fe) Alloy for Ductile High Pressure Die Casting Parts for the Automotive Industry. KEM 2016, 710, 244–249. [Google Scholar] [CrossRef]
- Liu, G.; Gong, M.; Xie, D.; Wang, J. Structures and Mechanical Properties of Al-Al2Cu Interfaces. JOM 2019, 71, 1200–1208. [Google Scholar] [CrossRef]
- Hurtalová, L.; Tillová, E.; Chalupová, M. Identification and Analysis of Intermetallic Phases in Age-Hardened Recycled AISi9Cu3 Cast Alloy. Arch. Mech. Eng. 2012, 59, 385–396. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Morri, A.; Seifeddine, S.; Svensson, I.L. Microstructure, Tensile and Fatigue Properties of the Al–10%Si–2%Cu Alloy with Different Fe and Mn Content Cast under Controlled Conditions. J. Mater. Process. Technol. 2009, 209, 5669–5679. [Google Scholar] [CrossRef]
- Lu, L.; Dahle, A.K. Iron-Rich Intermetallic Phases and Their Role in Casting Defect Formation in Hypoeutectic Al−Si Alloys. Metall Mater Trans A 2005, 36, 819–835. [Google Scholar] [CrossRef]
- Xiao, F.; Li, L.; Zhou, R.; Li, Y.; Jiang, Y.; Lu, D. Effect of Melt Treatment on Fe-Rich Phase in Al–25Si–2Fe–2Mn Alloy. In Advances in Materials Processing; Han, Y., Ed.; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2018; pp. 865–879. ISBN 978-981-13-0106-3. [Google Scholar]
- Seifeddine, S.; Svensson, I.L. The Influence of Fe and Mn Content and Cooling Rate on the Microstructure and Mechanical Properties of A380-Die Casting Alloys. Metall. Sci. Technol. 2009, 27, 11–20. [Google Scholar]
- Gowri, S.; Samuel, F.H. Effect of Alloying Elements on the Solidification Characteristics and Microstructure of Al-Si-Cu-Mg-Fe 380 Alloy. Metall. Mater. Trans. A 1994, 25, 437–448. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Cao, X.; Campbell, J. Overview of β-Al5FeSi Phase in Al-Si Alloys. Mater. Sci. Res. Trends 2008, 251–271. [Google Scholar]
- Samuel, A.M.; Samuel, E.; Songmene, V.; Samuel, F.H. A Review on Porosity Formation in Aluminum-Based Alloys. Materials 2023, 16, 2047. [Google Scholar] [CrossRef]
- Elwin, L.; Rooy, E. Mechanisms of Porosity Formation in Aluminium. Mod. Cast. 1992, 82, 5–8. [Google Scholar]
- Piątkowski, J.; Roskosz, S.; Stach, S. The Influence of Selected High—Pressure Die Casting Parameters on the Porosity of EN AB-46000 Alloy Castings. Adv. Sci. Technol. Res. J. 2024, 18, 361–371. [Google Scholar] [CrossRef]
- Peaceman, D.W. Fundamentals of Numerical Reservoir Simulation; Developments in Petroleum Science; Elsevier Scientific Pub. Co.: Amsterdam, The Netherlands; New York, NY, USA, 2010; ISBN 978-0-08-086860-8. [Google Scholar]
- Carlson, K.D.; Lin, Z.; Beckermann, C. Modeling the Effect of Finite-Rate Hydrogen Diffusion on Porosity Formation in Aluminum Alloys. Metall. Mater. Trans. B 2007, 38, 541–555. [Google Scholar] [CrossRef]
- Ambat, R.; Dwarakadasa, E.S. Effect of Hydrogen in Aluminium and Aluminium Alloys: A Review. Bull. Mater. Sci. 1996, 19, 103–114. [Google Scholar] [CrossRef]
- Cais, J.; Weiss, V.; Svobodova, J. Relation between Porosity and Mechanical Properties of Al-Si Alloys Produced by Low- Pressure Casting. Arch. Foundry Eng. 2014, 14, 97–102. [Google Scholar]
- Ludwig, T.; Di Sabatino, M.; Arnberg, L.; Dispinar, D. Influence of Oxide Additions on the Porosity Development and Mechanical Properties of A356 Aluminium Alloy Castings. Inter. Metalcast. 2012, 6, 41–50. [Google Scholar] [CrossRef]
- Piątkowski, J.; Hejne, M.; Wieszała, R. Influence of Manganese Content on the Microstructure and Properties of AlSi10MnMg(Fe) Alloy for Die Castings. Arch. Mater. Sci. Eng. 2023, 123, 5–12. [Google Scholar] [CrossRef]
- Mahta, M.; Emamy, M.; Daman, A.; Keyvani, A.; Campbell, J. Precipitation of Fe Rich Intermetallics in Cr- and Co-Modified A413 Alloy. Int. J. Cast Met. Res. 2005, 18, 73–79. [Google Scholar] [CrossRef]
- Shabestari, S.G.; Mahmudi, M.; Emamy, M.; Campbell, J. Effect of Mn and Sr on Intermetallics in Fe-Rich Eutectic Al-Si Alloy. Int. J. Cast Met. Res. 2002, 15, 17–24. [Google Scholar] [CrossRef]
- García-Hinojosa, J. Structure and Properties of Al–7Si–Ni and Al–7Si–Cu Cast Alloys Nonmodified and Modified with Sr. J. Mater. Process. Technol. 2003, 143–144, 306–310. [Google Scholar] [CrossRef]
- Baldan, R.; Malavazi, J.; Couto, A.A. Microstructure and Mechanical Behavior of Al9Si0.8Fe Alloy with Different Mn Contents. Mater. Sci. Technol. 2017, 33, 1192–1199. [Google Scholar] [CrossRef]
- Kral, M.V. A Crystallographic Identification of Intermetallic Phases in Al–Si Alloys. Mater. Lett. 2005, 59, 2271–2276. [Google Scholar] [CrossRef]
- Shabestari, S. The Effect of Iron and Manganese on the Formation of Intermetallic Compounds in Aluminum–Silicon Alloys. Mater. Sci. Eng. A 2004, 383, 289–298. [Google Scholar] [CrossRef]
- Taylor, J.A. Iron-Containing Intermetallic Phases in Al-Si Based Casting Alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Couture, A. Iron in Aluminum Casting Alloys. AFS Int. Cast Met. J. 1980, 5–6, 9–17. [Google Scholar]
- Ferraro, S.; Fabrizi, A.; Timelli, G. Evolution of Sludge Particles in Secondary Die-Cast Aluminum Alloys as Function of Fe, Mn and Cr Contents. Mater. Chem. Phys. 2015, 153, 168–179. [Google Scholar] [CrossRef]
- Bösch, D.; Pogatscher, S.; Hummel, M.; Fragner, W.; Uggowitzer, P.J.; Göken, M.; Höppel, H.W. Secondary Al-Si-Mg High-Pressure Die Casting Alloys with Enhanced Ductility. Metall. Mater. Trans. A 2015, 46, 1035–1045. [Google Scholar] [CrossRef]
- EN 1706:2010; Aluminium and Aluminium Alloys. Castings. Chemical Composition and Mechanical Properties. British Standards Institution: London, UK, 2021.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Piątkowski, J.; Roskosz, S.; Sapota, W.; Stach, S. The Influence of Iron Content on the Porosity of AlSi9 Alloy Intended for Alfining Piston Ring Inserts. Materials 2024, 17, 5181. [Google Scholar] [CrossRef]
- Vivas, J.; Fernández-Calvo, A.I.; Aldanondo, E.; Irastorza, U.; Álvarez, P. Friction Stir Weldability at High Welding Speed of Two Structural High Pressure Die Casting Aluminum Alloys. JMMP 2022, 6, 160. [Google Scholar] [CrossRef]
- Niklas, A.; Bakedano, A.; Orden, S.; Da Silva, M.; Nogués, E.; Fernández-Calvo, A.I. Effect of Microstructure and Casting Defects on the Mechanical Properties of Secondary AlSi10MnMg(Fe) Test Parts Manufactured by Vacuum Assisted High Pressure Die Casting Technology. Mater. Today Proc. 2015, 2, 4931–4938. [Google Scholar] [CrossRef]
- Lu, Y.; Godlewski, L.A.; Zindel, J.W.; Lee, A. Use of Reactive Nanostructured Chemicals for Refinement of Si Eutectic in an Aluminum Casting Alloy. J. Mater. Sci. 2019, 54, 12818–12832. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Manganese in Aluminium Alloys; Manganese Centre: Neuilly-sur-Seine, France, 1978; ISBN 978-2-901109-01-3. [Google Scholar]
- Taylor, J.A. The Effect of Iron in Al-Si Casting Alloys; Australian Foundry Institute (AFI): Adelaide, Australia, 2004; pp. 148–157. [Google Scholar]
- Taylor, J.A.; Schaffer, G.B.; StJohn, D.H. The Role of Iron in the Formation of Porosity in Al-Si-Cu-Based Casting Alloys: Part I. Initial Experimental Observations. Metall. Mater. Trans. A 1999, 30, 1643–1650. [Google Scholar] [CrossRef]
- Campbell, J.; Tiryakioğlu, M. Review of Effect of P and Sr on Modification and Porosity Development in Al–Si Alloys. Mater. Sci. Technol. 2010, 26, 262–268. [Google Scholar] [CrossRef]
- Campbell, J. Complete Casting Handbook; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 978-0-444-63509-9. [Google Scholar]



| Sample No. | Element Content, wt.% 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Mn | Mg | Cu | Cr | Zn | Ti | Sr | Other 2 | |
| Without Added Manganese | ||||||||||
| 1 | 9.64 | 0.32 | 0.18 | 0.49 | 0.03 | <0.01 | 0.014 | 0.059 | 0.015 | <0.02 |
| 2 | 9.88 | 0.44 | 0.20 | 0.52 | 0.03 | <0.01 | 0.012 | 0.062 | 0.014 | <0.02 |
| 3 | 9.97 | 0.48 | 0.22 | 0.48 | 0.02 | <0.01 | 0.014 | 0.058 | 0.014 | <0.02 |
| 4 | 9.91 | 0.51 | 0.21 | 0.52 | 0.04 | <0.01 | 0.013 | 0.061 | 0.013 | <0.02 |
| 5 | 9.76 | 0.60 | 0.21 | 0.54 | 0.04 | <0.01 | 0.014 | 0.064 | 0.014 | <0.02 |
| 6 | 9.69 | 0.71 | 0.19 | 0.54 | 0.03 | <0.01 | 0.013 | 0.060 | 0.013 | <0.02 |
| After the addition of manganese (AlMn) | ||||||||||
| 1Mn. | 9.58 | 0.32 | 0.18 | 0.44 | 0.02 | <0.01 | 0.013 | 0.053 | 0.014 | <0.02 |
| 2Mn. | 9.92 | 0.43 | 0.22 | 0.48 | 0.02 | <0.01 | 0.013 | 0.060 | 0.014 | <0.02 |
| 3Mn. | 9.77 | 0.47 | 0.25 | 0.46 | 0.02 | <0.01 | 0.012 | 0.060 | 0.013 | <0.02 |
| 4Mn. | 9.90 | 0.53 | 0.25 | 0.50 | 0.03 | <0.01 | 0.014 | 0.058 | 0.014 | <0.02 |
| 5Mn. | 9.56 | 0.58 | 0.30 | 0.49 | 0.03 | <0.01 | 0.011 | 0.061 | 0.013 | <0.02 |
| 6Mn. | 9.83 | 0.70 | 0.36 | 0.50 | 0.02 | <0.01 | 0.011 | 0.061 | 0.015 | <0.02 |
| Sample No. | Scrap Content, % | Characteristic Crystallization Temperatures and Parameters 2, °C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Point X TE(Fe) | Point A Tliq(α) | Point B TEmin(α+β) | Point C TE(α+β) | Point Y TE(AlFeMn) | Point D TE(Mg) | Point E Tsol. | Eutectic Recalescence α + β TEmin. − TE(α+β) | Crystallization Range Tliq. − Tsol. | ||
| Without added manganese | ||||||||||
| 1 | 50 | ---- | 565 | 556 | 560 | ---- | 542 | 529 | 6 | 36 |
| 2 | 55 | ---- | 566 | 556 | 561 | ---- | 543 | 530 | 5 | 36 |
| 3 | 60 | ---- | 568 | 555 | 560 | ---- | 542 | 530 | 5 | 38 |
| 4 | 65 | 564 | 568 | 556 | 560 | ---- | 544 | 530 | 6 | 38 |
| 5 | 70 | 637 | 569 | 558 | 564 | ---- | 543 | 529 | 6 | 40 |
| 6 | 75 | 661 | 570 | 560 | 566 | ---- | 545 | 528 | 6 | 52 |
| After the addition of manganese (AlMn20) | ||||||||||
| 1Mn. | 50 | ---- | 569 | 555 | 561 | 540 | 436 | 526 | 6 | 43 |
| 2Mn. | 55 | ---- | 571 | 556 | 561 | 543 | 539 | 525 | 5 | 46 |
| 3Mn. | 60 | ---- | 570 | 555 | 560 | 547 | 539 | 527 | 5 | 43 |
| 4Mn. | 65 | ---- | 569 | 557 | 563 | 550 | 540 | 530 | 6 | 39 |
| 5Mn. | 70 | ---- | 570 | 556 | 562 | 551 | 538 | 522 | 6 | 48 |
| 6Mn. | 75 | ---- | 568 | 556 | 562 | 555 | 540 | 521 | 6 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatkowski, J.; Nowinska, K.; Matula, T.; Siwiec, G.; Szucki, M.; Oleksiak, B. Microstructure and Mechanical Properties of AlSi10MnMg Alloy with Increased Content of Recycled Scrap. Materials 2025, 18, 1119. https://doi.org/10.3390/ma18051119
Piatkowski J, Nowinska K, Matula T, Siwiec G, Szucki M, Oleksiak B. Microstructure and Mechanical Properties of AlSi10MnMg Alloy with Increased Content of Recycled Scrap. Materials. 2025; 18(5):1119. https://doi.org/10.3390/ma18051119
Chicago/Turabian StylePiatkowski, Jaroslaw, Katarzyna Nowinska, Tomasz Matula, Grzegorz Siwiec, Michal Szucki, and Beata Oleksiak. 2025. "Microstructure and Mechanical Properties of AlSi10MnMg Alloy with Increased Content of Recycled Scrap" Materials 18, no. 5: 1119. https://doi.org/10.3390/ma18051119
APA StylePiatkowski, J., Nowinska, K., Matula, T., Siwiec, G., Szucki, M., & Oleksiak, B. (2025). Microstructure and Mechanical Properties of AlSi10MnMg Alloy with Increased Content of Recycled Scrap. Materials, 18(5), 1119. https://doi.org/10.3390/ma18051119








