3.1. Study by Differential Scanning Calorimetry
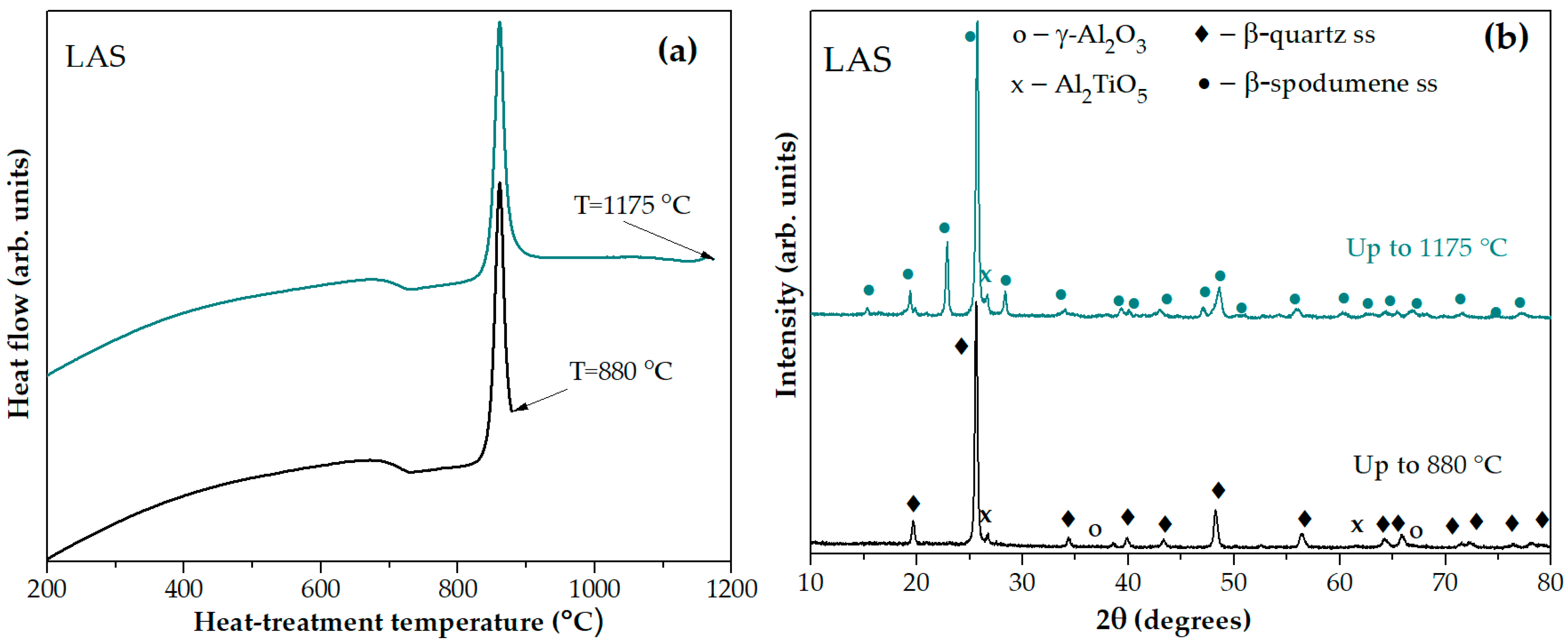
Figure 3 shows the DSC scans of the quenched LAS and LAS
ox glasses, as well as these glasses heat-treated at 680 °C, 700 °C and 720 °C for 6 h. The shapes of the corresponding DCS curves of LAS and LAS
ox glasses are nearly similar, being different in the values of characteristic crystallization temperatures, see
Figure 3 and
Table 1. The DSC curve of the quenched LAS
ox glass shows three peaks with markedly different intensities. The first one is a broad peak of a low intensity with T
max = 802 °C, the second one is a narrow and intense peak with crystallization onset temperature T
on = 851 °C, and crystallization maximum temperature T
max = 874 °C. The third one is a weak exothermic peak with T
max = 1186 °C. The first peak is usually assigned to nucleation of the main crystalline phase, the second one to the crystallization of the lithium aluminosilicate solid solution (ss) with β-quarts structure (β-quarts ss), and the third one to the transformation of β-quartz ss into β-spodumene ss [
38,
39]. The DSC curve of the LAS glass exhibits a plateau instead of the first peak, see
Figure 3 and
Table 1. All characteristic crystallization temperatures of the LAS glass are lower than those for the LAS
ox glass, while the glass transition temperatures of both glasses are similar, see
Table 1. Therefore, the redox conditions of glass melting affect crystallization temperatures and do not affect the transition temperature of glasses.
Preliminary heat treatments lead to a drastic change in the appearance of DSC curves, see
Figure 3. The character of this change for LAS and LAS
ox samples is the same, differing in the temperatures of thermal effects. Instead of a single narrow intense peak, two broad exothermic peaks of lower intensities appear. The characteristic temperatures for both glasses preliminarily heat-treated at 680 °C, 700 °C, and 720 °C are shown in
Figure 3 and
Table 1. T
max of the second broad exothermic peak is 35–40 °C higher than this temperature for the initial quenched glass. With the increase in the temperature of preliminary heat treatment, there is a redistribution of intensities of these two peaks in favor of the second one, see
Figure 3. T
max of the second peak and T
g gradually increase, see
Table 1.
The reason for this behavior will be explained below while discussing the phase composition of the samples. The characteristic Tg, Ton and Tmax temperatures of glasses preliminary heat-treated at 680 °C are similar, being somewhat higher for the LAS glass than for the corresponding LASox samples.
The XRD pattern of the glass-ceramic obtained by heating the quenched LAS glass in the furnace of the DSC instrument up to the temperature of the sharp exothermic peak at 880 °C, see
Figure 4a, testifies to the crystallization of β-quarts ss, traces of tieilite, Al
2TiO
5, and spinel, see
Figure 4b. On the XRD pattern of the sample heated up to the temperature of the third exothermic effect at 1175 °C, additional diffraction peaks appear as compared with the pattern of the sample heated up to 880 °C, manifesting in a transformation of the β-quarts ss into the β-spodumene ss while spinel traces disappeared. Thus, the small high-temperature peak evolving at ~1175 °C is due to crystallization of β-spodumene ss, see
Figure 4b.
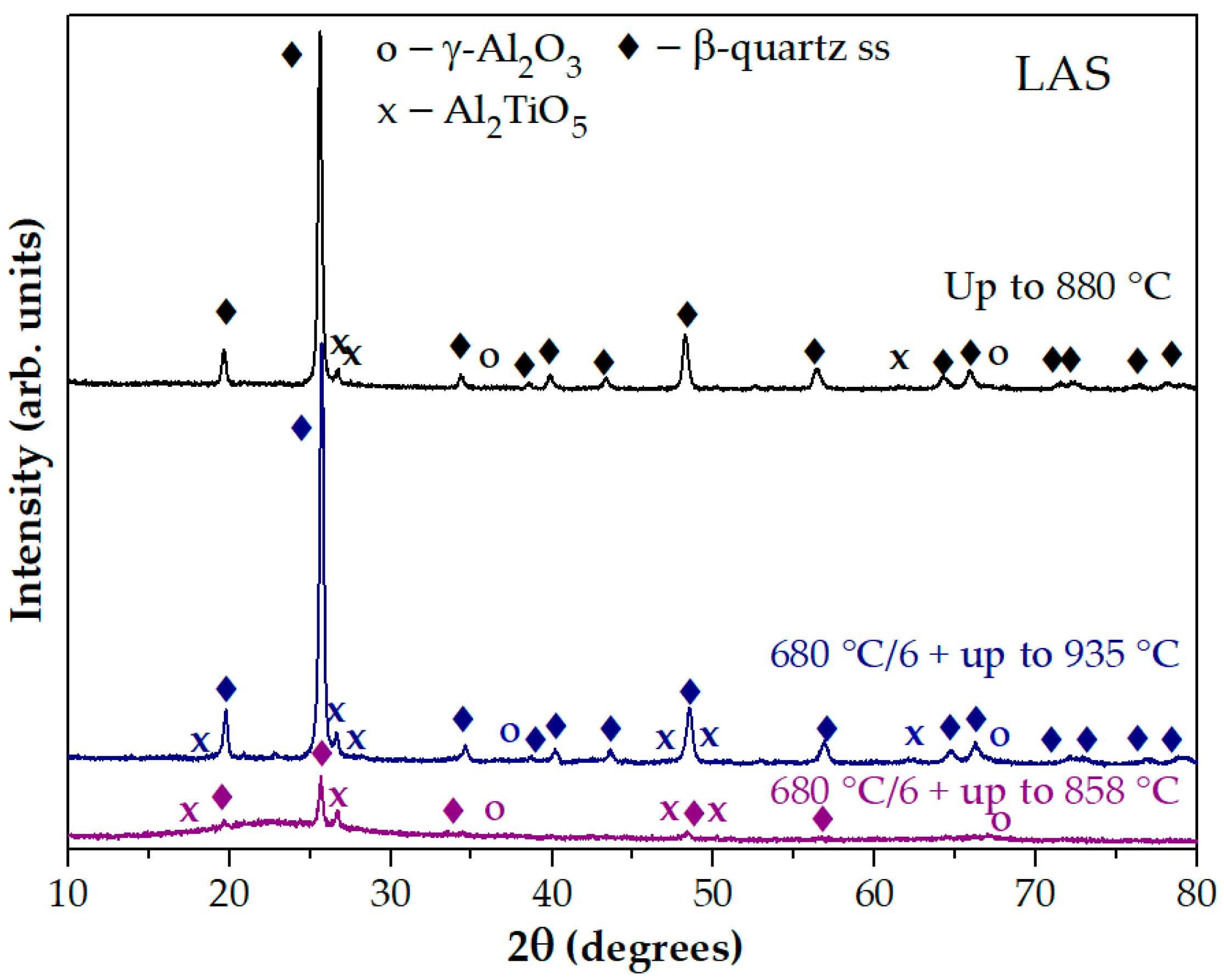
Figure 5a shows the DSC signals of the LAS glass preliminarily heat-treated at 680 °C for 6 h with stops at temperatures of 787 °C, 838 °C, 858 °C, and 935 °C. The corresponding XRD patterns are presented in
Figure 5b. The material obtained by the heat treatment at 680 °C, 6 h + 787 °C, 0 h is X-ray amorphous. The sample obtained by the heat treatment at 680 °C, 6 h + 838 °C, 0 h contains nanocrystals of γ-Al
2O
3 with a spinel structure and traces of β-quarts ss. It is speculated that this exothermic peak is caused by the crystallization of spinel, while traces of β-quarts ss appear during the cooling of the sample in the furnace, because the samples heated to the specified temperature could not be immediately removed from the hot furnace of the DSC instrument. The XRD pattern of the sample obtained by the heat treatment at 680 °C, 6 h + 858 °C, 0 h, corresponding to the end of the first exothermic peak and the beginning of the second one, contains peaks of nanocrystals of γ-Al
2O
3, Al
2TiO
5 and β-quarts ss. The XRD pattern of glass-ceramic obtained by the heat treatment at 680 °C, 6 h + 935 °C, 0 h proves the crystallization of β-quarts ss and Al
2TiO
5. The presence of γ-Al
2O
3 cannot be ruled out on this XRD pattern because the most intense peak of γ-Al
2O
3 overlaps the peaks of β-quarts ss and is therefore difficult to detect.
The XRD pattern of the glass preliminarily heat-treated at 680 °C for 6 h and heated in the DSC furnace up to 858 °C is significantly different from the XRD pattern of the quenched glass heated in the DSC furnace up to 880 °C. The XRD pattern of the glass preliminarily heat-treated at 680 °C for 6 h and heated in the DSC furnace up to 935 °C is similar to the XRD pattern of the quenched glass heated in the DSC furnace up to 880 °C, see
Figure 6. Thus, the first peak on the DSC curve of the preliminary heat-treated sample is associated with the crystallization of the phase with the spinel structure, and the second peak is caused by the crystallization of β-quartz ss and Al
2TiO
5. On the DSC curve of the quenched sample there is no exothermic peak associated with the crystallization of the phase with spinel structure. Therefore, preliminary heat treatments provoke spinel crystallization and increase the crystallization temperature of β-quartz ss and Al
2TiO
5.
3.2. XRD Study
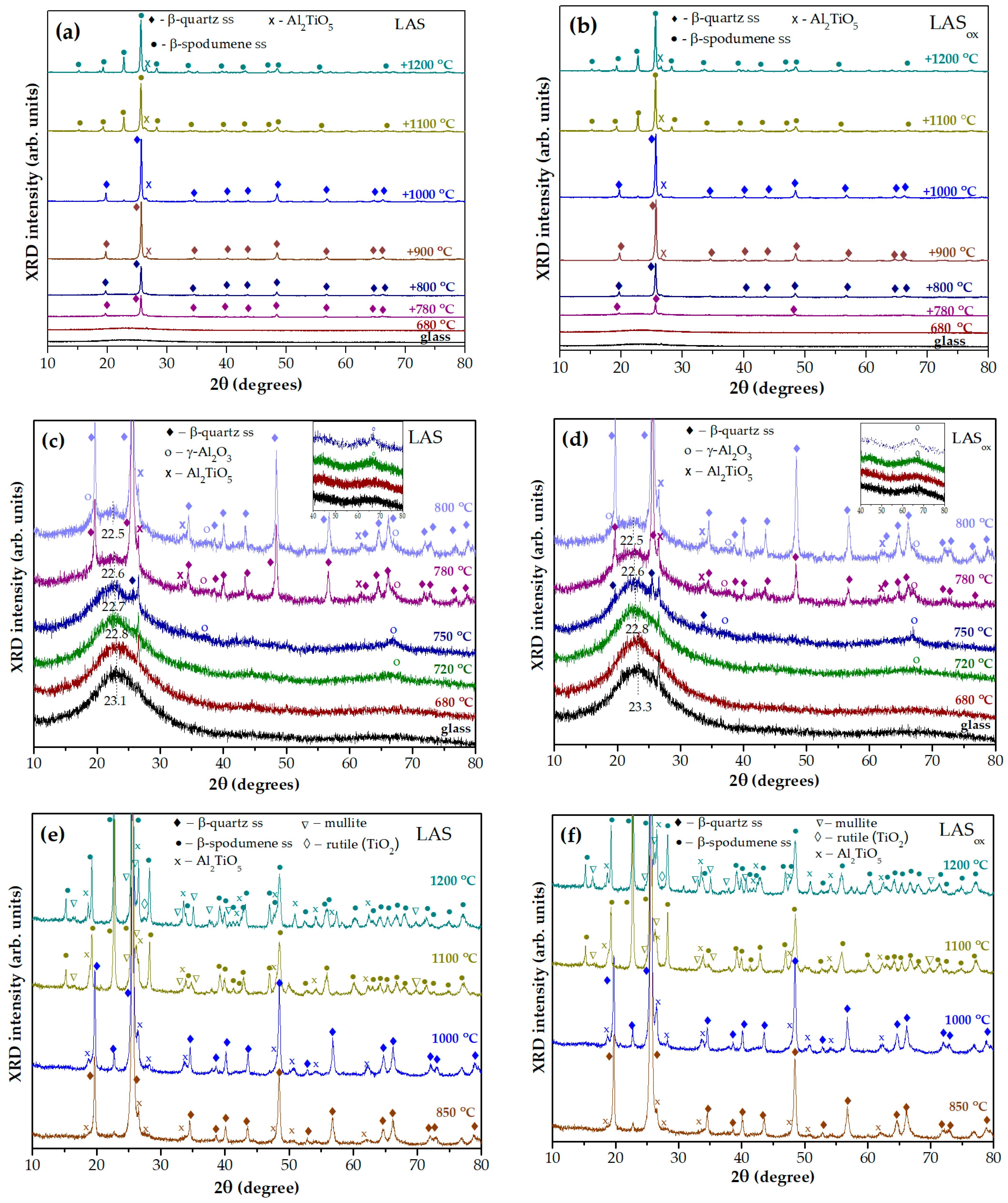
Figure 7a,b shows XRD patterns of the LAS and LAS
ox glasses and glasses of the same compositions, which underwent heat treatment at 680 °C and two-stage heat treatments with a temperature at the second stage between 720 °C and 1200 °C. The holding time at each stage was 6 h. The figures allow the following of the formation of the main crystalline phase, β-quartz ss, at temperatures between 780 °C and 1000 °C, and crystallization of β-spodumene ss at temperatures between 1100 °C and 1200 °C.
Figure 7c,d gives a closer look at the XRD patterns of the glasses and glass-ceramics fabricated by heat treatments at the second stage up to 800 °C. Initial LAS and LAS
ox glasses are X-ray amorphous with a maximum amorphous halo located at 2θ = 23.1° and 23.3°, respectively. Glasses heat-treated at the nucleation stage of 680 °C for 6 h are X-ray amorphous, and their XRD patterns are similar to those of glass precursors. The two-stage heat treatment with the second stage at 720 °C for 6 h leads to crystallization of a small fraction of crystallites with spinel structure, manifested by an appearance of a broad peak with Miller indices
hkl (440) at 2θ ≅ 66.8°.
Its low intensity prevents estimation of the lattice parameter and the size of the spinel crystallites. The position of the amorphous halo shifts to a smaller angle 2θ = 22.8° for both samples. After the second stage heat treatment at 750 °C for 6 h, spinel peaks become more pronounced, which indicates an increase in the volume fraction of this phase. The lattice parameters of spinel nanocrystals are a
norm = 7.918 Å and a
ox = 7.916 Å, and sizes are D
norm = 4.5 nm and D
ox = 7.3 nm, respectively. Traces of β-quartz ss are also seen on the patterns. The appearance of spinel and traces of β-quartz ss cause a further change in the composition of the residual glass phase, which is manifested by a shift in the maximum of the amorphous halo to smaller angles, indicating that the residual glass becomes enriched in silica. After heat treatments at the second stage at 780 °C and 800 °C, the β-quartz ss becomes the predominant crystalline phase, while spinel and tieilite, Al
2TiO
5, nanocrystals are also found in the XRD patterns. The position of the amorphous halo of residual glass is shifted to 2θ = 22.6°and then to 2θ = 22.5° with increasing heat-treatment temperature, see
Figure 7c,d.
The lattice parameters and mean crystallite sizes of spinel obtained at different heat-treatment temperatures are presented in
Table 2. It is believed that spinel has the composition and structure of γ-Al
2O
3. The lattice parameter
a of the unit cell of the cubic modification of Al
2O
3 usually takes the value
a = 7.900–7.908 Å [
40]. With a slight oxygen deficiency, the parameter
a of γ-Al
2O
3 becomes equal to 7.911 Å (ICDD PDF card #79-1558) and 7.914 Å (ICDD PDF card 79-1557) [
41]. The lattice parameter
a of γ-Al
2O
3 crystallites in glass-ceramics changes from 7.915 Å to 7.925 Å and increases with increasing the heat-treatment temperature. The difference in the parameter
a of γ-Al
2O
3 nanocrystals in LAS and LAS
ox glass-ceramics is very small. Nevertheless, the lattice parameter
a of γ-Al
2O
3 in LAS glass-ceramics is slightly higher than in LAS
ox ones. The sizes of spinel crystallites increase with increasing the heat-treatment temperature ranging from 4.5 to 14.0 nm, see
Table 2.
Figure 8a shows that in spite of the same phase assemblage of glass-ceramics obtained by two-stage heat treatment at 680 °C, 6 h + 780 °C, 6 h, the crystallinity fractions in the LAS glass-ceramic is much higher than in the LAS
ox one. This difference is levelled out by the heat treatment at 680 °C, 6 h + 800 °C, 6 h, see
Figure 8b.
Figure 7e,f show the XRD patterns of glass-ceramics obtained by two-stage heat treatments with a second-stage temperature between 850 °C and 1200 °C. After the heat treatment at 850 °C the crystallinity fraction increases to such extent that the amorphous halo disappears. Volume fractions of β-quartz ss and Al
2TiO
5 gradually increase. β-spodumene ss crystallizes at the expense of β-quartz ss at 1100 °C. Mullite (ICDD PDF card # 79-1458) appears at the same heat-treatment temperature. The lattice parameters and mean crystallite sizes of β-quartz ss and β-spodumene ss are presented in
Table 2. Their variation with heat-treatment temperature is similar for the LAS and the LAS
ox glasses.
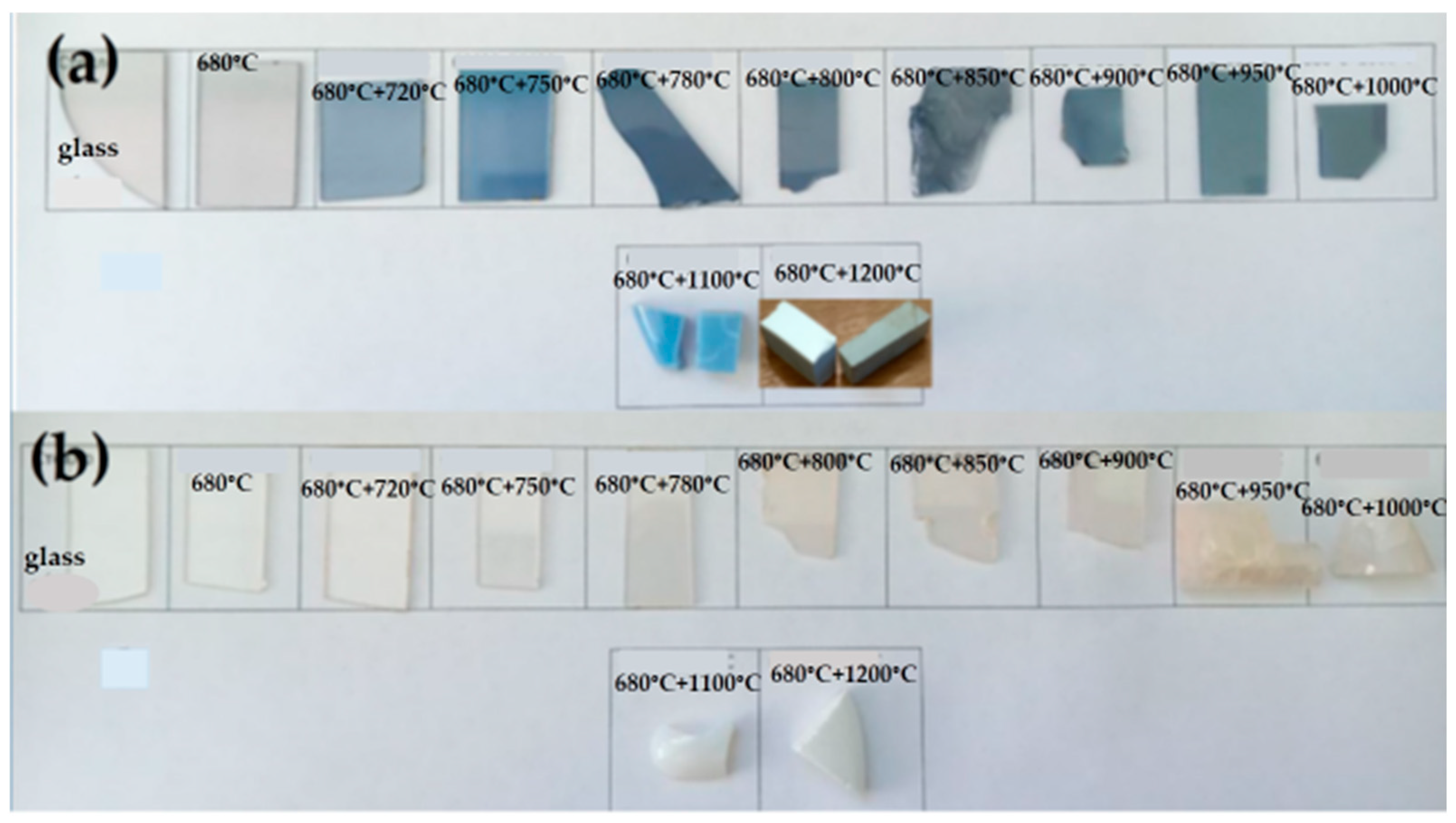
As was mentioned above, the appearance of the surface and the volume of the LAS glass-ceramics obtained by the heat treatment at 1200 °C are different. The surface is a dense, white-colored layer while the volume is grey colored, see
Figure 2. Traces of rutile (ICDD PDF card #78-1509) are found on the XRD patterns taken from the grey part of the LAS glass-ceramic. The appearance of rutile is accompanied by some decrease in the tieilite fraction. We failed to find a difference in the phase assemblages of the surface and volume of LAS
ox glass-ceramic obtained by the same heat treatment. The XRD pattern shows a tiny fraction of rutile in this sample.
Let us discuss the features of crystallization of tieilite, Al
2TiO
5. As mentioned above, the onset of the crystallization of β-quartz solid solution in glasses at a temperature of 780 °C is accompanied by the appearance of a small fraction of Al
2TiO
5 crystallites with mean sizes of ~6 and 8 nm for LAS and LAS
ox glass-ceramics, respectively, see
Table 3. Increasing the heat-treatment temperature up to 1200 °C leads to an increase in the size of Al
2TiO
5 crystallites up to ~40 nm, a slow increase in their fraction and a change in their lattice parameters, see
Table 3. The values of the lattice parameter do not change with the heat-treatment temperature within the measurement error and are independent of the redox conditions of glass melting. Lattice parameters b, c and the volume increase with the heat-treatment temperature. An analysis of the evolution of lattice parameters shows that the lattice of a Al
2TiO
5 unit cell, which has the shape of a rectangular parallelepiped elongated in [010] and [001] directions, becomes even more elongated in the same directions under the influence of high temperature. The lattice parameters of tieilite in LAS and LAS
ox glass-ceramics are similar. The character of their variation with temperature is also similar. The redox conditions of glass melting do not significantly affect the structural transformations in tieilite crystallites. A pronounced change in lattice parameters after the heat treatment at 1200 °C found in the present study can be a prerequisite to decomposition of tieilite with exsolution of rutile [
42]. Indeed, as mentioned above, traces of rutile are found in LAS
ox and in the volume of the LAS glass-ceramics obtained by the heat treatment at 1200 °C. The comparison of XRD patterns of the LAS and the LAS
ox glass-ceramics obtained by the heat treatment at 1200 °C demonstrates that LAS
ox glass-ceramic contains a smaller fraction of rutile and a higher fraction of mulite than the LAS glass-ceramic, compare
Figure 7e,f.
The sequence of phase transformations revealed by XRD study is similar for both glasses: precursor glasses and glasses subjected to the nucleation treatment are X-ray amorphous; nanocrystals of γ-Al2O3 with spinel structure and sizes ranging from 4.5 nm to 14 nm evolve during heat treatments in the second stage in the temperature range from 720 °C to 800 °C; β-quartz ss and the crystalline phase of the nucleating agent, tieilite, appear additionally to spinel during the heat treatment at 780 °C; β-quartz ss are obtained by heating in the interval between 780 °C and 1000 °C; the glass-ceramic prepared at 1100 °C contains β-spodumene ss instead of β-quartz ss and traces of mullite; β-spodumene ss is the main crystalline phase of glass-ceramics produced in the temperature range between 1100 °C and 1200 °C; tieilite nanocrystals with a mean size ranging from ~6 nm to ~45 nm are found in glass-ceramics prepared in the temperature interval from 800 °C to 1200 °C; during the heat treatment at 1200 °C, the rutile crystallites appear in the phase assemblage of glass-ceramics.
In spite of the same phase assemblage of glass-ceramics prepared from glasses melted in different redox conditions, the kinetics of phase transformations and spinel lattice parameters are slightly different. The spinel lattice parameters in the LAS glass-ceramics are larger than in the LASox glass-ceramics. The crystallinity fractions in the LAS glass-ceramic obtained by two-stage heat treatment at 680 °C, 6 h + 780 °C, 6 h is much higher than in the LASox one. However, this difference is levelled out by the heat treatment at 680 °C, 6 h + 800° C, 6 h. The rutile crystallinity fraction in the LAS glass-ceramics is larger than in the LASox one, while the mullite fraction is smaller.
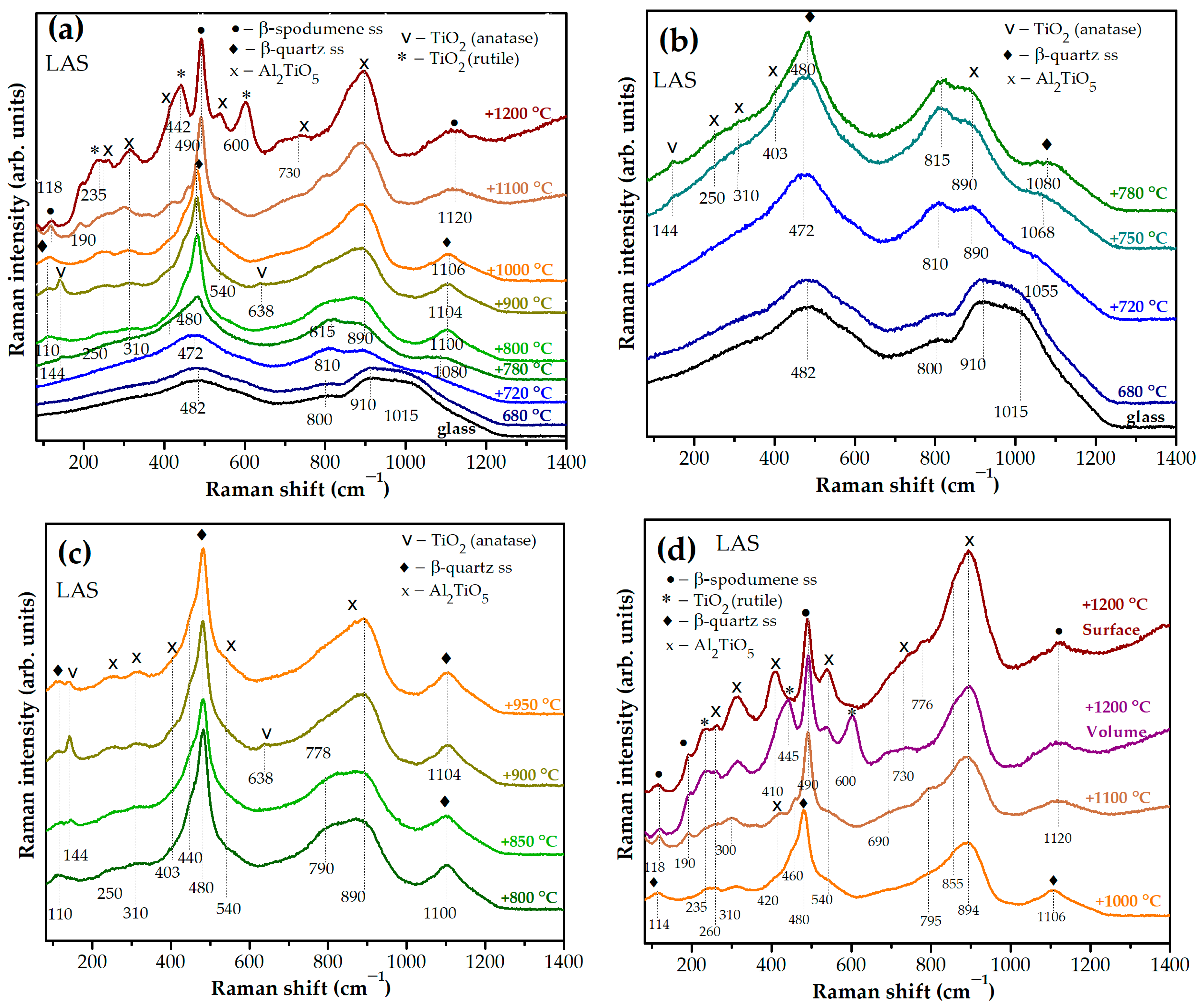
3.3. Study by Raman Spectroscopy
Figure 9a,b show Raman spectra of the initial and heat-treated LAS glass. Raman spectra of initial LAS and LAS
ox glasses are similar and contain broad bands with maxima at 482 cm
−1, 800 cm
−1, 910 cm
−1 and ~1015 cm
−1. The wing of the latter band extends to 1200 cm
−1. A similar spectrum was obtained in ref. [
19] for the glass of the same composition nucleated by 7 mol% TiO
2 and melted in oxidizing conditions. The bands at 482 cm
−1, 800 cm
−1, and ~1000–1200 cm
−1 are due to vibrations of bonds in the tetrahedrons of the aluminosilicate network in the glass structure [
43], and the band at ~910 cm
−1 is due to vibrations of [TiO
4] tetrahedra embedded in this network [
44]. After the heat treatment at the nucleation stage at a temperature of 680 °C, minor changes are noticed in the Raman spectrum, see
Figure 9a,b. The high-frequency band slightly broadens and the band with a maximum at 800 cm
−1 somewhat increases in intensity compared to the band with a maximum at 910 cm
−1, which indicates the development of liquid–liquid phase separation of the precursor glass [
19]. The position of the band at 482 cm
−1 is not influenced by this heat treatment.
After the two-stage heat treatment with a temperature of 720 °C at the second stage, significant changes are observed in the Raman spectrum, see
Figure 9a,b. The bands with maxima at 910 cm
−1, 800 cm
−1, and 482 cm
−1 move their positions to 890 cm
−1, 810 cm
−1, and 472 cm
−1, respectively. Instead of the band with a maximum at ca. 1015 cm
−1, a band at 1055 cm
−1 appears. The intensity of the band at 472 cm
−1 increases as compared with the similar band in spectra of precursor glass and glass heat treated at the nucleation stage. The intensity of the band at ~810 cm
−1 increases relatively to the band at ca. 890 cm
−1. The increase in the intensity of this band at the expense of the band with a maximum at ca. 890 cm
−1 is due to the superposition of vibrations of the [TiO
5] and [TiO
6] groups in amorphous aluminotitanate regions on a weak band in the 800 cm
−1 region, corresponding to vibrations of the tetrahedrons of the aluminosilicate network [
17,
19,
20]. These changes are associated with a further development of the liquid–liquid phase separation with the formation of aluminotitanate amorphous regions [
19,
20] and a change in the composition of the aluminosilicate glass network, which is in accordance with a change in the position of the amorphous halo in the corresponding XRD pattern, see
Figure 7c. Crystallization of γ-Al
2O
3 with spinel structure revealed by XRD analysis does not show itself in the Raman spectrum of this glass-ceramic. Running ahead, one will say that spectral features of γ-Al
2O
3, were not detected in Raman spectra of samples obtained by heat treatments between 750 °C and 800 °C as well, in spite of the fact that XRD analysis reliably identified them. The reason is that bond vibrations in aluminate spinel crystals are very weak compared to bond vibrations in titanium-containing compounds. According to refs [
45,
46], γ-Al
2O
3 exhibits narrow peaks with maxima at 315, 410, 520, 713 and 835 cm
−1 in the Raman spectrum of γ-Al
2O
3 corresponding to vibrations of the Al–O bond in tetrahedral structural units of AlO
4 [
45]. Based on calculations presented in ref. [
47], the strongest Raman peak for γ-Al
2O
3 with a spinel structure is located at ~401 cm
−1. There are also several bands of medium intensities ranging from 100 cm
−1 to 900 cm
−1 [
47]. In Raman spectra presented in
Figure 9b there is a band with a maximum at 403 cm
−1, which could be the spectroscopic sign of γ-Al
2O
3. However, this band can be also caused by vibrations in tieilite crystals, because this band appears simultaneously with other bands at 250 cm
−1, 310 cm
−1, and 890 cm
−1, characteristic of tieilite, which crystallizes at a larger temperature interval of heat treatments than γ-Al
2O
3 (see below). It is worth mentioning that the spectroscopic signs of crystals with a spinel structure were found only in Raman spectra of spinel-based glass-ceramics of magnesium [
48] and zinc aluminosilicate systems [
34], where the spinel crystallinity fraction was significantly higher than in the present case.
In the Raman spectrum of the glass-ceramic obtained by the heat treatment at the second stage at 750 °C, the bands with maxima at 472 cm
−1 and 815 cm
−1 are enhanced, positions of the band maxima change from 810 cm
−1 to 815 cm
−1 and from 1055 cm
−1 to 1068 cm
−1, and a very weak band appears at ~144 cm
−1. The latter peak can be attributed to the most intense vibration in the Raman spectrum of the metastable modification of TiO
2, anatase [
17].
In the Raman spectrum of the sample obtained by the heat treatment at the second stage at a temperature of 780 °C, the band at ~144 cm
−1, caused by vibrations in anatase nanocrystals, narrows, the band at 480 cm
−1 narrows and intensifies, and the band at ~1080 cm
−1 appears. The last two bands are related to vibrations in nanocrystals of β-quartz ss [
17,
19,
49,
50,
51]. As mentioned above, in the spectrum of these glass-ceramics there is a number of bands with maxima at 250 cm
−1, 310 cm
−1, 403 cm
−1, 540 cm
−1, and 890 cm
−1. They appear simultaneously, and their intensities increase continuously with the increasing temperature of heat treatment. These bands belong to vibrations in tieilite nanocrystals [
17,
19], which is in accordance with the XRD data. The Raman spectrum of glass-ceramic obtained by heat treatment with a temperature of 900 °C at the second stage shows two anatase bands at ~144 cm
−1 and ~638 cm
−1 manifesting the maximum anatase crystallinity fraction achieved by this heat treatment, see
Figure 9a,c. After increasing the temperature of the second hold to 1000 °C, the bands related to anatase disappear. Note that the XRD peaks of anatase coincide in position with the main peaks of β-quartz ss and its small amount cannot be detected by the XRD data [
17,
19].
As the temperature at the second stage of heat treatment increases, the intensities of the bands at ~460 cm
−1 and ~1080 cm
−1 increase, and their peaks become narrower, see
Figure 9c, which indicates the development of β-quartz ss crystallization. Their positions constantly move to longer wave numbers manifesting enrichment of these crystals with silica [
50]. After the second stage heat treatment at 1100 °C, these bands shift to 490 cm
−1 and 1120 cm
−1, which can be interpreted as recrystallization of β-quartz ss into β-spodumene ss [
17,
19]. Starting from the heat treatment at 800 °C, a weak band appears at 110 cm
−1 simultaneously with increasing intensity and narrowing of the bands attributed to β-quartz ss. This band can correspond to external vibrations in nanocrystals of β-quartz ss [
51]. In Raman spectra of glass-ceramics obtained by heat treatments at 1100 °C and at 1200 °C, weak bands at ~118 cm
−1 and ~190 cm
−1 were observed that can be attributed to external vibrations in nanocrystals of β-spodumene ss [
49], see
Figure 9d.
Figure 9d shows the bands at ~260 cm
−1, ~445 cm
−1 and ~600 cm
−1, which are related to crystallization of rutile [
17] at the expense of tieilite in the bulk of glass-ceramic obtained by the heat treatment at a second stage at 1200 °C. The signs of rutile crystals were not found in the spectrum from the white surface of this sample, which is in accordance with XRD data. XRD patterns of glass-ceramics obtained by heat treatments at 1100 °C and 1200 °C contain small mullite fractions, see
Figure 7e,f. Raman spectra of synthetic mullites of different compositions have similar spectra with the strongest bands located at 415 cm
−1, 600 cm
−1, and 980 cm
−1 [
52]. They can be hidden in the contours of the corresponding broad bands in Raman spectra, see
Figure 10f.
Raman spectra of glass-ceramics obtained by the same heat treatments that differ most strongly from each other according to Raman spectroscopy data are presented in
Figure 10a–f. Raman spectra of glass precursors, as well as the spectra of glasses heat-treated at 680 °C for 6 h are similar to each other. The spectra of glass-ceramics obtained by the two-stage heat treatment at 680 °C and at 720 °C for 6 h differ from each other by the ratio of band intensities in the region of high wave numbers, see
Figure 10a. The increase in the relative intensity of the band with a maximum at 800 cm
−1 compared to the intensity of the band at 890 cm
−1 in the spectrum of the LAS glass-ceramic suggests that the rate of liquid–liquid phase separation in this glass during this heat treatment is higher than that in the LAS
ox glass, i.e., neutral conditions of glass melting speed up the development of liquid–liquid phase separation in this glass. Crystallization of the titanate phases of anatase and tieilite are sped up in the LAS glass ceramized by the heat treatment at the second stage at 780 °C as compared with the LAS
ox glass, see
Figure 10b. The same tendency remains true for glass-ceramics obtained by heat treatment with the second stage at 900 °C, see
Figure 10c. The higher intensity of the anatase peaks in the Raman spectrum of the LAS
ox glass-ceramic obtained by heat treatment at 950 °C, see
Figure 10d, means that anatase crystallization in the LAS
ox glass reaches its maximum and will decrease at higher temperatures while the maximum anatase content in the LAS glass-ceramics was reached at a previous holding temperature. The traces of anatase crystals remain in the LAS
ox glass-ceramic obtained by heat treatment at 1000 °C while the LAS glass-ceramic does not show spectral features of this phase, and intensities of peaks assigned to tieilite crystals are higher for the LAS glass-ceramic than for the LAS
ox one, see
Figure 10e.
Figure 10f shows that the LAS glass-ceramic prepared by heat treatment at 1200 °C demonstrates rutile bands of higher intensities as compared with the LAS
ox glass-ceramic.
Though Raman spectra of precursor glasses and glasses heat-treated at 680 °C for 6 h are similar to each other, a comparison of the Raman spectra of the materials obtained by two-stage heat treatments unambiguously indicates that, despite the fact that the sequence of transformations in the titania-containing phase is independent of the redox conditions of glass melting, the rate of these transformations is significantly higher at ceramming of the LAS glass.
3.4. Morphology Characterization by SEM
SEM analysis (
Figure 11a) reveals the presence of inhomogeneous regions within the bulk of the initial amorphous LAS glass. The calculated size distribution shows a broad profile, with the mean size of the inhomogeneous regions being approximately 27 nm (
Figure 12). These inhomogeneities may indicate liquid–liquid phase separation during glass formation. The broad size distribution may arise from overlapping size distributions of chemically distinct regions. Such regions are likely precursors to the crystallization of various phases upon subsequent heat treatments.
Following the single-step heat treatment at 680 °C for 6 h, the LAS glass remains X-ray amorphous. However, its SEM image (
Figure 11b) shows increased inhomogeneity, clearly distinguishing two types of regions. The first type consists of small, spherical, bright regions with a narrow size distribution averaging 16 nm (
Figure 12). The second predomonant type features larger, irregular, dark regions with an average size of 74 nm (
Figure 12).
After two-stage heat treatment at 680 °C for 6 h and 750 °C for 6 h, the large inhomogeneous regions are not seen on the SEM image anymore. Numerous spherical particles appear in the SEM image of the LAS glass-ceramic, averaging 23 nm in size (
Figure 11c). The broad shape of the size distribution suggests overlap from multiple chemically distinct particle populations, see
Figure 12. XRD analysis confirms the crystallization of the spinel phase with a mean crystallite size of 4.5 nm.
The heat treatment at 680 °C for 6 h and 780 °C for 6 h promotes significant crystallization of the LAS glass. The SEM image of this glass-ceramic is shown in
Figure 11d. XRD analysis confirms the formation of three crystalline phases, β-quartz ss, spinel, and Al
2TiO
5, see
Figure 7c, with mean crystallite sizes of 8 nm, 26 nm and ~6 nm, respectively, see
Table 2 and
Table 3. The particle size distribution suggests the possible overlap of multiple size distributions corresponding to different phases, see
Figure 12. The mean particle size is approximately 21 nm, which is close to the mean size of the predominant crystalline phase of β-quartz ss.
The two-step heat treatment at 680 °C and 800 °C for 6 h results in more extensive crystallization of the LAS glass, see
Figure 7e. XRD analysis confirms the presence of crystalline phases of β-quartz ss, spinel, and Al₂TiO₅. The SEM image reveals significant etching of the material, characterized by numerous large caverns, which may indicate silica depletion, see
Figure 11f. The mean particle size is 31 nm, see
Figure 12, which is also close to the mean size of the predominant crystalline phase of β-quartz ss, which is 25 nm, see
Table 2.
Following the two-step heat treatment at 680 °C and 1000 °C for 6 h, XRD analysis shows the formation of β-quartz ss, mullite, and Al
2TiO
5. Signs of the residual glass phase become less prominent. The particle size distribution, similar to previous samples, exhibits a broad profile, likely due to overlapping distributions from different phases, with an average particle size of 30 nm. The size of the predominant crystalline phase of β-quartz ss is 28 nm, see
Table 2.
Finally, after two-step heat treatment at 680 °C and 1200 °C for 6 h, the SEM image of the opaque LAS sample shows the presence of micron-sized, needle-like crystals and agglomerates of spherical crystals, see
Figure 11h. The needle-like crystals are very similar to crystals of β-spodumene ss that were crystallized in the lithium aluminosilicate glass of a different composition during its heat treatment at 1350 °C [
12]. XRD analysis confirms the crystallization of β-spodumene (ss), mullite, and Al
2TiO
5 phases, see
Figure 7e.
The influence of redox conditions of glass melting on the phase assamblage of glass-ceramics is revealed by the comparison of SEM images of LAS and LAS
ox glass-ceramics obtained by two-stage heat treatment at 680 °C and 780 °C for 6 h. In accordance with XRD data, see
Figure 8a, the SEM image of the LAS
ox glass-ceramic shows a significantly reduced crystallinity fraction, see
Figure 11e. The particle size distribution for this sample also indicates overlapping distributions, with a mean particle size of 16 nm, which is smaller than the mean particle size of the LAS glass-ceramic. Considering that the main contribution to the increase in mean crystallite size comes from larger crystals of β-quartz ss, the smaller mean crystallite size in the LAS
ox glass-ceramic indicates a smaller fraction of these crystals.
3.5. Optical Spectroscopy
Light losses in phase separated glasses and glass-ceramics are determined by absorption due to coloring ions and light scattering on the interfaces of regions of inhomogeneity inherent to such materials. It is generally accepted that the coloration of glass-ceramics containing titania as a nucleating agent is mainly caused by intervalent charge transfer transitions between titanium and iron impurity ions in different oxidation states [
8,
53]. Though the influence of absorption due to the Fe
3+-Ti
3+ and Fe
2+-Ti
4+ intervalence charge transitions on absorption spectra of our materials cannot be excluded, only absorption due to titanium ions was considered in this study. A similar approach was suggested in [
53]. Titanium ions in glasses exist in two oxidation states. The presence of Ti
3+ and Ti
4+ ions, and Ti
3+-Ti
4+ pairs defines the absorption of glasses under study. Ti
4+ ion has an electronic configuration of 3d
0 and does not demonstrate absorption bands due to d–d transitions in the ligand field. Ti
4+ ions participate in O-Ti
4+ charge transfer bands positioning in the UV range of spectrum at ~300 nm [
54] and in intervalence charge transfer Ti
4+-Ti
3+ transitions responsible for coloration in the visible spectral range [
55]. Ti
3+ ions have an electronic configuration of 3d
1 and demonstrate one broad band in the visible range of spectrum due to the
2T
2g → E
g transition of the Ti
3+ ions in octahedral symmetry. The band often has a shoulder at the long-wavelength side of the absorption band caused by the Jahn–Teller effect [
23,
25,
26,
27,
55]. The absorption band of Ti
3+ ion in tetrahedral (T
d) symmetry caused by the
2T
2g → E
g transition is located in the near-infrared region of the spectra [
55]. The O-Ti
3+ charge transfer band is expected in UV range of spectrum at ~240 nm [
54].
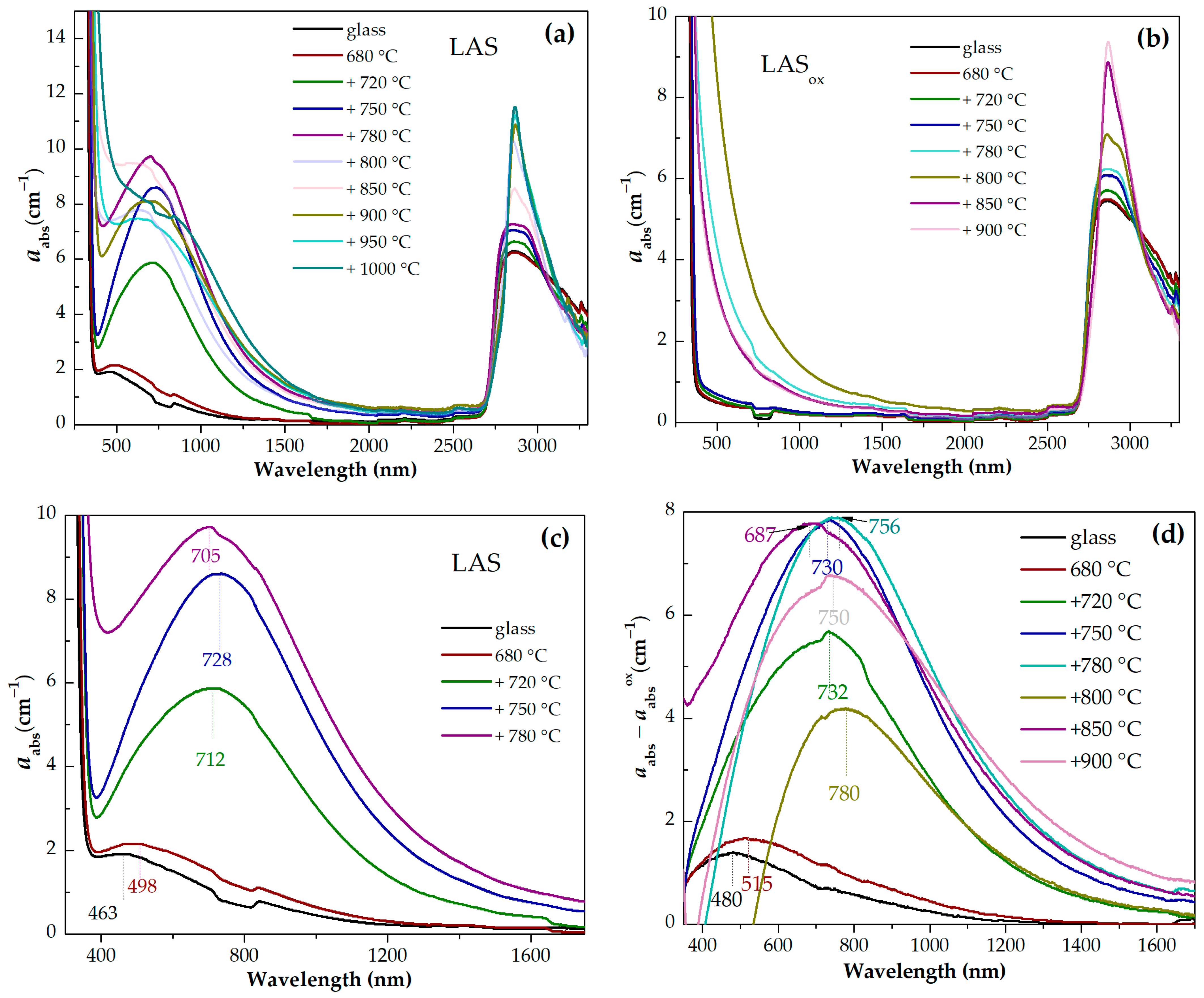
Absorption spectra of initial and heat-treated glasses are shown in
Figure 13a–h. For the convenience of comparison, the spectra of the LAS and LAS
ox glasses and glass-ceramics obtained by the same heat-treatment schedule are presented in
Figure 14a–h. The spectra are formed by the absorption edge in the UV spectral range, see
Figure 13e,f, intense absorption of OH
− groups spanning from 2700 nm to 3300 nm, see
Figure 13g,h, and light losses in the visible and near-IR spectral range, see
Figure 13a–c, which have different origin in the LAS and the LAS
ox glass-ceramics. Note that the LAS
ox glass-ceramics obtained by heat treatments at 950 °C and 1000 °C at the second stage cracked during heat treatment, and their spectra were not recorded.
For the initial LAS glass, the UV absorption edge is observed at ~330 nm. In the spectrum of the glass heat-treated at the nucleation stage at 680 °C for 6 h it moves to longer wavelengths by 5 nm. In the spectrum of glass-ceramics obtained by the two-stage heat treatment with a temperature of 720 °C at the second stage, the position of the absorption edge is found at ~343 nm and it is nearly the same after heat treatments at 750 °C and 780 °C. After heat treatment with a temperature of 800 °C at the second stage, the absorption edge shifts to shorter wavelengths, to 334 nm. After increasing the temperature of the second stage to 850 °C and 900 °C, the absorption edge shifts again to longer wavelengths. Its position is equal to 347 nm and 351 nm, respectively. After heat treatments at 950 °C and 1000 °C, the position of the absorption edge shifts again to shorter wavelengths, see
Figure 13e.
The position of the absorption edge for the LAS
ox glass and its variation with the heat-treatment temperature are surprisingly similar to those of the LAS glass, see
Figure 13e,f and
Figure 14a–e,g,h. The position of the absorption edge differs only for glass-ceramics obtained by the heat treatment at the second stage at 800 °C, see
Figure 14f. The absorption edge in the spectrum of this LAS
ox glass-ceramic is shifted to longer wavelengths compared to its position in the spectrum of the LAS sample. The nonmonotonic transmittance variation during the crystallization of glasses inclined to liquid–liquid phase separation was described in ref. [
56] and assigned to incoherent scattering that takes place in a material containing amorphous and crystallized regions of inhomogeneity. The authors of ref. [
56] demonstrated that the extinction coefficient can reach a maximum when the crystallinity fraction is 0.5–1.0 and then decrease due to the presence of elements of ordering in the relative position of the crystallites.
The same raw materials were used for the preparation of both glasses, which means that the iron content in both glasses was similar. The absorption edge in precursor glass and in glass-ceramics originates from O
2−→Ti
4+ and O
2−→Ti
3+ charge transfer bands [
54]. Taking into account that the O
2−→Ti
4+ charge transfer band is located at longer wavelengths than the O
2−→Ti
3+ one, it can be suggested that the content of Ti
4+ ions in the LAS and the LAS
ox glasses is very similar, i.e., the content of Ti
3+ in the LAS glass is rather low. In glass-ceramics containing β-quarts ss, scattering losses are superimposed with the absorption edge.
A broad absorption band is found in the spectrum of the LAS precursor glass. Its intensity increases after heat treatment at the nucleation stage and with the increasing of the heat-treatment temperature at the second stage up to 780 °C, see
Figure 13a,c. After further heat treatments the absorption band becomes broader, and its intensity somewhat decreases. The appearance of this absorption band can be explained by Ti
3+ ions distributed between different amorphous and crystalline phases. Since the absorption edge and light scattering in the obtained multiphase materials are superimposed on the short-wave part of the absorption spectrum, absorption due to these losses was subtracted from the experimental absorption spectra of the LAS samples using the corresponding absorption spectra of LAS
ox samples for substraction. The difference spectra are shown in
Figure 13d. The spectrum of the precursor glass is a typical absorption spectrum of Ti
3+ ions in silicate glasses with a broad peak with a maximum at ~480 nm attributed to d–d transition
2T
2g→
2E
g of 3d
1 electron of Ti
3+ ions in octahedral coordination in the ligand field affected by the Jahn–Teller effect [
25,
26,
27]. The tail with a maximum at ~800 nm is assigned to intervalence charge transfer in Ti
3+-Ti
4+ pairs [
57,
58]. The absorption intensity increases after the preliminary heat treatment, the band maximum shifts to ~500 nm, which is connected with the participation of Ti
3+ ions in the liquid–liquid phase separation and entering into the aluminotitanate amorphous regions. With the increase in the heat-treatment temperature at the second stage, the broadband absorption in the visible and near-IR region intensifies, and its maximum shifts to ~730 nm in the spectra of glass-ceramics obtained by heat treatments at 750 °C and 780 °C at the second stage. Similar to the absorption of Ti
3+ ions in corrund crystals [
59], this absorption is attributed to Ti
3+ ions in the ligand field of octahedral symmetry (the shoulder at ~480 nm) and Ti
3+-Ti
4+ pairs in crystals of γ-Al
2O
3. In the spectrum of corrundum, the Ti
4+ ions in Al
3+ sites are considered to be charge-compensated by Al vacancies with one vacancy for every three Ti
4+ ions [
60]. An increase of the lattice parameters of γ-Al
2O
3 with heat-treatment temperature found by the XRD analysis can be due to the entering of the crystals of γ-Al
2O
3 by Ti
3+ ions (ionic radius in octahedral coordination is 0.81 Å) and Ti
4+ ions (ionic radius in octahedral coordination is 0.745 Å). Note that the ionic radii of Al
3+ in octahedral coordination is 0.675 Å.
The absorption band shape changes with the raising of the heat-treatment temperature. The spectrum is still mainly formed by the absorption of Ti3+-Ti4+ pairs. Taking into account the crystallization of β-quartz ss and tieilite, Al2TiO5, this change in the shape of the absorption band can be connected with the distribution of titanium ions between the crystalline phases. Spinel does not crystallize in glass-ceramics obtained by heat treatments at 850–1000 °C at the second stage. So the broadband spectra of glass-ceramics obtained by these heat treatments are connected with titanium ions in β-quartz ss and tieilite.
The light losses in the LASox glass-ceramics are mostly caused by light scattering, which has a non-monotoneous dependence on the heat-treatment temperature, as was mentioned above.
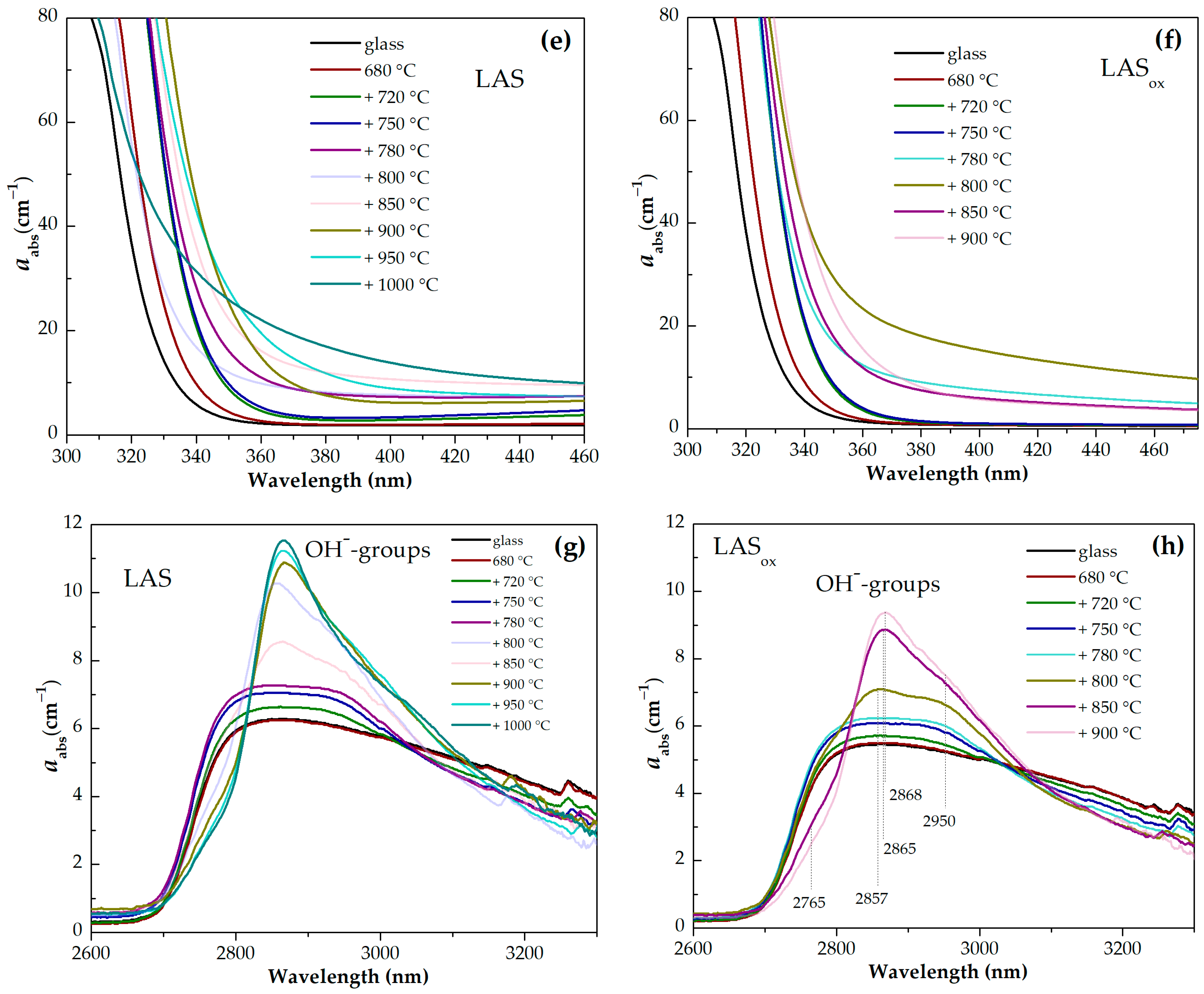
Figure 13g,h show wide asymmetric absorption bands in the spectral region from ~2700 nm to 3300 nm caused by the presence of OH-groups in glasses and glass-ceramics. Heat treatment at the nucleation stage at 680 °C for 6 h has no effect on the shape and intensity of this band. Two-stage heat treatments with a temperature from 720 °C to 780 °C at the second stage cause a successive change in the spectrum of OH-groups. The intensity in the range from 2750 nm to 3050 nm increases and the intensity in the range from 3050 nm to 3300 nm decreases. Further narrowing of the OH-groups absorption band and growth of its intensity is observed in spectra of glass-ceramics obtained during heat treatments in the range of crystallization temperatures from 800 °C to 1000 °C. Comparison of the absorption bands of OH-groups in glasses melted in different redox conditions and in corresponding glass-ceramics showed that the intensities of the absorption bands of OH-groups are higher in inital and heat-treated LAS glass than in LAS
ox samples. The absorption band shape is the same for the materials obtained by the same heat-treatment shedule, see
Figure 14a–h, which is explained by the similarity of their phase compositions.