Exploring Selenium-Functionalized Hydroxyapatite Using Organic Selenocystine for Antitumor Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
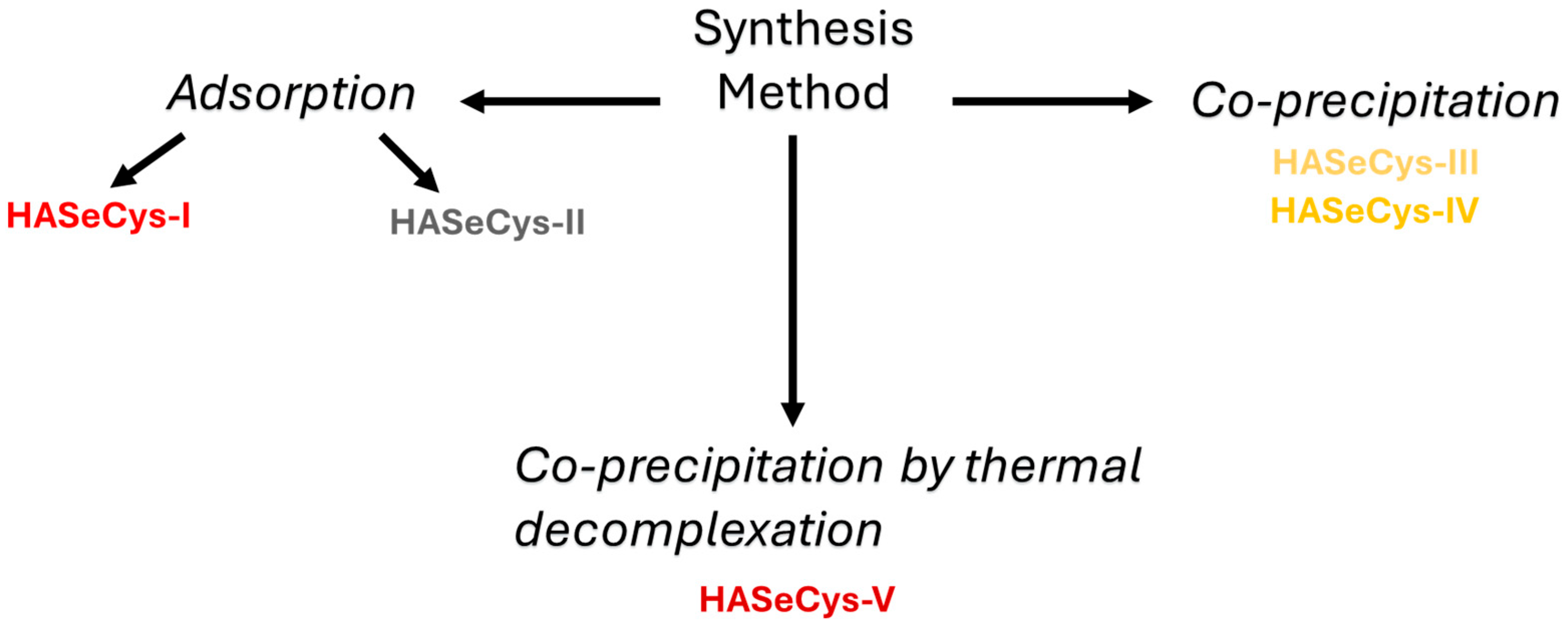
2.2. Synthesis of HASeCys Nanoparticles
2.2.1. Adsorption Method
2.2.2. Co-Precipitation Method
2.2.3. Co-Precipitation by Thermal Decomplexation Method
2.3. Characterization of HASeCys
2.4. Biology
2.4.1. Cell Culture
2.4.2. In Vitro Cell Viability
3. Results
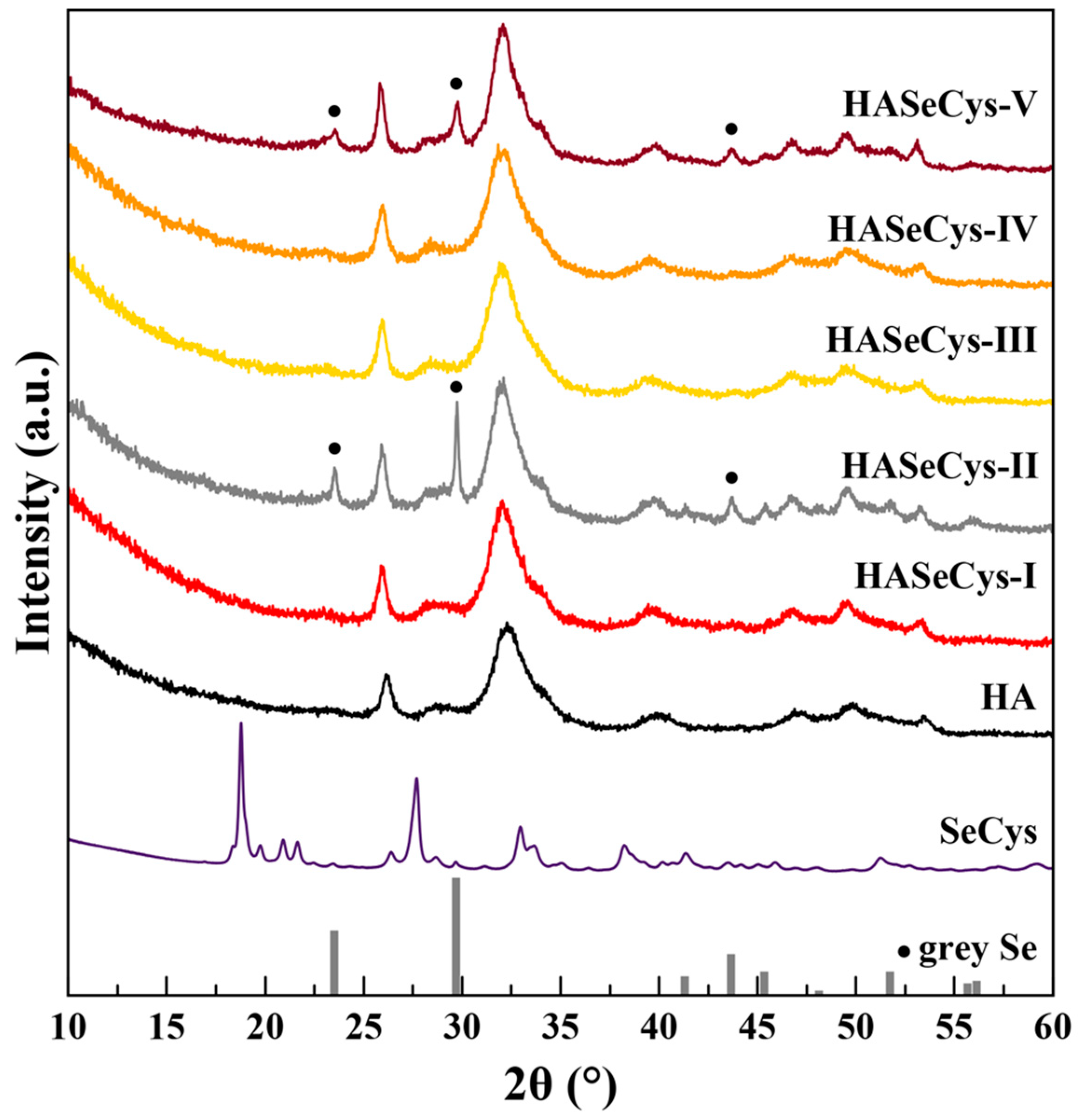
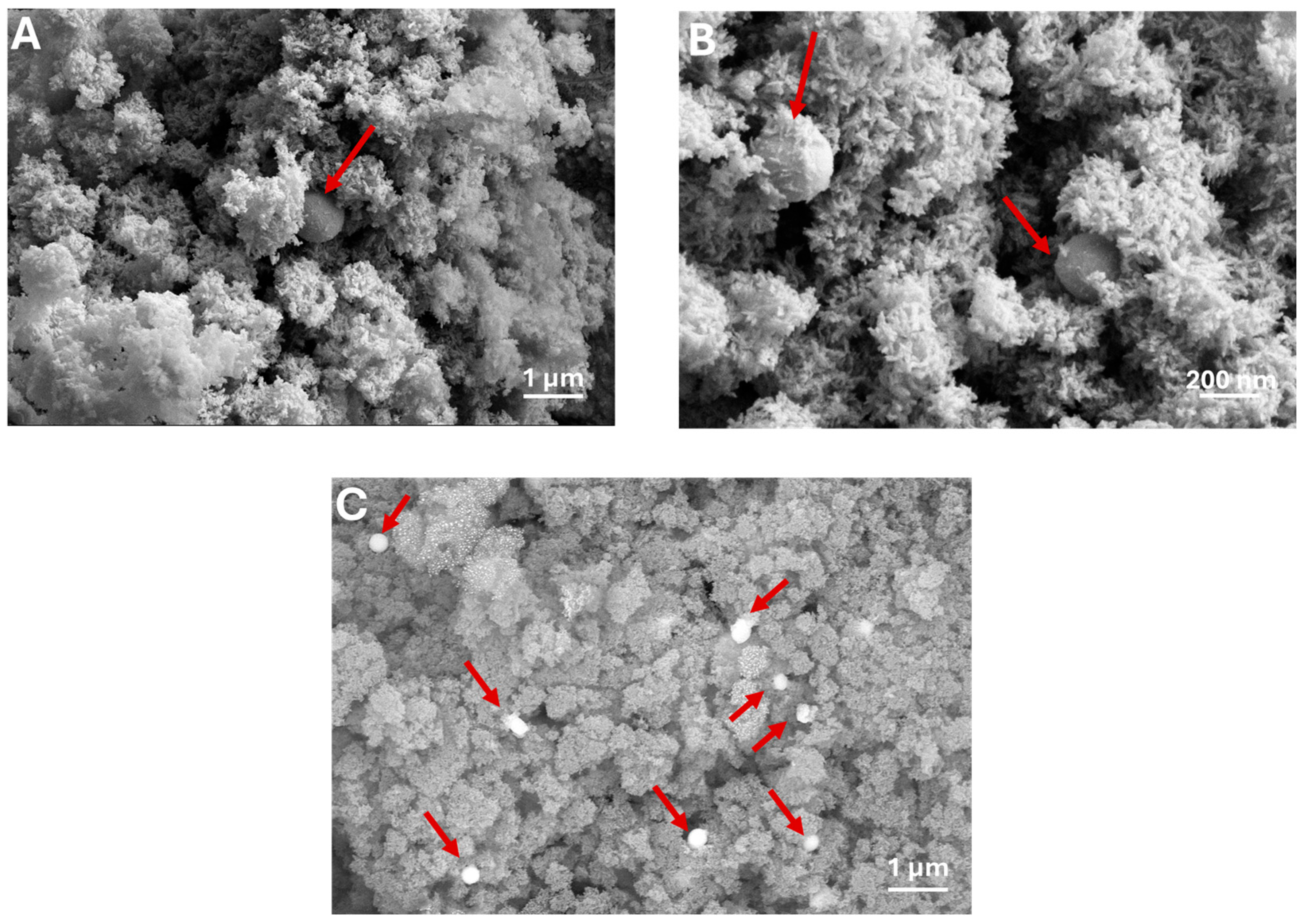
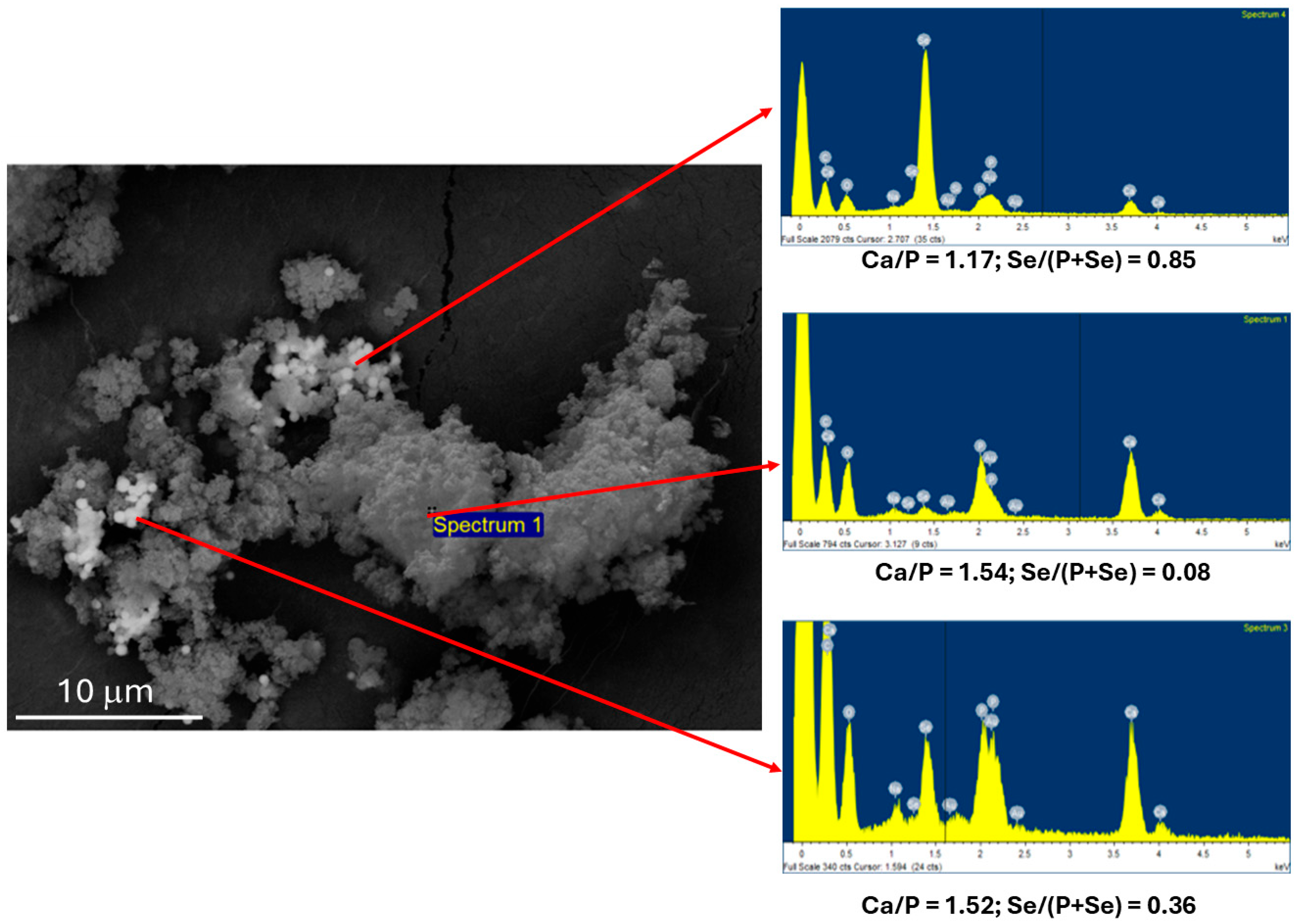
3.1. Synthesis and Characterization of HASeCys
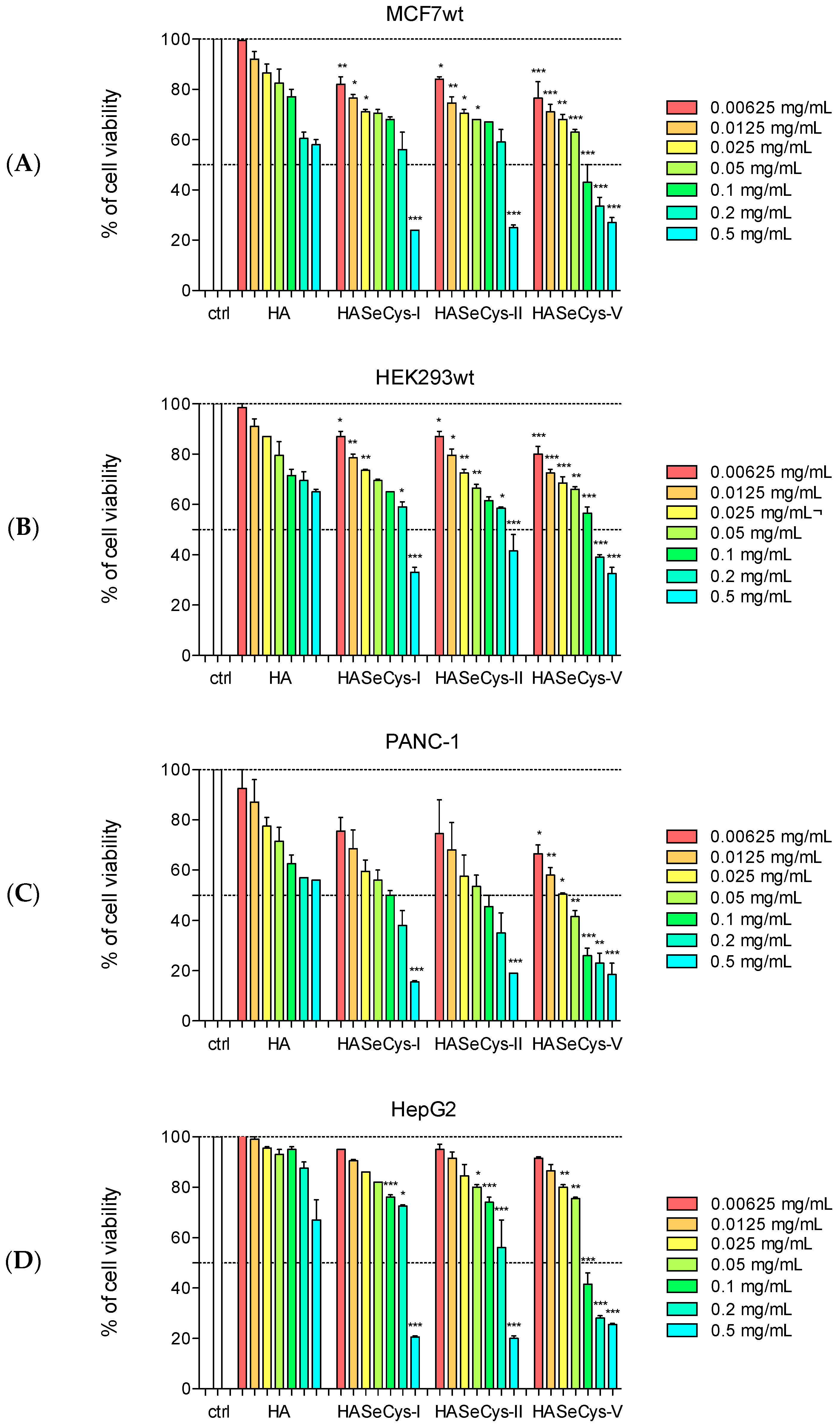
3.2. Cytotoxic Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronai, Z.; Tillotson, J.K.; Traganos, F.; Darzynkiewicz, Z.; Conaway, C.C.; Upadhyaya, P.; El-Bayoumy, K. Effects of Organic and Inorganic Selenium Compounds on Rat Mammary Tumor Cells. Int. J. Cancer 1995, 63, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Peláez, C.; et al. Scientific Opinion on the Tolerable Upper Intake Level for Selenium. EFSA J. 2023, 21, e07704. [Google Scholar] [CrossRef] [PubMed]
- Barbanente, A.; Palazzo, B.; Esposti, L.D.; Adamiano, A.; Iafisco, M.; Ditaranto, N.; Migoni, D.; Gervaso, F.; Nadar, R.; Ivanchenko, P.; et al. Selenium-Doped Hydroxyapatite Nanoparticles for Potential Application in Bone Tumor Therapy. J. Inorg. Biochem. 2021, 215, 111334. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yuan, Q.; Gao, L.; Cai, P.; Zhu, H.; Liu, R.; Wang, Y.; Wei, Y.; Huang, G.; Liang, J.; et al. Cytotoxicity and Therapeutic Effect of Irinotecan Combined with Selenium Nanoparticles. Biomaterials 2014, 35, 8854–8866. [Google Scholar] [CrossRef]
- Menon, S.; Ks, S.D.; Santhiya, R.; Rajeshkumar, S.; Kumar, V. Selenium Nanoparticles: A Potent Chemotherapeutic Agent and an Elucidation of Its Mechanism. Colloids Surf. B. Biointerfaces 2018, 170, 280–292. [Google Scholar] [CrossRef]
- Storkey, C.; Davies, M.J.; White, J.M.; Schiesser, C.H. Synthesis and Antioxidant Capacity of 5-Selenopyranose Derivatives. Chem. Commun. 2011, 47, 9693. [Google Scholar] [CrossRef]
- Jariwalla, R.J.; Gangapurkar, B.; Nakamura, D. Differential Sensitivity of Various Human Tumour-Derived Cell Types to Apoptosis by Organic Derivatives of Selenium. Br. J. Nutr. 2008, 101, 182–189. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Tsai, M.-L.; Kuo, F.-L.; Lai, C.-S.; Badmaev, V.; Ho, C.-T.; Pan, M.-H. Se-Methyl-L-Selenocysteine Induces Apoptosis via Endoplasmic Reticulum Stress and the Death Receptor Pathway in Human Colon Adenocarcinoma COLO 205 Cells. J. Agric. Food Chem. 2015, 63, 5008–5016. [Google Scholar] [CrossRef]
- Domracheva, I.; Kanepe-Lapsa, I.; Jackevica, L.; Vasiljeva, J.; Arsenyan, P. Selenopheno Quinolinones and Coumarins Promote Cancer Cell Apoptosis by ROS Depletion and Caspase-7 Activation. Life Sci. 2017, 186, 92–101. [Google Scholar] [CrossRef]
- Fan, C.; Zheng, W.; Fu, X.; Li, X.; Wong, Y.-S.; Chen, T. Strategy to Enhance the Therapeutic Effect of Doxorubicin in Human Hepatocellular Carcinoma by Selenocystine, a Synergistic Agent That Regulates the ROS-Mediated Signaling. Oncotarget 2014, 5, 2853–2863. [Google Scholar] [CrossRef]
- Deepagan, V.G.; Kwon, S.; You, D.G.; Nguyen, V.Q.; Um, W.; Ko, H.; Lee, H.; Jo, D.-G.; Kang, Y.M.; Park, J.H. In Situ Diselenide-Crosslinked Polymeric Micelles for ROS-Mediated Anticancer Drug Delivery. Biomaterials 2016, 103, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sakallı Çetin, E.; Nazıroğlu, M.; Çiğ, B.; Övey, İ.S.; Aslan Koşar, P. Selenium Potentiates the Anticancer Effect of Cisplatin against Oxidative Stress and Calcium Ion Signaling-Induced Intracellular Toxicity in MCF-7 Breast Cancer Cells: Involvement of the TRPV1 Channel. J. Recept. Signal Transduct. 2017, 37, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Carrier, L.; Belame, A.; Thiyagarajah, A.; Salvo, V.A.; Burow, M.E.; Rowan, B.G. Combination of Methylselenocysteine with Tamoxifen Inhibits MCF-7 Breast Cancer Xenografts in Nude Mice through Elevated Apoptosis and Reduced Angiogenesis. Breast Cancer Res. Treat. 2009, 118, 33–43. [Google Scholar] [CrossRef]
- Fan, C.; Fu, X.; Zhang, Z.; Cao, M.; Sun, J.; Yang, M.; Fu, X.; Zhao, S.; Shao, L.; Zhang, H.; et al. Selenocysteine Induces Apoptosis in Human Glioma Cells: Evidence for TrxR1-Targeted Inhibition and Signaling Crosstalk. Sci. Rep. 2017, 7, 6465. [Google Scholar] [CrossRef]
- Soriano-Garcia, M. Organoselenium Compounds as Potential Therapeutic and Chemopreventive Agents: A Review. Curr. Med. Chem. 2004, 11, 1657–1669. [Google Scholar] [CrossRef]
- Gandin, V.; Khalkar, P.; Braude, J.; Fernandes, A.P. Organic Selenium Compounds as Potential Chemotherapeutic Agents for Improved Cancer Treatment. Free Radic. Biol. Med. 2018, 127, 80–97. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.-S. Selenocystine Induces Reactive Oxygen Species–Mediated Apoptosis in Human Cancer Cells. Biomed. Pharmacother. 2009, 63, 105–113. [Google Scholar] [CrossRef]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.; Björnstedt, M. Redox-Active Selenium Compounds—From Toxicity and Cell Death to Cancer Treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Wallenberg, M.; Misra, S.; Wasik, A.M.; Marzano, C.; Björnstedt, M.; Gandin, V.; Fernandes, A.P. Selenium Induces a Multi-targeted Cell Death Process in Addition to ROS Formation. J. Cell. Mol. Med. 2014, 18, 671–684. [Google Scholar] [CrossRef]
- Poerschke, R.L.; Moos, P.J. Thioredoxin Reductase 1 Knockdown Enhances Selenazolidine Cytotoxicity in Human Lung Cancer Cells via Mitochondrial Dysfunction. Biochem. Pharmacol. 2011, 81, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wong, Y.S. Selenocystine Induces Apoptosis of A375 Human Melanoma Cells by Activating ROS-Mediated Mitochondrial Pathway and P53 Phosphorylation. Cell. Mol. Life Sci. 2008, 65, 2763–2775. [Google Scholar] [CrossRef]
- Fan, C.; Chen, J.; Wang, Y.; Wong, Y.-S.; Zhang, Y.; Zheng, W.; Cao, W.; Chen, T. Selenocystine Potentiates Cancer Cell Apoptosis Induced by 5-Fluorouracil by Triggering Reactive Oxygen Species-Mediated DNA Damage and Inactivation of the ERK Pathway. Free Radic. Biol. Med. 2013, 65, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential Apoptotic Response of Human Cancer Cells to Organoselenium Compounds. Cancer Chemother. Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Husbeck, B.; Nonn, L.; Peehl, D.M.; Knox, S.J. Tumor-Selective Killing by Selenite in Patient-Matched Pairs of Normal and Malignant Prostate Cells. Prostate 2006, 66, 218–225. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.-S. Selenocystine Induces Caspase-Independent Apoptosis in MCF-7 Human Breast Carcinoma Cells with Involvement of P53 Phosphorylation and Reactive Oxygen Species Generation. Int. J. Biochem. Cell Biol. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The Mechanical Properties and Osteoconductivity of Hydroxyapatite Bone Scaffolds with Multi-Scale Porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kalinkevich, O.V.; Pogorelov, M.V.; Kalinkevich, A.N.; Sklyar, A.M.; Kalinichenko, T.G.; Ilyashenko, V.Y.; Starikov, V.V.; Bumeyster, V.I.; Sikora, V.Z.; et al. Characterization and in Vivo Evaluation of Chitosan-hydroxyapatite Bone Scaffolds Made by One Step Coprecipitation Method. J. Biomed. Mater. Res. Part A 2011, 96, 639–647. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, H.; Shi, T.; Xie, D.; Chen, R.; Han, X.; Shen, L.; Wang, C.; Tian, Z. Additive Manufacturing of Hydroxyapatite Bone Scaffolds via Digital Light Processing and in Vitro Compatibility. Ceram. Int. 2019, 45, 11079–11086. [Google Scholar] [CrossRef]
- Barbanente, A.; Nadar, R.A.; Esposti, L.D.; Palazzo, B.; Iafisco, M.; Van Den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Margiotta, N. Platinum-Loaded, Selenium-Doped Hydroxyapatite Nanoparticles Selectively Reduce Proliferation of Prostate and Breast Cancer Cells Co-Cultured in the Presence of Stem Cells. J. Mater. Chem. B 2020, 8, 2792–2804. [Google Scholar] [CrossRef]
- Lelli, M.; Roveri, N.; Marzano, C.; Hoeschele, J.D.; Curci, A.; Margiotta, N.; Gandin, V.; Natile, G. Hydroxyapatite Nanocrystals as a Smart, PH Sensitive, Delivery System for Kiteplatin. Dalt. Trans. 2016, 45, 13187–13195. [Google Scholar] [CrossRef] [PubMed]
- Zakhireh, S.; Adibkia, K.; Beygi-Khosrowshahi, Y.; Barzegar-Jalali, M. Osteogenesis Promotion of Selenium-Doped Hydroxyapatite for Application as Bone Scaffold. Biol. Trace Elem. Res. 2021, 199, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Palazzo, B.; Martra, G.; Margiotta, N.; Piccinonna, S.; Natile, G.; Gandin, V.; Marzano, C.; Roveri, N. Nanocrystalline Carbonate-Apatites: Role of Ca/P Ratio on the Upload and Release of Anticancer Platinum Bisphosphonates. Nanoscale 2012, 4, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, Y.; Ivanchenko, P.; Iafisco, M.; Tampieri, A.; Martra, G. A Step toward Control of the Surface Structure of Biomimetic Hydroxyapatite Nanoparticles: Effect of Carboxylates on the {010} P-Rich/Ca-Rich Facets Ratio. J. Phys. Chem. C 2015, 119, 5928–5937. [Google Scholar] [CrossRef]
- Martínez-Casado, F.J.; Iafisco, M.; Delgado-López, J.M.; Martínez-Benito, C.; Ruiz-Pérez, C.; Colangelo, D.; Oltolina, F.; Prat, M.; Gómez-Morales, J. Bioinspired Citrate–Apatite Nanocrystals Doped with Divalent Transition Metal Ions. Cryst. Growth Des. 2016, 16, 145–153. [Google Scholar] [CrossRef]
- Kopecka, J.; Barbanente, A.; Vitone, D.; Arnesano, F.; Margiotta, N.; Berchialla, P.; Niso, M.; Riganti, C.; Abate, C. Cytotoxic Pathways Activated by Multifunctional Thiosemicarbazones Targeting Sigma-2 Receptors in Breast and Lung Carcinoma Cells. Pharmacol. Rep. 2023, 75, 1588–1596. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.; Kinneary, J. (Eds.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 12th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 1996. [Google Scholar]
- Thomas Webster, T.J. Differential Effects of Nanoselenium Doping on Healthy and Cancerous Osteoblasts in Coculture on Titanium. Int. J. Nanomed. 2010, 13, 351–358. [Google Scholar] [CrossRef]
- Webster, T.; Hassan, C. The Effect of Red-Allotrope Selenium Nanoparticles on Head and Neck Squamous Cell Viability and Growth. Int. J. Nanomed. 2016, 11, 3641–3654. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, X.; Zhang, L.; Bao, Y. Biological Effects of a Nano Red Elemental Selenium. BioFactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Webster, T.J. Ramos Cytotoxicity of Selenium Nanoparticles in Rat Dermal Fibroblasts. Int. J. Nanomed. 2012, 7, 3907–3914. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in Food and the Human Body: A Review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mejía Jaramillo, A.; Pavon, J.J.; Webster, T.J. Red Selenium Nanoparticles and Gray Selenium Nanorods as Antibacterial Coatings for PEEK Medical Devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1352–1358. [Google Scholar] [CrossRef] [PubMed]



| Sample | Ca wt.% | P wt.% | Se wt.% | Ca/P | Ca/(P + Se) | Se/(P + Se) |
|---|---|---|---|---|---|---|
| HASeCys-I | 30.0 ± 1.1 | 14.9 ± 0.5 | 0.34 ± 0.04 | 1.55 ± 0.01 | 1.54 ± 0.01 | 0.009 ± 0.001 |
| HASeCys-II | 29.9 ± 0.7 | 14.5 ± 0.2 | 0.39 ± 0.02 | 1.59 ± 0.01 | 1.57 ± 0.01 | 0.010 ± 0.001 |
| HASeCys-III | 32.1 ± 0.3 | 16.6 ± 0.1 | 0.25 ± 0.01 | 1.49 ± 0.01 | 1.48 ± 0.01 | 0.006 ± 0.001 |
| HASeCys-IV | 31.4 ± 1.3 | 15.9 ± 0.7 | 0.51 ± 0.02 | 1.52 ± 0.01 | 1.50 ± 0.01 | 0.012 ± 0.01 |
| HASeCys-V | 25.3 ± 0.9 | 12.9 ± 0.4 | 0.91 ± 0.55 | 1.51 ± 0.01 | 1.48 ± 0.02 | 0.027 ± 0.015 |
| Antiproliferative Effect (EC50 mg/mL ± SD) | ||||
|---|---|---|---|---|
| Samples | MCF7wt | PANC-1 | HepG2 | HEK293wt |
| HA | n.d. | n.d. | n.d. | n.d. |
| HASeCys-I | 0.38 ± 0.03 | 0.13 ± 0.02 | 0.48 ± 0.06 | 0.35 ± 0.06 |
| HASeCys-II | 0.27 ± 0.03 | 0.07 ± 0.01 | 0.27 ± 0.03 | 0.20 ± 0.04 |
| HASeCys-V | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.02 | 0.13 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbanente, A.; Di Cosola, A.M.; Degli Esposti, L.; Iafisco, M.; Niso, M.; Margiotta, N. Exploring Selenium-Functionalized Hydroxyapatite Using Organic Selenocystine for Antitumor Applications. Materials 2025, 18, 1043. https://doi.org/10.3390/ma18051043
Barbanente A, Di Cosola AM, Degli Esposti L, Iafisco M, Niso M, Margiotta N. Exploring Selenium-Functionalized Hydroxyapatite Using Organic Selenocystine for Antitumor Applications. Materials. 2025; 18(5):1043. https://doi.org/10.3390/ma18051043
Chicago/Turabian StyleBarbanente, Alessandra, Anna Maria Di Cosola, Lorenzo Degli Esposti, Michele Iafisco, Mauro Niso, and Nicola Margiotta. 2025. "Exploring Selenium-Functionalized Hydroxyapatite Using Organic Selenocystine for Antitumor Applications" Materials 18, no. 5: 1043. https://doi.org/10.3390/ma18051043
APA StyleBarbanente, A., Di Cosola, A. M., Degli Esposti, L., Iafisco, M., Niso, M., & Margiotta, N. (2025). Exploring Selenium-Functionalized Hydroxyapatite Using Organic Selenocystine for Antitumor Applications. Materials, 18(5), 1043. https://doi.org/10.3390/ma18051043









