Enhancing Mechanical and Biodegradation Properties of Zn-0.5Fe Alloys Through Rotary Forging
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Mechanical Test
2.4. Electrochemical Measurement
2.5. In Vitro Degradation Test
2.6. Characterization of Cytocompatibility
3. Results
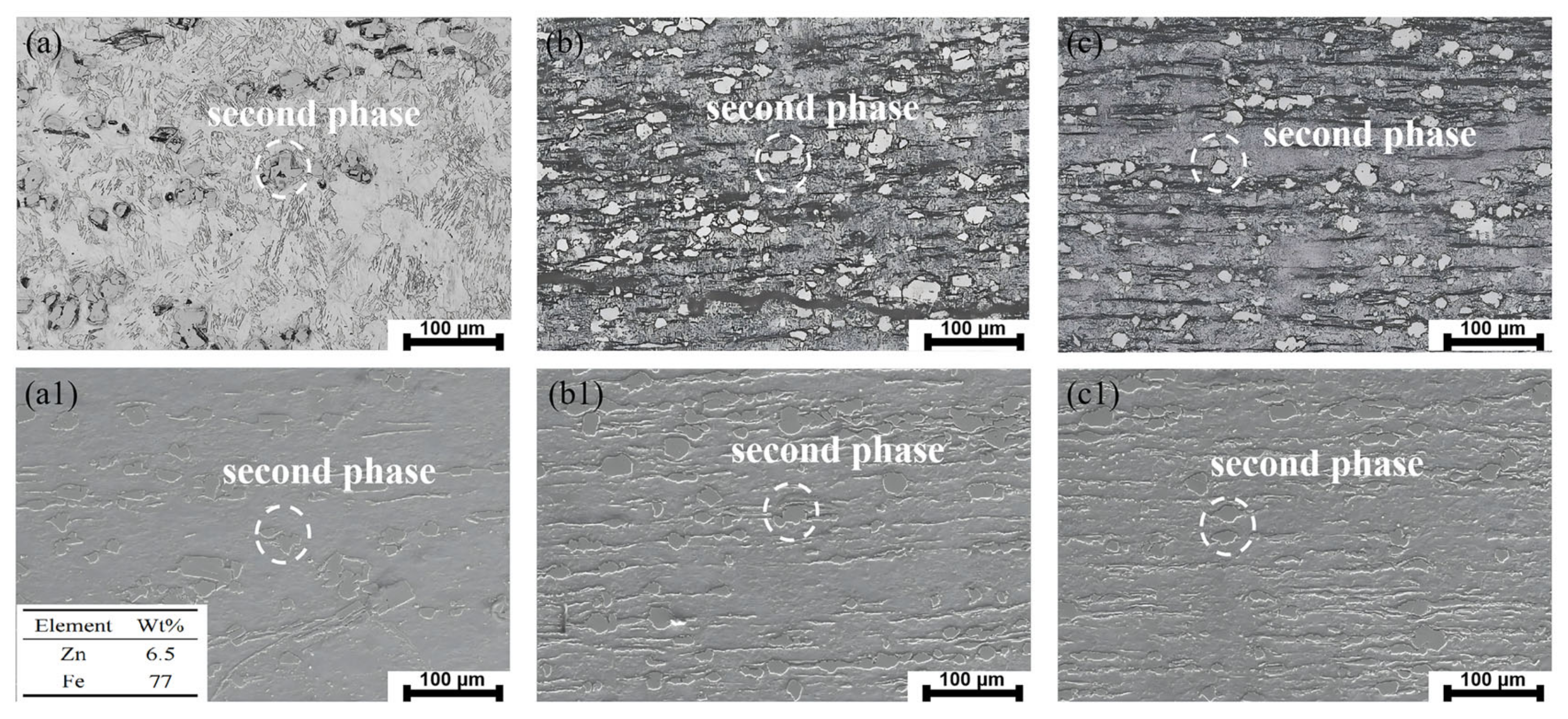
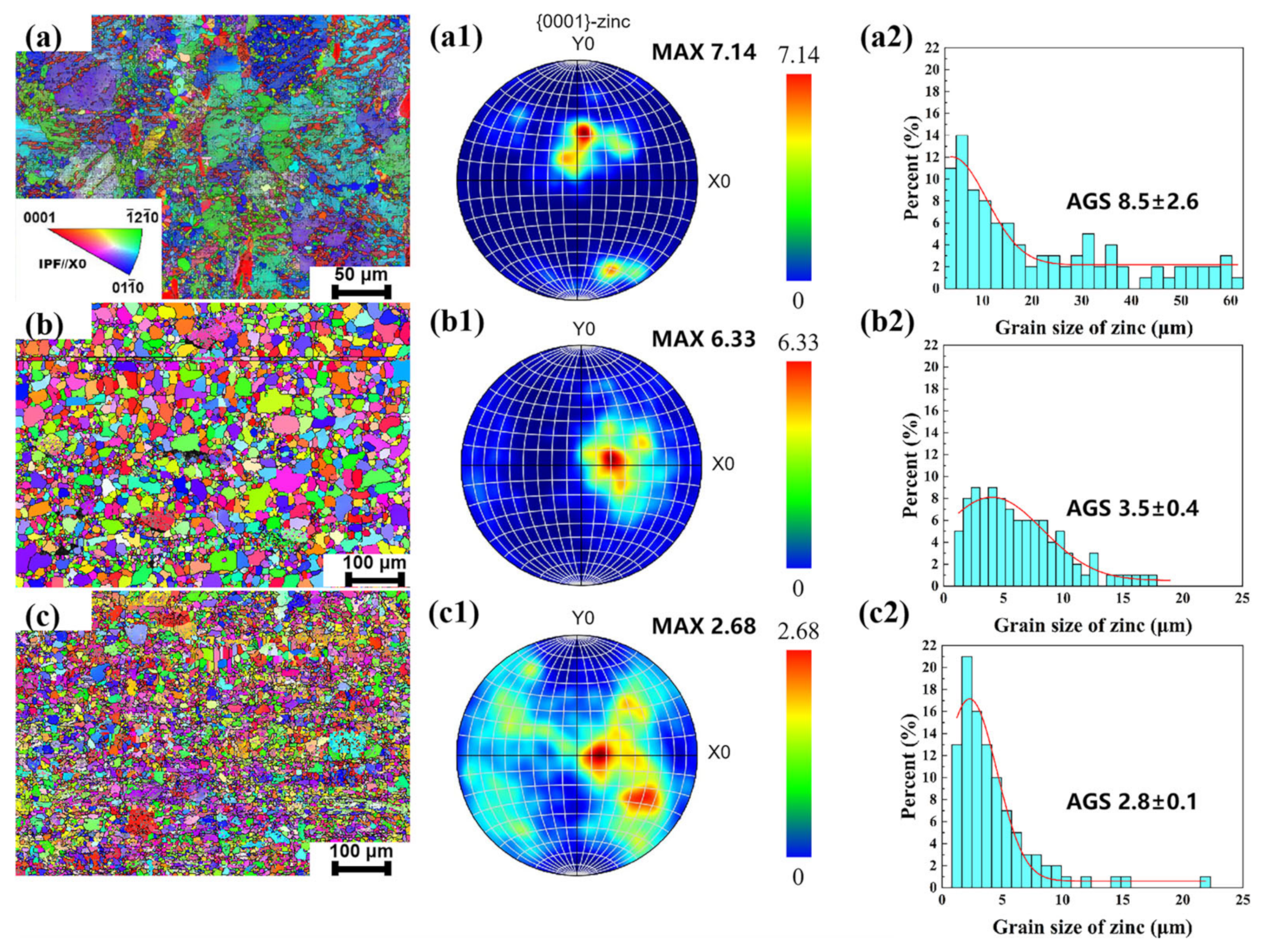
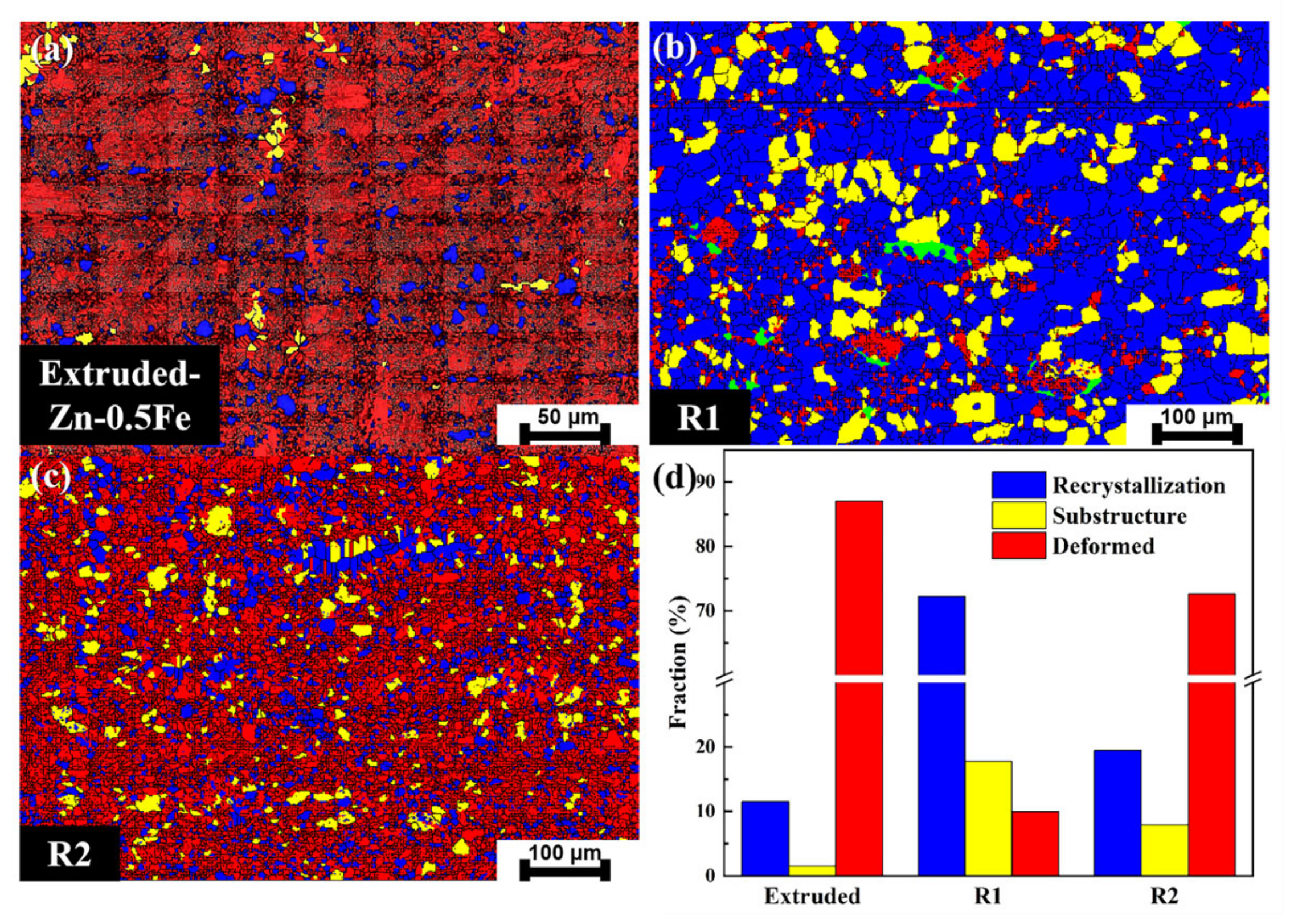
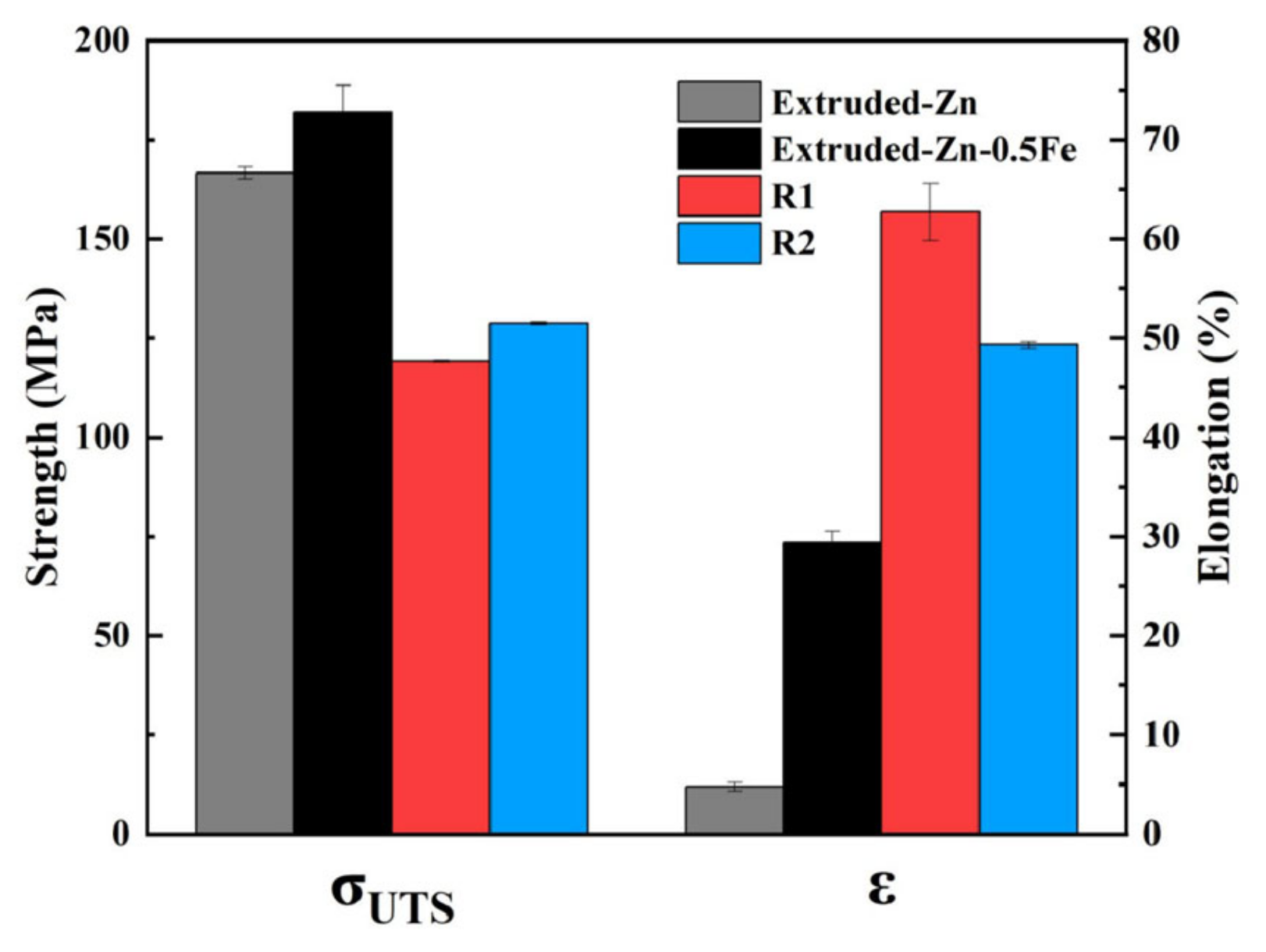
3.1. Microstructural Evolution and Mechanical Performance
3.2. Characterization of Degradation Properties
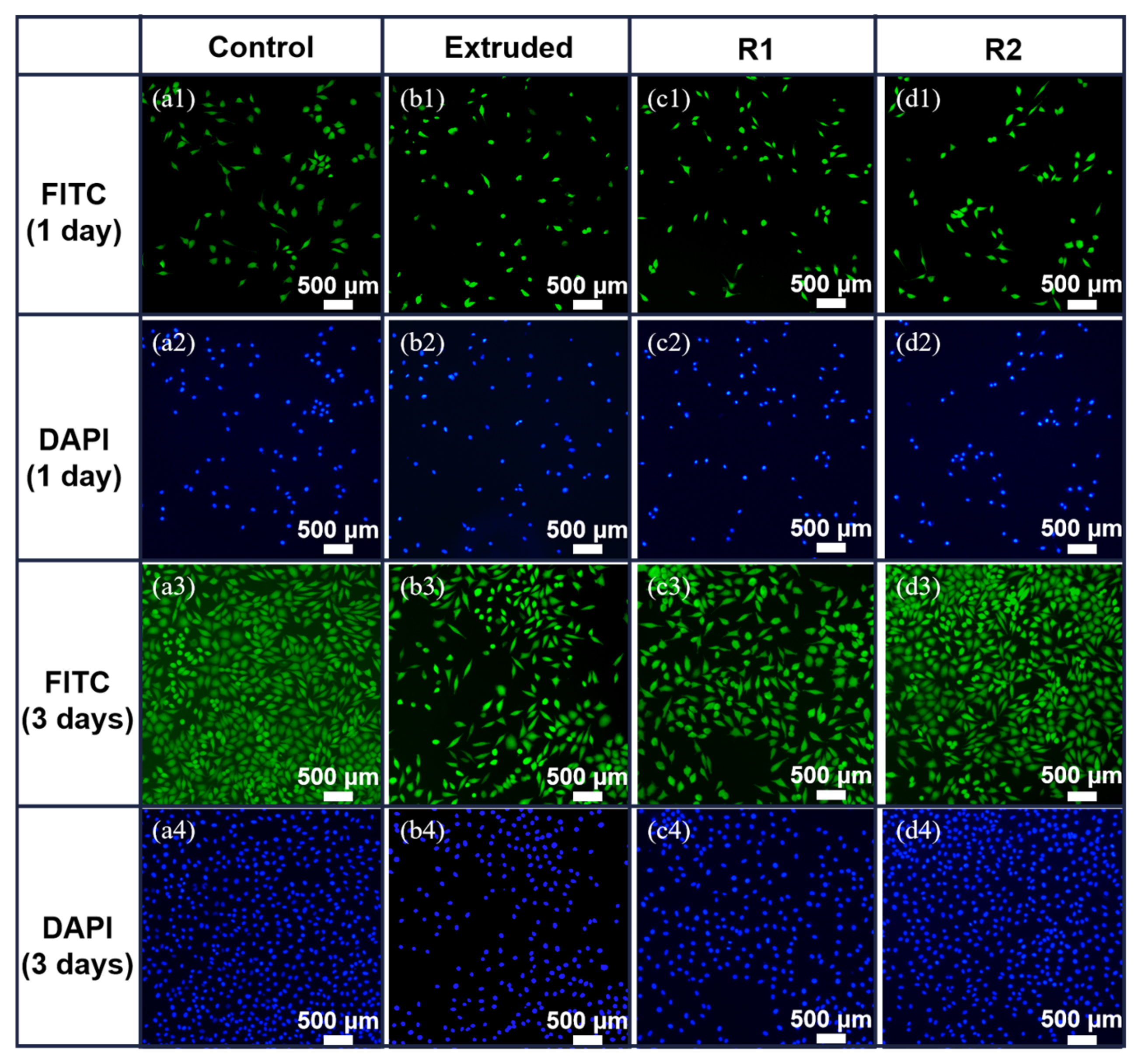
3.3. Cytotoxicity Test and Fluorescence Staining
4. Conclusions
- (1)
- Microstructural refinement: Rotary forging significantly refined the grain structure and optimized the texture distribution of Zn-0.5Fe alloys. The dynamic recrystallization and deformation-induced thermal effects contributed to grain refinement and improved homogeneity.
- (2)
- Mechanical properties: The elongation of the Zn-0.5Fe alloy increased by 114% after rotary forging, reaching 60%. The process facilitated the formation of fine-grain structures, indicating improved ductility and toughness.
- (3)
- Degradation behavior: Electrochemical and immersion tests demonstrated that rotary forging enhances the formation of a dense and uniform corrosion product layer. The Zn-0.5Fe alloy exhibited a stable degradation rate within acceptable limits for biodegradable implants.
- (4)
- Biocompatibility: Cytotoxicity tests and fluorescence staining revealed excellent biocompatibility of the Zn-0.5Fe alloy after rotary forging, with favorable cell viability, especially in samples subjected to higher deformation levels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H.F.; Huang, Y.; Ji, X.J.; Wen, C.E.; Wang, L.N. Fatigue and corrosion fatigue behaviors of biodegradable Zn-Li and Zn-Cu-Li under physiological conditions. J. Mater. Sci. Technol. 2022, 131, 48–59. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent progress on coatings of biomedical magnesium alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Jia, B.; Yang, H.T.; Zhang, Z.C.; Qu, X.H.; Jia, X.F.; Wu, Q.; Han, Y.; Zheng, Y.F.; Dai, K.R. Biodegradable Zn-Sr alloy for bone regeneration in rat femoral condyle defect model: In vitro and in vivo studies. Bioact. Mater. 2021, 6, 1588–1604. [Google Scholar] [CrossRef]
- Xiao, Y.K.; Chen, H.; Bian, Z.Y.; Sun, T.T.; Ding, H.; Yang, Q.; Wu, Y.; Lian, Q.; Chen, Z.; Wang, H.W. Enhancing strength and ductility of AlSi10Mg fabricated by selective laser melting by TiB2 nanoparticles. J. Mater. Sci. Technol. 2022, 109, 254–266. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Ebrahimi, S.; Yarmand, B. In-vitro corrosion and bioactivity behavior of tailored calcium phosphate-containing zinc oxide coating prepared by plasma electrolytic oxidation. Corros. Sci. 2020, 173, 108781. [Google Scholar] [CrossRef]
- Li, P.; Qian, J.Y.; Zhang, W.T.; Schille, C.; Schweizer, E.; Heiss, A.; Klotz, U.E.; Scheideler, L.; Wan, G.J.; Geis-Gerstorfer, J. Improved biodegradability of zinc and its alloys by sandblasting treatment. Surf. Coat. Technol. 2021, 405, 126678. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Xiong, X.Y.; Qian, K.; Gao, Z.D.; Ye, Y.J.; Chu, C.L.; Xue, F.; Bai, J. The antitumor effect of biodegradable metals (Mg, Zn, and Fe) on colon cancer. Mater. Lett. 2024, 360, 136049. [Google Scholar] [CrossRef]
- Králová, Z.O.; Gorejová, R.; Orinaková, R.; Petráková, M.; Orinak, A.; Kupková, M.; Hrubovcáková, M.; Sopcák, T.; Baláz, M.; Maskalova, I.; et al. Biodegradable zinc-iron alloys: Complex study of corrosion behavior, mechanical properties and hemocompatibility. Prog. Nat. Sci. Mater. Int. 2021, 31, 279–287. [Google Scholar] [CrossRef]
- Chen, C.; Niu, J.L.; Huang, H.; Zhu, D.H.; Nie, J.F.; Yuan, G.Y. Basal-plane stacking fault energies of biodegradable Zn-based alloys: A first-principles study of alloying effects. Mater. Lett. 2022, 309, 131413. [Google Scholar] [CrossRef]
- Su, Y.C.; Fu, J.Y.; Lee, W.; Du, S.K.; Qin, Y.X.; Zheng, Y.F.; Wang, Y.D.; Zhu, D.H. Improved mechanical, degradation, and biological performances of Zn-Fe alloys as bioresorbable implants. Bioact. Mater. 2022, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Nan, X.L.; Wang, H.Y.; Wu, Z.Q.; Xue, E.S.; Zhang, L.; Jiang, Q.C. Effect of c/a axial ratio on Schmid factors in hexagonal close-packed metals. Scr. Mater. 2013, 68, 530–533. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Gao, X.X.; Chen, H.T.; Liu, X.F.; Li, A.; Zhang, H.J.; Wang, L.N. Enhancement in mechanical and corrosion resistance properties of a biodegradable Zn-Fe alloy through second phase refinement. Mater. Sci. Eng. C 2020, 116, 111197. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, P.; Li, F.; Yang, L.; Zhu, X.; Xu, C.; Zhang, Q.; Shi, Y.; Song, Z.; Sun, W.; et al. Ultrafine-grained Zn-0.45Li alloy with enhanced mechanical property, degradation behavior and cytocompatibility prepared by hot extrusion and multi-pass drawing. Mater. Werkst. 2021, 52, 991–996. [Google Scholar] [CrossRef]
- Kuncická, L. Structural Phenomena Introduced by Rotary Swaging: A Review. Materials 2024, 17, 466. [Google Scholar] [CrossRef]
- Illarionov, A.; Mukanov, G.; Stepanov, S.; Kuznetsov, V.; Karelin, R.; Andreev, V.; Yusupov, V.; Korelin, A. Microstructure and Physico-Mechanical Properties of Biocompatible Titanium Alloy Ti-39Nb-7Zr after Rotary Forging. Metals 2024, 14, 497. [Google Scholar] [CrossRef]
- Yi, Q.P.; Dai, Y.X.; Hu, Y.B.; Jiang, B.; Pan, F.S. Nanocrystallization and strengthening of Mg-Dy-Zr alloys by room temperature rotary swaging. J. Mater. Res. Technol. 2024, 30, 6777–6786. [Google Scholar] [CrossRef]
- Martynenko, N.; Anisimova, N.; Rybalchenko, G.; Rybalchenko, O.; Serebryany, V.; Zheleznyi, M.; Shinkareva, M.; Gorbenko, A.; Temralieva, D.; Lukyanova, E.; et al. Effect of Rotary Swaging on Mechanical and Operational Properties of Zn-1%Mg and Zn-1%Mg-0.1%Ca Alloys. Metals 2023, 13, 1386. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, Y.; Zhu, X.L.; Guo, P.S.; Yang, L.J.; Zhang, Q.K.; Xu, C.; Sun, W.S.; Song, Z.L. Strengthening Mechanism of Rotary-Forged Deformable Biodegradable Zn-0.45Li Alloys. Materials 2023, 16, 3003. [Google Scholar] [CrossRef]
- Peron, M.; Bertolini, R.; Ghiotti, A.; Torgersen, J.; Bruschi, S.; Berto, F. Enhancement of stress corrosion cracking of AZ31 magnesium alloy in simulated body fluid thanks to cryogenic machining. J. Mech. Behav. Biomed. Mater. 2020, 101, 103429. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Hu, C.L.; Hu, F.P.; Liu, X.F.; Kong, F.X.; Xie, W.D.; Wei, G.B.; Yang, Y.; Peng, X.D.; Huang, Y.D.; et al. Effect of rotary swaging on the microstructure and mechanical properties of high-strength Mg-Mn-Al-Ca-Zn alloys. Mater. Charact. 2023, 196, 112575. [Google Scholar] [CrossRef]
- Zhang, W.T.; Li, P.; Shen, G.; Mo, X.S.; Zhou, C.; Alexander, D.; Rupp, F.; Geis-Gerstorfer, J.; Zhang, H.J.; Wan, G.J. Appropriately adapted properties of hot-extruded Zn-0.5Cu-xFe alloys aimed for biodegradable guided bone regeneration membrane application. Bioact. Mater. 2021, 6, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.Q.; Zhou, C.; Zhang, H.W.; Bai, J.Q.; Jiang, B.; Jiang, C.Y.; Ming, W.Y.; Zhang, H.J.; Long, H.G.; Huang, X.G.; et al. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023, 17, 56. [Google Scholar] [CrossRef]
- Panov, D.O.; Kudryavtsev, E.A.; Chernichenko, R.S.; Naumov, S.V.; Klimenko, D.N.; Stepanov, N.D.; Zherebtsov, S.V.; Salishchev, G.A.; Sanin, V.V.; Pertsev, A.S. Excellent strength-ductility combination of interstitial non-equiatomic middle-entropy alloy subjected to cold rotary swaging and post-deformation annealing. Mater. Sci. Eng. A 2024, 898, 146121. [Google Scholar] [CrossRef]
- ISO 6892-1:2019; Metallic materials — Tensile testing — Part 1: Method of test at room temperature. International Organization for Standardization: Geneva, Switzerland, 2019.
- Su, Y.C.; Guo, Y.T.; Huang, Z.L.; Zhang, Z.H.; Li, G.Y.; Lian, J.S.; Ren, L.Q. Preparation and corrosion behaviors of calcium phosphate conversion coating on magnesium alloy. Surf. Coat. Technol. 2016, 307, 99–108. [Google Scholar] [CrossRef]
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 1972.
- Huang, S.; Wang, L.; Zheng, Y.; Qiao, L.; Yan, Y. In vitro degradation behavior of novel Zn-Cu-Li alloys: Roles of alloy composition and rolling processing. Mater. Des. 2021, 212, 110288. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Zhang, J.D.; Zhu, X.L.; Guo, P.S.; Zhang, Y.; Xu, D.Y.; Pang, Y.; Song, Z.L.; Yang, L.J. Effects of Li addition on the properties of biodegradable Zn-Fe-Li alloy: Microstructure, mechanical properties, corrosion behavior, and cytocompatibility. Mater. Today Commun. 2024, 39, 108661. [Google Scholar] [CrossRef]
- Glezer, A.M.; Sundeev, R.V. General view of severe plastic deformation in solid state. Mater. Lett. 2015, 139, 455–457. [Google Scholar] [CrossRef]
- Miller, V.M.; Nie, J.F.; Pollock, T.M. Nucleation of recrystallization in magnesium alloy grains of varied orientation and the impacts on texture evolution. J. Magnesium Alloys 2022, 10, 3041–3053. [Google Scholar] [CrossRef]
- Rogachev, S.O.; Andreev, V.A.; Yusupov, V.S.; Bondareva, S.A.; Khatkevich, V.M.; Nikolaev, E. Effect of Rotary Forging on Microstructure Evolution and Mechanical Properties of Aluminum Alloy/Copper Bimetallic Material. Met. Mater. Int. 2022, 28, 1038–1046. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Li, M.; Li, X.M.; Wang, L.N. Surface-Roughness-Induced Plasticity in a Biodegradable Zn Alloy. Adv. Mater. 2023, 35, 2207570. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.S.; Zhou, X.X.; Feng, Y.J.; Qian, K.; Liu, H.; Lu, M.M.; Chu, C.L.; Xue, F.; Bai, J. Insights into self-healing behavior and mechanism of dicalcium phosphate dihydrate coating on biomedical Mg. Bioact. Mater. 2021, 6, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Shi, Z.Z.; Yan, Y.; Zhang, D.W.; Yang, K.; Li, H.F.; Zhang, H.J.; Wang, L.N. Suppression mechanism of initial pitting corrosion of pure Zn by Li alloying. Corros. Sci. 2021, 189, 109564. [Google Scholar] [CrossRef]
- MacDonald, M.A.; Andreas, H.A. Method for equivalent circuit determination for electrochemical impedance spectroscopy data of protein adsorption on solid surfaces. Electrochim. Acta 2014, 129, 290–299. [Google Scholar] [CrossRef]
- Liu, L.J.; Meng, Y.; Volinsky, A.A.; Zhang, H.J.; Wang, L.N. Influences of albumin on in vitro corrosion of pure Zn in artificial plasma. Corros. Sci. 2019, 153, 341–356. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Yu, J.; Liu, X.F.; Wang, L.N. Fabrication and characterization of novel biodegradable Zn-Mn-Cu alloys. J. Mater. Sci. Technol. 2018, 34, 1008–1015. [Google Scholar] [CrossRef]


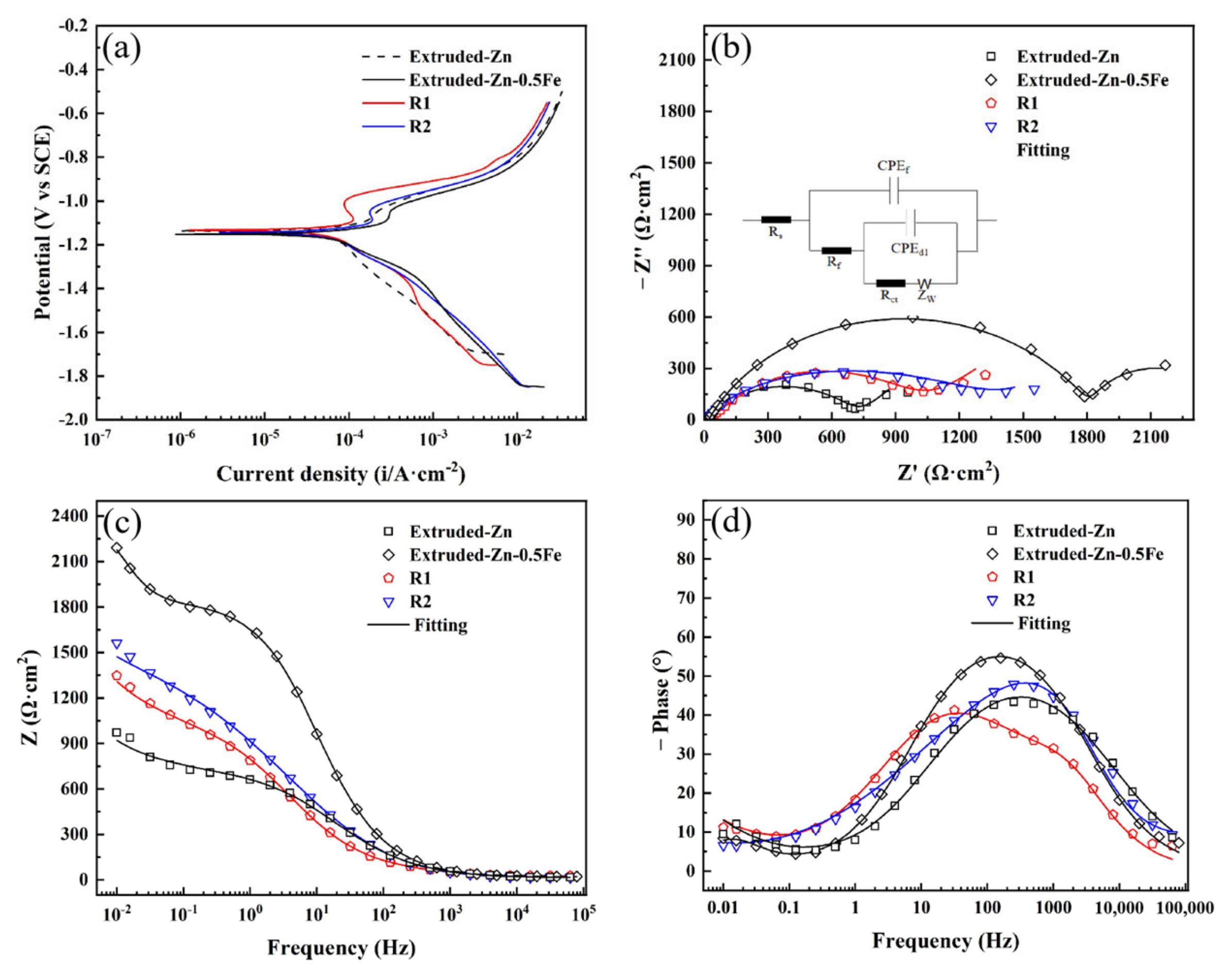
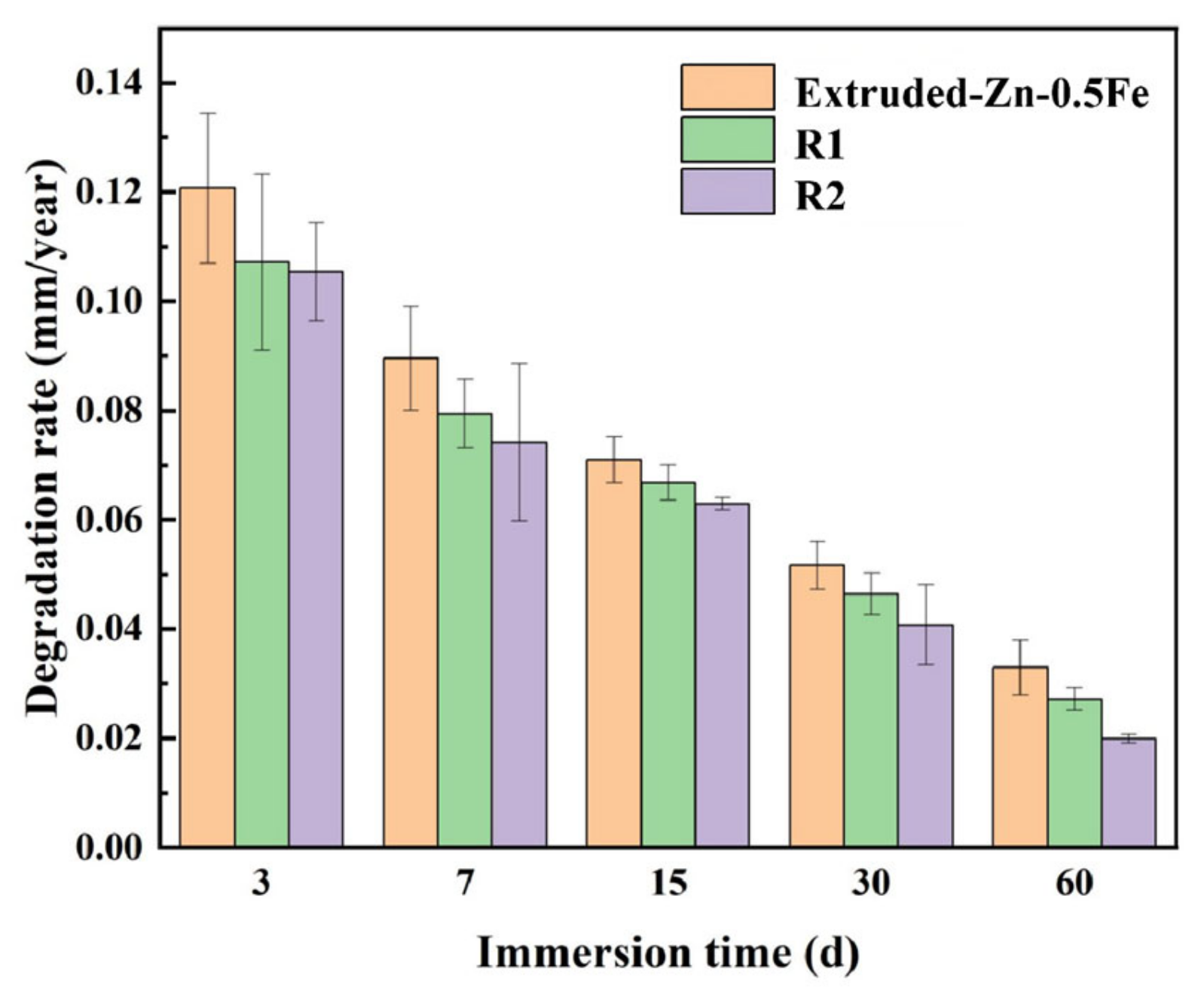
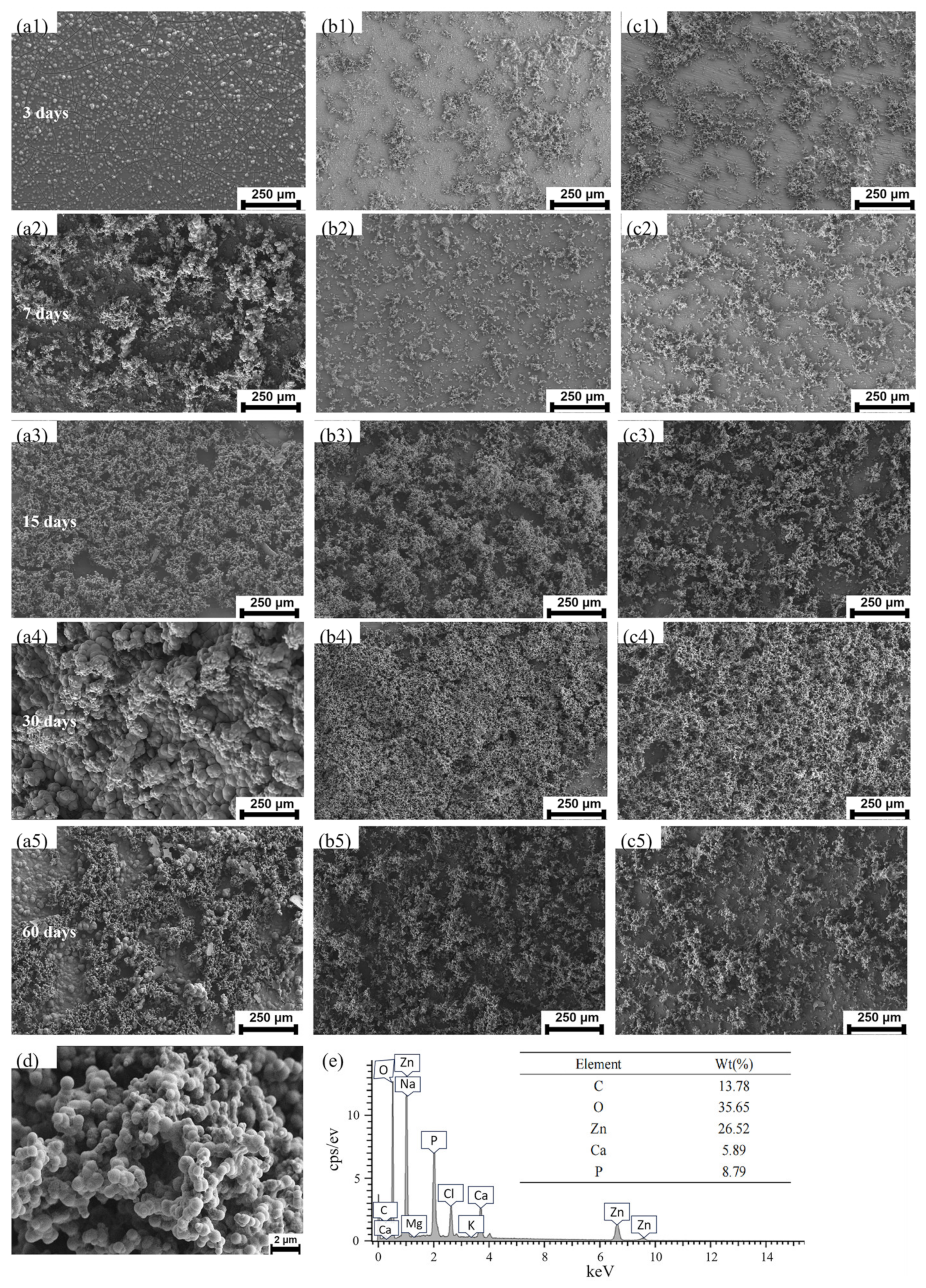
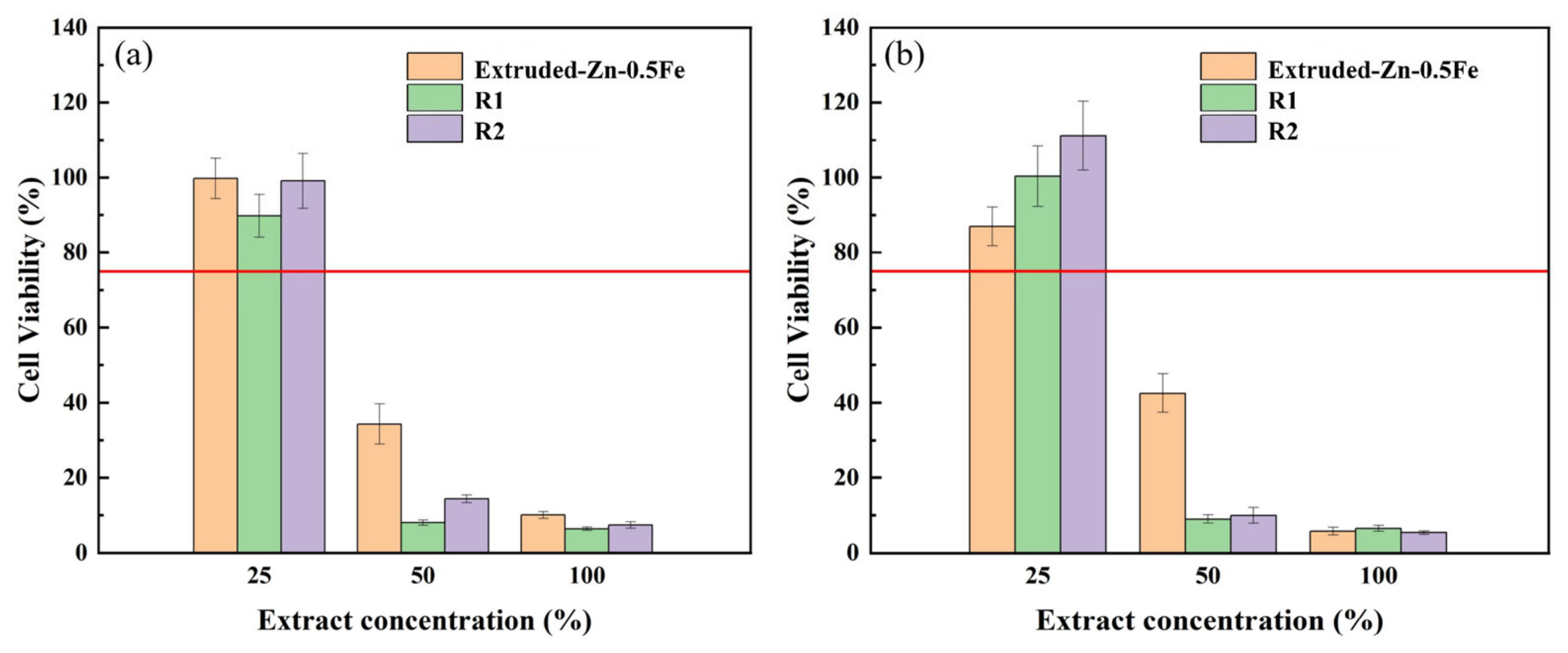
| Alloy | Ecorr (v) | icorr (μA/cm2) | Corrosion Rate (mm/Year) |
|---|---|---|---|
| Extruded Zn | −1.14 | 34.97 | 0.41 |
| Extruded Zn-0.5Fe | −1.13 | 53.03 | 0.62 |
| R1 | −1.14 | 34.72 | 0.40 |
| R2 | −1.15 | 48.07 | 0.56 |
| Alloy | Rs (Ω·cm2) | Rf (Ω·cm2) | Rcf (Ω·cm2) | χ−2 (×10−4) |
|---|---|---|---|---|
| Extruded Zn | 13.93 | 710.6 | 2.83 × 107 | 9.43 |
| Extruded Zn-0.5Fe | 21.21 | 1828 | 518.5 | 4.32 |
| R1 | 26.46 | 60.25 | 952.3 | 8.01 |
| R2 | 8.18 | 13.81 | 1468 | 7.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Chen, H.; Zhu, X.; Zubair, M.; Sun, T.; Yang, L.; Lu, X.; Song, Z. Enhancing Mechanical and Biodegradation Properties of Zn-0.5Fe Alloys Through Rotary Forging. Materials 2025, 18, 722. https://doi.org/10.3390/ma18030722
Tang L, Chen H, Zhu X, Zubair M, Sun T, Yang L, Lu X, Song Z. Enhancing Mechanical and Biodegradation Properties of Zn-0.5Fe Alloys Through Rotary Forging. Materials. 2025; 18(3):722. https://doi.org/10.3390/ma18030722
Chicago/Turabian StyleTang, Lebin, Hailing Chen, Xinglong Zhu, Muhammad Zubair, Tao Sun, Lijing Yang, Xiang Lu, and Zhenlun Song. 2025. "Enhancing Mechanical and Biodegradation Properties of Zn-0.5Fe Alloys Through Rotary Forging" Materials 18, no. 3: 722. https://doi.org/10.3390/ma18030722
APA StyleTang, L., Chen, H., Zhu, X., Zubair, M., Sun, T., Yang, L., Lu, X., & Song, Z. (2025). Enhancing Mechanical and Biodegradation Properties of Zn-0.5Fe Alloys Through Rotary Forging. Materials, 18(3), 722. https://doi.org/10.3390/ma18030722






