Molecular Dynamics Investigation of the Diffusion Mechanisms and Thermodynamic Behaviors in Warm Mix Recycled Asphalt Binders with and Without Rejuvenators
Abstract
1. Introduction
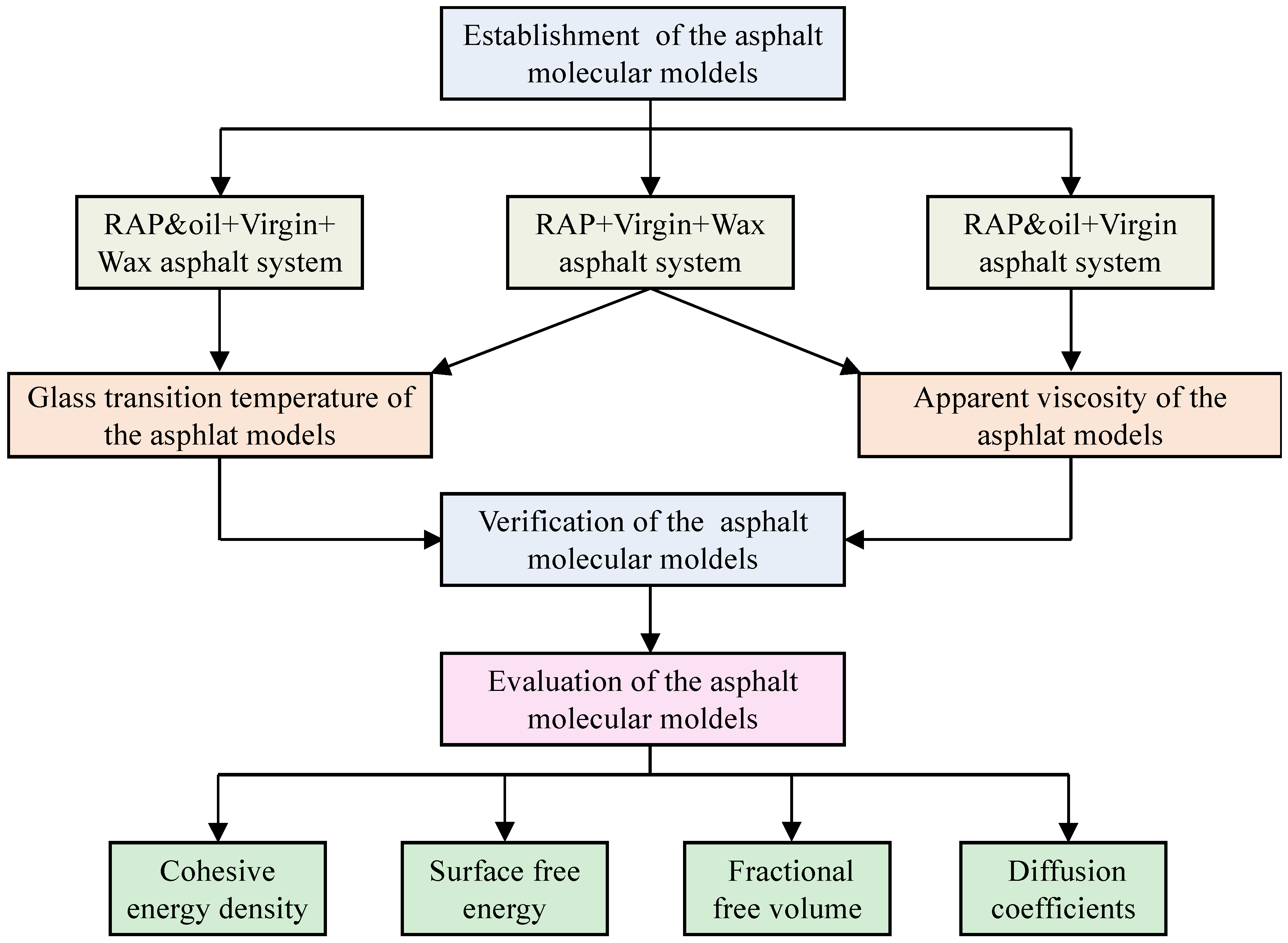
2. Details of Molecular Dynamics (MD) Simulations
2.1. Establishment of the Asphalt Diffusion System
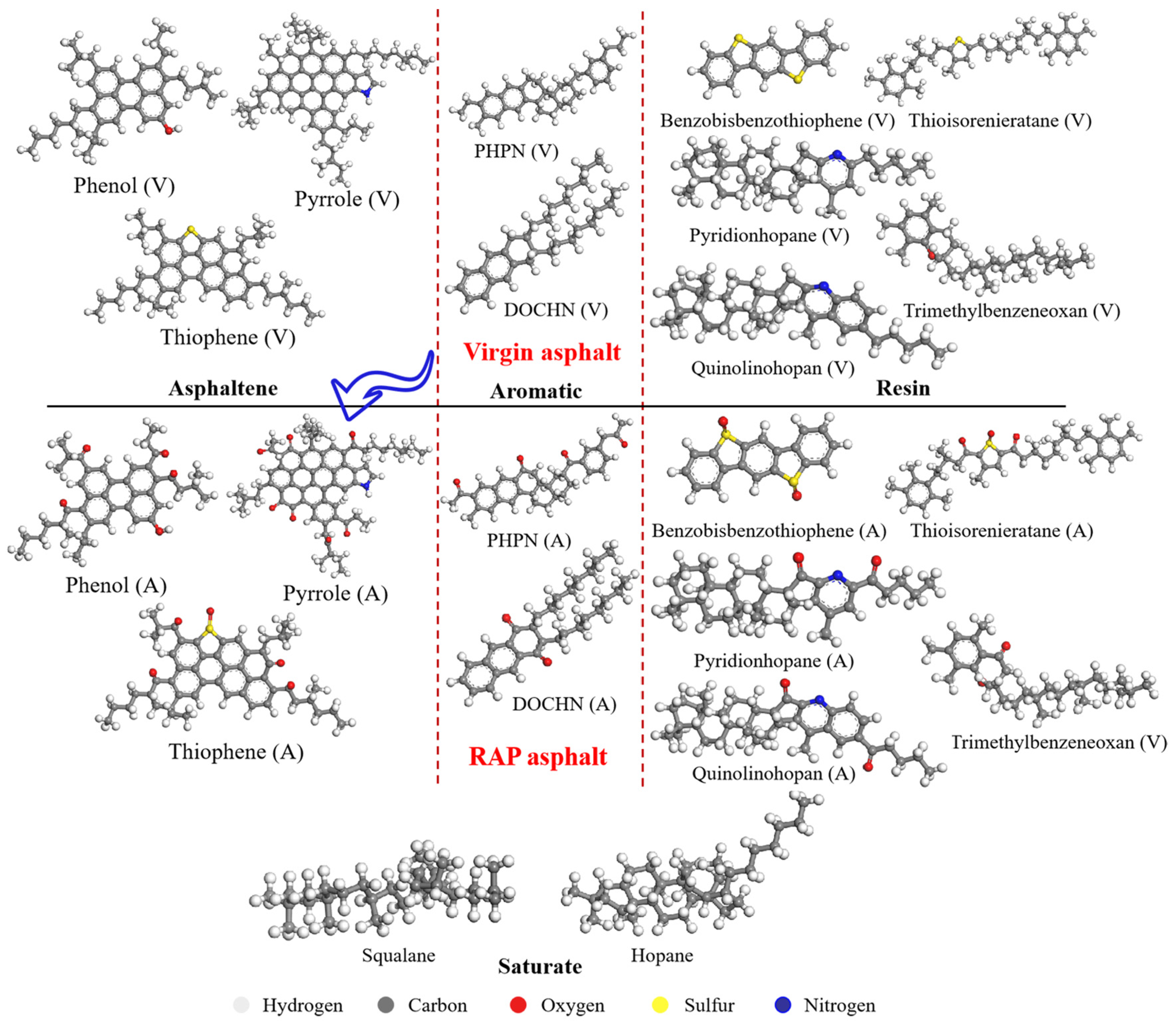
2.1.1. Virgin Asphalt Binders
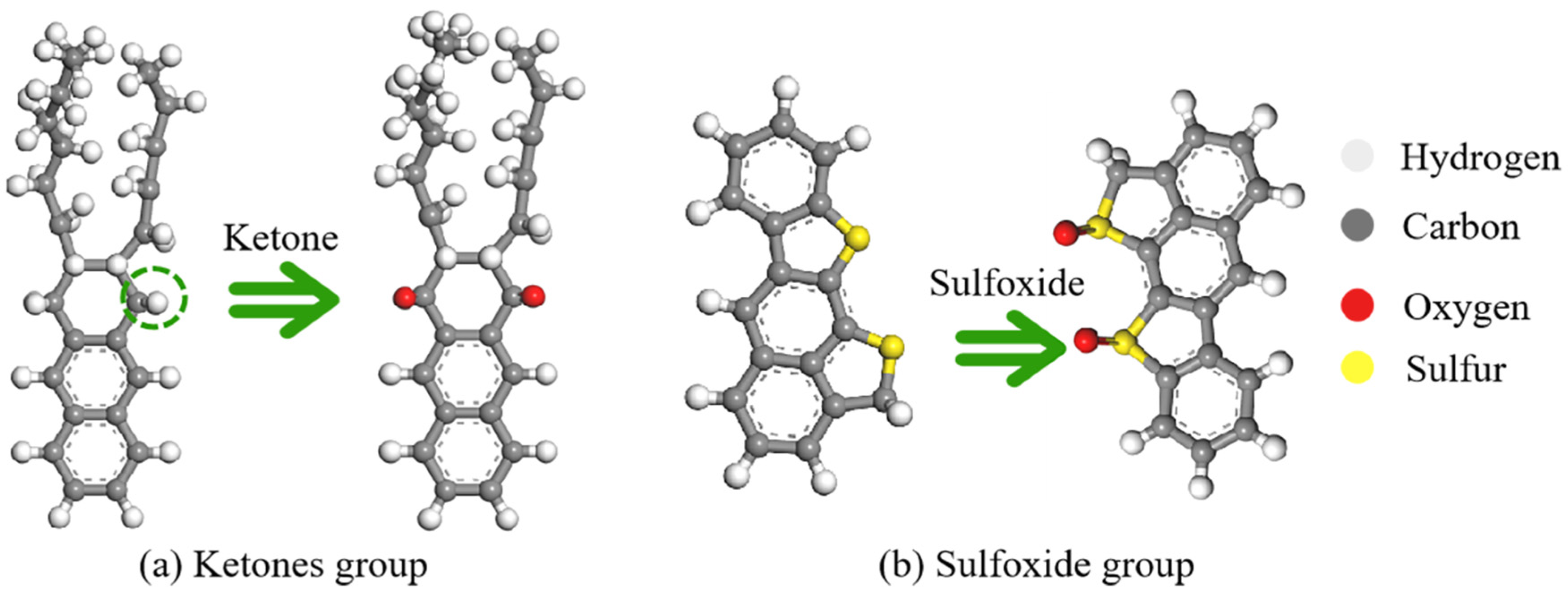
2.1.2. Aged Asphalt Binders
| SARA | Model Numbers | Molecule Name | Virgin Asphalt | Aged Asphalt | ||
|---|---|---|---|---|---|---|
| Molecule Formula | Mass Fraction (%) | Molecule Formula | Mass Fraction (%) | |||
| Saturate | 4 | Hopane | C35H62 | 11.1 | C35H62 | 10.3 |
| 4 | Squalane | C30H62 | C30H62 | |||
| Aromatic | 13 | DOCHN | C30H46 | 31.9 | C30H42O2 | 32.4 |
| 11 | PHPN | C35H44 | C35H36O4 | |||
| Resin | 4 | Thioisorenieratane | C40H60S | 39.8 | C40H56O3S | 39.6 |
| 4 | Pyridionhopane | C36H57N | C36H53NO2 | |||
| 4 | Quinolinohopane | C40H59N | C40H55NO2 | |||
| 15 | Benzobisbenzothiophene | C18H10S2 | C18H10O2S2 | |||
| 5 | Trimethylbenzeneoxane | C29H50O | C29H48O2 | |||
| Asphaltene | 2 | Pyrrole | C66H81N | 17.3 | C66H67NO7 | 17.7 |
| 3 | Phenol | C42H54O | C42H46O5 | |||
| 3 | Thiophene | C51H62S | C51H54O5S | |||
2.1.3. Warm Mix Asphalt (WMA) Additives
2.1.4. Rejuvenators
2.1.5. Mutual Diffusion System
2.2. MD Simulation Procedures
3. Evaluation Indexes
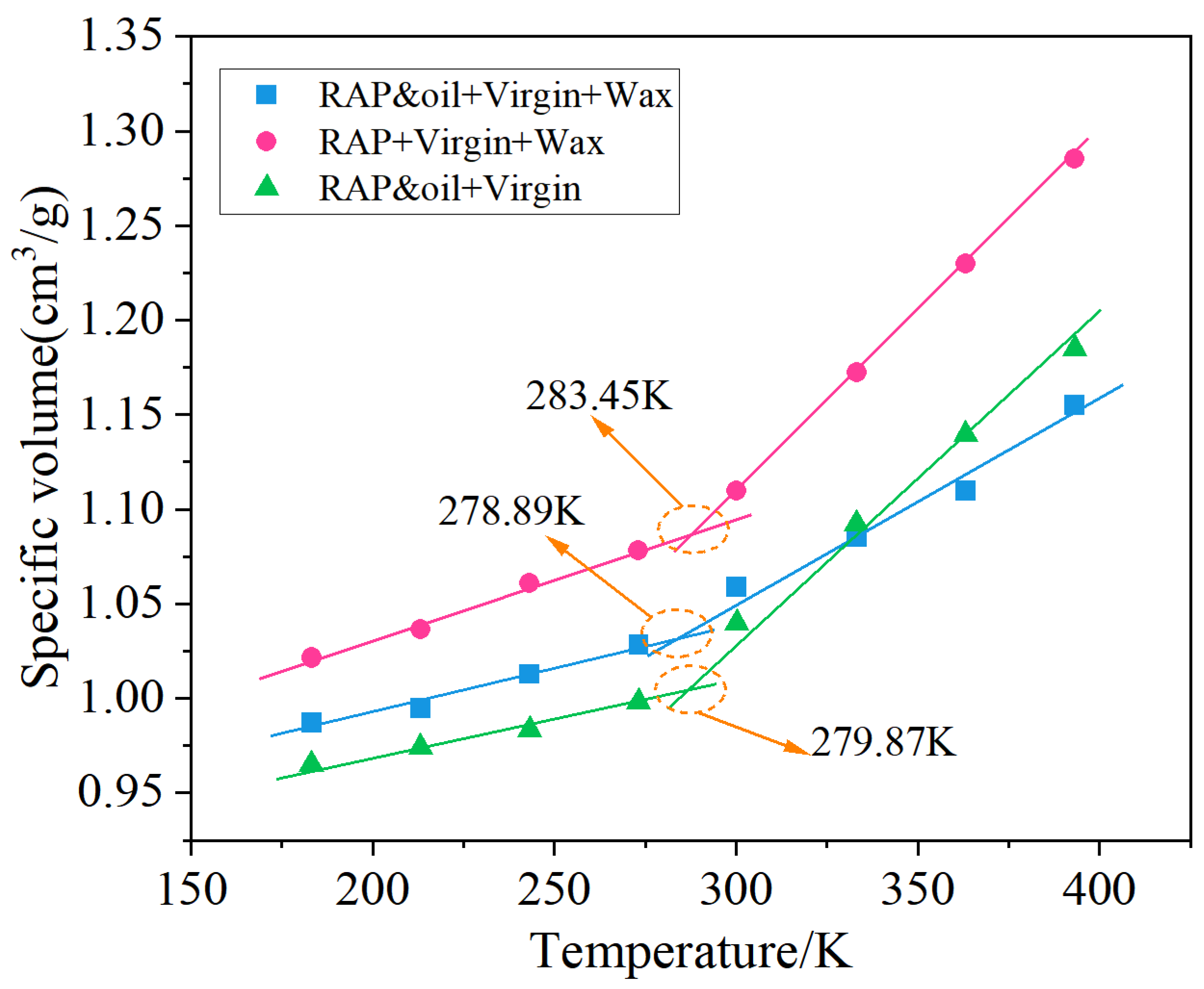
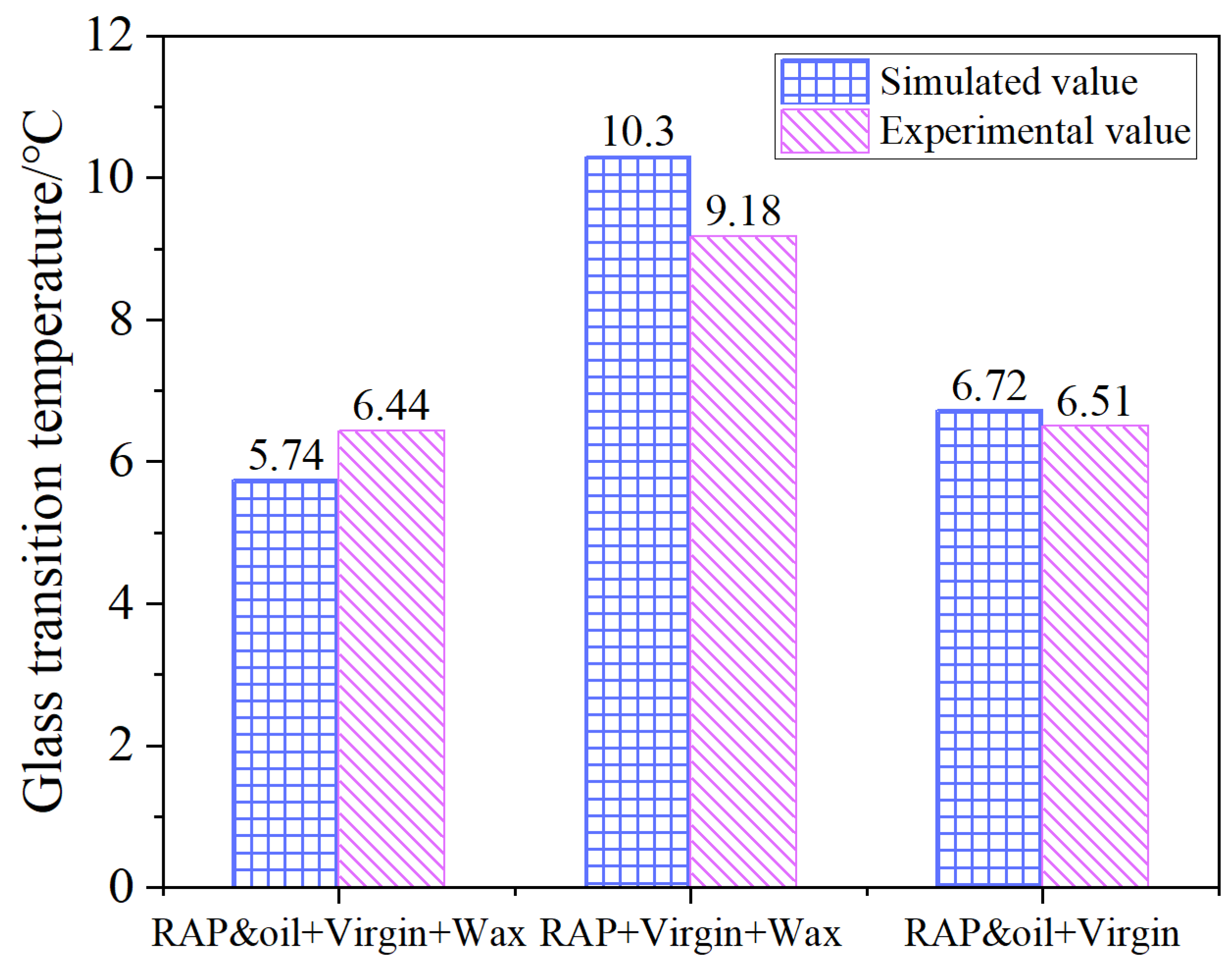
3.1. Glass Transition Temperature
3.2. Asphalt Binder Viscosity
3.3. Cohesive Energy Density (CED) and Surface Free Energy (SFE)
3.4. Asphalt Binder Density
3.5. Fractional Free Volume
3.6. Diffusion Coefficients
4. Results and Discussion
4.1. Glass State Transition Behavior
4.2. Viscosity Simulation Prediction
4.3. Thermal Properties
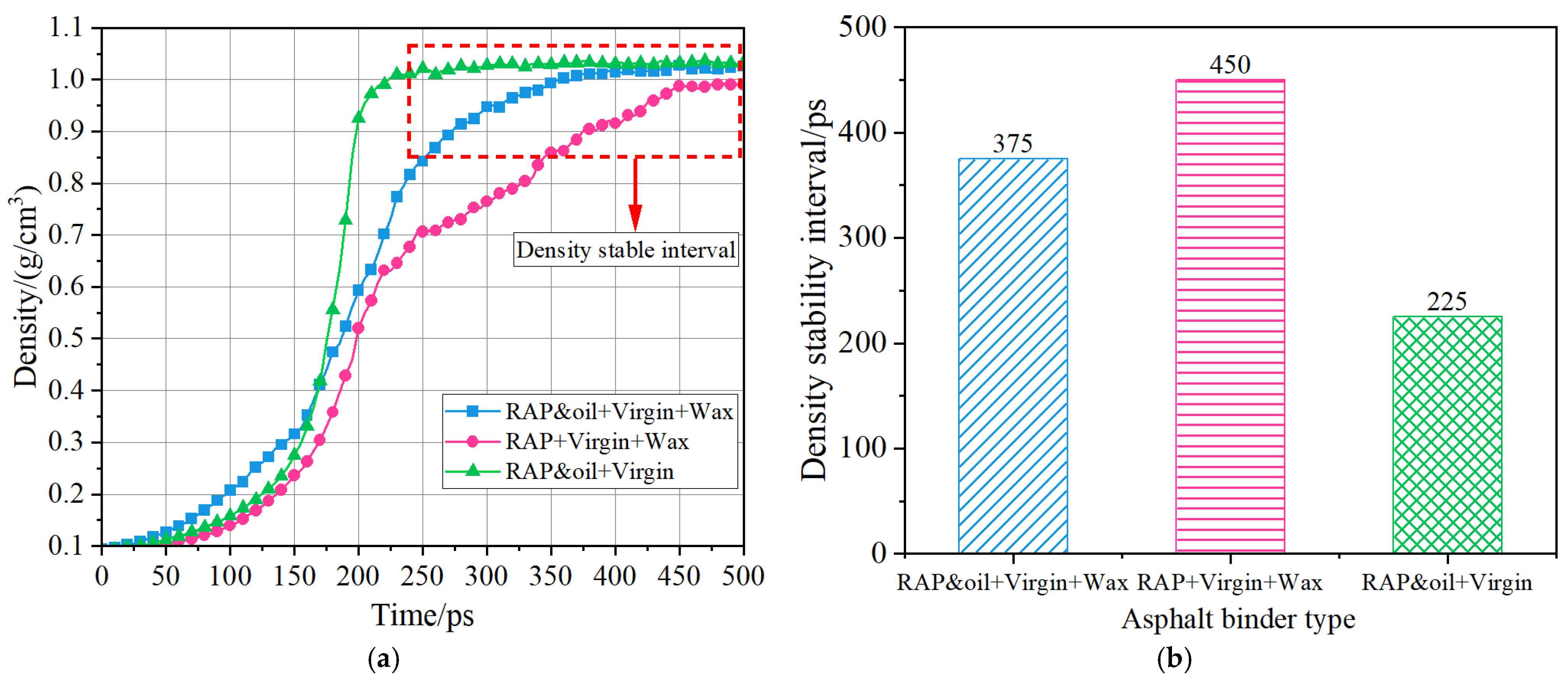
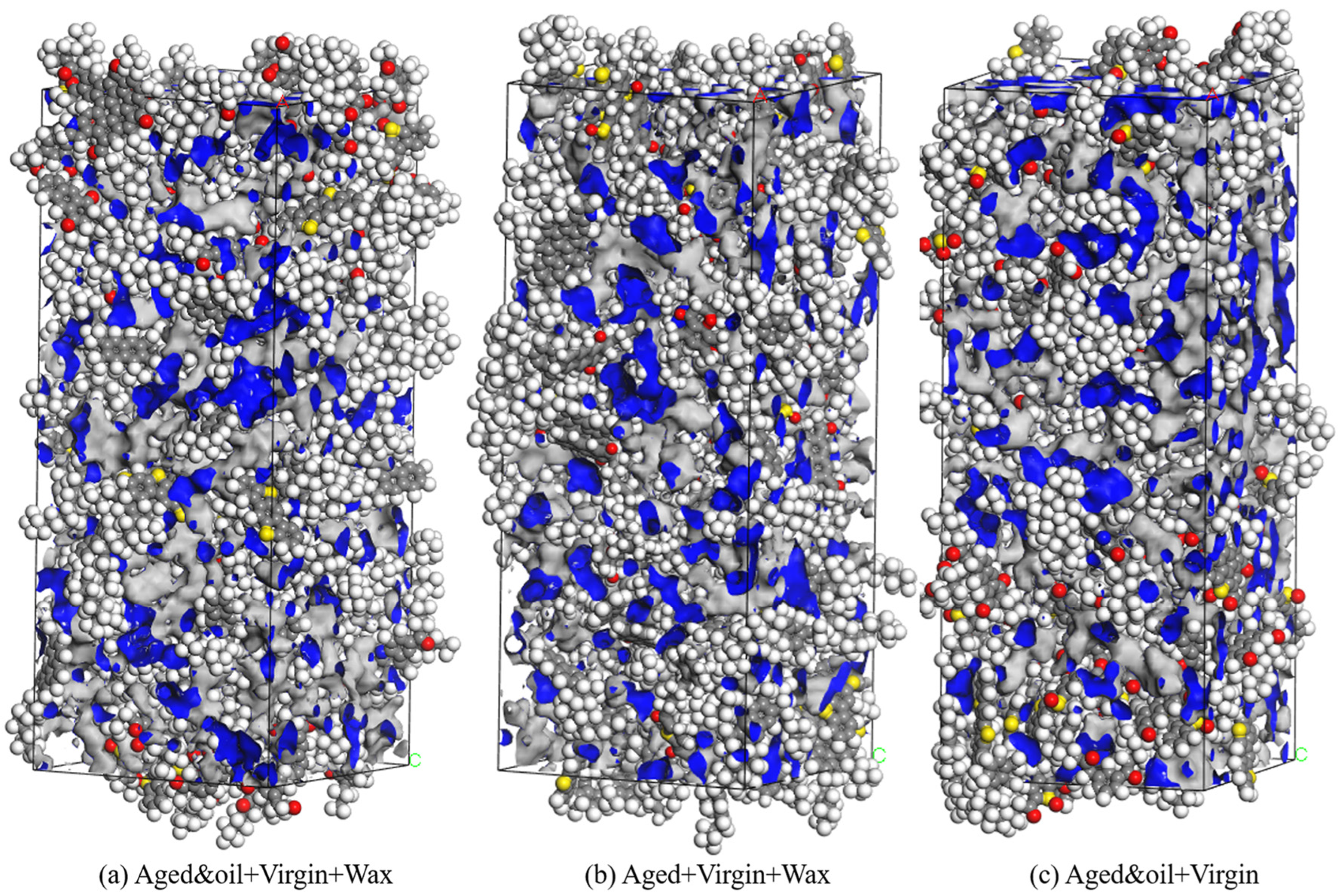
4.4. Self-Healing Ability
4.5. Changes in Free Volume
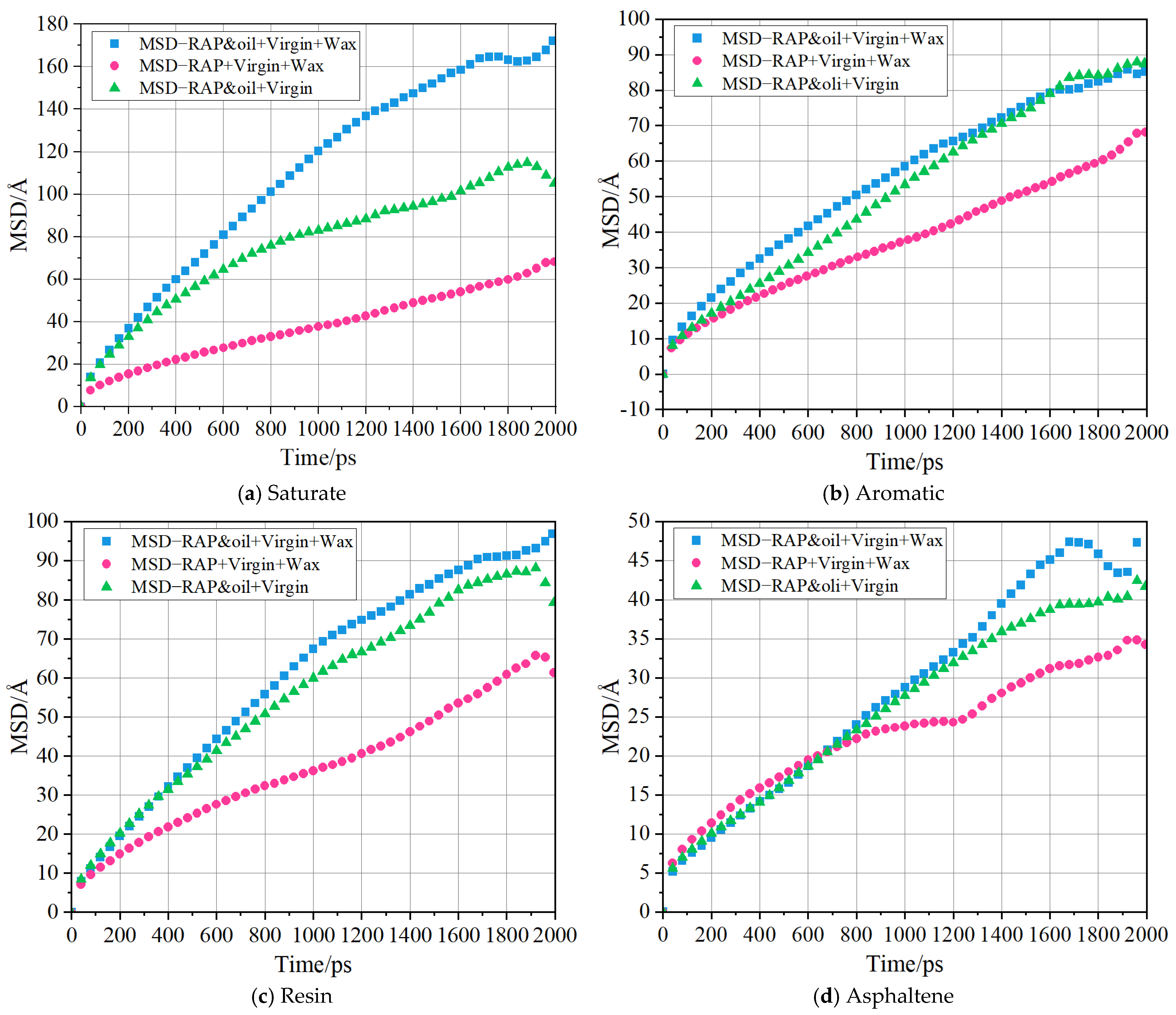
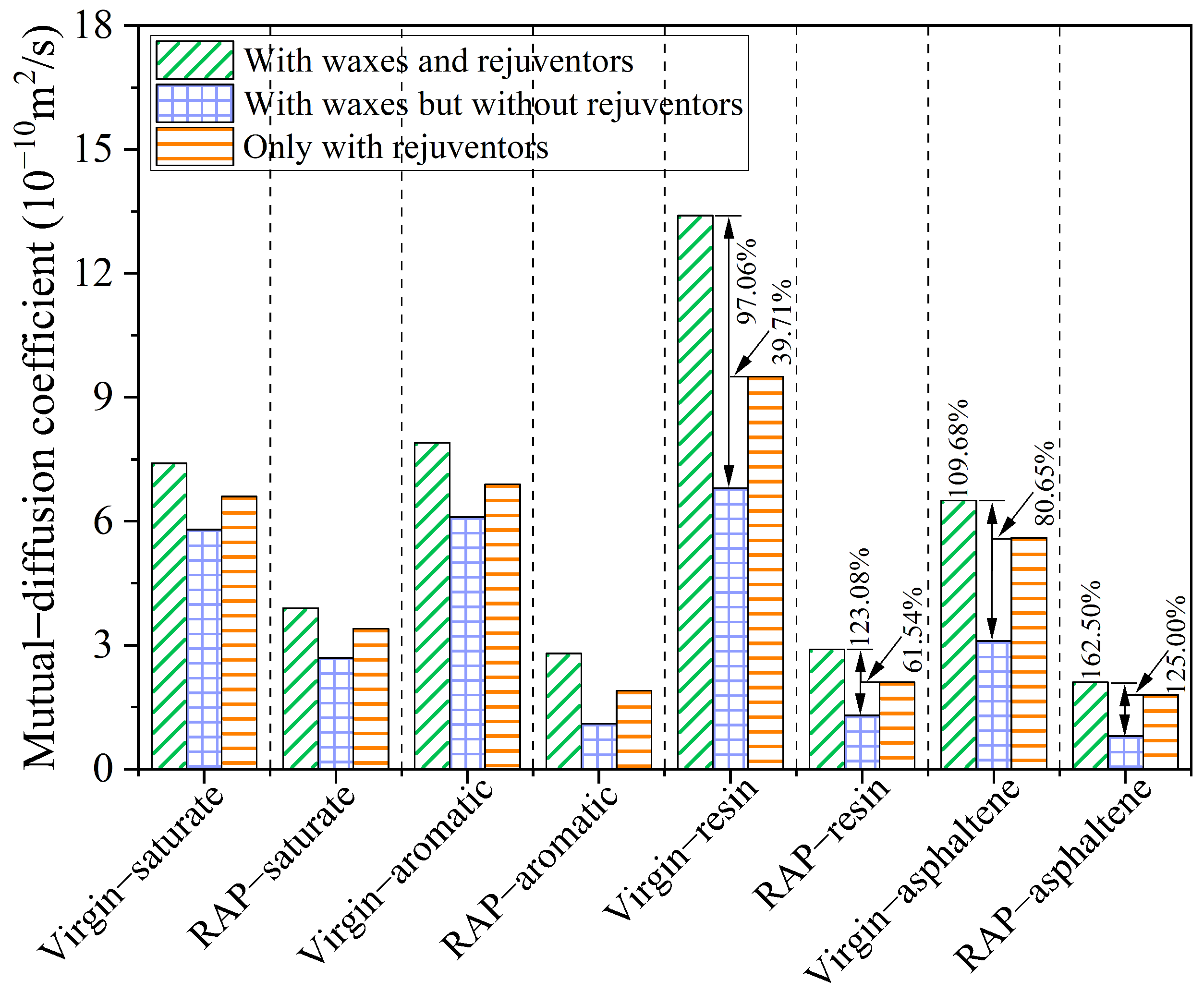
4.6. Diffusion of Four SARA Components
5. Conclusions
- The RAP + virgin + wax asphalt system manifested the highest glass transition temperature and apparent viscosity among the three asphalt molecule systems, which were markedly decreased under the dual action of organic waxes and rejuvenators. The simulated glass transition temperature and apparent viscosity keep good consistency with the experimental values, illustrating the feasibility of the asphalt molecule simulations in this study;
- The presence of RAP binders and organic waxes slightly increased the density, CED, and solubility parameters (δ, δvdw, and δele) while highly decreasing the surface free energy (SFE), resulting in an enhanced stiffness and poor cracking resistance. The incorporation of rejuvenators was beneficial to revise these thermal properties to improve the performance of the asphalt binders;
- The different asphalt binders had different self-healing times, and the self-healing performance of the Aged + virgin + wax asphalt binder was poorer than the other two asphalt binders. The incorporation of aromatic oils into the Aged + virgin + wax asphalt binder enhances its self-healing capabilities, as indicated by the earlier attainment of density stabilization, from 450 ps to 375 ps;
- The free volume of the asphalt binder with organic waxes but without rejuvenators slightly decreased (from 22.02% to 21.9%) in comparison to the asphalt binder with organic waxes and rejuvenators, leading to a greater volume of van der Waals and a decrease in the gaps between unoccupied molecules. And this fact was alleviated after adding a proper dosage of light aromatic oils;
- The blending process of the virgin and RAP binders was fundamentally achieved through the diffusion of virgin binders. Under the concurrent presence of organic waxes and rejuvenators, the diffusion of macromolecules (resins and asphaltenes) was accelerated, and the de-aggregation of aged asphaltenes was improved, which are the critical factors in effectively promoting the blending of virgin binders with the overall structure of RAP binders.
6. On-Going Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Birgisson, B.; Luo, X.; Onifade, I. A new long-term aging model for asphalt pavements using morphology-kinetics based approach. Constr. Build. Mater. 2019, 229, 117032. [Google Scholar] [CrossRef]
- Zhang, D.; Birgisson, B.; Luo, X.; Onifade, I. Correction to: A new short-term aging model for asphalt binders based on rheological activation energy. Mater. Struct. 2020, 53, 85. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, Z.; Miao, Y.; Zhang, D.; Fu, C. Field Aging Characterization of Asphalt Pavement Based on the Artificial Neural Networks and Gray Relational Analysis. J. Mater. Civ. Eng. 2023, 35, 04023188. [Google Scholar] [CrossRef]
- Sun, W.; Wang, H. Molecular dynamics simulation of diffusion coefficients between different types of rejuvenator and aged asphalt binder. Int. J. Pavement Eng. 2020, 21, 966–976. [Google Scholar] [CrossRef]
- Fan, J.; Ma, T.; Zhu, X.Y. Macro-micro testing and molecular simulation on the aging mechanism and behavior of sbs-modified high viscosity asphalt. J. Mater. Civ. Eng. 2024, 36, 4023561.1–4023561.18. [Google Scholar] [CrossRef]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M.C. Understanding the bitumen ageing phenomenon: A review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Shi, K.; Ma, F.; Liu, J.; Fu, Z.; Song, R.; Yuan, D.; Li, C. Rejuvenation effect of aged SBS-modified asphalt utilizing molecule analysis. J. Clean. Prod. 2023, 405, 136964.1–136964.16. [Google Scholar] [CrossRef]
- Behnood, A. Application of rejuvenators to improve the rheological and mechanical properties of asphalt binders and mixtures: A review. J. Clean. Prod. 2019, 231, 171–182. [Google Scholar] [CrossRef]
- Guduru, G.; Kumara, C.; Gottumukkala, B.; Kuna, K.K. Effectiveness of Different Categories of Rejuvenators in Recycled Asphalt Mixtures. J. Transp. Eng. Part B Pavements 2021, 147, 04021006. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, N.; Jamal, M.; Badin, G.; Suleman, M. Investigation into possibility of rejuvenating aged asphalt binder using mustard oil. Int. J. Pavement Eng. 2020, 23, 1738–1753. [Google Scholar] [CrossRef]
- Pouranian, M.R.; Shishehbor, M. Sustainability Assessment of Green Asphalt Mixtures: A Review. Environments 2019, 6, 73. [Google Scholar] [CrossRef]
- Mattinzioli, T.; Sol-Sánchez, M.; Carrión, A.J.d.B.; Moreno-Navarro, F.; Rubio-Gámez, M.d.C.; Martínez, G. Analysis of the GHG savings and cost-effectiveness of asphalt pavement climate mitigation strategies. J. Clean. Prod. 2021, 320, 128768. [Google Scholar] [CrossRef]
- Rodríguez-Alloza, A.M.; Gallego, J.; Pérez, I. Study of the effect of four warm mix asphalt additives on bitumen modified with 15% crumb rubber. Constr. Build. Mater. 2013, 43, 300–308. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, H.; Sun, L.; Sun, Y.; Liu, N. Multi-performance evaluation of recycled warm-mix asphalt mixtures with high reclaimed asphalt pavement contents. J. Clean. Prod. 2022, 377, 134209. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, J.; Gao, J.; Xu, J.; Zhang, Y.; Yu, S. Effect of design parameters on low-temperature cracking resistance of recycled hot-mix asphalt mixtures. Mater. Struct. 2025, 58, 24. [Google Scholar] [CrossRef]
- Obaid, H.A.; Hashim, T.M.; Al-Abody, A.A.M.; Nasr, M.S.; Abbas, G.H.; Kadhim, A.M.; Sadique, M. Properties of Modified Warm-Mix Asphalt Mixtures Containing Different Percentages of Reclaimed Asphalt Pavement. Energies 2022, 15, 7813. [Google Scholar] [CrossRef]
- Stimilli, A.; Virgili, A.; Canestrari, F. Warm recycling of flexible pavements: Effectiveness of Warm Mix Asphalt additives on modified bitumen and mixture performance. J. Clean. Prod. 2017, 156, 911–922. [Google Scholar] [CrossRef]
- Araujo, D.L.V.; Santos, J.; Martinez-Arguelles, G. Environmental performance evaluation of warm mix asphalt with recycled concrete aggregate for road pavements. Int. J. Pavement Eng. 2023, 24, 2064999. [Google Scholar] [CrossRef]
- Shi, K.; Fu, Z.; Ma, F.; Liu, J.; Song, R.; Li, J.; Dai, J.; Li, C.; Wen, Y. Development on the Rheological Properties and Micromorphology of Active Reagent-Rejuvenated SBS-Modified Asphalt. ACS Sustain. Chem. Eng. 2022, 10, 16734–16751. [Google Scholar] [CrossRef]
- Mirhosseini, A.F.; Tahami, A.; Hoff, I.; Dessouky, S.; Kavussi, A.; Fuentes, L.; Walubita, L.F. Performance Characterization of Warm-Mix Asphalt Containing High Reclaimed-Asphalt Pavement with Bio-Oil Rejuvenator. J. Mater. Civ. Eng. 2022, 32, 04020382. [Google Scholar] [CrossRef]
- Valdés-Vidal, G.; Calabi-Floody, A.; Sanchez-Alonso, E.; Díaz, C.; Fonseca, C. Highway trial sections: Performance evaluation of warm mix asphalt and recycled warm mix asphalt. Constr. Build. Mater. 2020, 262, 120069. [Google Scholar] [CrossRef]
- Mondal, A.; Ransinchung, R.N.G.D.; Choudhary, J. Sustainable recycling of industrial waste fillers and reclaimed asphalt pavement to produce environmentally feasible warm mix asphalt. Innov. Infrastruct. Solut. 2022, 8, 34. [Google Scholar] [CrossRef]
- Muñoz Perez, S.P.; Onofre Maicelo, P.A. Use of recycled asphalt as an aggregate for asphalt mixtures: Literary review. Innov. Infrastruct. Solut. 2021, 6, 146. [Google Scholar] [CrossRef]
- Hettiarachchi, C.; Hou, X.; Wang, J.; Xiao, F. A comprehensive review on the utilization of reclaimed asphalt material with warm mix asphalt technology. Constr. Build. Mater. 2019, 227, 117096. [Google Scholar] [CrossRef]
- Barraj, F.; Hatoum, A.; Assaad, J.J.; Alhakim, G.; Khatib, J.; Assaf, Y.; Elkordi, A. Characterisation of Superpave recycled asphalt mixtures utilising optimum contents of fatty amine-based asphalt additive. Int. J. Mason. Res. Innov. 2025, 10, 76–99. [Google Scholar] [CrossRef]
- Behnood, A.; Karimi, M.M.; Cheraghian, G. Coupled effects of warm mix asphalt (WMA) additives and rheological modifiers on the properties of asphalt binders. Clean. Eng. Technol. 2020, 1, 100028. [Google Scholar] [CrossRef]
- Xu, H.; Sun, Y.; Chen, J.; Li, J.; Yu, B.; Qiu, G.; Zhang, Y.; Xu, B. Investigation into Rheological Behavior of Warm-Mix Recycled Asphalt Binders with High Percentages of RAP Binder. Materials 2023, 16, 1599. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Sun, Y.; Zhu, X.; Wang, W.; Liu, J. Rheological and physico-chemical properties of warm-mix recycled asphalt mastic containing high percentage of RAP binder. J. Clean. Prod. 2021, 289, 125134. [Google Scholar] [CrossRef]
- Wang, W.; Huang, S.; Qin, Y.; Sun, Y.; Chen, J. Multi-scale study on the high percentage warm-mix recycled asphalt binder based on chemical experiments. Constr. Build. Mater. 2020, 252, 119124. [Google Scholar] [CrossRef]
- Lu, D.X.; Saleh, M.; Nguyen, N.H. Effect of rejuvenator and mixing methods on behaviour of warm mix asphalt containing high RAP content. Constr. Build. Mater. 2019, 197, 792–802. [Google Scholar] [CrossRef]
- Guo, N.; You, Z.; Tan, Y.; Zhao, Y. Performance evaluation of warm mix asphalt containing reclaimed asphalt mixtures. Int. J. Pavement Eng. 2016, 18, 981–989. [Google Scholar] [CrossRef]
- Farooq, M.A.; Mir, M.S.; Sharma, A. Laboratory study on use of RAP in WMA pavements using rejuvenator. Constr. Build. Mater. 2018, 168, 61–72. [Google Scholar] [CrossRef]
- Li, Q.; Sun, G.; Lu, Y.; Meng, Y.; Luo, S.; Gao, L. Effects of warm-mix asphalt technologies and modifiers on pavement performance of recycled asphalt binders. J. Clean. Prod. 2021, 282, 125435. [Google Scholar] [CrossRef]
- Jiao, B.; Gong, M. Multiscale enhancement mechanism of low-temperature performance for degraded recycled waste rubber asphalt binders: MD simulation and microscopic investigation. J. Clean. Prod. 2024, 461, 142647. [Google Scholar] [CrossRef]
- Fallah, F.; Khabaz, F.; Kim, Y.R.; Kommidi, S.R.; Haghshenas, H.F. Molecular dynamics modeling and simulation of bituminous binder chemical aging due to variation of oxidation level and saturate-aromaticresin-asphaltene fraction. Fuel 2019, 237, 71–80. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Gao, Y.; Erkens, S. Review on the diffusive and interfacial performance of bituminous materials: From a perspective of molecular dynamics simulation. J. Mol. Liq. 2022, 366, 120363. [Google Scholar] [CrossRef]
- Li, D.D.; Greenfield, M.L. High Internal Energies of Proposed Asphaltene Structures. Energy Fuels 2011, 25, 3698–3705. [Google Scholar] [CrossRef]
- Xiao, M.M.; Fan, L. Ultraviolet aging mechanism of asphalt molecular based on microscopic simulation. Constr. Build. Mater. 2022, 319, 126157. [Google Scholar] [CrossRef]
- Xu, G.; Wang, H.; Sun, W. Molecular dynamics study of rejuvenator effect on RAP binder: Diffusion behavior and molecular structure. Constr. Build. Mater. 2018, 158, 1046–1054. [Google Scholar] [CrossRef]
- Yu, C.; Hu, K.; Yang, Q.; Chen, Y. Multi–scale observation of oxidative aging on the enhancement of high–temperature property of sbs–modified asphalt. Constr. Build. Mater. 2021, 313, 125478. [Google Scholar] [CrossRef]
- Zadshir, M.; Oldham, D.J.; Hosseinnezhad, S.; Fini, E.H. Investigating bio-rejuvenation mechanisms in asphalt binder via laboratory experiments and molecular dynamics simulation. Constr. Build. Mater. 2018, 190, 392–402. [Google Scholar] [CrossRef]
- Guangji, X.; Hao, W. Diffusion and interaction mechanism of rejuvenating agent with virgin and recycled asphalt binder: A molecular dynamics study. Mol. Simul. 2018, 44, 1433–1443. [Google Scholar]
- Zhang, D.; Xu, P.; Yu, P.; Hu, Q.; Luan, D. Influence of acidity and alkalinity of water environment on water stability of asphalt mixture: Phase II-microscopic erosion mechanism. Int. J. Adhes. Adhes. 2024, 132, 103738. [Google Scholar] [CrossRef]
- Samieadel, A.; Oldham, D.; Fini, E.H. Multi-scale characterization of the effect of wax on intermolecular interactions in asphalt binder. Constr. Build. Mater. 2017, 157, 1163–1172. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Erkens, S.; Gao, Y. Chemical characterizations and molecular dynamics simulations on different rejuvenators for aged bitumen recycling. Fuel 2022, 324, 124550. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, D.; Liu, Z.; Fu, C.; Lv, H. Chemical and rheological properties evaluation of a novel synchronous rejuvenated aged SBS modified asphalt. J. Clean. Prod. 2022, 381, 135213. [Google Scholar] [CrossRef]
- Chen, L.; Shen, X.; Zhang, Y.F. Recent advances in self-healing polymer-modified asphalt utilizing dynamic covalent bonds. J. Polym. Res. 2025, 32, 21. [Google Scholar] [CrossRef]
- Peng, C.; Lu, L.; You, Z.; Xu, F.; Zhou, L.; Ma, H.; Hu, Y.; Liu, Y. Influence of Waste Polyethylene on the Performances of Asphalt before and after Oxidative Aging Based on the Molecular Dynamics Simulation. J. Mater. Civ. Eng. 2022, 34, 04022274. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Wang, S.; Zhang, X.; Xiao, C.; Yu, B. Molecular dynamics evaluation of activation mechanism of rejuvenator in reclaimed asphalt pavement (RAP) binder. Constr. Build. Mater. 2021, 298, 123898. [Google Scholar] [CrossRef]
- Liu, K.; Xu, P.; Wang, F.; You, L.; Zhang, X.; Fu, C. Assessment of automatic induction self-healing treatment applied to steel deck asphalt pavement. Autom. Constr. 2022, 133, 104011. [Google Scholar] [CrossRef]
- Gong, M.; Jiao, B. Thermodynamic properties analysis of warm-mix recycled asphalt binders using molecular dynamics simulation. Road Mater. Pavement Des. 2024, 25, 239–258. [Google Scholar] [CrossRef]
- Notani, M.A.; Nejad, F.M.; Fini, E.H.; Hajikarimi, P. Low-Temperature Performance of Toner-Modified Asphalt Binder. J. Transp. Eng. Part B Pavements 2019, 145, 04019022. [Google Scholar] [CrossRef]

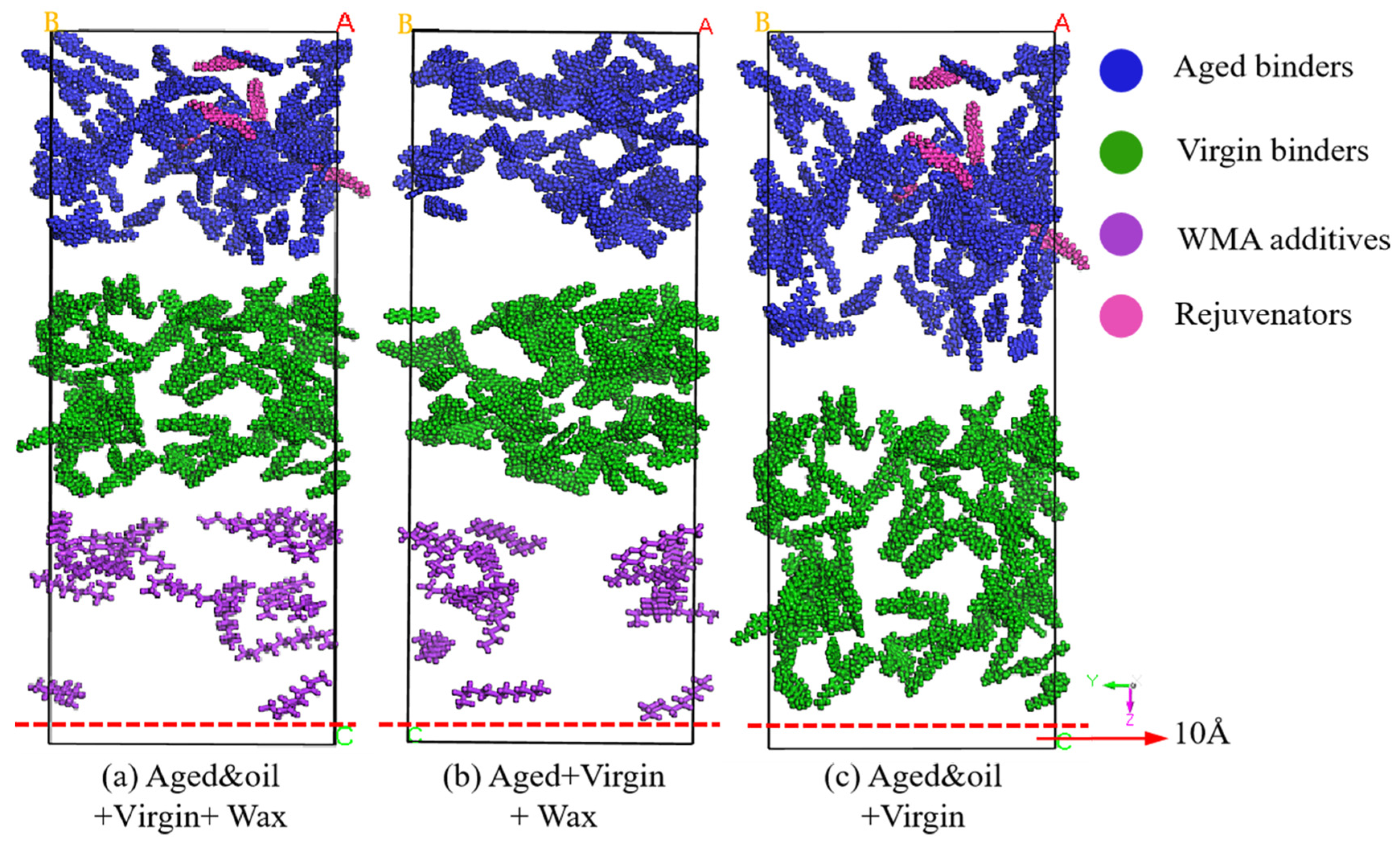
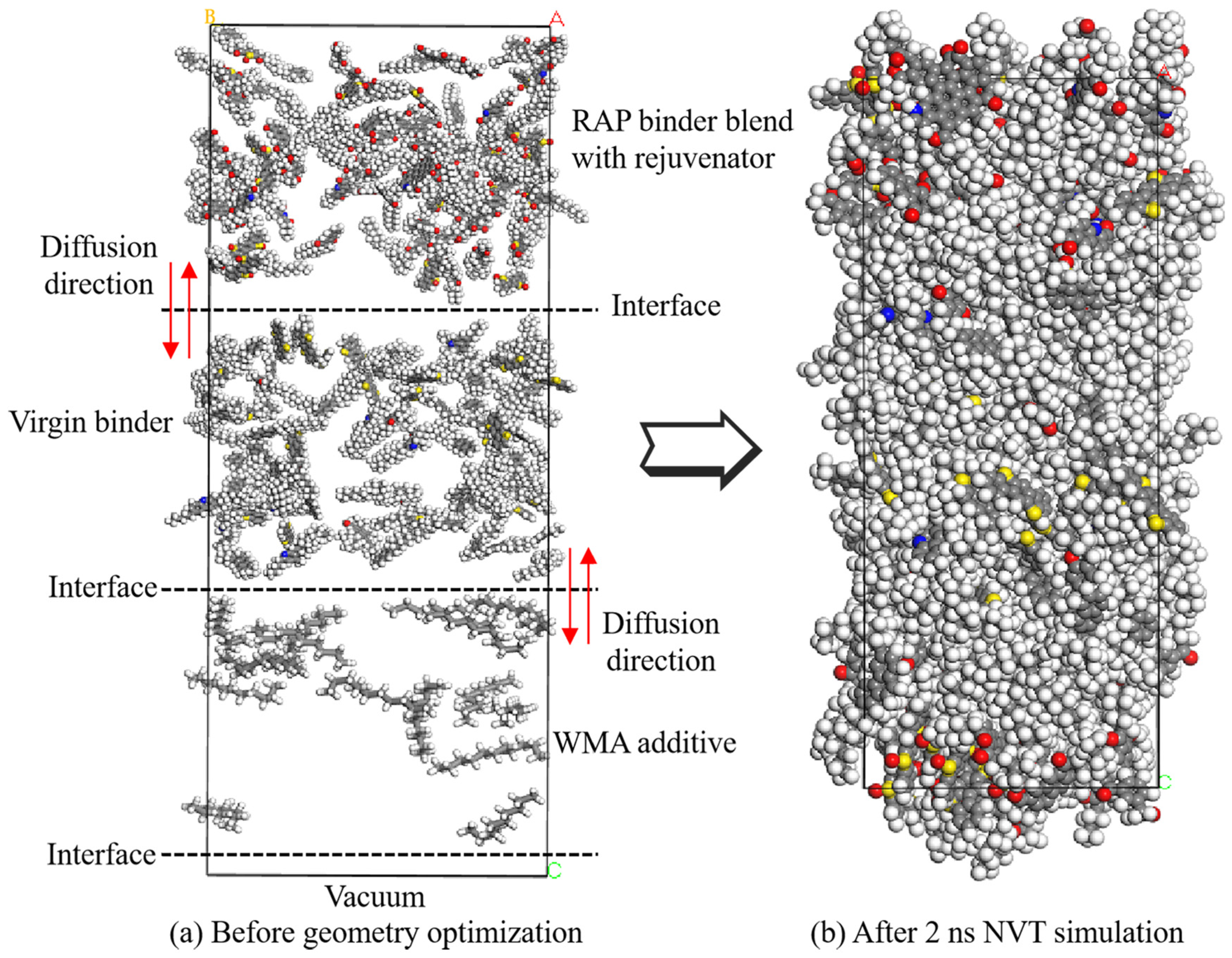




| Basic Property | Value | Reference Standard |
|---|---|---|
| Density | 0.890 ± 0.006 g/cm3 | ASTM D891-2018 |
| Appearance | Dark, viscous liquid | / |
| Saturated hydrocarbon content | >10% | ASTM D5580-2015 |
| Flash point | 170–200 °C | ASTM D92-2018 |
| Boiling point | 150–200 °C | ASTM D2887-2022 |
| Aniline point | 36 °C | ASTM D611-2016 |
| Engler viscosity (60 °C) | 12–15 °E | ASTM D1665-2020 |
| Temperature/°C | Aged and Oil + Virgin + Wax | Aged + Virgin + Wax | Aged and Oil + Virgin | |||
|---|---|---|---|---|---|---|
| Simulated | Experimental | Simulated | Experimental | Simulated | Experimental | |
| 115 | 1426.69 | 1510 | 2077.51 | 2180 | 1543.91 | 1490 |
| 135 | 343.32 | 350 | 461.73 | 480 | 365.43 | 360 |
| 155 | 109.71 | 120 | 139.42 | 150 | 115.40 | 130 |
| 175 | 43.11 | 50 | 52.53 | 60 | 44.95 | 50 |
| Evaluation Indexes | Aged and Oil + Virgin + Wax | Aged + Virgin + Wax | Aged and Oil + Virgin | Experimental Values |
|---|---|---|---|---|
| Density (433.15 K, g/cm3) | 0.9690 | 0.9893 | 0.9673 | 0.95–1.08 at 298.15 K |
| CED (108 J/m3) | 3.074 | 3.162 | 3.045 | 2.80–3.32 |
| δ ((J/cm3)1/2) | 17.53 | 17.79 | 17.45 | 13.30–22.50 |
| δvdw ((J/cm3)1/2) | 17.09 | 17.33 | 16.71 | / |
| δele ((J/cm3)1/2) | 3.27 | 3.32 | 3.18 | / |
| SFE (mJ/m2) | 36.4 | 18.5 | 38.2 | 13.0–47.6 |
| Binder Types | Occupied Volume/Å3 | Free Volume/Å3 | FFV (%) |
|---|---|---|---|
| Aged and oil + virgin + wax | 96,275.24 | 27,193.11 | 22.02 |
| Aged + virgin + wax | 88,956.98 | 25,034.61 | 21.96 |
| Aged and oil + virgin | 92,411.38 | 24,603.08 | 21.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Zhang, D.; Xu, P. Molecular Dynamics Investigation of the Diffusion Mechanisms and Thermodynamic Behaviors in Warm Mix Recycled Asphalt Binders with and Without Rejuvenators. Materials 2025, 18, 703. https://doi.org/10.3390/ma18030703
Hu Q, Zhang D, Xu P. Molecular Dynamics Investigation of the Diffusion Mechanisms and Thermodynamic Behaviors in Warm Mix Recycled Asphalt Binders with and Without Rejuvenators. Materials. 2025; 18(3):703. https://doi.org/10.3390/ma18030703
Chicago/Turabian StyleHu, Qisheng, Derun Zhang, and Peixin Xu. 2025. "Molecular Dynamics Investigation of the Diffusion Mechanisms and Thermodynamic Behaviors in Warm Mix Recycled Asphalt Binders with and Without Rejuvenators" Materials 18, no. 3: 703. https://doi.org/10.3390/ma18030703
APA StyleHu, Q., Zhang, D., & Xu, P. (2025). Molecular Dynamics Investigation of the Diffusion Mechanisms and Thermodynamic Behaviors in Warm Mix Recycled Asphalt Binders with and Without Rejuvenators. Materials, 18(3), 703. https://doi.org/10.3390/ma18030703







