Abstract
Carbonaceous-based metal-free catalysts are promising aspirants for effective electrocatalytic hydrogen generation. Herein, we synthesized mesoporous-activated carbon nanosheets (ELC) from biomass eucalyptus leaves through KOH activation. The microstructure, structural, and textural characteristics of the prepared materials were characterized by FE-SEM, Raman, XRD, and BET measurements. The high temperature (700 °C) KOH-activated ELC nanosheets exhibited an interconnected nanosheet morphology with a large specific surface area (1436 m2/g) and high mesoporosity. The ELC-700 catalyst exhibited an excellent electrocatalytic HER performance with a low overpotential (39 mV at 10 mA/cm2), excellent durability, and a Trivial Tafel slope (36 mV/dec) in 0.5 M H2SO4 electrolyte. These findings indicate a new approach for developing excellent biomass-derived electrocatalysts for substantially efficient green hydrogen production.
1. Introduction
Environmental changes and global warming driven by the utilization of fossil fuels have intensified substantial interest in pursuing sustainable, clean, and green renewable energy sources [1,2,3,4]. Green hydrogen is considered one of the most versatile alternative energy sources due to its zero-carbon emission, sustainability, eco-friendliness, and excellent energy density [5,6,7,8]. Among the various production techniques, electrocatalytic water splitting is the most efficient, clean, and scalable approach for producing green hydrogen via hydrogen evolution reaction (HER) [9,10,11,12]. However, the insignificant reaction kinetics and the high overpotential of HER restrict the catalytic efficiency of water splitting [13,14]. Consequently, developing highly efficient electrocatalysts is essential to lower the energy barrier and accelerate hydrogen production. Currently, platinum (Pt)-based derivates are used as efficient catalysts for HER, but their shortage and expensiveness impede their large-scale industrial application [15,16,17]. Recently, various alternatives have been classified as promising high-performance HER electrocatalysts to replace Pt-based catalysts. For instance, the transition of metal dichalcogenides [18,19,20], noble metal alloys [21,22], carbides [23,24], phosphides [25,26], oxides [27,28,29], nitrides [30], metal–organic frameworks [31,32,33], single-atom catalysts [34], layered double hydroxides [35,36], and perovskite- [37] and spinel-structured materials [8,38] are distinctive catalysts that exhibit excellent electrocatalytic HER performances. However, due to complex and expensive synthesis methods, insignificant electrical conductivity, and poor durability, efforts have been made to avoid using metal-based electrocatalysts [39]. Consequently, developing inexpensive, highly effective, metal-free, and acid-stable HER catalysts from cost-effective natural resources is vital.
Currently, different kinds of carbonaceous materials (e.g., porous carbon [40,41,42], graphitic carbon [11,43], carbon fiber [44], activated carbon (AC) [45,46,47], carbon nanotube [48], graphene oxide [49], and graphene [15,50,51]) have been investigated as HER electrocatalysts. Owing to the large textural area, natural abundance, high conductivity, low cost, tunable porous structure, and robust stability, biomass-derived AC has attracted significant attention as HER electrocatalysts [40,41,42,52]. For example, Liu et al. [40] synthesized N,S-doped porous carbon nanosheets from human hair using ZnCl2 activation under a nitrogen atmosphere. These nanosheets exhibited excellent HER performance, achieving a small overpotential of 12 mV with remarkable durability. Cao et al. [41] fabricated N-porous carbon from bean sprouts using a SiO2 template method and demonstrated the overpotential of 413 mV with a Tafel value of 98 mV/dec. Sun et al. [45] used waste paper for preparing Co and N co-doped carbon through hydrothermal and pyrolysis methods, retaining a low overpotential of 223 mV at 10 mA/cm2. Prabu et al. [42] prepared hierarchical porous carbon nanosheets from palm plants and showed an overpotential of 330 mV with a Tafel value of 63 mV/dec. Among various biomass resources, eucalyptus leaves have attracted attention as a sustainable biomass precursor for the fabrication of AC nanostructures due to their cheap, natural abundance, environmental friendliness, and high carbon content (~75%) [53,54,55,56]. Therefore, salvaging abundant biomass eucalyptus leaves can be a favorable material for preparing high-performance AC nanostructures. Despite such benefits, no studies on biomass eucalyptus leaves-derived mesoporous AC (ELC) nanostructures for HER electrocatalysts have been reported so far.
Despite all the above, herein, we fabricated mesoporous AC nanosheets from biomass eucalyptus leaves by using the KOH activation process at 600–700 °C. The ELC-700 catalyst exhibited a small overpotential of 39 mV with a low Tafel value of 36 mV/dec at 10 mA/cm2 in 0.5 M H2SO4. The material preparation, material characterization, textural characteristics, and the electrocatalytic HER activities of the prepared catalysts were systematically evaluated and deliberated in detail.
2. Experimental Section
2.1. Preparation of ELC Nanosheets
Figure 1 illustrates the schematic process for synthesizing ELC nanosheets. Biomass eucalyptus leaves (EL) were collected from Tholudur, Tamil Nadu, India, and served as the carbonaceous precursor. Initially, the EL was washed with deionized (DI) water and air-dried for five days. Then, the parched ELs were carbonized at 300 °C for 60 min in a muffle furnace to produce carbonized ashes (ELAs). After carbonization, the ELA-KOH mixture was prepared using ELA (4 g) and KOH (16 g) in a mortar. Next, the prepared mixture was stimulated at two various temperatures (600 and 700 °C) for 120 min in an air atmosphere. During the KOH activation, oxygen- and carbon-containing groups react with KOH; then, carbonaceous chemicals (e.g., carbon monoxide (CO), potassium carbonate (K2CO3), etc.) are formed. The activation process was carried out at high temperatures and is illustrated by the following chemical reactions [57,58]:
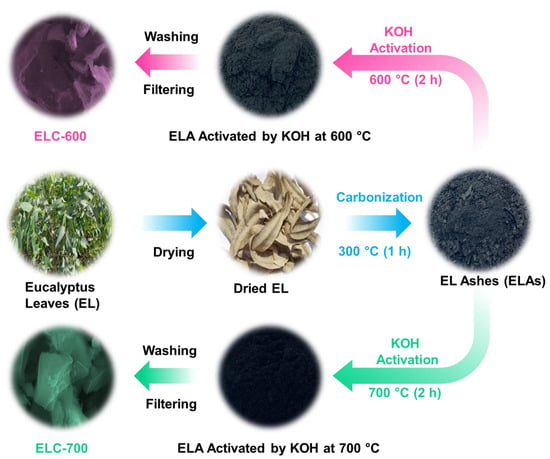
Figure 1.
Schematic description of the experimental procedure for ELC-600 and ELC-700 nanosheets from eucalyptus leaves through the KOH activation.
Next, the KOH-treated ELA was blended in DI water (100 mL) and stirred for 720 min to eliminate unreacted potassium components. After that, the soaked colloidal mixture was filtered, rinsed, and dried at 150 °C for 720 min. Finally, the powder form of ELC nanosheets was observed and labeled as ‘ELC-600’ and ‘ELC-700’ for KOH-activated at 600 and 700 °C, respectively.
2.2. Physicochemical Characterizations
The surface morphology of the prepared ELC-600 and ELC-700 catalysts was inspected by using scanning electron microscopy (FE-SEM, Clara LMH, Tescan Brno, Brno-Kohoutovice, Czech Republic). The vibrational and structural characteristics of the fabricated catalysts were assessed using Raman scattering spectroscopy (LabRAM HR800, Jobin Yvon, Longjumeau, France) and X-ray diffraction (XRD, D8-Advance, Bruker, Madison, WI, USA), respectively. The porosity and textural characteristics were investigated using Barrett–Joyner–Halenda (BJH) and Brunauer–Emmett–Teller (BET) measurements (BELSORP-mini II system, MicrotracBEL, Osaka, Japan).
2.3. Electrochemical HER Measurements
The electrocatalytic HER performances of the ELC-600 and ELC-700 catalysts were assessed using a VersaSTAT3 workstation (Ametek Scientific Company, Berwyn, PA, United States of America) with a typical three-electrode system. Initially, we assembled the two working electrodes using activated carbon (ELC-600 or ELC-700) mixed with an N-methyl-2-pyrrolidinone solvent. After that, the mixed slurries were smeared on the stainless-steel substrates (1 cm × 1 cm) and desiccated at 160 °C for 720 min. Additionally, the saturated calomel electrode (SCE) and the coiled Pt wire functioned as the reference and counter electrodes, respectively. All the HER measurements (i.e., linear sweep voltammetry (LSV), chronopotentiometry (CP), cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS)) were conducted in 0.5 M H2SO4. CV characteristics were obtained at various current densities (10–100 mV/s) with a window range of 0–1.0 V. LSV characteristics of the ELC-600 and ELC-700 catalysts were examined in the window range of 0.1 to 1.2 V at 5 mV/s. The HER rate performances were evaluated via CP measurements at several current densities of -10 to 100 mA/cm2 for 10 min. EIS characteristics were assessed at a frequency range of 1 Hz to 10 kHz. Electrochemical double-layer capacitances (Cdl) of the fabricated catalysts were calculated from the non-Faradic CV region to assess the electrochemically active surface area (ECSA) using the following equations [8,59,60,61]:
where A is the fabricated electrode area, JDL is the non-Faradaic current density, Ce is the capacitance of the electrolyte (1 M H2SO4 = 0.035 mF/cm2), v is the scan rate, and Cdl is the non-Faradaic capacitance. The overpotential (η) and the Tafel slope (ST) values of the fabricated catalysts were derived using the following equations [11,15,46]:
where c, E0SCE, ERHE, and J are the fitting parameters, SCE’s standard potential, RHE’s standard potential, and the current density, respectively.
3. Results and Discussion
The topography insights of the biomass EL-derived ELC-600 and ELC-700 were inspected via FE-SEM measurement. Figure 2 displays the FE-SEM images of the prepared catalysts. The ELC-600 sample exhibits irregular and aggregated stacked sheets-like morphology (Figure 2a,b). Conversely, the ELC-700 clearly shows a structure of agglomerated and interconnected nanosheets (Figure 2c,d). However, the interconnected nanosheet textures are clearly more visible in ELC-700 than in ELC-600. This structural enhancement in ELC-700 could potentially improve the catalytic active sites and porosity, which is beneficial for enhancing the HER activities. The EDX analysis (see Figure S1) revealed that both samples predominantly consisted of carbon, confirming the high purity of the materials.
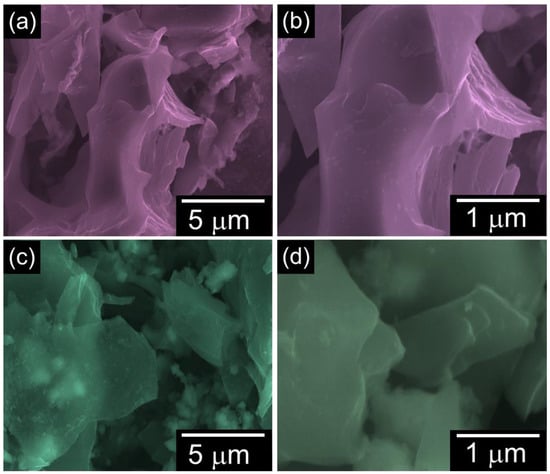
Figure 2.
Low- and high-magnification FE-SEM images of (a,b) ELC-600 and (c,d) ELC-700 nanosheets.
The structural characteristics of the ELC-600 and ELC-700 samples were investigated by XRD measurements. Figure 3a demonstrates the XRD pattern of the ELC-600 and ELC-700. Both materials exhibited the two diffraction peaks at 24.2° and 43.5° are associated with the (002) and (100) crystalline planes of the disordered carbon structure with the amorphous nature of the materials [57,62,63,64], respectively. Compared to ELC-700, the ELC-600 sample exhibited broader (002) diffraction peaks, demonstrating a high degree of disordered structure. This result indicates that as the KOH activation temperature increases, the carbon materials become more graphitic, which enhances the electrical conductivity of the materials [65]. No other impurities were observed, suggesting the high purity of the samples. Furthermore, the disordered structures of the carbonaceous materials were investigated by Raman measurements. Figure 3b illustrates the Raman spectra of the ELC-600 and ELC-700 samples. Both samples indicate three characteristic peaks at 1346 cm−1 (D), 1579 cm−1 (G), and 2889 cm−1 (2D), demonstrating the graphitic carbon nature of the prepared materials [11,53,66]. The D band is accompanied by the disorder vibration with dangling bonds in terminal planes (A1g symmetry) of graphitic carbon [67,68]. G band is ascribed to the E2g vibration mode of the sp2 hybridized carbon electronic configuration [40]. The 2D band acts as a typical signature of activated carbon [57,69]. The band intensity ratio (ID/IG) of the ELC-600 and ELC-700 samples were determined to be 0.99 and 0.97, respectively. All of the above indicates that, compared to ELC-600, the ELC-700 sample exhibits a trivial disordered lattice structure and a high level of graphitization. Furthermore, the surface functional characteristics of the ELC-600 and ELC-700 samples were investigated by FTIR instruments (Figure S2). The wider peak in the region of 3010–3647 cm−1 is attributed to the O-H stretching vibrations because of the vibrations of the water molecules or surface-adsorbed moisture [47]. The peak at 2916 cm−1 is correlated with the C-H stretching vibrations (i.e., CH3, CH2, and CH groups) [70]. The peaks at 2356, 1553, and 986 cm−1 are correlated to the C = O, C = C, and C-C stretching vibration of the activated carbon, respectively [46,71,72]. While increasing the activation temperature, the water molecules or surface-adsorbed moisture are reduced due to modified hydrogen bonding networks, and the samples showed a slight intensity and peak shift in the FTIR spectra.

Figure 3.
(a) XRD patterns, (b) Raman spectra, (c) Nitrogen absorption–desorption isotherms, and (d) Pore size distributions of ELC-600 and ELC-700 nanosheets.
The textural characteristics and the porosity nature of the ELC-600 and ELC-700 samples were investigated by BET and BJH measurements. As displayed in Figure 3c, both materials distinctly display a Type-IV physisorption isotherm (verified by IUPAC), demonstrating the mesoporous characteristics of the AC nanosheets [15,57,64,66,67]. However, the coinciding adsorption and desorption branches accentuate the absence of a hysteresis loop, suggesting a uniform and adequate pore structure without significant capillary condensation effects. From the BET measurements, the surface area of the ELC-600 and ELC-700 were estimated to be 1022 and 1436 m2/g, respectively. Figure 3d depicts the pore characteristics of the ELC-600 and ELC-700. The pore surface area of ELC-600 and ELC-700 are 132 and 507 m2/g, respectively. In addition, the total pore volume of the ELC-700 (0.5122 cm3/g) sample is higher than that of the ELC-600 (0.3358 cm3/g) sample. In addition, the average pore size of the ELC-600 and ELC-700 samples was estimated to be 2.27 and 2.18 nm, respectively. From the t-plot (Figure S3), we calculated the mesoporous surface area of the ELC-600 and ELC-700 are 217 and 433 m2/g, respectively. The micropore volumes of the ELC-600 and ELC-700 were determined to be 0.028 and 0.036 cm3/g, respectively. Compared to ELC-600 (0.307 cm3/g), the ELC-700 sample (0.476 cm3/g) exhibited a high mesopore volume, indicating the high mesoporosity of the ELC-700 material. This result clearly indicates that the ELC-700 sample has a high mesoporosity nature with significant micropores compared to ELC-600, which could be favorable for high electrocatalytic HER performance.
After confirming the material characteristics of the prepared catalysts, we evaluated the electrocatalytic HER performances of ELC-600 and ELC-700 catalysts. Figure 4a,b show the CV characteristics of the ELC-600 and ELC-700 catalysts at different scan rates of 10–100 mV/s. Both catalysts showed distinctive rectangular-shaped CV curves, indicating the effective electrical double-layer capacitive behavior of the materials [11,53,56]. When the scan rate rose, the current density also improved owing to the small diffusion resistance of the active catalysts. Compared to the ELC-600, the ELC-700 catalyst exhibited a substantial CV loop area and a higher current response that demonstrated the ELC-700 has more active sites than the ELC-600 catalyst. The electrocatalytic HER activities are correlated with the electrochemical double-layer capacitance (Cdl) and electrochemically active surface area (ECSA) of the catalyst material. The Cdl values of the ELC-600 and ELC-700 catalysts were calculated to be 0.971 and 4.92 mF/cm2 from the non-Faradic CV region (0.05V, see Figure 4c,d, and Figure S4), respectively. The ECSA values are 33 cm2 (ELC-600) and 158 cm2 (ELC-700) using Equations (6) and (7). Compared to ELC-600, the ELC-700 catalyst has higher Cdl and ECSA values, demonstrating the ELC-700 revealed larger catalytic active sites and higher electrical conductivity.
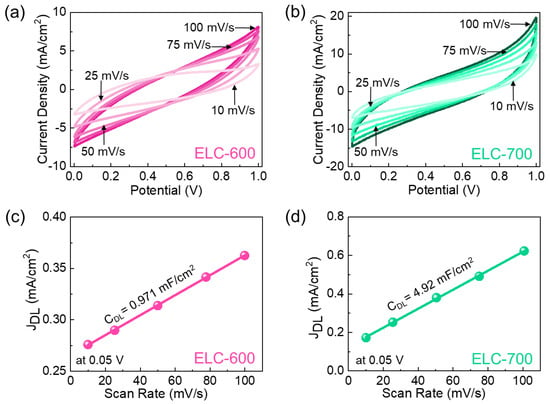
Figure 4.
CV curves of the (a) ELC-600 and (b) ELC-700 catalysts. Non-Faradaic JDL at 0.05 V as a function of the scan rate of the (c) ELC-600 and the (d) ELC-700 catalysts.
The ELC-600 and ELC-700 catalysts’ HER performances were assessed using an LSV measurement conducted at 5 mV/s in a 0.5 M H2SO4 acidic solution. Figure 5a depicts the iR-corrected LSV slopes of the ELC-600 and ELC-700 catalysts. The η values of the ELC-600 and ELC-700 catalysts were measured to be 55 mV and 39 mV at 10 mA/cm2 (from Equations (8) and (9)), respectively. The ELC-700 catalyst exhibited lower η values due to its larger catalytic-active sites (i.e., higher ECSA) and higher conductivity than the ELC-600 catalyst. As shown in Figure 5b, the ST value of the ELC-600 and ELC-700 catalysts were measured to be 67 and 36 mV/dec, respectively, by utilizing Equation (10). The lower ST value of the ELC-700 catalyst exhibited the more efficient HER kinetics ensuing the Volmer–Heyrovsky mechanism. Therefore, it is widely recognized that the HER mechanism unfolds on the cathode surface through a multistep electrochemical reaction process. Specifically, in an acidic electrolyte medium, the multistep HER process proceeds via the following reaction steps [73,74].
where the M-H and M are related to the absorbed hydrogen atoms and vacant surface site of the catalysts. The strength of the M-H bond is unanimously critical for determining the HER kinetics of catalyst materials [75]. Compared to the other electrocatalysts, the ELC-700 catalyst exhibited excellent HER activities (i.e., lower η and ST values) because of the higher ECSA and high conductivity (Table S1).
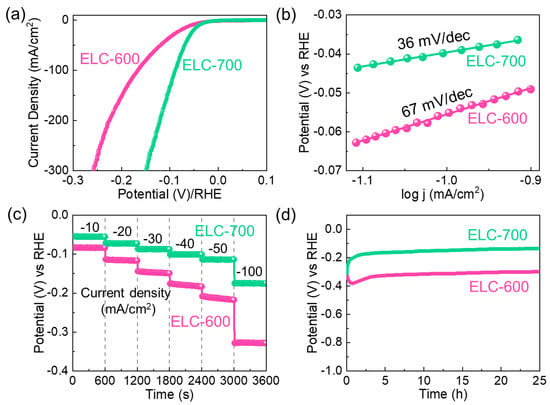
Figure 5.
HER activities of the ELC-600 and ELC-700 catalysts. (a) iR-corrected LSV curves, (b) Tafel plots, (c) chronopotentiometric profiles at different current densities (−10 to −100 mA/cm2), and (d) long-term stability characteristics.
The superb HER performances of the ELC-600 and ELC-700 catalysts were further clarified by using CP measurements. Figure 5c shows the CP characteristics of the ELC-600 and ELC-700 catalysts. The CP measurements clearly show that the ELC-700 catalyst has a lower overpotential at each current density (i.e., −10, −20, −30, −40, −50, and −100 mA/cm2) than that of the ELC-600 catalyst. Figure 5d indicates the long-term HER stability measurements of the catalysts at −10 mA/cm2 for 25 h. Compared to the ELC-600 catalyst, the ELC-700 catalyst demonstrated stable and long-term HER stability because of the high ECSA, large porosity, and low resistance of the material. Moreover, the LSV slopes of the catalysts are nearly the same before and after the long-term stability test (Figure S5). These indicate that the ELC-700 catalyst can serve as a stable and excellent electrocatalyst for HER. After the HER stability, we carried out the FE-SEM, Raman, and FTIR measurements to examine the changes in both the microstructural and vibrational characteristics of the fabricated catalysts. From FE-SEM measurements, the ELC-600 catalyst exhibited the aggregated sheet structure (see Figure S6a). However, the ELC-700 catalyst still maintained its original agglomerate nanosheet structure (see Figure S6b). From Raman measurements (see Figure S7), both catalysts still maintain their original nature, indicating the highly stable nature of the materials. Furthermore, the FTIR spectra (see Figure S8) of both catalysts clearly exhibited the original structural vibration of the material. Compared to the ELC-700 catalyst, the FTIR spectra of the ELC-600 catalyst are slightly intensity and peak shift demonstrating the ELC-700 catalyst has a high stable nature of the materials.
To further clarify the enhanced HER kinetics of the ELC-700 catalyst, EIS measurements were conducted. Figure 6 displays the Nyquist plot of the ELC-600 and ELC-700 catalysts with their subsequent equivalent circuit (Figure 6, inset). Both catalysts displayed a linear line at lower frequencies due to the distribution of the ionic solution across the catalyst surface [76,77]. However, the absence of a semicircle in both catalysts indicates the high ionic diffusivity and extraordinary electronic conductivity [78,79]. The serial resistance (Rs) values of the ELC-600 and ELC-700 catalysts are 1.27 Ω and 0.85 Ω, respectively, by using an equivalent circuit model. Compared to the ELC-600 catalyst, the ELC-700 catalyst showed a low Rs value and steeper slope because of the high active sites, large porosity, enhanced ionic diffusion, and high conductivity. From all the above results, the biomass-derived ELC-700 nanosheets are a more sustainable catalyst material for excellent HER electrocatalyst for future green hydrogen production.
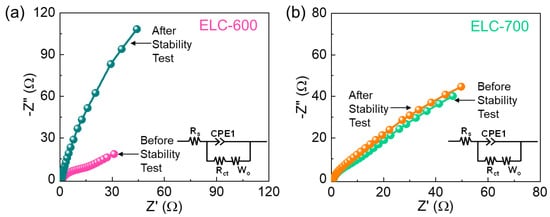
Figure 6.
Nyquist plots of (a) ELC-600 and (b) ELC-700 catalysts before and after the stability test (Inset in the equivalent circuit of the active catalysts).
4. Conclusions
Mesoporous EL-AC nanosheets were effectively derived from biomass eucalyptus leaves via KOH activation. The ELC-700 nanosheets exhibited mesoporous agglomerated and interconnected nanosheet morphology with a high specific textural area. Owing to the mesoporous nature and the superior graphitization of ELC-700, the catalyst revealed excellent HER performances with a significantly smaller η (i.e., 39 mV at 10 mA/cm2) and a lower ST (i.e., 36 mV/dec) in a 0.5 M H2SO4 solution, compared to those of ELC-700 (i.e., η: 55 mV and ST: 67 mV/dec). These outcomes indicate that mesoporous EL-AC nanosheets hold promise as a tremendous HER electrocatalyst for future green hydrogen production.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ma18030670/s1. Figure S1: EDX spectra of the (a) ELC-600 and (b) ELCC-700 samples; Figure S2: FTIR spectra of the ELC-600 and ELC-700 samples; Figure S3: t-plot of the ELC-600 and ELC-700 samples; Figure S4: Non-Faradaic CV curves at 0.05 V of the (a) ELC-600 and (b) ELC-700 catalysts; Figure S5: LSV curves of the (a) ELC-600 and (b) ELC-700 catalysts before and after the HER stability test; Figure S6: FE-SEM images of the (a) ELC-600 and the (b) ELC-700 catalysts after the stability test; Figure S7: Raman spectra of the (a) ELC-600 and the (b) ELC-700 catalysts after the stability test; Figure S8: FTIR spectra of the (a) ELC-600 and the (b) ELC-700 catalysts after the stability test; Table S1: Comparison of the HER performances between biomass ELC nanosheets and other carbonaceous electrocatalysts reported in previous works.
Author Contributions
S.S.: Methodology, Formal analysis, Investigation, Writing—original draft. A.S.: Formal analysis, G.S.: Formal analysis. K.T.: Formal analysis. H.J.: Data curation, Validation. Y.L.: Data curation, Validation, Supervision. S.L.: Conceptualization, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation (NRF) of Korea through the basic science research programs (2021R1I1A1A01049638; RS-2023-NR076644) funded by the Korean Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data were used for the research described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ji, X.; Lin, Y.; Zeng, J.; Ren, Z.; Lin, Z.; Mu, Y.; Qiu, Y.; Yu, J. Graphene/MoS2/FeCoNi(OH)x and Graphene/MoS2/FeCoNiPx multilayer-stacked vertical nanosheets on carbon fibers for highly efficient overall water splitting. Nat. Commun. 2021, 12, 1380. [Google Scholar] [CrossRef] [PubMed]
- Achakulwisut, P.; Erickson, P.; Guivarch, C.; Schaeffer, R.; Brutschin, E.; Pye, S. Global fossil fuel reduction pathways under different climate mitigation strategies and ambitions. Nat. Commun. 2023, 14, 5425. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, Q.; Sainio, J.; Saveleva, V.A.; Jiang, H.; Shi, J.; Ali, B.; Kallio, A.-J.; Huotari, S.; Sundholm, D.; et al. Amorphous carbon modulated-quantum dots NiO for efficient oxygen evolution in anion exchange membrane water electrolyzer. Appl. Catal. B Environ. 2024, 358, 124437. [Google Scholar] [CrossRef]
- Baraneedharan, P.; Sekar, S.; Murugesan, S.; Ahamada, D.; Mohamed, S.A.B.; Lee, Y.; Lee, S. Recent Advances and Remaining Challenges in Perovskite Solar Cell Components for Innovative Photovoltaics. Nanomaterials 2024, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Q.; Algburi, S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. Green hydrogen: A pathway to a sustainable energy future. Int. J. Hydrogen Energy 2024, 50, 310–333. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, E.; Yun, J.; Arumugasamy, S.K.; Choi, M.-J.; Lee, Y.; Lee, S. High-performance electrocatalyst of activated carbon-decorated molybdenum trioxide nanocomposites for effective production of H2 and H2O2. Sep. Purif. Technol. 2025, 361, 131614. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Shanmugam, A.; Saravanan, S.; Pugazhendi, I.; Lee, Y.; Kim, D.Y.; Manikandan, R.; Chang, S.-C.; Lee, S. Enhanced bifunctional water electrolysis performance of spherical ZnMn2O4 nanoparticles. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Kazemi, A.; Manteghi, F.; Tehrani, Z. Metal Electrocatalysts for Hydrogen Production in Water Splitting. ACS Omega 2024, 9, 7310–7335. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Y.; Wang, Y.; Zhao, L.; Zhao, X.; Du, J.; Wu, H.; Chen, A. Next-Generation Green Hydrogen: Progress and Perspective from Electricity, Catalyst to Electrolyte in Electrocatalytic Water Splitting. Nano-Micro Lett. 2024, 16, 237. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Sim, D.H.; Lee, S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials 2022, 12, 531. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Fei, L.; Zhou, W.; Shao, Z. Electrochemical Oxidation of Small Molecules for Energy-Saving Hydrogen Production. Adv. Energy Mater. 2024, 14, 2401242. [Google Scholar] [CrossRef]
- Du, Y.; Li, Q.; Han, L.; Yang, P.; Xin, L.; Jin, W.; Xiao, W.; Li, Z.; Wang, J.; Wu, Z.; et al. Metallic Mo, Ru coupled with graphitic carbon dots to activate inertness 1D copper-based nanowires as efficient electrocatalyst for pH-universal hydrogen evolution reaction. Appl. Catal. B Environ. 2024, 344, 123617. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy. 2022, 2, 100144. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Sim, D.H.; Lee, S. Extraordinarily high hydrogen-evolution-reaction activity of corrugated graphene nanosheets derived from biomass rice husks. Int. J. Hydrogen Energy 2022, 47, 40317–40326. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Nguyen, N.-A.; Ho, T.H.; Nguyen, T.P.; Dang, H.T.; Pham, D.D.; Nguyen, T.L.; Thi, L.L.D.; Tran, T.N.; Tran, M.X.; et al. Pumpkin shell-derived activated carbon-supported S-incorporated transition metal oxide electrocatalyst for hydrogen evolution reaction. Fuel 2024, 373, 132357. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Zhong, Y.; Ge, L.; Jiang, S.P.; Shao, Z. From scheelite BaMoO4 to perovskite BaMoO3: Enhanced electrocatalysis toward the hydrogen evolution in alkaline media. Compos. B Eng. 2020, 198, 108214. [Google Scholar] [CrossRef]
- Mehta, S.; Thakur, R.; Rani, S.; Nagaraja, B.M.; Mehla, S.; Kainthla, I. Recent advances in ternary transition metal dichalcogenides for electrocatalytic hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 82, 1061–1080. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, Y.; Huang, Z.; Qiao, H.; Qi, X. Transition Metal Dichalcogenides in Electrocatalytic Water Splitting. Catalysts 2024, 14, 689. [Google Scholar] [CrossRef]
- Lee, D.J.; Kumar, G.M.; Sekar, S.; Ganesh, V.; Jeon, H.C.; Kim, D.Y.; Kang, T.W.; Ilanchezhiyan, P. Re1-xNixS2 nanosheets for high efficient electrocatalytic hydrogen evolution functions. Surf. Interfaces. 2023, 40, 103014. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Karuturi, S.; Catchpole, K.; Zhang, Q.; Zhao, C. Design and operando/in situ characterization of precious-metal-free electrocatalysts for alkaline water splitting. Carbon Energy 2020, 2, 582–613. [Google Scholar] [CrossRef]
- Gao, G.; Zhao, G.; Zhu, G.; Sun, B.; Sun, Z.; Li, S.; Lan, Y.-Q. Recent advancements in noble-metal electrocatalysts for alkaline hydrogen evolution reaction. Chin. Chem. Lett 2025, 36, 109557. [Google Scholar] [CrossRef]
- Chen, W.-F.; Muckerman, J.T.; Fujita, E. Recent developments in transition metal carbides and nitrides as hydrogen evolution electrocatalysts. Chem. Commun. 2013, 49, 8896–8909. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhang, H.; Ma, J.; Huang, Z.; Li, J.; Wang, Y. Transition-Metal Carbides as Hydrogen Evolution Reduction Electrocatalysts: Synthetic Methods and Optimization Strategies. Chem. Eur. J. 2021, 27, 5074–5090. [Google Scholar] [CrossRef]
- Du, M.; Li, D.; Liu, S.; Yan, J. Transition metal phosphides: A wonder catalyst for electrocatalytic hydrogen production. Chin. Chem. Lett 2023, 34, 108156. [Google Scholar] [CrossRef]
- Yin, H.; Rong, F.; Xie, Y. A review of typical transition metal phosphides electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2024, 52, 350–375. [Google Scholar] [CrossRef]
- Danish, M.S.S. Exploring metal oxides for the hydrogen evolution reaction (HER) in the field of nanotechnology. RSC Sustain. 2023, 1, 2180–2196. [Google Scholar] [CrossRef]
- Jamadar, A.S.; Sutar, R.; Patil, S.; Khandekar, R.; Yadav, J.B. Progress in metal oxide-based electrocatalysts for sustainable water splitting. Mater. Rep. Energy. 2024, 4, 100283. [Google Scholar] [CrossRef]
- Manikandan, R.; Sadhasivam, S.; Lee, S.; Seung-Cheol, C.; Ashok Kumar, K.; Bathula, C.; Gopalan Sree, V.; Young Kim, D.; Sekar, S. Deep eutectic solvents assisted synthesis of AC-decorated NiO nanocomposites for hydrogen evolution reaction. J. Mol. Liq. 2023, 375, 121338. [Google Scholar] [CrossRef]
- Meng, Z.; Zheng, S.; Luo, R.; Tang, H.; Wang, R.; Zhang, R.; Tian, T.; Tang, H. Transition Metal Nitrides for Electrocatalytic Application: Progress and Rational Design. Nanomaterials 2022, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.; Šljukić, B.; Gago, S.; Santos, D.M.F. The current state of transition metal-based electrocatalysts (oxides, alloys, POMs, and MOFs) for oxygen reduction, oxygen evolution, and hydrogen evolution reactions. Front. Energy Res. 2024, 12, 1373522. [Google Scholar] [CrossRef]
- Liao, P.-Q.; Shen, J.-Q.; Zhang, J.-P. Metal–organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sekar, S.; Khadtare, S.S.; Rochman, N.T.; Chinna, B.; Ansari, A.S. Anion-exchange synthesis of an MnCo2S4 electrocatalyst towards facilitated ultralong hydrogen evolution reaction in acidic and alkaline media. CrystEngComm 2024, 26, 215–222. [Google Scholar] [CrossRef]
- Tong, S.; Fu, B.; Gan, L.; Zhang, Z. Single atom catalysts for boosting electrocatalytic and photoelectrocatalytic performances. J. Mater. Chem. A 2021, 9, 10731–10738. [Google Scholar] [CrossRef]
- Alves, D.; Kasturi, P.R.; Collins, G.; Barwa, T.N.; Ramaraj, S.; Karthik, R.; Breslin, C.B. 2D layered double hydroxides and transition metal dichalcogenides for applications in the electrochemical production of renewable hydrogen. Mater. Adv. 2023, 4, 6478–6497. [Google Scholar] [CrossRef]
- He, H.; Xiao, J.; Liu, Z.; Yang, B.; Wang, D.; Peng, X.; Zeng, L.; Li, Z.; Lei, L.; Qiu, M.; et al. Boosting the hydrogen evolution of layered double hydroxide by optimizing the electronic structure and accelerating the water dissociation kinetics. Chem. Eng. J. 2023, 453, 139751. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.; Cao, Y.; Wang, F.; Qyyum, M.A.; Han, N. Recent advancements in perovskite electrocatalysts for clean energy-related applications: Hydrogen production, oxygen electrocatalysis, and nitrogen reduction. Int. J. Hydrogen Energy 2024, 52, 1104–1126. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, P.; Cheng, Y.; Mo, Y.; Luo, X.; Liu, P.; Guo, R.; Liu, X. Recent progress on NiFe2O4 spinels as electrocatalysts for the oxygen evolution reaction. J. Electroanal. Chem. 2023, 946, 117703. [Google Scholar] [CrossRef]
- Begum, H.; Jung, S. Electronically tuned defective Prussian-blue on graphene for electrochemical water splitting. Int. J. Hydrogen Energy 2022, 47, 28752–28762. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, W.; Yang, L.; Li, L.; Zhang, Z.; Ke, Y.; Chen, S. Nitrogen and sulfur co-doped porous carbon derived from human hair as highly efficient metal-free electrocatalysts for hydrogen evolution reactions. J. Mater. Chem. A 2015, 3, 8840–8846. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Chen, H.; Zhang, C.; Zhang, Y.; Gu, C.; Xu, X.; Li, Q. Synthesis of biomass porous carbon materials from bean sprouts for hydrogen evolution reaction electrocatalysis and supercapacitor electrode. Int. J. Hydrogen Energy 2021, 46, 18887–18897. [Google Scholar] [CrossRef]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Shinde, S.S.; Sami, A.; Lee, J.-H. Electrocatalytic hydrogen evolution using graphitic carbon nitride coupled with nanoporous graphene co-doped by S and Se. J. Mater. Chem. A 2015, 3, 12810–12819. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Yang, W.; Yang, Y.; Fu, Q.; Zhang, L.; Liao, Q.; Zhu, X. An open-structured carbon fiber brush electrode for efficient hydrogen evolution by inducing oriented bubble transport. Chem. Eng. J. 2021, 407, 127159. [Google Scholar] [CrossRef]
- Sun, H.; Xue, L.; Shi, Y.; Dong, J.; Wu, Q.; Yao, W. Waste paper derived Co, N co-doped carbon as an efficient electrocatalyst for hydrogen evolution. Reac. Kinet. Mech. Cat. 2021, 132, 1137–1150. [Google Scholar] [CrossRef]
- Saravanan, K.R.A.; Prabu, N.; Sasidharan, M.; Maduraiveeran, G. Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 489, 725–733. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Ren, T.-Z.; Yuan, Z.-Y.; Bandosz, T.J. Activated carbon with heteroatoms from organic salt for hydrogen evolution reaction. Microporous Mesoporous Mater. 2020, 297, 110033. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Chanda, D.; Hnát, J.; Dobrota, A.S.; Pašti, I.A.; Paidar, M.; Bouzek, K. The effect of surface modification by reduced graphene oxide on the electrocatalytic activity of nickel towards the hydrogen evolution reaction. Phys. Chem. Chem. Phys. 2015, 17, 26864–26874. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ola, O.; Zhao, J.; Yang, Z.; Tiwari, S.K.; Wang, N.; Zhu, Y. Recent Progress in Graphene-Based Electrocatalysts for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1806. [Google Scholar] [CrossRef]
- Wu, H.; Chen, Z.; Wang, Y.; Cao, E.; Xiao, F.; Chen, S.; Du, S.; Wu, Y.; Ren, Z. Regulating the allocation of N and P in codoped graphene via supramolecular control to remarkably boost hydrogen evolution. Energy Environ. Sci. 2019, 12, 2697–2705. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, A.; Gupta, R.K. Nanostructured carbon materials derived from biomass waste for electrocatalytic hydrogen production. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Bejjanki, D.; Banothu, P.; Kumar, V.B.; Kumar, P.S. Biomass-Derived N-Doped Activated Carbon from Eucalyptus Leaves as an Efficient Supercapacitor Electrode Material. C 2023, 9, 24. [Google Scholar] [CrossRef]
- Grima-Olmedo, C.; Ramírez-Gómez, Á.; Gómez-Limón, D.; Clemente-Jul, C. Activated carbon from flash pyrolysis of eucalyptus residue. Heliyon 2016, 2, e00155. [Google Scholar] [CrossRef]
- Fennila, J.; Vijayalakshmi, K.A. Renewable cold plasma exposed activated carbon-derived eucalyptus leaves (EL) as electrode in fabricating low-cost energy storage devices. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 6743–6760. [Google Scholar] [CrossRef]
- Jain, D.; Kanungo, J.; Tripathi, S.K. Enhancement in performance of supercapacitor using eucalyptus leaves derived activated carbon electrode with CH3COONa and HQ electrolytes: A step towards environment benign supercapacitor. J. Alloys Compd. 2020, 832, 154956. [Google Scholar] [CrossRef]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Ansari, A.S.; Sree, V.G.; Jana, A.; Meena, A.; Sekar, S.; Cho, S.; Kim, H.; Im, H. Nitrogen-Doped CuO@CuS Core–Shell Structure for Highly Efficient Catalytic OER Application. Nanomaterials 2023, 13, 3160. [Google Scholar] [CrossRef]
- Sekar, S.; Ilanchezhiyan, P.; Ahmed, A.T.A.; Lee, Y.; Lee, S. Substantial Electrocatalytic Oxygen Evolution Performances of Activated Carbon-Decorated Vanadium Pentoxide Nanocomposites. Int. J. Energy Res. 2024, 2024, 9953038. [Google Scholar] [CrossRef]
- Sekar, S.; Yun, J.-S.; Park, S.; Kim, D.Y.; Lee, Y.; Lee, S. Excellent Bifunctional Water Electrolysis Activities of α-MoO3/AC Nanocomposites. Int. J. Energy Res. 2024, 2024, 3167699. [Google Scholar] [CrossRef]
- Manasa, P.; Lei, Z.J.; Ran, F. Biomass Waste Derived Low Cost Activated Carbon from Carchorus Olitorius (Jute Fiber) as Sustainable and Novel Electrode Material. J. Energy Storage 2020, 30, 101494. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Inamdar, A.I.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Activated carbon-decorated spherical silicon nanocrystal composites synchronously-derived from rice husks for anodic source of lithium-ion battery. Nanomaterials 2019, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Xiao, P.-W.; Meng, Q.; Zhao, L.; Li, J.-J.; Wei, Z.; Han, B.-H. Biomass-derived flexible porous carbon materials and their applications in supercapacitor and gas adsorption. Mater. Des. 2017, 129, 164–172. [Google Scholar] [CrossRef]
- Homayounfard, A.M.; Maleki, M.; Ghanbari, H.; Kahnamouei, M.H.; Safaei, B. Growth of few-layer flower-like MoS2 on heteroatom-doped activated carbon as a hydrogen evolution reaction electrode. Int. J. Hydrogen Energy 2024, 55, 1360–1370. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Raghavan, V. Facile synthesis of activated carbon derived from Eucalyptus globulus seed as efficient electrode material for supercapacitors. Diam. Relat. Mater 2020, 109, 108038. [Google Scholar] [CrossRef]
- Fu, P.; Zhou, L.; Sun, L.; Huang, B.; Yuan, Y. Nitrogen-doped porous activated carbon derived from cocoon silk as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2017, 7, 13383–13389. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nano. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol adsorption on high microporous activated carbons prepared from oily sludge: Equilibrium, kinetic and thermodynamic studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef]
- Amalanathan, M.; Aravind, M.; Ahmed, N.; Sony Michel Mary, M.; Velusamy, P.; Kumaresubitha, T.; Noreen, R.; Ali, S. The influence of activated carbon annealing temperature on sunlight-driven photocatalytic dye degradation and biological activity. Inorg. Chem. Commun. 2022, 146, 110149. [Google Scholar] [CrossRef]
- Prabu, N.; Saravanan, R.S.A.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. An efficient palm waste derived hierarchical porous carbon for electrocatalytic hydrogen evolution reaction. Carbon 2019, 152, 188–197. [Google Scholar] [CrossRef]
- Bao, F.; Kemppainen, E.; Dorbandt, I.; Bors, R.; Xi, F.; Schlatmann, R.; van de Krol, R.; Calnan, S. Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel-Molybdenum in Acidic, Near-Neutral, and Alkaline Conditions. ChemElectroChem 2021, 8, 195–208. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef]
- Strmcnik, D.; Lopes, P.P.; Genorio, B.; Stamenkovic, V.R.; Markovic, N.M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 2016, 29, 29–36. [Google Scholar] [CrossRef]
- Ullah, N.; Zhao, W.; Lu, X.; Oluigbo, C.J.; Shah, S.A.; Zhang, M.; Xie, J.; Xu, Y. In situ growth of M-MO (M = Ni, Co) in 3D graphene as a competent bifunctional electrocatalyst for OER and HER. Electrochim. Acta 2019, 298, 163–171. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dinh, K.N.; Gomes, V.G. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: A sustainable bifunctional electrocatalyst for efficient water splitting. Carbon 2020, 157, 515–524. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M.; Xu, W.; Wu, X.; Jiang, J. Catalytically Active Carbon From Cattail Fibers for Electrochemical Reduction Reaction. Front. Chem. 2019, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Zheng, Y.; Zhang, X.; Davey, K.; Dai, S.; Qiao, S.Z. Promotion of Electrocatalytic Hydrogen Evolution Reaction on Nitrogen-Doped Carbon Nanosheets with Secondary Heteroatoms. ACS Nano 2017, 11, 7293–7300. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).