Catalytic Combustion of Biodiesel Wastewater on Red Mud Catalyst
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Characterization
2.2.1. Characterizations of Biodiesel Wastewater
2.2.2. Characterizations of Catalyst
2.3. Catalytic Test
3. Results and Discussion
3.1. Analysis of Biodiesel Wastewater
3.2. Composition of RM
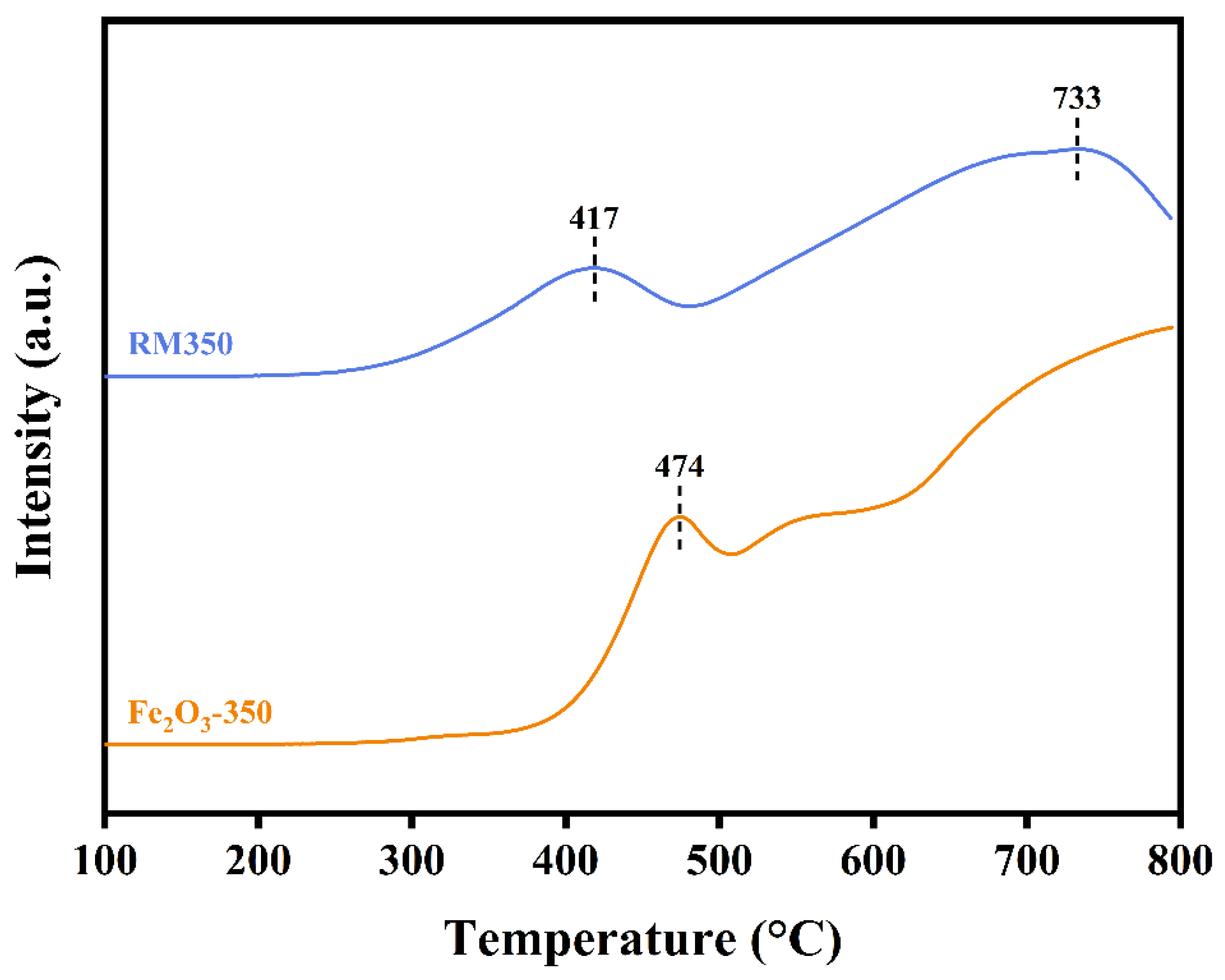
3.3. TG-DSC
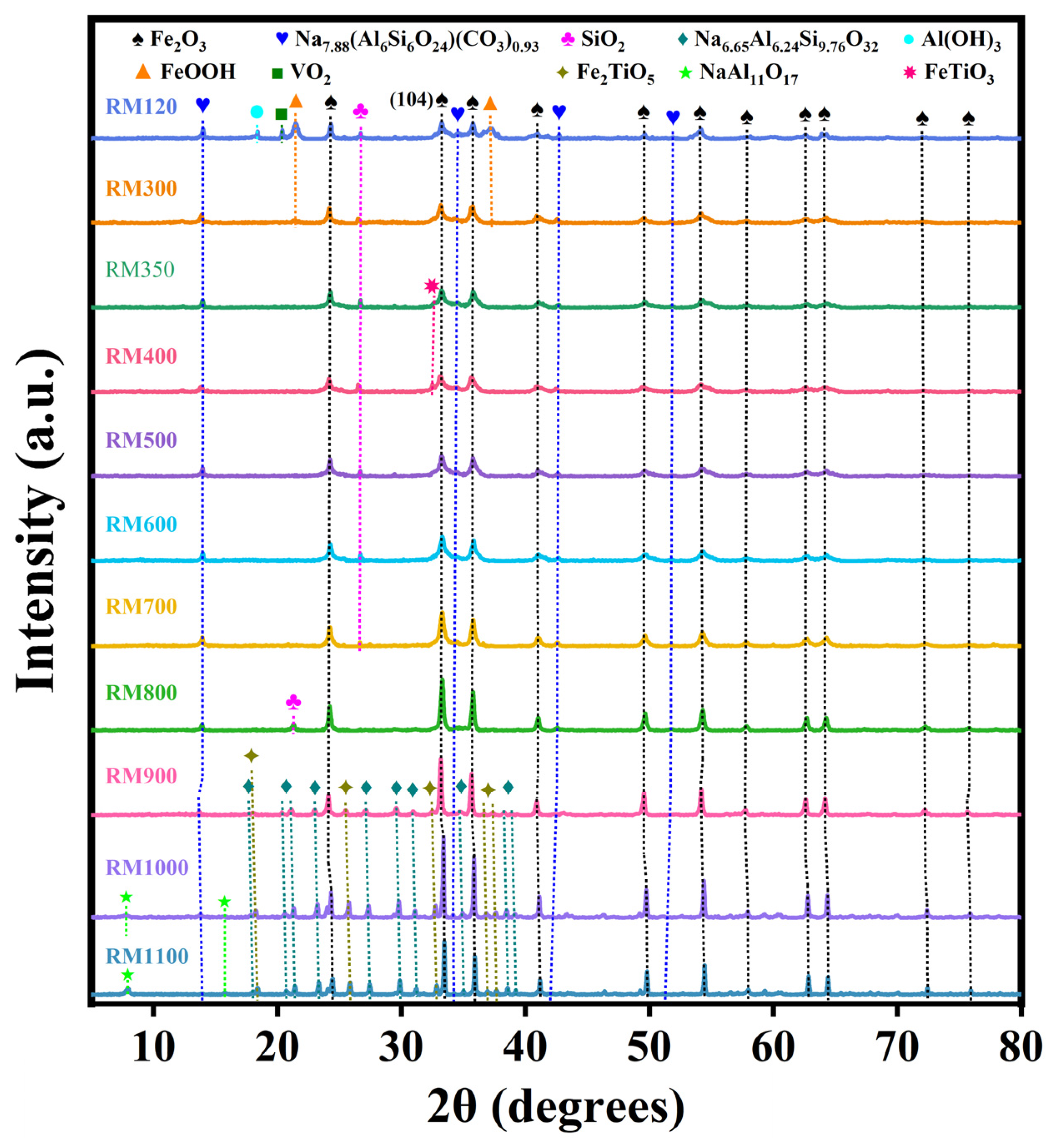
3.4. Crystal Phase Composition
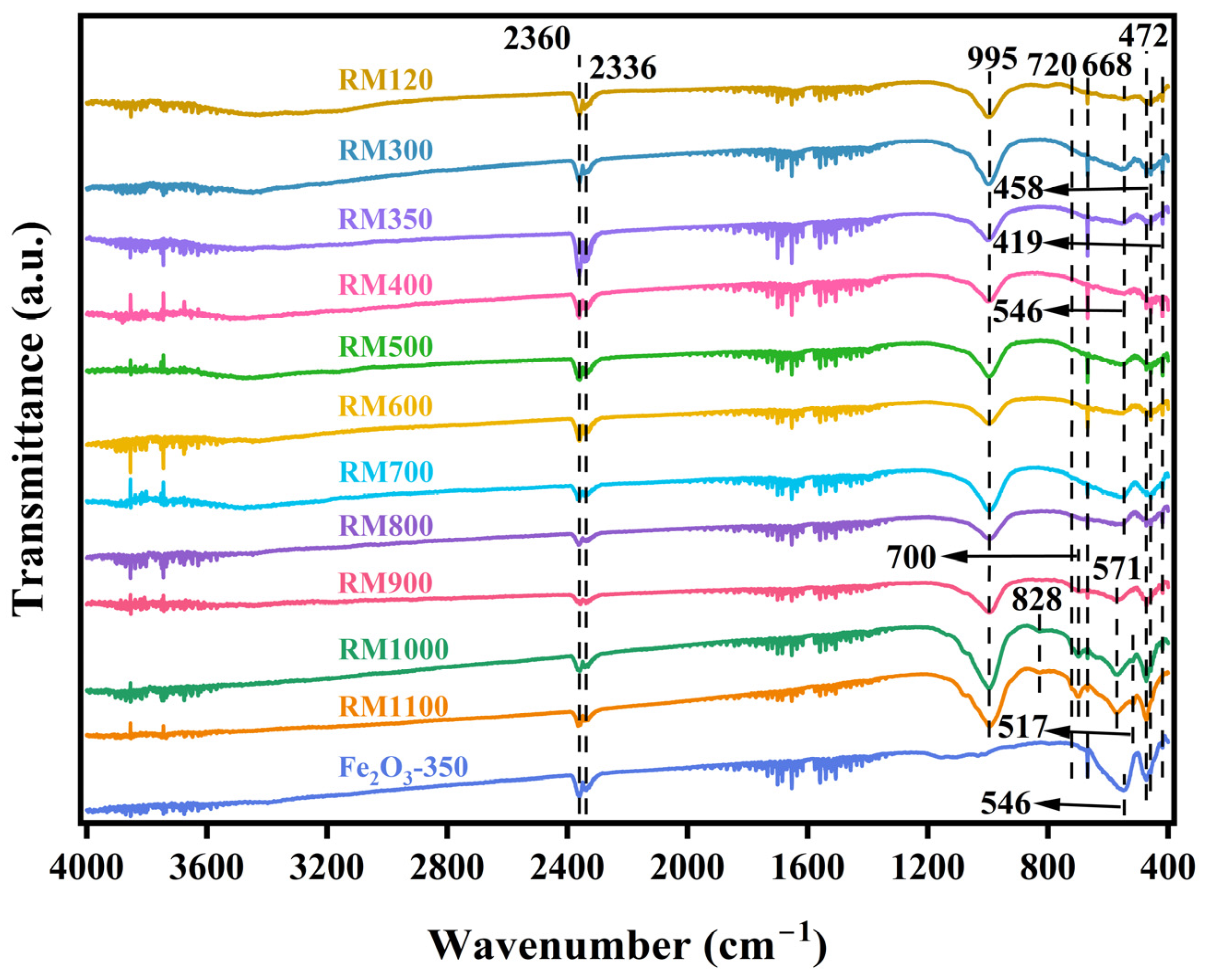
3.5. FTIR Spectra
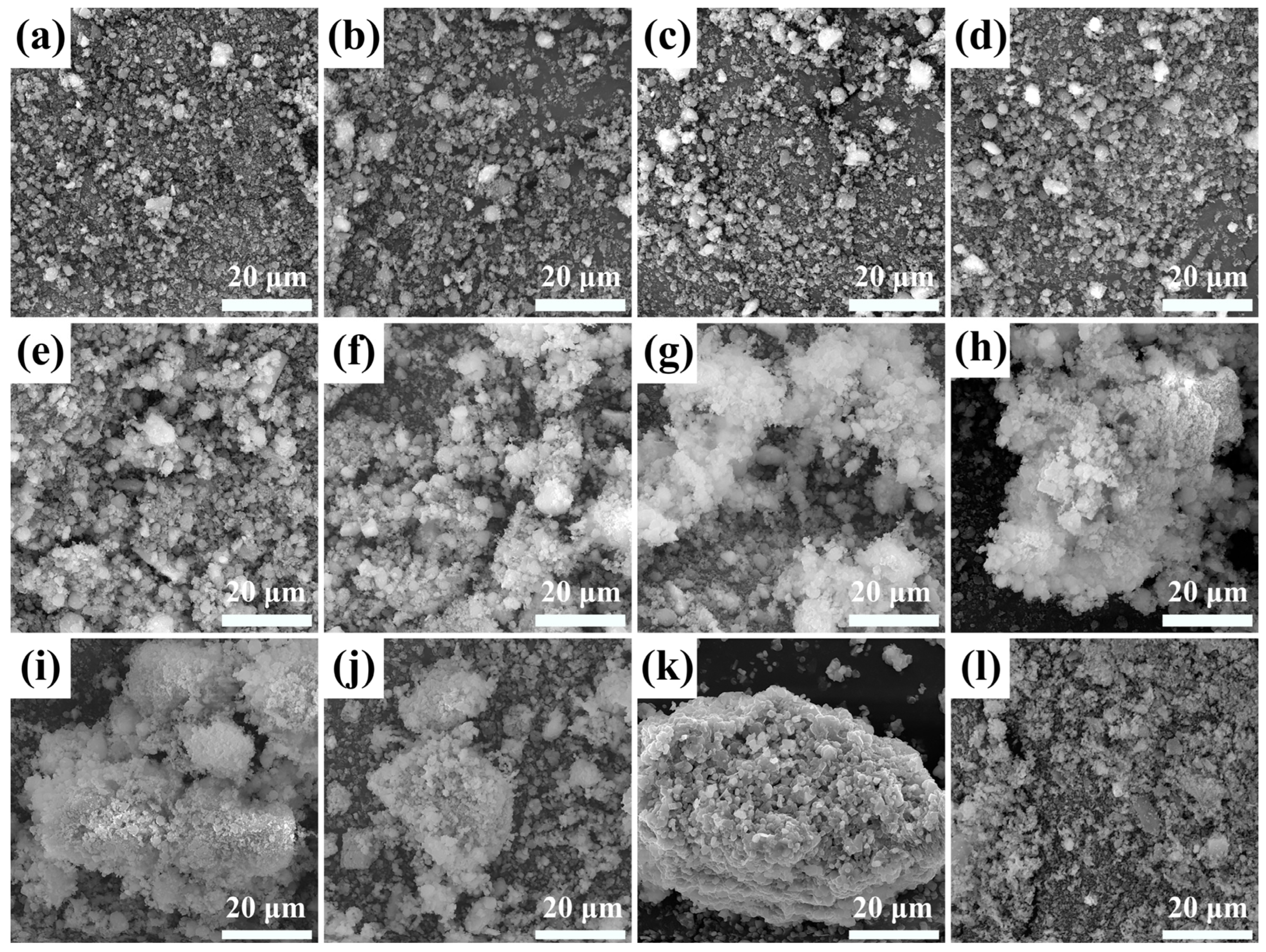
3.6. Catalyst Morphology
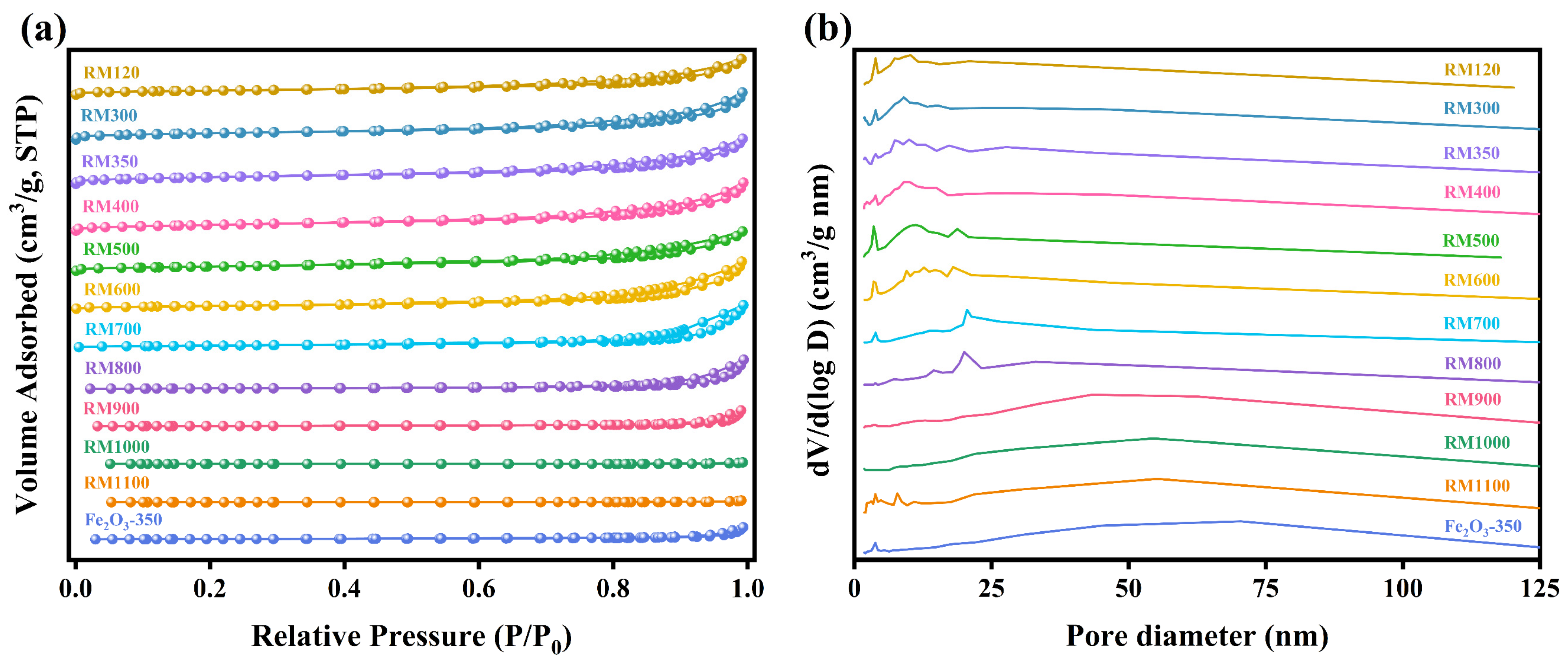
3.7. Pore Structure Analysis
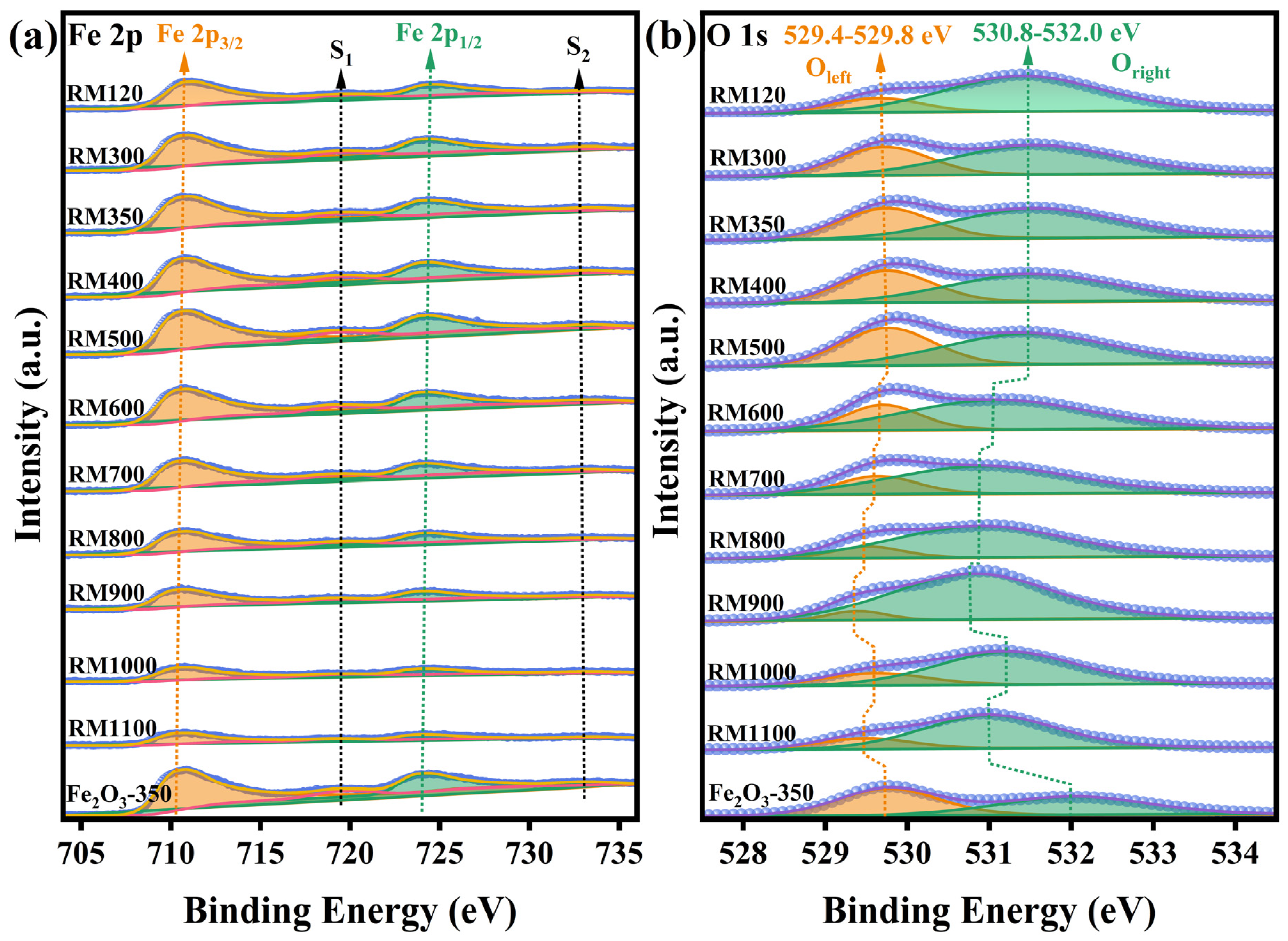
3.8. Surface Chemical Composition and State
3.9. Reducibility
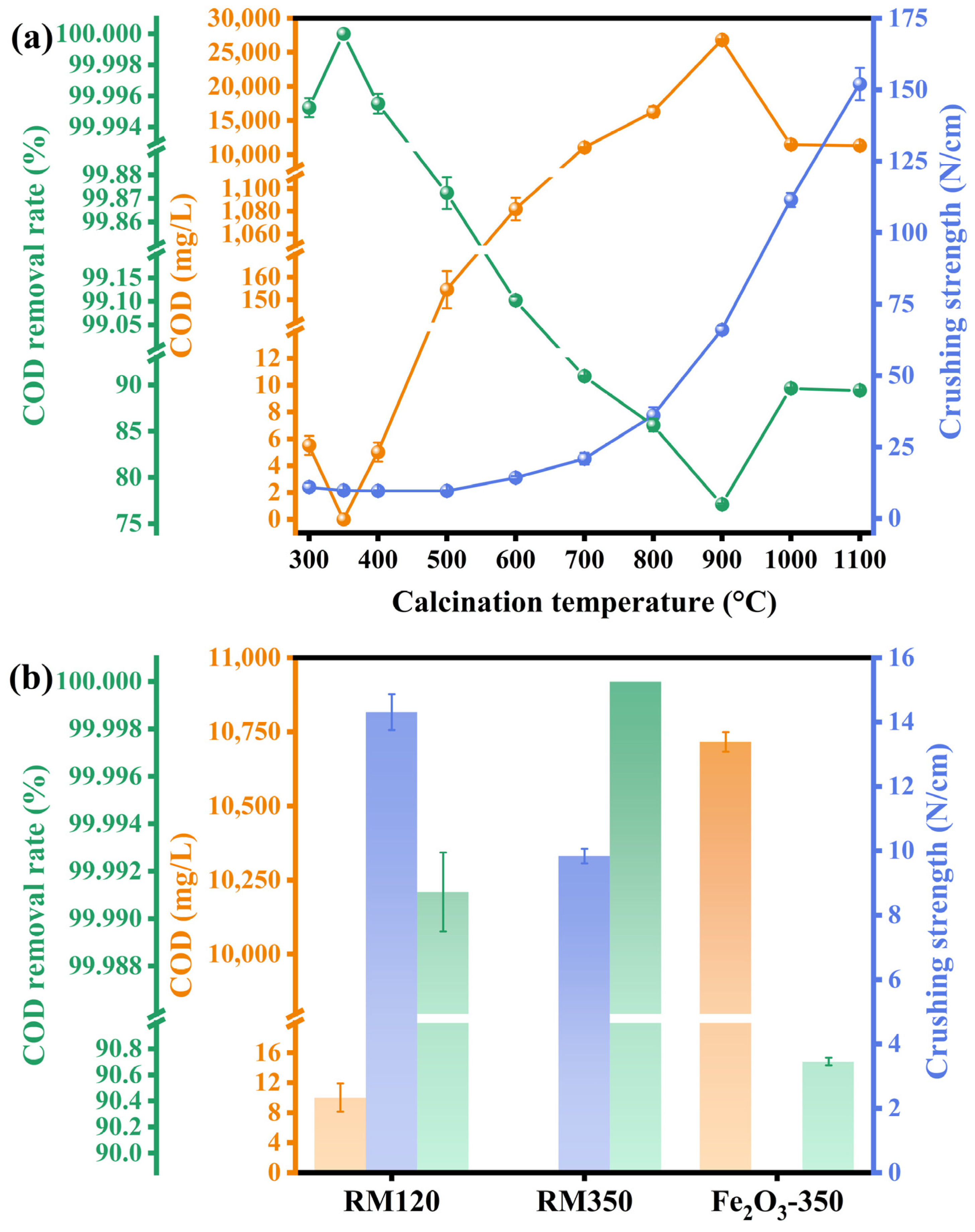
3.10. Catalytic Activity and Mechanical Strength
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. A Critical Review of Bio-Diesel as a Vehicular Fuel. Energy Convers. Manag. 2008, 49, 2727–2741. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and Recent Trends in Biodiesel Fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Berrios, M.; Skelton, R.L. Comparison of Purification Methods for Biodiesel. Chem. Eng. J. 2008, 144, 459–465. [Google Scholar] [CrossRef]
- Veljković, V.B.; Stamenković, O.S.; Tasić, M.B. The Wastewater Treatment in the Biodiesel Production with Alkali-Catalyzed Transesterification. Renew. Sustain. Energy Rev. 2014, 32, 40–60. [Google Scholar] [CrossRef]
- European Biodiesel Board. Statistical Report 2023; European Biodiesel Board: Etterbeek, Belgium, 2024; Available online: https://ebb-eu.org/wp-content/uploads/2024/03/EBB_Statistical_Report2023-Final.pdf (accessed on 20 December 2024).
- Jaruwat, P.; Kongjao, S.; Hunsom, M. Management of Biodiesel Wastewater by the Combined Processes of Chemical Recovery and Electrochemical Treatment. Energy Convers. Manag. 2010, 51, 531–537. [Google Scholar] [CrossRef]
- Ngamlerdpokin, K.; Kumjadpai, S.; Chatanon, P.; Tungmanee, U.; Chuenchuanchom, S.; Jaruwat, P.; Lertsathitphongs, P.; Hunsom, M. Remediation of Biodiesel Wastewater by Chemical- and Electro-Coagulation: A Comparative Study. J. Environ. Manag. 2011, 92, 2454–2460. [Google Scholar] [CrossRef]
- Daud, N.M. Production of Biodiesel and Its Wastewater Treatment Technologies: A Review. Process Saf. Environ. 2015, 94, 487–508. [Google Scholar] [CrossRef]
- Torres, J.J.; Cuello, M.; Ochoa, N.A.; Pagliero, C. Biodiesel Wastewater Treatment Using Nanofiltration Membranes. Process Saf. Environ. Prot. 2021, 148, 825–833. [Google Scholar] [CrossRef]
- Yu, S.; Bai, J.; Xie, Q.; Liang, X.; Nie, Y. Efficient Treatment of Biodiesel Wastewater by Catalytic Combustion. J. Water Process Eng. 2021, 43, 102207. [Google Scholar] [CrossRef]
- Nie, Y.; Yu, S.; Liang, X.; Xie, Q.; Wu, Z.; Bai, J. Process and Device for Continuous Treatment of High-Concentration Organic Wastewater. US Patent US11767232B2, 26 September 2023. [Google Scholar]
- Yu, S.; Yuan, W.; Bai, J.; Xie, Q.; Liang, X.; Nie, Y. Catalytic Combustion of Biodiesel Wastewater over the Fe2O3 Catalyst Coupled with a Pt-Based Catalyst. Environ. Sci. Water Res. Technol. 2024, 10, 2366–2380. [Google Scholar] [CrossRef]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and Iron Oxides as Combustion Catalysts of Volatile Organic Compounds. Appl. Catal. B Environ. 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Deng, W.; Han, J.; Qin, L.; Zhao, B.; Guo, L.; Xing, F. Study on the Structure-Activity Relationship of Fe-Mn Oxide Catalysts for Chlorobenzene Catalytic Combustion. Chem. Eng. J. 2020, 395, 125172. [Google Scholar] [CrossRef]
- Soltan, W.B.; Sun, J.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y.; Zhang, Z. Discovering the Key Role of MnO2 and CeO2 Particles in the Fe2O3 Catalysts for Enhancing the Catalytic Oxidation of VOC: Synergistic Effect of the Lattice Oxygen Species and Surface-Adsorbed Oxygen. Sci. Total Environ. 2022, 819, 152844. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Y.; Jia, Z.; Liu, F.; Song, Z. Properties and Mechanisms of Low Concentration Methane Catalytic Combustion in Porous Media Supported with Transition Metal Oxides. Appl. Energy 2023, 350, 121811. [Google Scholar] [CrossRef]
- Samal, S. Utilization of Red Mud as a Source for Metal Ions—A Review. Materials 2021, 14, 2211. [Google Scholar] [CrossRef] [PubMed]
- Shoppert, A.; Loginova, I.; Napol’skikh, J.; Valeev, D. High-Selective Extraction of Scandium (Sc) from Bauxite Residue (Red Mud) by Acid Leaching with MgSO4. Materials 2022, 15, 1343. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Wu, C.; Zhang, W.; Chen, Y.; Li, Y. Preparation and Research on Mechanical Properties of Eco-Friendly Geopolymer Grouting Cementitious Materials Based on Industrial Solid Wastes. Materials 2024, 17, 3874. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Xue, B.; Chen, L.; Wang, G.; Wang, J.; Wan, H.; Lin, X.; Zhu, G. Simulation of Red Mud/Phosphogypsum-Based Artificial Soil Engineering Applications in Vegetation Restoration and Ecological Reconstruction. Sci. Total Environ. 2024, 951, 175656. [Google Scholar] [CrossRef]
- Paredes, J.R.; Ordóñez, S.; Vega, A.; Díez, F.V. Catalytic Combustion of Methane over Red Mud-Based Catalysts. Appl. Catal. B 2004, 47, 37–45. [Google Scholar] [CrossRef]
- Sushil, S.; Scholz, P.; Pollok, K.; Ondruschka, B.; Batra, V.S. Application of Industrial Waste Based Catalysts for Total Oxidation of Propane. Chem. Eng. J. 2011, 166, 568–578. [Google Scholar] [CrossRef]
- Kim, S.C.; Nahm, S.W.; Park, Y.-K. Property and Performance of Red Mud-Based Catalysts for the Complete Oxidation of Volatile Organic Compounds. J. Hazard. Mater. 2015, 300, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Shim, W.G.; Nah, J.W.; Jung, H.-Y.; Park, Y.-K.; Jung, S.C.; Kim, S.C. Recycling of Red Mud as a Catalyst for Complete Oxidation of Benzene. J. Ind. Eng. Chem. 2018, 60, 259–267. [Google Scholar] [CrossRef]
- Ha, H.K.P.; Tri, N.; Anh, N.P.; Van, N.T.T.; Hieu, D.T.M.; Anh, N.T. CuO-Doped Catalyst Synthesized from Red Mud and Rice Husk Ash Using Urea-Nitrate Combustion Technique for p-Xylene Deep Oxidation. Mater. Trans. 2019, 60, 2470–2474. [Google Scholar] [CrossRef]
- Pande, G.; Selvakumar, S.; Ciotonea, C.; Giraudon, J.-M.; Lamonier, J.-F.; Batra, V.S. Modified Red Mud Catalyst for Volatile Organic Compounds Oxidation. Catalysts 2021, 11, 838. [Google Scholar] [CrossRef]
- Yu, S.; Duan, Y.; Mao, X.; Xie, Q.; Zeng, G.; Lu, M.; Nie, Y.; Ji, J. Pyrolysis of Methyl Ricinoleate by Microwave-Assisted Heating Coupled with Atomization Feeding. J. Anal. Appl. Pyrolysis 2018, 135, 176–183. [Google Scholar] [CrossRef]
- GB/T 30202.3-2013; Test Method for Granular Activated Carbon from Coal for Desulfurization and Denitration Process—Part3: Abrasive Resistance and Compression Strength. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Li, Y.; Wei, G.; Shao, L.; Li, Z.; Yu, F.; Liu, J.; Yang, X.; Lu, Q.; Li, A.; Huang, Y.; et al. Green Synthesis of Red Mud Based ZnO Fe2O3 Composite Used for Photo-Fenton Reaction under Visible Light. J. Clean. Prod. 2019, 207, 717–727. [Google Scholar] [CrossRef]
- Zou, X.; Ma, Z.; Deng, J.; Zhong, J.; He, Y.; Liu, J. Core-Shell PdO@SiO2/Al2O3 with Sinter-Resistance and Water-Tolerance Promoting Catalytic Methane Combustion. Chem. Eng. J. 2020, 396, 125275. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Jing, H.; Xiao, J.; Xie, H.; Hong, F.; Ta, N.; Zhang, X.; Zhu, J.; Li, C. Blocking the Reverse Reactions of Overall Water Splitting on a Rh/GaN–ZnO Photocatalyst Modified with Al2O3. Nat. Catal. 2023, 6, 80–88. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The Composition, Recycling and Utilisation of Bayer Red Mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Zhong, Q.; Cao, M.; Hu, H.; Yang, D.; Chen, M.; Li, P.; Wu, L.; Zhang, Q. One-Pot Synthesis of Highly Stable CsPbBr3@SiO2 Core–Shell Nanoparticles. ACS Nano 2018, 12, 8579–8587. [Google Scholar] [CrossRef]
- Zhao, S.; Hu, F.; Li, J. Hierarchical Core–Shell Al2O3@Pd-CoAlO Microspheres for Low-Temperature Toluene Combustion. ACS Catal. 2016, 6, 3433–3441. [Google Scholar] [CrossRef]
- Sushil, S.; Batra, V.S. Catalytic Applications of Red Mud, an Aluminium Industry Waste: A Review. Appl. Catal. B 2008, 81, 64–77. [Google Scholar] [CrossRef]
- Geng, S.; Zhang, Z.; Li, J.; Qian, J.; Liu, J.; Yu, J.; Xu, G. Catalytic Behavior in CH4 Decomposition of Catalysts Derived from Red Mud: Impact of Residual Na2O. Int. J. Hydrogen Energy 2022, 47, 7836–7845. [Google Scholar] [CrossRef]
- Meng, L.; Zhao, H. Low-Temperature Complete Removal of Toluene over Highly Active Nanoparticles CuO-TiO2 Synthesized via Flame Spray Pyrolysis. Appl. Catal. B 2020, 264, 118427. [Google Scholar] [CrossRef]
- Kurtoğlu, S.F.; Soyer-Uzun, S.; Uzun, A. Tuning Structural Characteristics of Red Mud by Simple Treatments. Ceram. Int. 2016, 42, 17581–17593. [Google Scholar] [CrossRef]
- Lin, S.; Gu, Z.; Zhu, X.; Wei, Y.; Long, Y.; Yang, K.; He, F.; Wang, H.; Li, K. Synergy of Red Mud Oxygen Carrier with MgO and NiO for Enhanced Chemical-Looping Combustion. Energy 2020, 197, 117202. [Google Scholar] [CrossRef]
- Abdulvaliyev, R.A.; Akcil, A.; Gladyshev, S.V.; Tastanov, E.A.; Beisembekova, K.O.; Akhmadiyeva, N.K.; Deveci, H. Gallium and Vanadium Extraction from Red Mud of Turkish Alumina Refinery Plant: Hydrogarnet Process. Hydrometallurgy 2015, 157, 72–77. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, J.; Cao, Q.; Zhou, Z.; Hu, W.; Liu, X. Highly Efficient Removal of Toluene over Cu-V Oxides Modified γ-Al2O3 in the Presence of SO2. J. Hazard. Mater. 2022, 436, 129041. [Google Scholar] [CrossRef]
- Wang, X. Enhanced Sequestration of CO2 from Simulated Electrolytic Aluminum Flue Gas by Modified Red Mud. J. Environ. Manag. 2023, 346, 118972. [Google Scholar] [CrossRef]
- Liu, S. Upcycling Sintering Red Mud Waste for Novel Superfine Composite Mineral Admixture and CO2 Sequestration. Cem. Concr. Compos. 2022, 129, 104497. [Google Scholar] [CrossRef]
- Ottonello-Briano, F.; Errando-Herranz, C.; Rödjegård, H.; Martin, H.; Sohlström, H.; Gylfason, K.B. Carbon Dioxide Absorption Spectroscopy with a Mid-Infrared Silicon Photonic Waveguide. Opt. Lett. 2020, 45, 109. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Sitarz, M.; Zajac, M.; Deja, J. The Effect of CaO/SiO2 Molar Ratio of CaO-Al2O3-SiO2 Glasses on Their Structure and Reactivity in Alkali Activated System. Spectrochim. Acta Part A 2018, 194, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing Amorphous Silica, Short-Range-Ordered Silicates and Silicic Acid Species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Strengthening Mechanism of Red Mud with Calcium Oxide. Constr. Build. Mater. 2024, 424, 135932. [Google Scholar] [CrossRef]
- Cui, W. Damage Degradation Pattern and Life Time Prediction of Solidified Red Mud under Coupled Environment of Corrosive Salt and Freeze-Thaw Cycles. Constr. Build. Mater. 2024, 440, 137455. [Google Scholar] [CrossRef]
- Song, Z.; Ke, G.; Qin, P.; Han, S.; Guo, X.; Zhang, Z. Solidification Mechanism of Bayer Red Mud under the Action of Calcium Hydroxide. Sustainability 2024, 16, 4770. [Google Scholar] [CrossRef]
- Palmer, S.J.; Frost, R.L.; Nguyen, T. Hydrotalcites and Their Role in Coordination of Anions in Bayer Liquors: Anion Binding in Layered Double Hydroxides. Coord. Chem. Rev. 2009, 253, 250–267. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, W.; Zhao, Y.; Zhang, G.; Zhang, W. Enhanced Catalytic Degradation of Methylene Blue by α-Fe2O3/Graphene Oxide via Heterogeneous Photo-Fenton Reactions. Appl. Catal. B 2017, 206, 642–652. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhao, Y.; Dionysiou, D.D. Aligned α-FeOOH Nanorods Anchored on a Graphene Oxide-Carbon Nanotubes Aerogel Can Serve as an Effective Fenton-like Oxidation Catalyst. Appl. Catal. B 2017, 213, 74–86. [Google Scholar] [CrossRef]
- Pang, J.; Fu, F.; Li, W.; Zhu, L.; Tang, B. Fe-Mn Binary Oxide Decorated Diatomite for Rapid Decolorization of Methylene Blue with H2O2. Appl. Surf. Sci. 2019, 478, 54–61. [Google Scholar] [CrossRef]
- Ma, X.; Feng, X.; Guo, J.; Cao, H.; Suo, X.; Sun, H.; Zheng, M. Catalytic Oxidation of 1,2-Dichlorobenzene over Ca-Doped FeOx Hollow Microspheres. Appl. Catal. B Environ. 2014, 147, 666–676. [Google Scholar] [CrossRef]
- Ordóñez, S.; Sastre, H.; Díez, F.V. Characterisation and Deactivation Studies of Sulfided Red Mud Used as Catalyst for the Hydrodechlorination of Tetrachloroethylene. Appl. Catal. B 2001, 29, 263–273. [Google Scholar] [CrossRef]
- GB 8978-1996; Integrated Wastewater Discharge Standard. Standardization Administration of the People’s Republic of China: Beijing, China, 1996.
- Tanattı, N.P. Optimizing TOC and COD Removal for the Biodiesel Wastewater by Electrocoagulation. Appl. Water Sci. 2018, 8, 58. [Google Scholar] [CrossRef]
- Costa, N.M.; Silva, V.M.; Damaceno, G.; Sousa, R.M.F.; Richter, E.M.; Machado, A.E.H.; Trovó, A.G. Integrating Coagulation-Flocculation and UV-C or H2O2/UV-C as Alternatives for Pre- or Complete Treatment of Biodiesel Effluents. J. Environ. Manag. 2017, 203, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Rattanapan, C.; Sawain, A.; Suksaroj, T.; Suksaroj, C. Enhanced Efficiency of Dissolved Air Flotation for Biodiesel Wastewater Treatment by Acidification and Coagulation Processes. Desalination 2011, 280, 370–377. [Google Scholar] [CrossRef]
- Ramírez, X.M.V.; Mejía, G.M.H.; López, K.V.P.; Vásquez, G.R.; Sepúlveda, J.M.M. Wastewater Treatment from Biodiesel Production via a Coupled Photo-Fenton–Aerobic Sequential Batch Reactor (SBR) System. Water Sci. Technol. 2012, 66, 824–830. [Google Scholar] [CrossRef]

| Parameter | Unit | Value |
|---|---|---|
| COD (Chemical Oxygen Demand) | mg/L | 114,500 ± 7600 |
| BOD5 (5-day Biochemical Oxygen Demand) | mg/L | 55,750 ± 3700 |
| SS (Suspended Solids) | mg/L | 59.3 ± 2 |
| EC (Electrical Conductivity) | μS/cm | 190 ± 5 |
| Colority | times | 11 |
| pH | — | 3.85 ± 0.05 |
| Appearance | — | Yellow |
| Element | RM | Error |
|---|---|---|
| Fe | 27.74 | 0.17 |
| Al | 12.64 | 0.11 |
| Si | 10.42 | 0.09 |
| Na | 7.36 | 0.13 |
| Ti | 2.73 | 0.06 |
| Ca | 0.398 | 0.02 |
| Mg | 0.174 | 0.009 |
| S | 0.157 | 0.008 |
| K | 0.116 | 0.006 |
| V | 0.0998 | 0.005 |
| P | 0.0939 | 0.0047 |
| Sample | SBET (m2/g) a | Vp (cm3/g) b | Dp (nm) c | Crystallite Size (nm) d | Fe 2p (eV) e | O 1s (eV) e | ||
|---|---|---|---|---|---|---|---|---|
| Fe 2p3/2 | Fe 2p1/2 | Oleft | Oright | |||||
| RM120 | 43.37 | 0.124 | 11.39 | 15 | 710.8 | 724.3 | 529.6 | 531.4 |
| RM300 | 61.55 | 0.152 | 9.87 | 15 | 710.5 | 724.0 | 529.7 | 531.6 |
| RM350 | 64.15 | 0.147 | 9.19 | 15 | 710.5 | 724.0 | 529.7 | 531.6 |
| RM400 | 60.03 | 0.150 | 9.98 | 15 | 710.5 | 724.0 | 529.8 | 531.5 |
| RM500 | 46.10 | 0.133 | 11.53 | 15 | 710.4 | 723.9 | 529.8 | 531.5 |
| RM600 | 33.63 | 0.157 | 18.65 | 16 | 710.4 | 723.9 | 529.7 | 531.1 |
| RM700 | 23.65 | 0.132 | 22.26 | 19 | 710.3 | 723.8 | 529.6 | 530.9 |
| RM800 | 12.21 | 0.084 | 27.64 | 32 | 710.4 | 723.9 | 529.5 | 530.9 |
| RM900 | 6.74 | 0.055 | 32.57 | 51 | 710.3 | 723.8 | 529.4 | 530.8 |
| RM1000 | 0.80 | 0.005 | 22.47 | >100 | 710.4 | 723.9 | 529.6 | 531.2 |
| RM1100 | 0.65 | 0.005 | 32.83 | 88 | 710.3 | 723.8 | 529.5 | 531.0 |
| Fe2O3-350 | 7.62 | 0.040 | 17.27 | — | 710.3 | 723.8 | 529.8 | 532.0 |
| Process | Biodiesel Wastewater | References | ||
|---|---|---|---|---|
| Original COD (mg/L) | Treated COD (mg/L) | COD Removal Rate (%) | ||
| Catalytic combustion | 109,159 | 0 | 100 | This work |
| Membrane treatment | 47,200 | 13,688 | 71 | [10] |
| Electrocoagulation | 399,800 | 35,982 | 91 | [58] |
| Coagulation-flocculation and photolysis | 5960 | 1311 | 78 | [59] |
| Acidification and coagulation | 60,000–150,000 | 10,000–20,000 | 80–90 | [60] |
| Biological treatment | 40,975 | 9793 | 76.1 | [61] |
| Chemical coagulation | 271,000–341,712 | 6480 | 97.6–98.1 | [8] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yuan, W.; Xie, Q.; Liang, X.; Nie, Y. Catalytic Combustion of Biodiesel Wastewater on Red Mud Catalyst. Materials 2025, 18, 652. https://doi.org/10.3390/ma18030652
Yu S, Yuan W, Xie Q, Liang X, Nie Y. Catalytic Combustion of Biodiesel Wastewater on Red Mud Catalyst. Materials. 2025; 18(3):652. https://doi.org/10.3390/ma18030652
Chicago/Turabian StyleYu, Shangzhi, Wenyu Yuan, Qinglong Xie, Xiaojiang Liang, and Yong Nie. 2025. "Catalytic Combustion of Biodiesel Wastewater on Red Mud Catalyst" Materials 18, no. 3: 652. https://doi.org/10.3390/ma18030652
APA StyleYu, S., Yuan, W., Xie, Q., Liang, X., & Nie, Y. (2025). Catalytic Combustion of Biodiesel Wastewater on Red Mud Catalyst. Materials, 18(3), 652. https://doi.org/10.3390/ma18030652







