Microscopic Mechanical Properties and Physicochemical Changes of Cement Paste Exposed to Elevated Temperatures and Subsequent Rehydration
Highlights
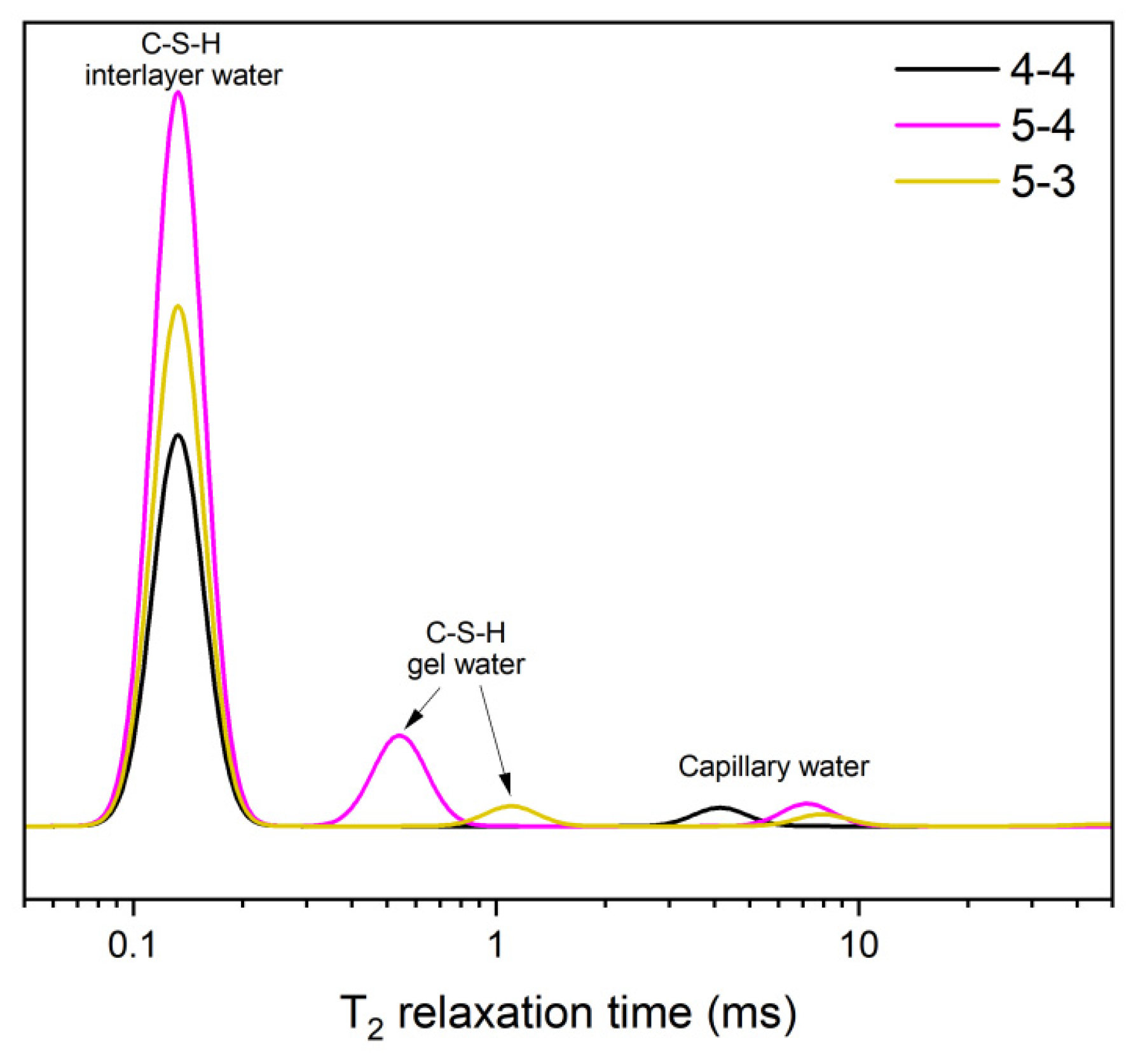
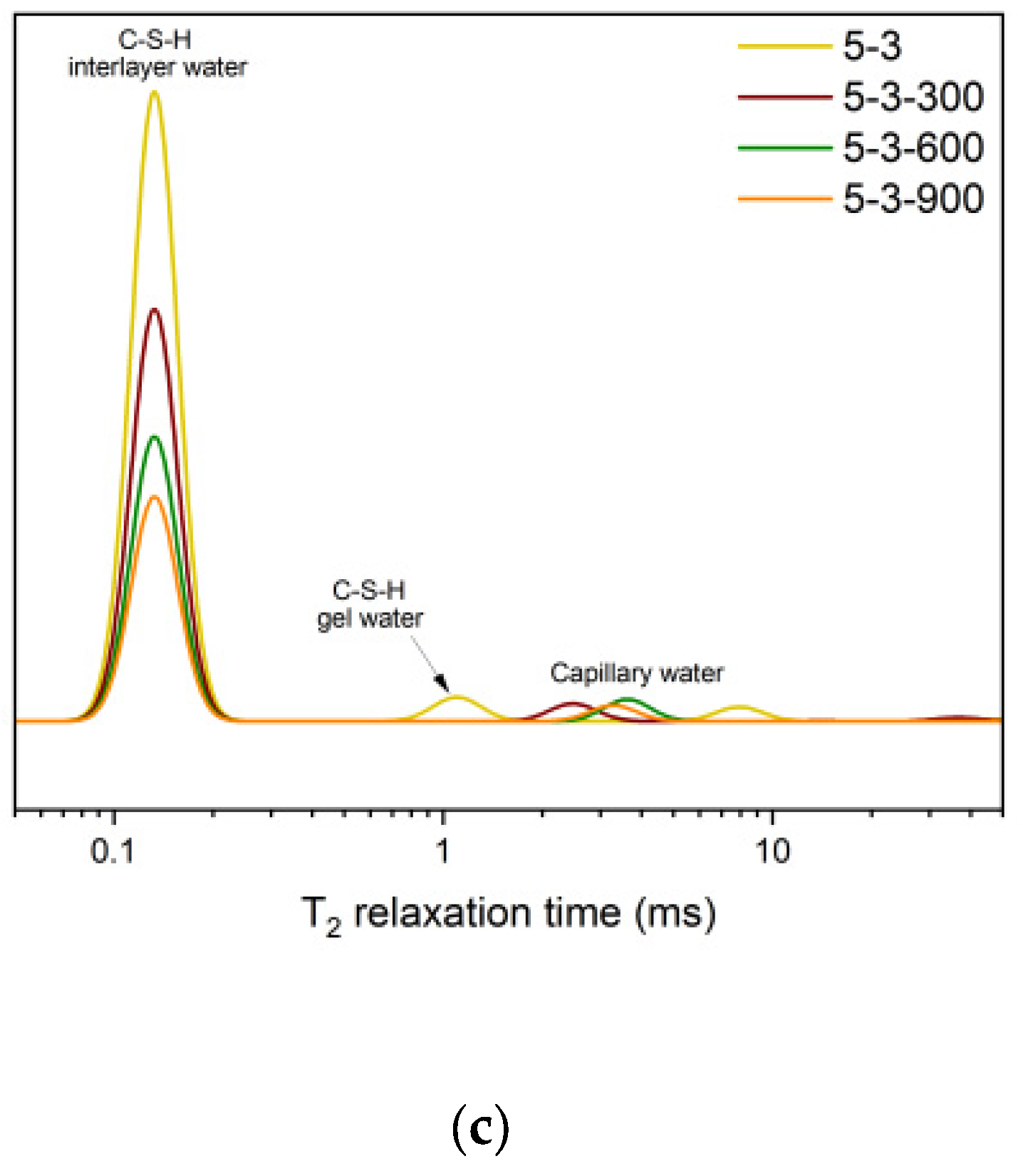
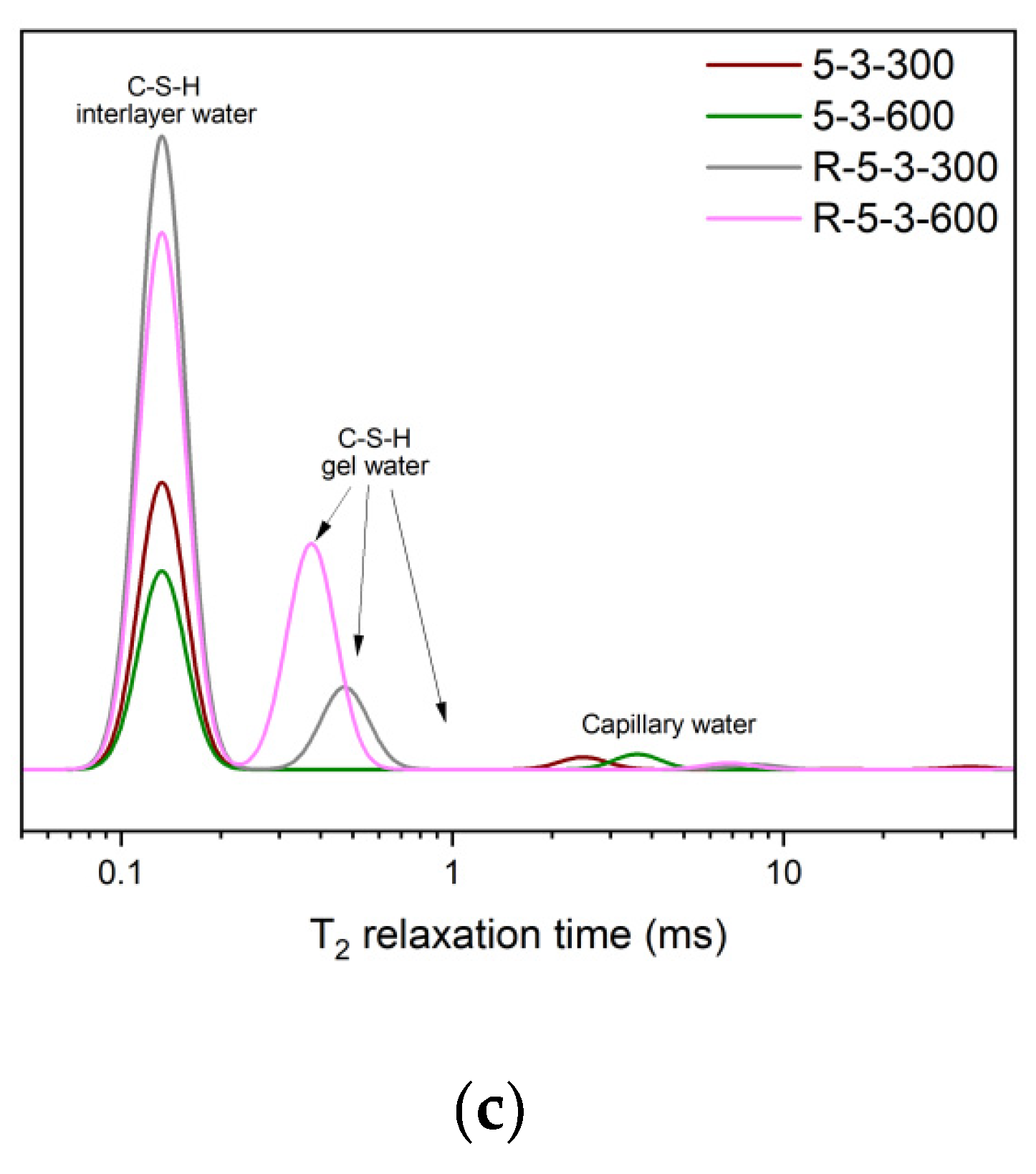
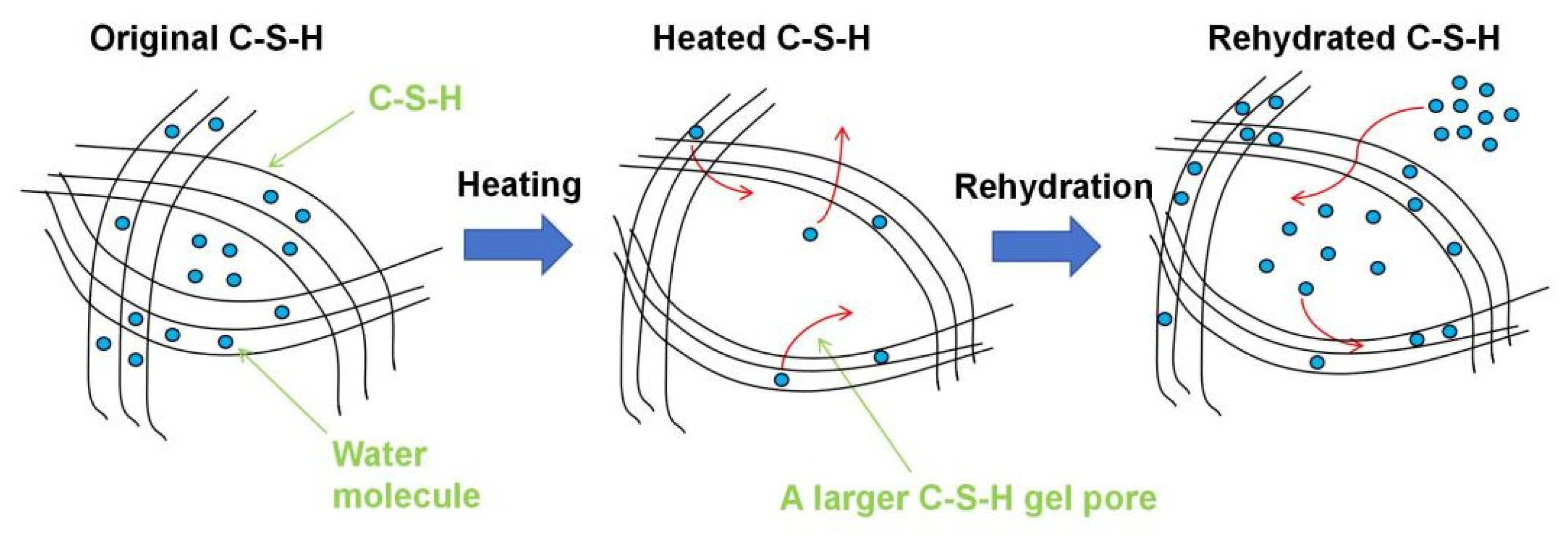
- Elevated temperatures lead to the structure rearrangement of C-S-H, involving the collapse of the interlayer structure of C-S-H and the enlargement of C-S-H gel pores.
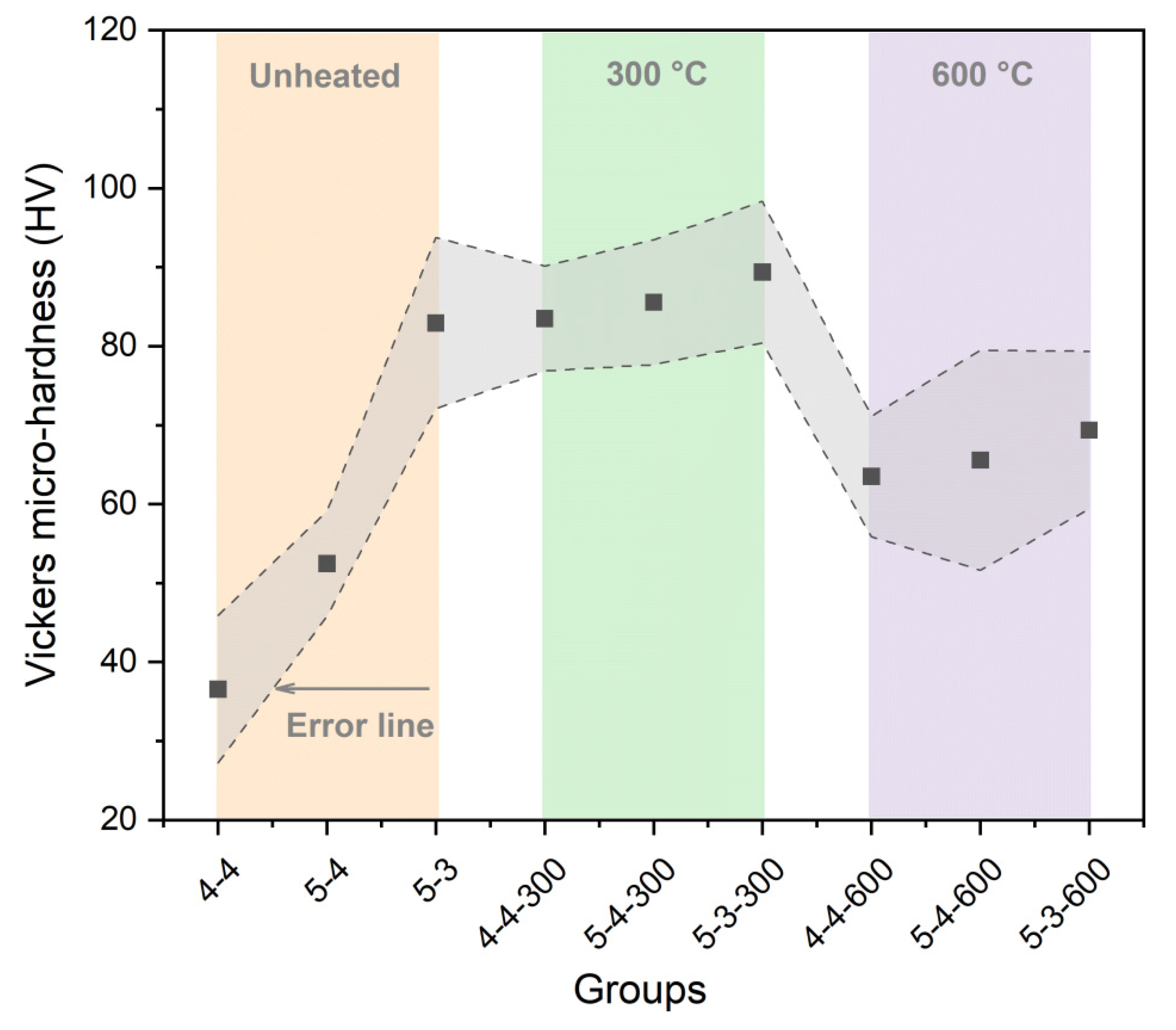
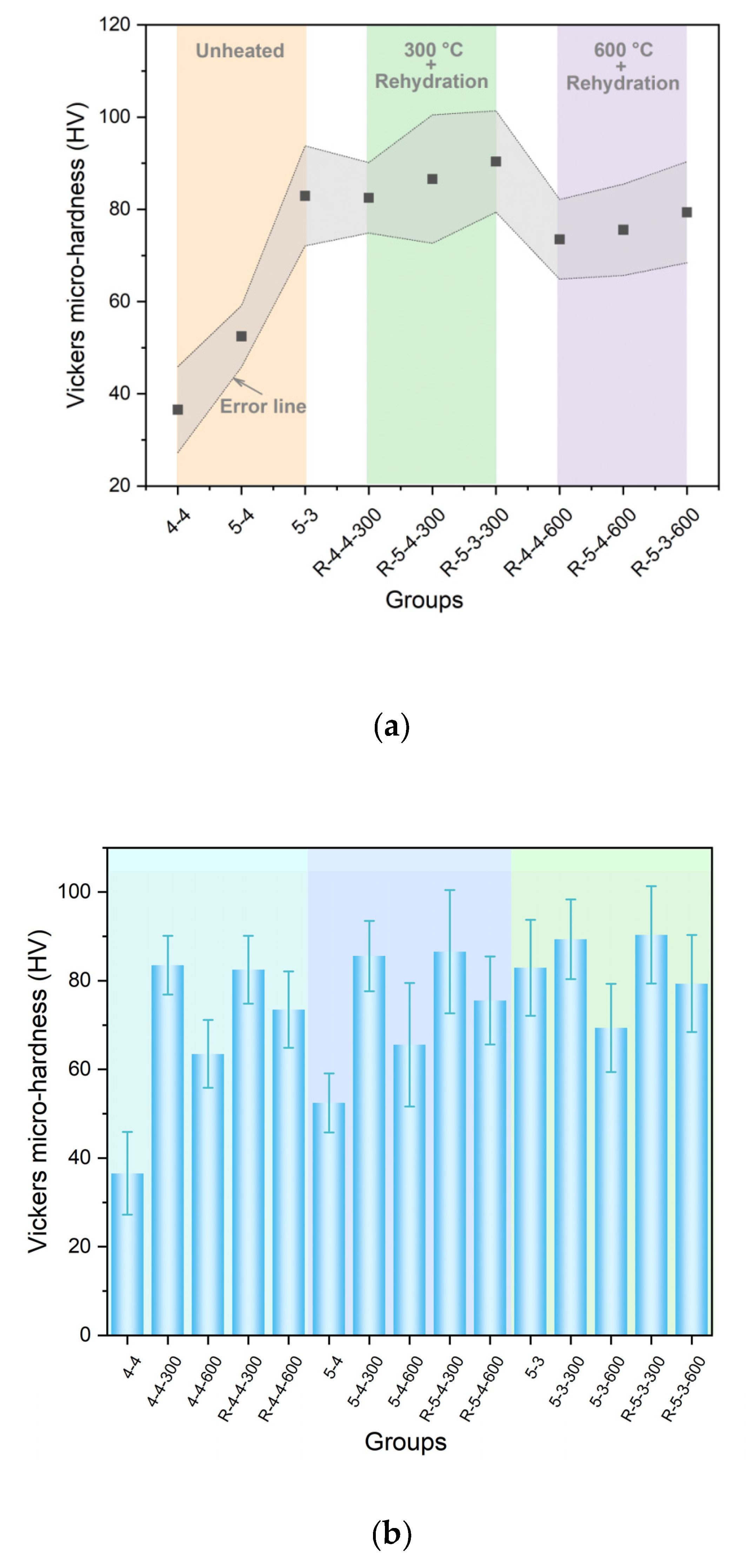
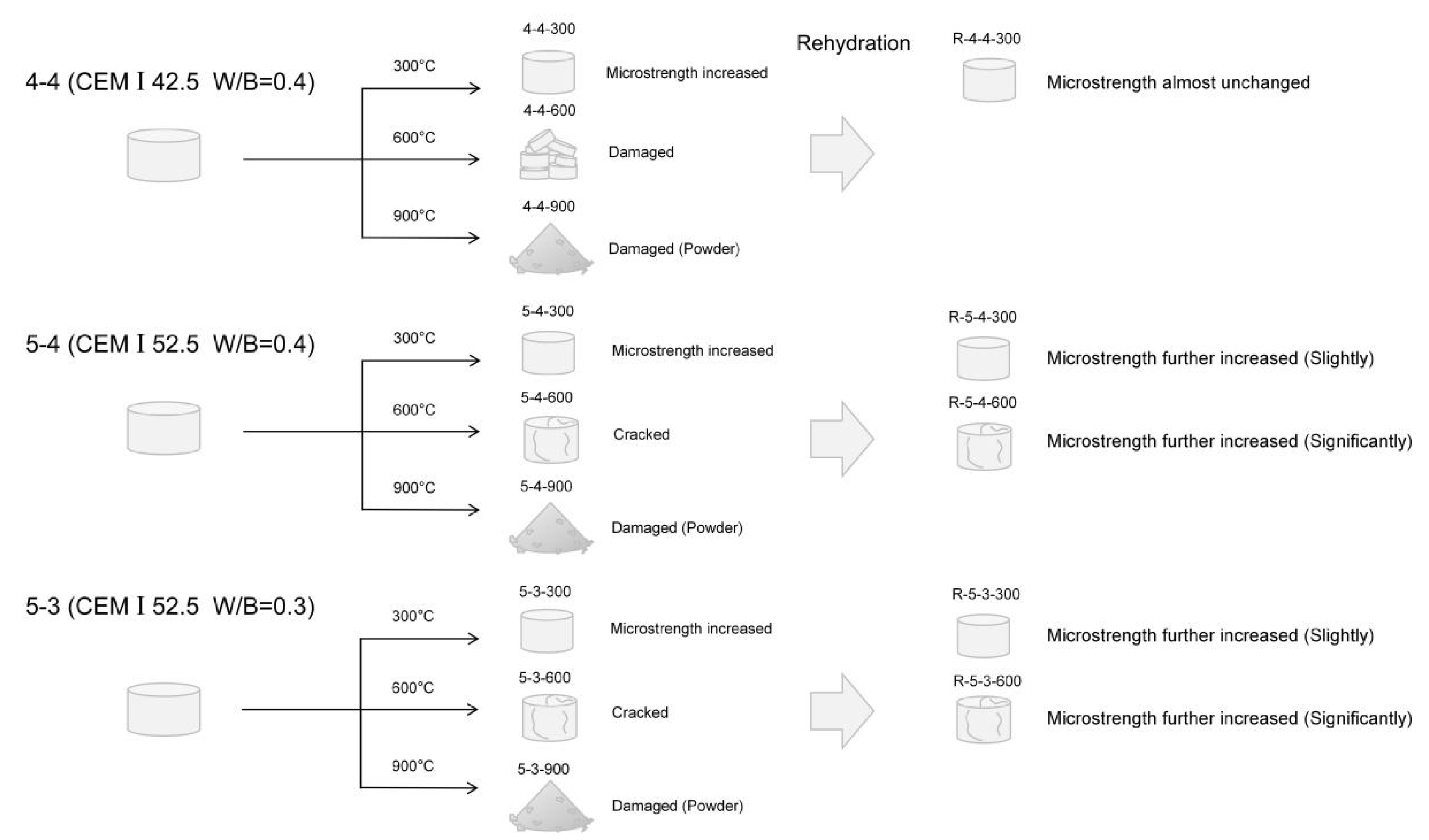
- Elevated temperatures (300 °C and 600 °C), followed by rehydration, enhance the Vickers microhardness of the cement pastes.
- Excessively high temperature (900 °C) weakens the micro-mechanical properties and may cause damage.
- Cement pastes heated to 600 °C show a more significant recovery in micro-mechanical properties, attributed to the rehydration of a new amorphous nesosilicate phase formed at 600 °C.
Abstract
1. Introduction
2. Experimental Program
2.1. Materials and Sample Preparation
2.2. Characterization Techniques
3. Results and Analyses
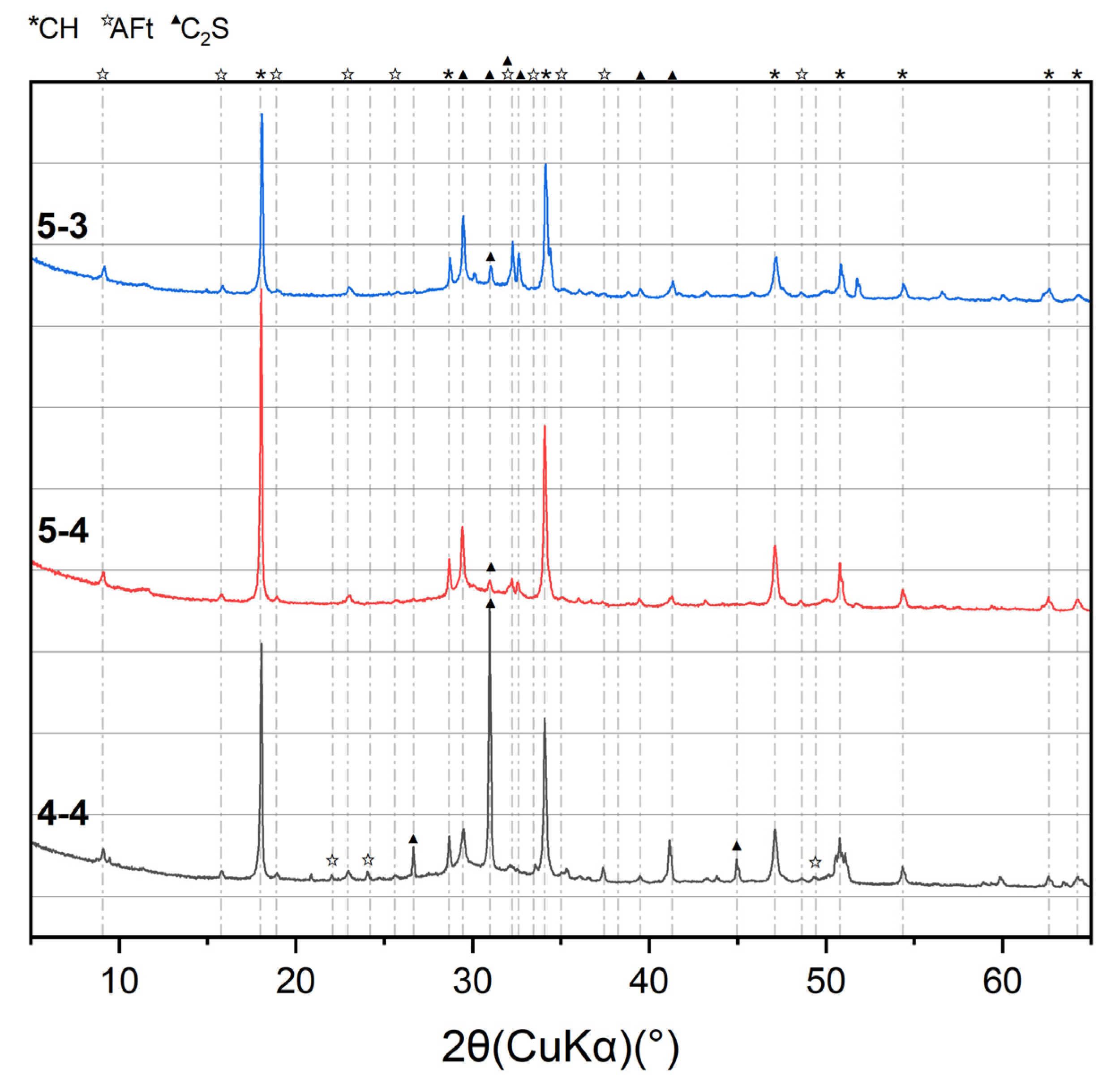
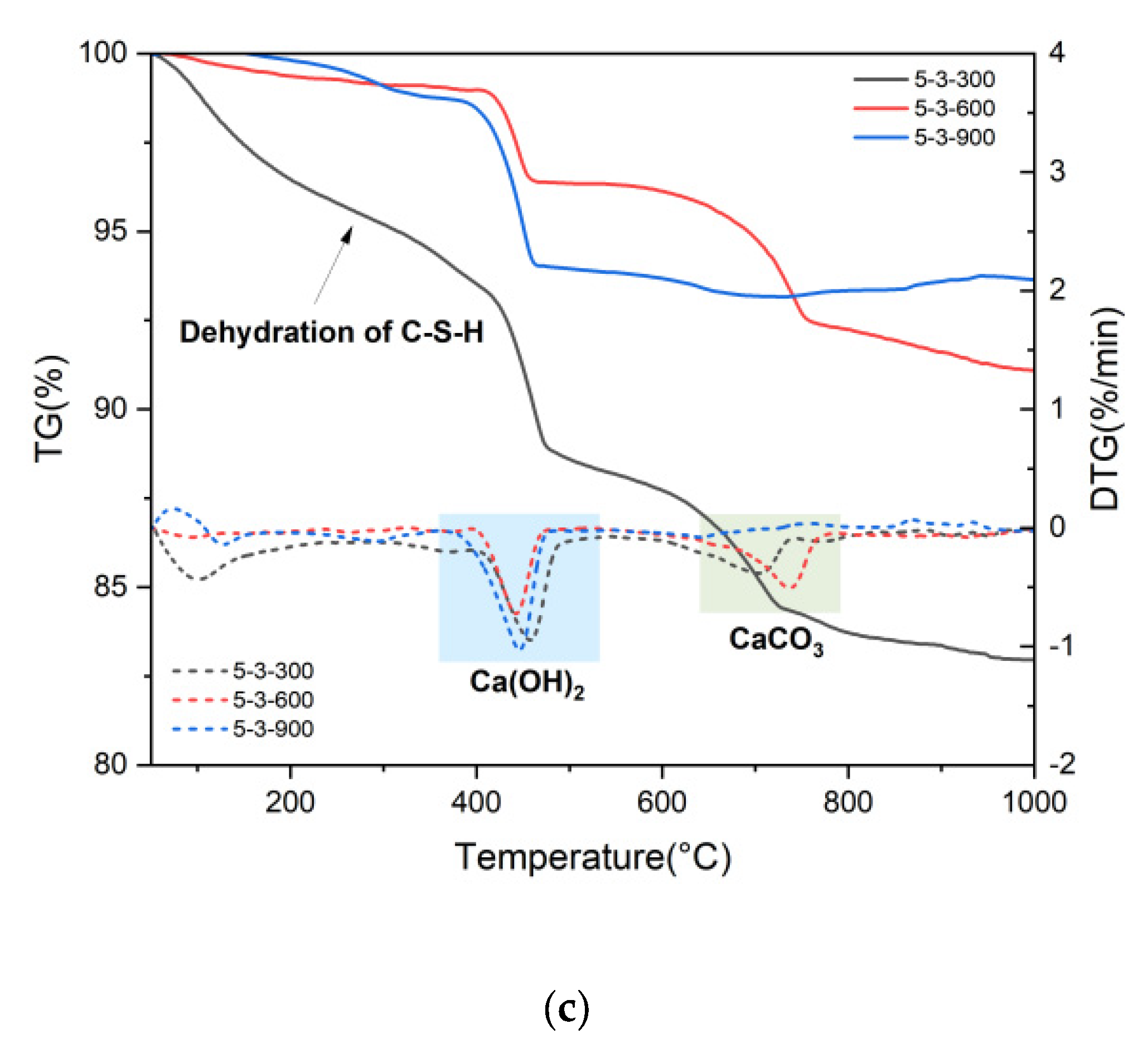
3.1. Macro–Meso–Micro Changes of Cement Pastes Subjected to Elevated Temperatures
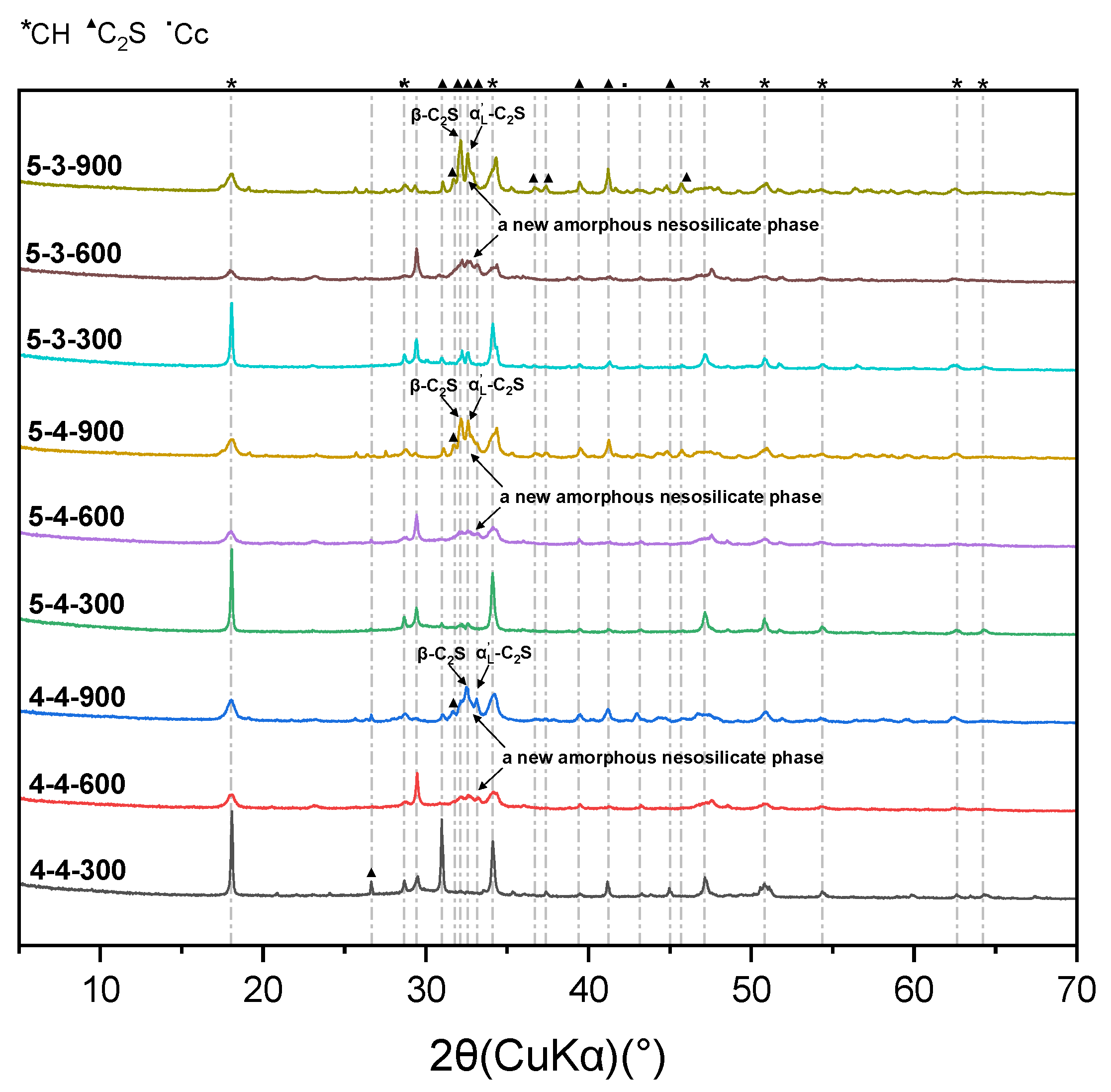
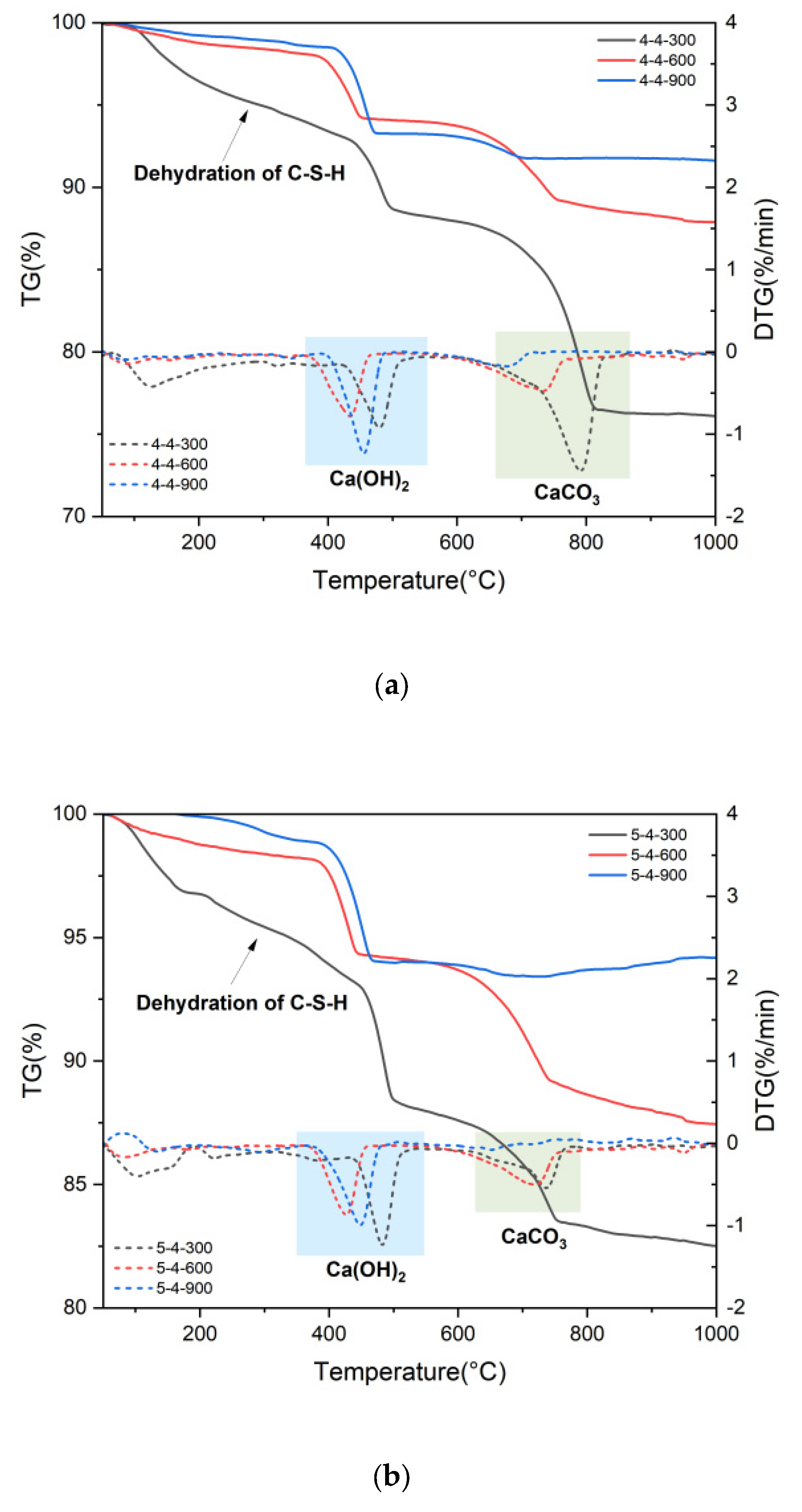
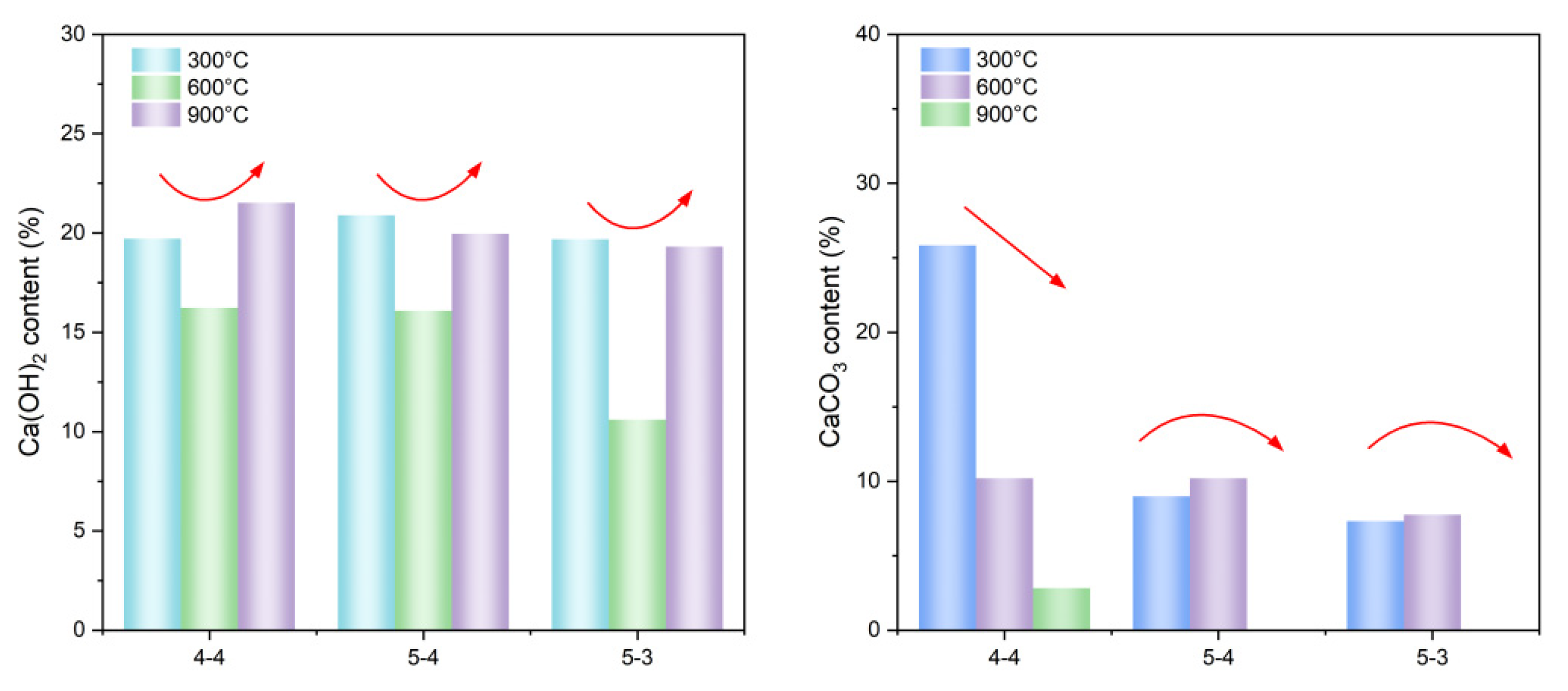
3.2. Physical and Chemical Changes of High-Temperature Dehydrated Cement Pastes After Rehydration
4. Discussion
5. Conclusions
- (1)
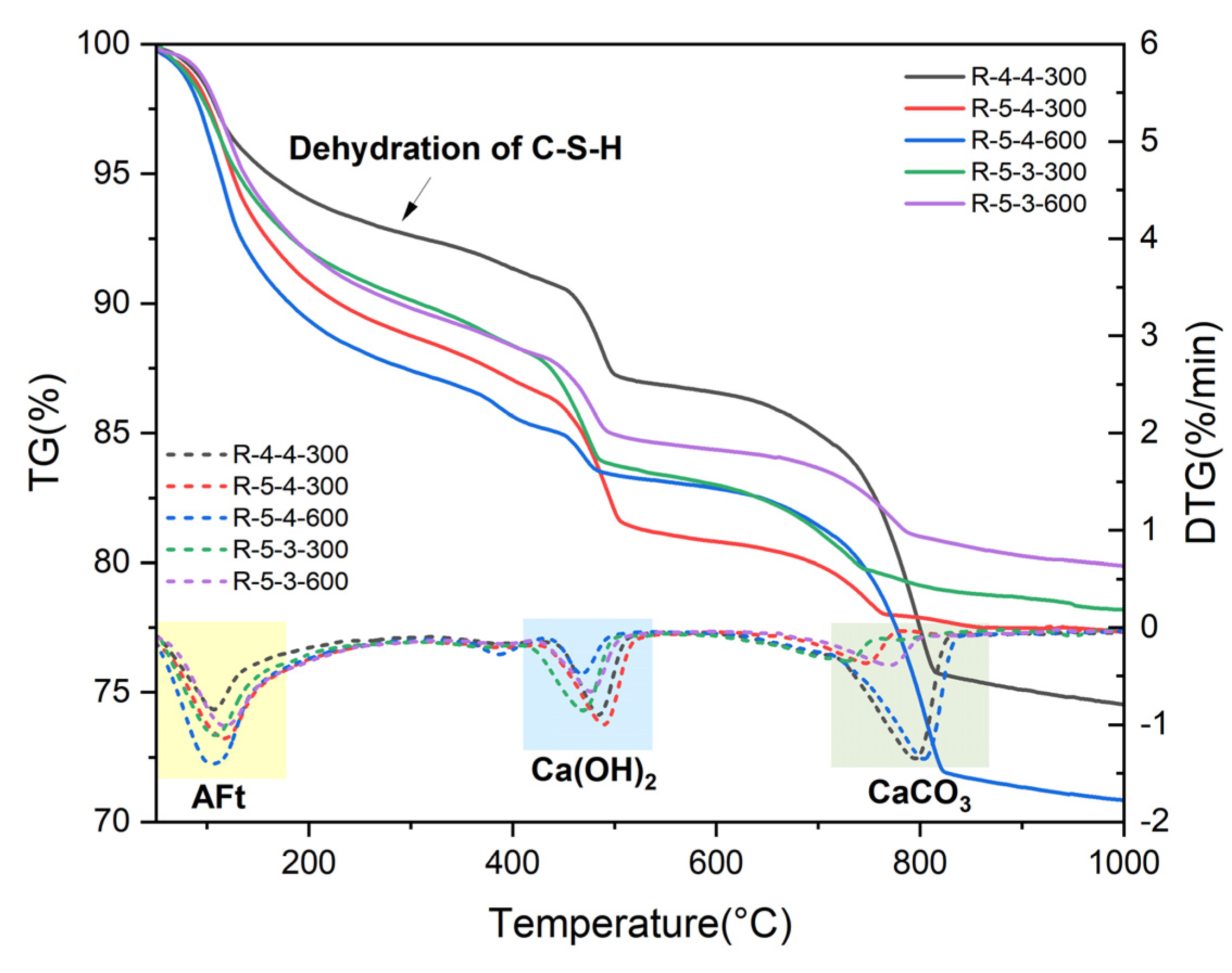
- Elevated temperatures significantly affect the structure and water distribution of C-S-H. During the heating process, the C-S-H interlayer water enters the C-S-H gel pores, subsequently migrates outward from the gel pores, and is eventually removed. This phenomenon leads to the structural rearrangement of C-S-H, which involves the collapse of the interlayer structure of C-S-H and the enlargement of the C-S-H gel pores.
- (2)
- Elevated temperatures can enhance the Vickers microhardness of cement pastes by removing the C-S-H interlayer water. Additionally, carbonation due to high-temperature (300 °C and 600 °C in this paper) treatment also increases the Vickers microhardness of cement pastes by refining their pore structures. However, excessively high temperatures (900 °C) and excessive interlayer water losses can lead to the collapse of the C-S-H structure, thereby weakening the Vickers microhardness of cement pastes and potentially leading to damage.
- (3)
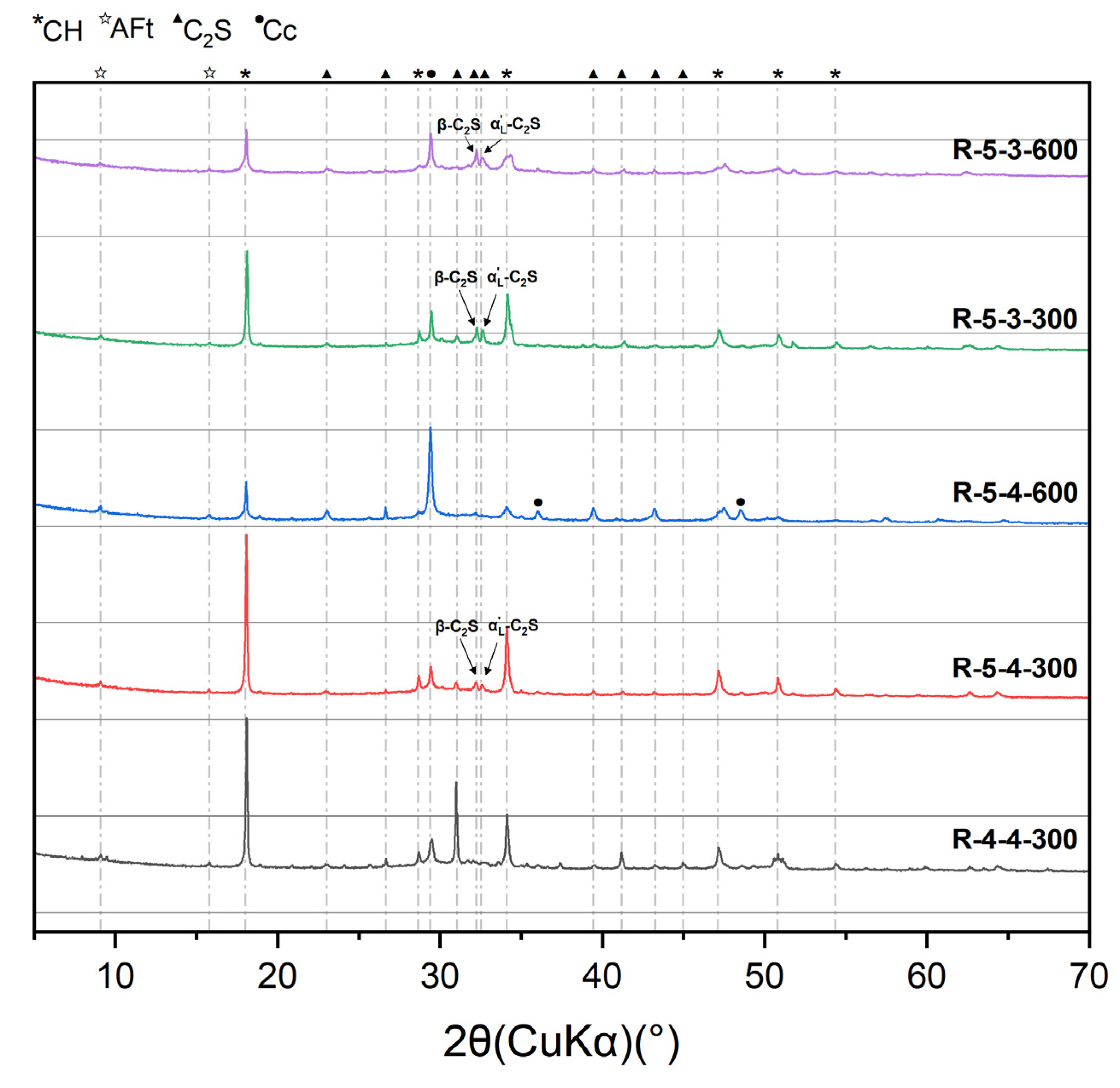
- Elevated temperatures lead to the formation of well-crystallized β-C2S and α’L-C2S with little hydration reactivity. Compared to samples heated at 300 °C, those heated at 600 °C demonstrate the more significant recovery of the micro-mechanical properties. This phenomenon is attributed to the rehydration of a new amorphous nesosilicate phase that is generated at 600 °C.
- (4)
- Samples with a lower water-to-cement ratio contain less C-S-H interlayer water, making the collapse of the C-S-H structure more likely to occur in these samples. Moreover, samples made from a high grade of cement exhibit better resistance to high temperatures. However, high-temperature treatment promotes cement hydration, thus eliminating the differences in the Vickers microhardness due to the variations in cement types to some extent.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wróblewska, J.; Kowalski, R. Assessing concrete strength in fire-damaged structures. Constr. Build Mater. 2020, 254, 119122. [Google Scholar] [CrossRef]
- Lin, J.; Dong, Y.; Duan, J.; Zhang, D.; Zheng, W. Experiment on single-tunnel fire in concrete immersed tunnels. Tunn. Undergr. Sp. Technol. 2021, 116, 104059. [Google Scholar] [CrossRef]
- Malik, M.; Bhattacharyya, S.; Barai, S. Temperature, porosity and strength relationship for fire affected concrete. Mater. Struct. 2022, 55, 72. [Google Scholar] [CrossRef]
- Henry, M.; Hashimoto, K.; Darma, I.; Sugiyama, T. Cracking and Chemical Composition of Cement Paste Subjected to Heating and Water Re-Curing. J. Adv. Concr. Technol. 2016, 14, 134–143. [Google Scholar] [CrossRef]
- Taylor, H. Cement Chemistry; Thomas Telford: London, UK, 1990. [Google Scholar]
- Bogas, J.A.; Carriço, A.; Pereira, M.F.C. Mechanical characterization of thermal activated low-carbon recycled cement mortars. J. Clean. Prod. 2019, 218, 377–389. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Li, K.; Li, M.; Lin, S.; Hao, T.; Wang, T.; Guo, Y.; Ling, Z. Investigations on the rehydration of recycled blended SCMs cement. Cem. Concr. Res. 2023, 163, 107036. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J.; Hu, X.; Ran, B.; Wu, T.; Zhou, X.; Xiong, Y. Physical performance, durability, and carbon emissions of recycled cement concrete and fully recycled concrete. Constr. Build Mater. 2024, 447, 138128. [Google Scholar] [CrossRef]
- Zhang, Q. Microstructure and Deterioration Mechanisms of Portland Cement Paste at Elevated Temperature. Ph.D. Thesis, Structural Engineering Department, University of Delft, Delft, The Netherlands, 2013. [Google Scholar]
- Cao, B. Study on the Thermal Properties of Concrete and the Technology of Concrete Reuse. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2006. [Google Scholar]
- Xuan, D.; Shui, Z. Rehydration activity of hydrated cement paste exposed to high temperature. Fire Mater. 2011, 35, 481–490. [Google Scholar] [CrossRef]
- Vyšvařil, M.; Bayer, P.; Chromá, M.; Rovnaníková, P. Physico-mechanical and microstructural properties of rehydrated blended cement pastes. Constr. Build Mater. 2014, 54, 413–420. [Google Scholar] [CrossRef]
- Zhang, L.; Ji, Y.; Huang, G.; Li, J.; Hu, Y. Modification and enhancement of mechanical properties of dehydrated cement paste using ground granulated blast-furnace slag. Constr. Build Mater. 2018, 164, 525–534. [Google Scholar] [CrossRef]
- Alonso, C.; Fernandez, L. Dehydration and rehydration processes of cement paste exposed to high temperature environments. J. Mater. Sci. 2004, 39, 3015–3024. [Google Scholar] [CrossRef]
- Shui, Z.; Xuan, D.; Chen, W.; Yu, R.; Zhang, R. Cementitious characteristics of hydrated cement paste subjected to various dehydration temperatures. Constr. Build Mater. 2009, 23, 531–537. [Google Scholar] [CrossRef]
- Bogas, J.; Carriço, A.; Tenza-Abril, A. Microstructure of thermoactivated recycled cement pastes. Cem. Concr. Res. 2020, 138, 106226. [Google Scholar] [CrossRef]
- Bogas, J.; Real, S.; Carriço, A.; Abrantes, J.; Guedes, M. Hydration and phase development of recycled cement. Cem. Concr. Comp. 2022, 127, 104405. [Google Scholar] [CrossRef]
- Real, S.; Carriço, A.; Bogas, J.; Guedes, M. Influence of the Treatment Temperature on the Microstructure and Hydration Behavior of Thermoactivated Recycled Cement. Materials 2020, 13, 3937. [Google Scholar] [CrossRef]
- Baldusco, R.; Nobre, T.; Angulo, S.; Quarcioni, V.; Cincotto, M. Dehydration and rehydration of blast furnace slag cement. J. Mater. Civ. Eng. 2019, 31, 1–13. [Google Scholar] [CrossRef]
- Xu, L.; Hu, X.; Yang, Q.; Ran, B.; Li, K.; Wang, J.; Zhang, X. Insight into multi-ionic adsorption behavior of recycled cement paste exposed to chloride solutions. Constr. Build. Mater. 2024, 426, 136142. [Google Scholar] [CrossRef]
- Lu, J.; Shen, P.; Zheng, H.; Zhan, B.; Ali, H.A.; He, P.; Poon, C. Synergetic recycling of waste glass and recycled aggregates in cement mortars: Physical, durability and microstructure performance. Cem. Concr. Comp. 2020, 113, 103632. [Google Scholar] [CrossRef]
- Letelier, V.; Tarela, E.; Muñoz, P.; Moriconi, G. Combined effects of recycled hydrated cement and recycled aggregates on the mechanical properties of concrete. Constr. Build. Mater. 2017, 132, 365–375. [Google Scholar] [CrossRef]
- Xi, X.; Zheng, Y.; Du, C.; Zhang, P.; Sun, M. Study on the hydration characteristics, mechanical properties, and microstructure of thermally activated low-carbon recycled cement. Constr. Build. Mater. 2024, 447, 138042. [Google Scholar] [CrossRef]
- Carriço, A.; Real, S.; Bogas, J. Durability performance of thermoactivated recycled cement concrete. Cem. Concr. Comp. 2021, 124, 104270. [Google Scholar] [CrossRef]
- Suh, H.; Im, S.; Bae, S.; Kim, J.; Bae, S. Instant mechanical recovery of heat-damaged nanosilica-incorporated cement composites under various rehydrations procedures. Mater. Struct. 2022, 55, 5. [Google Scholar] [CrossRef]
- Bordy, A.; Younsi, A.; Aggoun, S.; Fiorio, B. Cement substitution by a recycled cement paste fine: Role of the residual anhydrous clinker. Constr. Build. Mater. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Zhang, B.; Li, Q.; Shui, Z. Study on the high-temperature behavior and rehydration characteristics of hardened cement paste. Fire Mater. 2015, 39, 741–750. [Google Scholar] [CrossRef]
- Suh, H.; Jee, H.; Kim, J.; Kitagaki, R.; Ohki, S.; Woo, S.; Jeong, K.; Bae, S. Influences of rehydration conditions on the mechanical and atomic structural recovery characteristics of Portland cement paste exposed to elevated temperatures. Constr. Build. Mater. 2020, 235, 117453. [Google Scholar] [CrossRef]
- Hu, Z.; Wyrzykowski, M.; Scrivener, K.; Scrivener, K.; Lura, P. A novel method to predict internal relative humidity in cementitious materials by 1H NMR. Cem. Concr. Res. 2018, 104, 80–93. [Google Scholar] [CrossRef]
- Yin, J. Study on Moisture Distribution, Microstructure Changes and Macro-deformation Behaviors of Calcium Silicate Hydrate During Drying-Resaturation Cycles. Ph.D. Thesis, Tsinghua University, Beijing, China, 2023. [Google Scholar]
- Xu, L.; Wang, J.; Huang, R.; Li, B.; Ran, B.; Hu, X. Investigations on micro-mechanical properties of the ITZs between recycled aggregates and recycled cement paste. Constr. Build. Mater. 2024, 450, 138640. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Castellote, M.; Alonso, C.; Andrade, C.; Turrillas, X.; Campo, J. Composition and microstructural changes of cement pastes upon heating, as studied by neutron diffraction. Cem. Concr. Res. Cem. Concr. Res. 2004, 34, 1633–1644. [Google Scholar] [CrossRef]
- Chen, T.; Gao, X. Effect of carbonation curing regime on strength and microstructure of Portland cement paste. J. CO2 Util. 2019, 34, 74–86. [Google Scholar] [CrossRef]
- Phung, Q.; Maes, N.; Jacques, D.; Bruneel, E.; Van Driessche, I.; Ye, G.; De Schutter, G. Effect of limestone fillers on microstructure and permeability due to carbonation of cement pastes under controlled CO2 pressure conditions. Constr. Build. Mater. 2015, 82, 376–390. [Google Scholar] [CrossRef]
- Im, S.; Jee, H.; Suh, H.; Kanematsu, M.; Morooka, S.; Taku, K.; Yuhei, N.; Machida, A.; Kim, J.; Bae, S. Temperature effects on local structure, phase transformation, and mechanical properties of calcium silicate hydrates. J. Am. Ceram. Soc. 2021, 104, 4803–4818. [Google Scholar] [CrossRef]
- Pellenq, R.; Kushima, A.; Shahsavari, R.; Vliet, K.; Buehler, M.; Yip, S.; Ulm, F. A realistic molecular model of cement hydrates. Proc. Natl. Acad. Sci. USA 2009, 106, 16102–16107. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, W.; Wang, J.; Kong, X. Irreversible microstructural changes of calcium silicate hydrate during the first drying-resaturation cycle. Cem. Concr. Res. 2023, 163, 107032. [Google Scholar] [CrossRef]



| Oxide/Element | CaO | SiO2 | SO3 | Al2O3 | MgO | K2O | Na2O | Fe2O3 | TiO2 | SrO | P2O5 | MnO | NiO | CuO | Cl | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CEM I 42.5 | 60.990 | 19.838 | 4.535 | 2.417 | 9.726 | 0.697 | 0.375 | 0.310 | 0.053 | 0.035 | 0.048 | 0.028 | 0.016 | 0.015 | 0.046 | - | 0.872 |
| CEM I 52.5 | 78.305 | 13.400 | 2.841 | 2.156 | 1.321 | 0.411 | 0.305 | 0.233 | 0.150 | 0.072 | 0.019 | 0.010 | 0.009 | 0.008 | 0.003 | 0.091 | 0.666 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Hu, X.; Tang, R.; Zhang, X.; Xia, Y.; Ran, B.; Liu, J.; Zhuang, S.; Tian, W. Microscopic Mechanical Properties and Physicochemical Changes of Cement Paste Exposed to Elevated Temperatures and Subsequent Rehydration. Materials 2025, 18, 1050. https://doi.org/10.3390/ma18051050
Xu L, Hu X, Tang R, Zhang X, Xia Y, Ran B, Liu J, Zhuang S, Tian W. Microscopic Mechanical Properties and Physicochemical Changes of Cement Paste Exposed to Elevated Temperatures and Subsequent Rehydration. Materials. 2025; 18(5):1050. https://doi.org/10.3390/ma18051050
Chicago/Turabian StyleXu, Lei, Xiaochuan Hu, Ruifeng Tang, Xin Zhang, Yan Xia, Bo Ran, Jinlong Liu, Shiyu Zhuang, and Weichen Tian. 2025. "Microscopic Mechanical Properties and Physicochemical Changes of Cement Paste Exposed to Elevated Temperatures and Subsequent Rehydration" Materials 18, no. 5: 1050. https://doi.org/10.3390/ma18051050
APA StyleXu, L., Hu, X., Tang, R., Zhang, X., Xia, Y., Ran, B., Liu, J., Zhuang, S., & Tian, W. (2025). Microscopic Mechanical Properties and Physicochemical Changes of Cement Paste Exposed to Elevated Temperatures and Subsequent Rehydration. Materials, 18(5), 1050. https://doi.org/10.3390/ma18051050






