Enhanced Performance of Austenitic Oxide Dispersion-Strengthened 316L Steel: A Study on Y2O3 Reinforcement and Corrosion Behaviour
Abstract
1. Introduction
2. Materials and Methods
- D90 = particle size below which 90% of the powder volume is present,
- D10 = particle size below which 10% of the powder volume is present, and
- D50 = median particle size (mean of distribution).
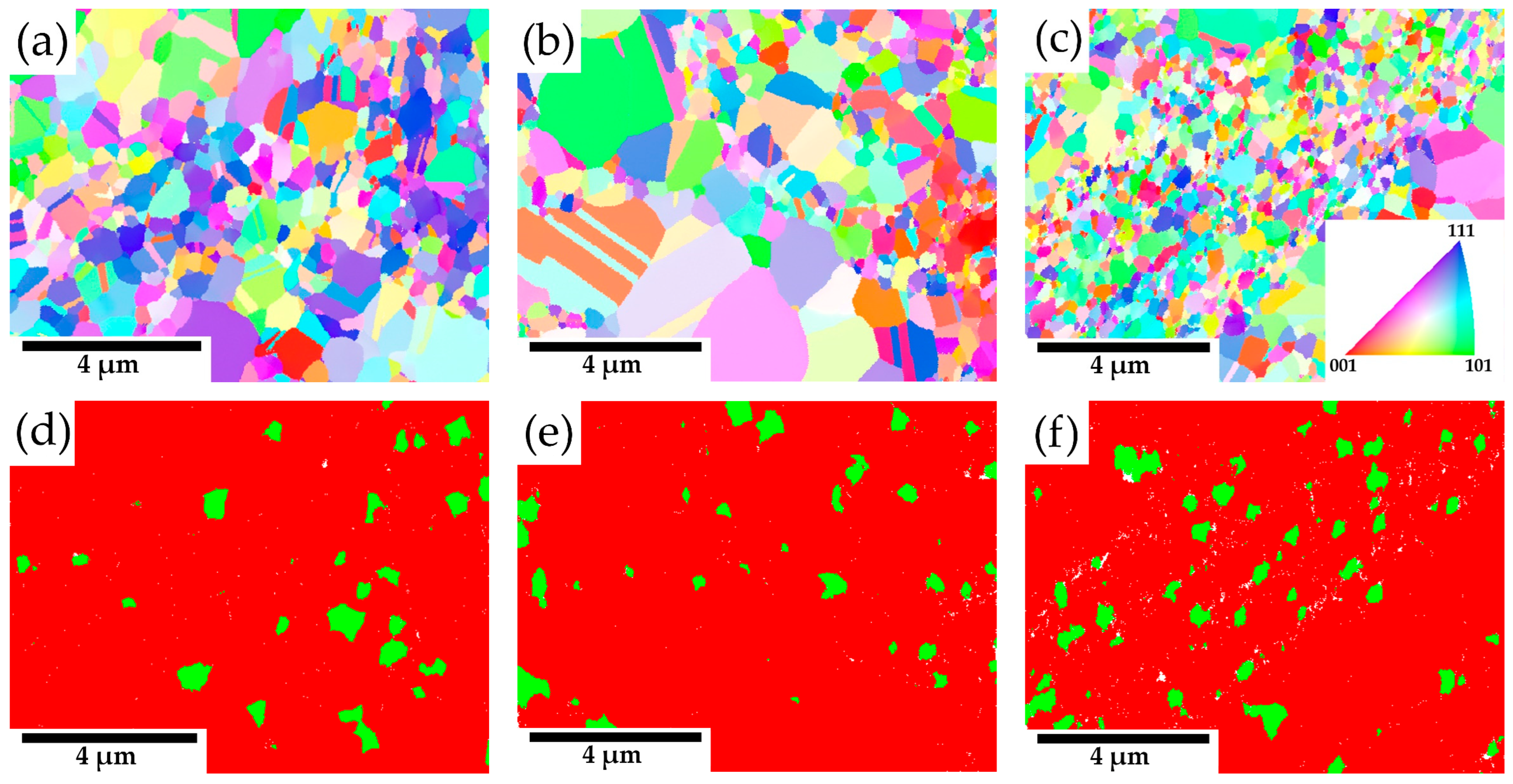
2.1. Microstructure
2.2. Mechanical Properties
2.3. Corrosion Tests
- Q = charge measured on current integration measuring instrument (C),
- X = As [5.1 × 10−3·e0.35·G],
- As = specimen area (cm2), and
- G = grain size number ().
3. Results
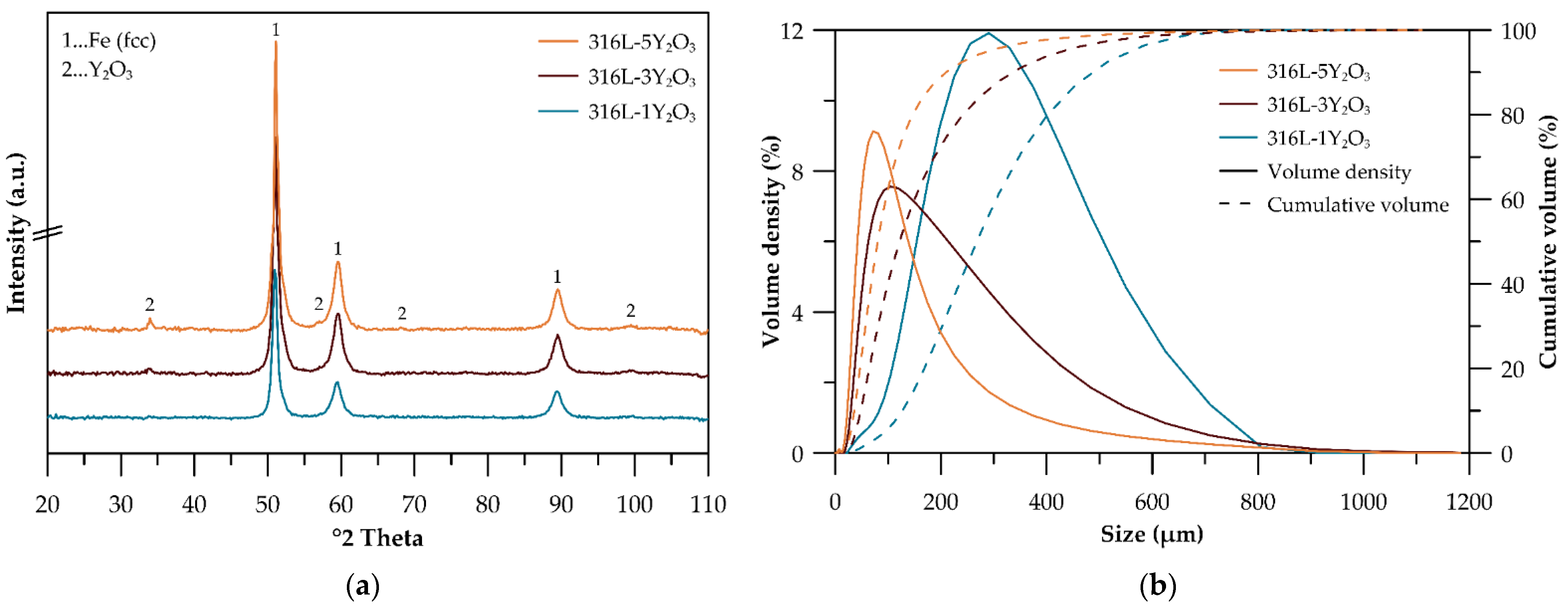
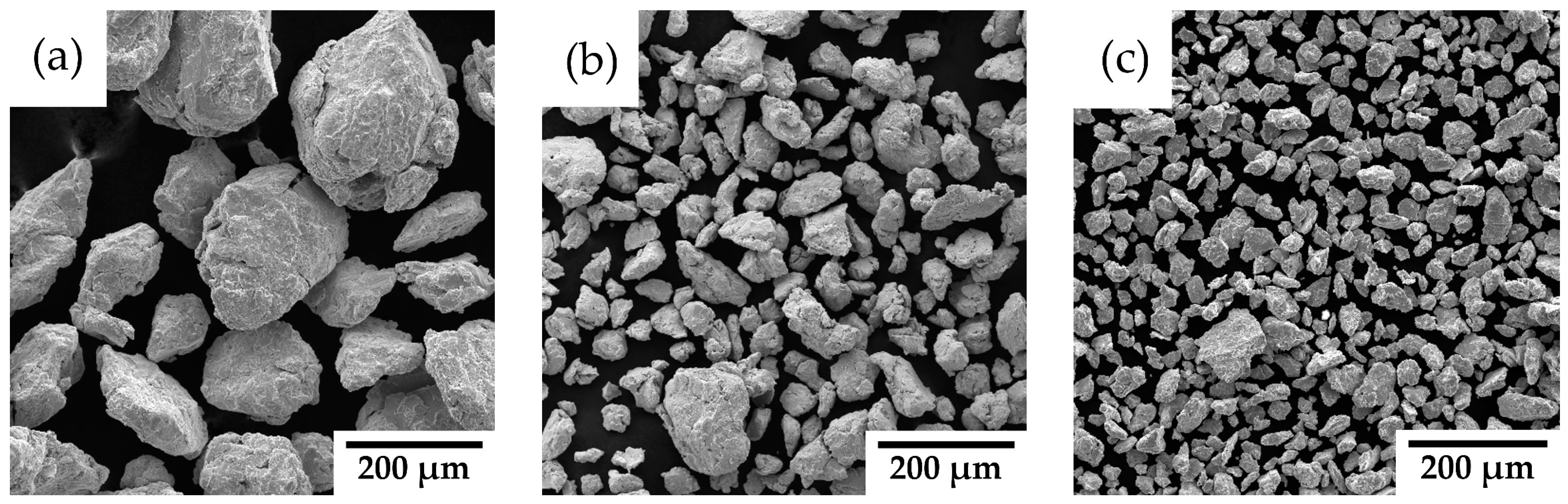
3.1. Powder Precursors
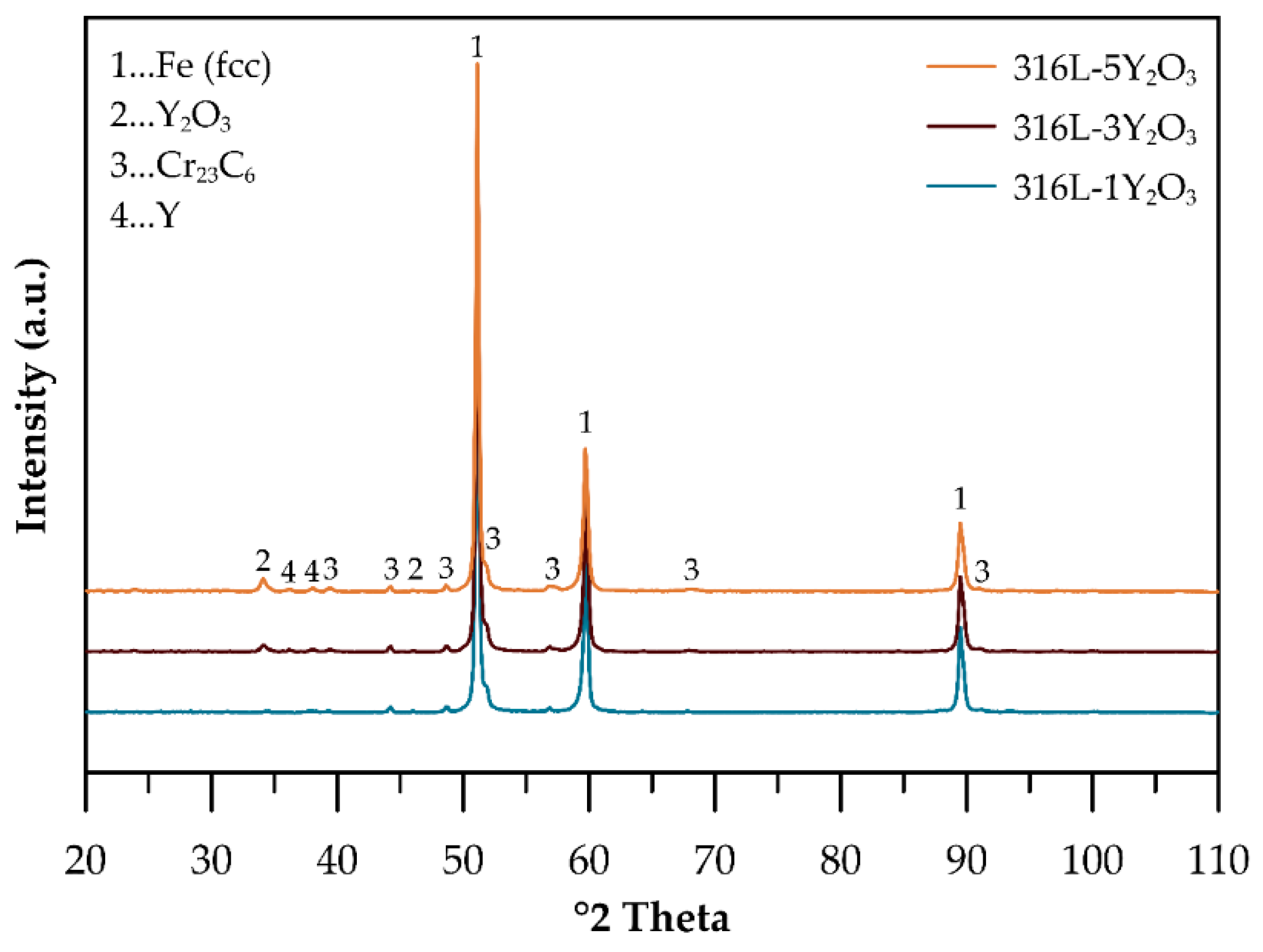
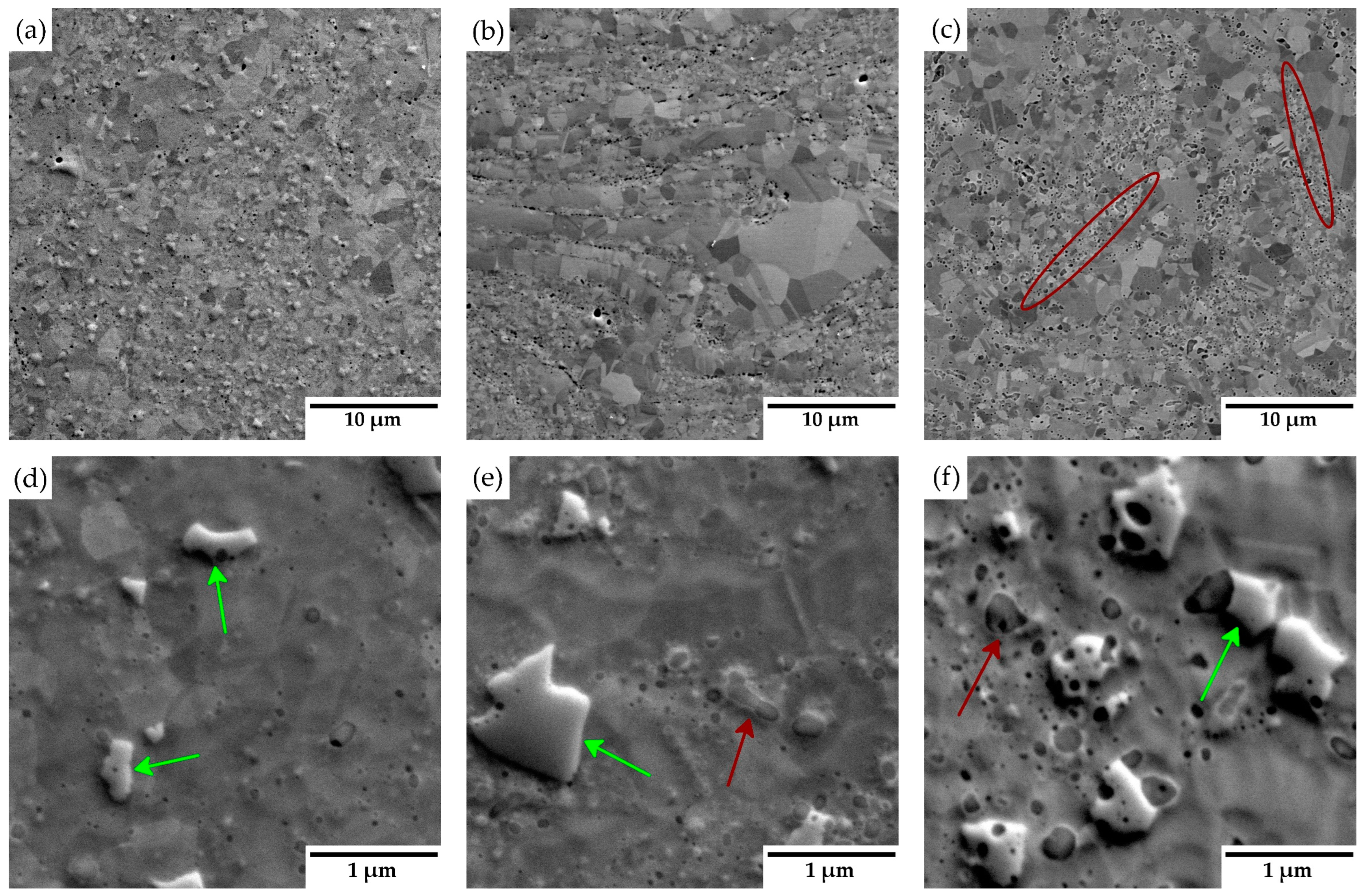
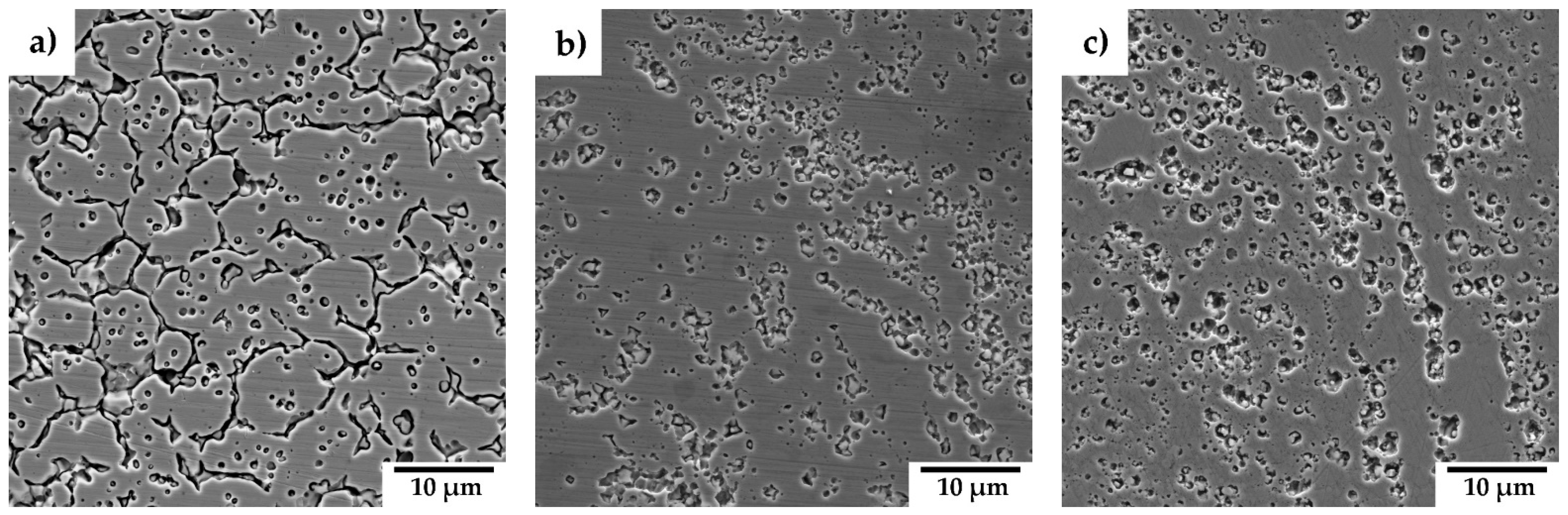
3.2. Consolidated Materials
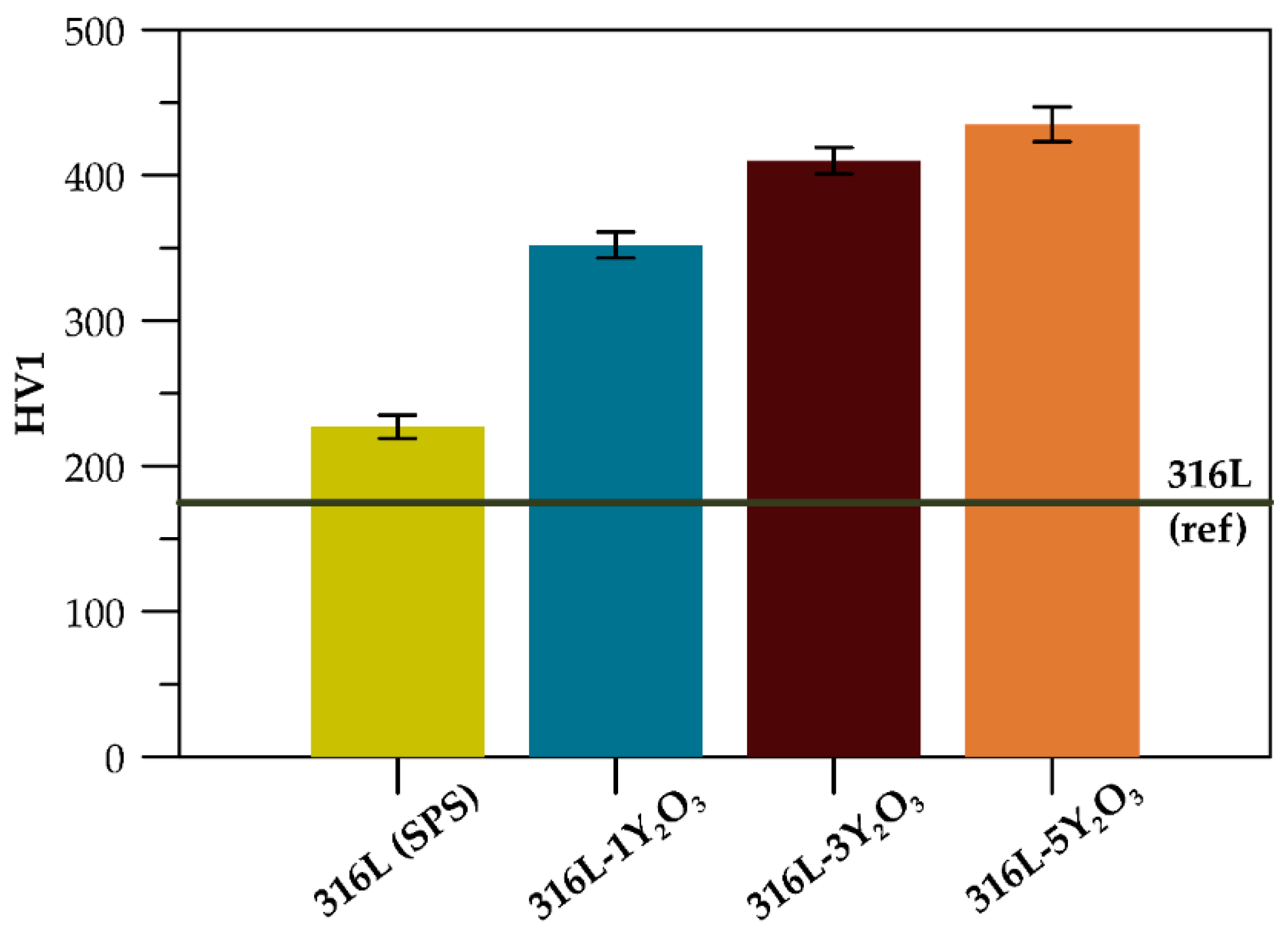
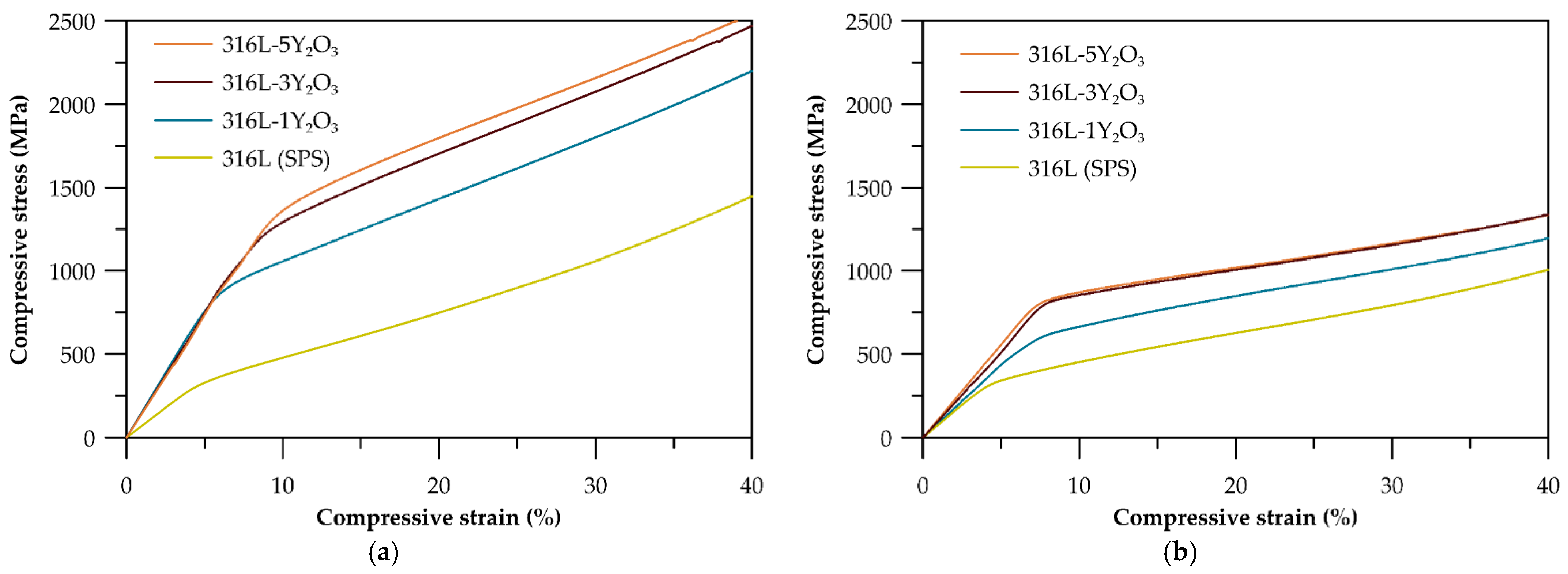
3.3. Mechanical Properties
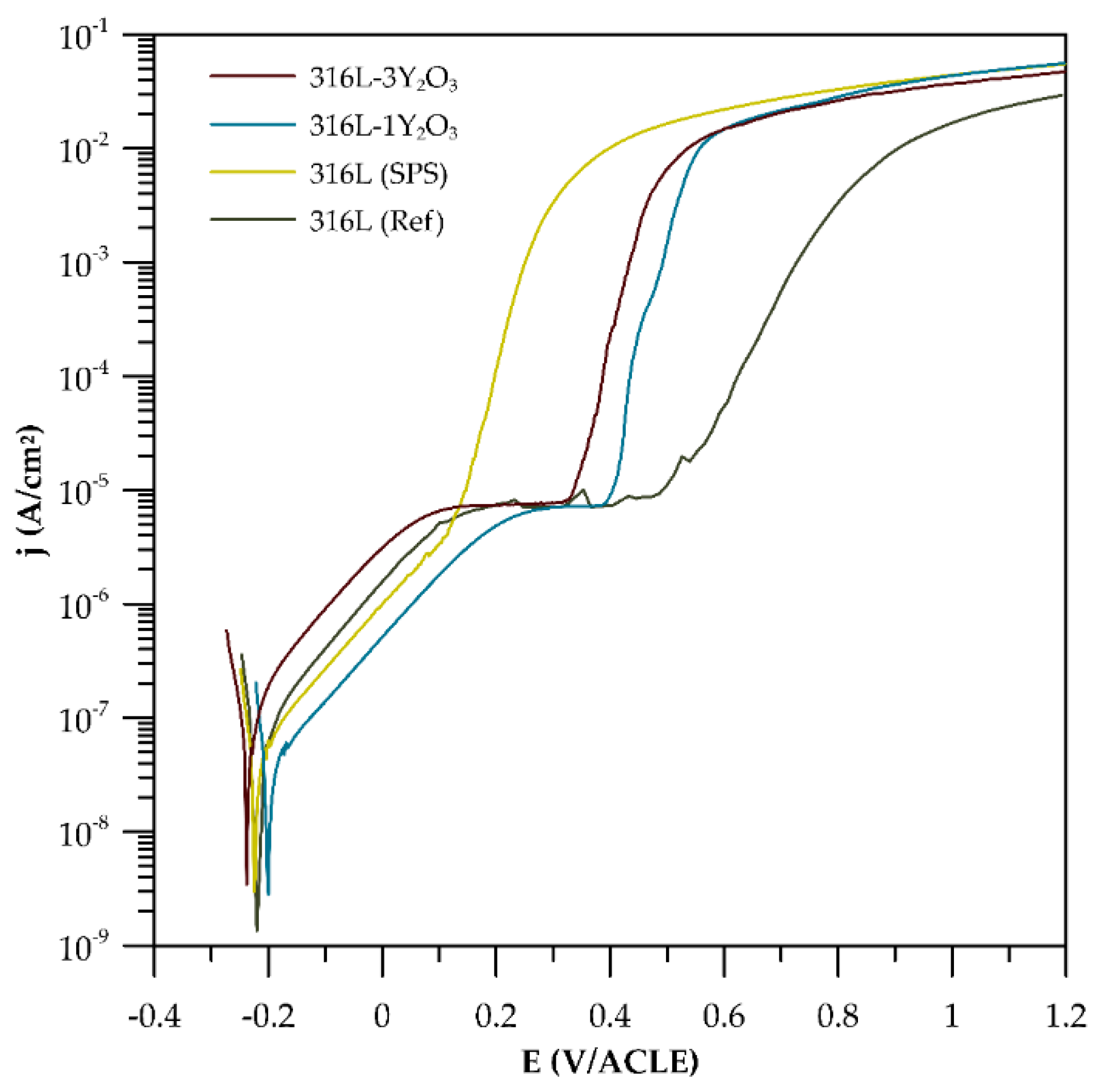
3.4. Corrosion Tests
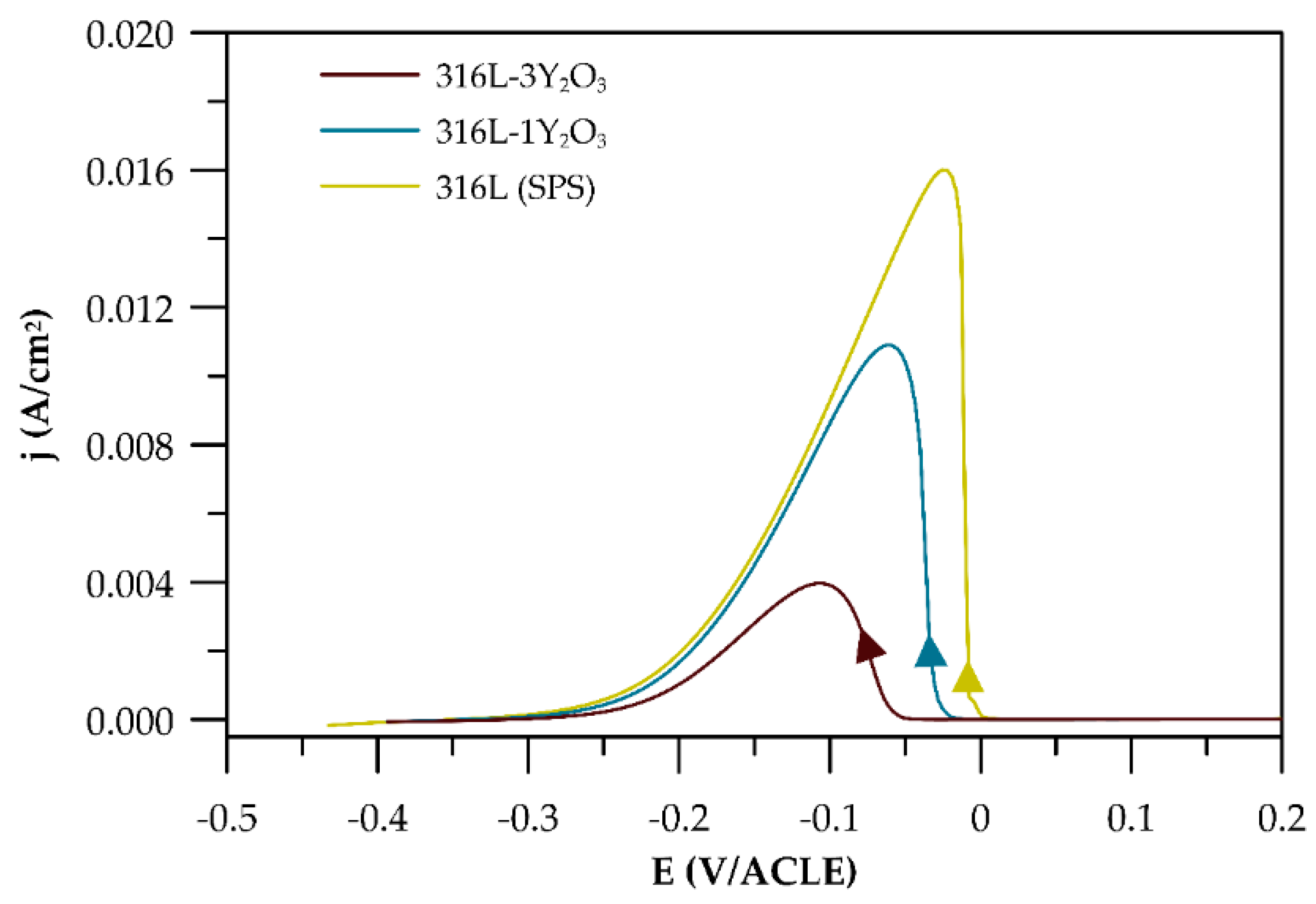
3.4.1. Potentiodynamic Curves
3.4.2. EPR-SL
4. Discussion
4.1. Powder Precursors
4.2. Consolidated Materials
4.3. Mechanical Properties
4.4. Corrosion Tests
5. Conclusions
- (1)
- Mechanical alloying effectively produced homogeneous 316L powders reinforced with Y2O3.
- (2)
- Spark plasma sintering preserved the FCC austenitic structure but introduced chromium carbides and microstructural heterogeneities at higher Y2O3 concentrations, influencing mechanical and corrosion behaviour.
- (3)
- Increasing Y2O3 contents enhanced hardness and compressive yield strength while maintaining compressive plasticity, with diminishing returns at contents above 3 wt%.
- (4)
- At elevated temperatures, mechanical performance decreased; however, composites still exhibited significantly higher compressive yield strength compared to the reference 316L steel.
- (5)
- Corrosion resistance was compromised by localized oxide-rich regions and Cr-depleted zones, particularly at higher Y2O3 contents.
- (6)
- Localized corrosion was more prominent in areas of Y2O3 clustering and carbide enrichment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gelles, D.S. Development of martensitic steels for high neutron damage applications. J. Nucl. Mater. 1996, 239, 99–106. [Google Scholar] [CrossRef]
- Kim, D.W. Influence of nitrogen-induced grain refinement on mechanical properties of nitrogen alloyed type 316LN stainless steel. J. Nucl. Mater. 2012, 420, 473–478. [Google Scholar] [CrossRef]
- Mathew, M.D.; Laha, K.; Ganesan, V. Improving creep strength of 316L stainless steel by alloying with nitrogen. Mater. Sci. Eng. A 2012, 535, 76–83. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Z. Processing and structure of a Nitrogen Alloyed Oxide Dispersion Strengthened Austenitic Stainless Steel by mechanical alloying. J. Phys. Conf. Ser. 2013, 419, 012052. [Google Scholar] [CrossRef]
- Murty, K.L.; Charit, I. Structural materials for Gen-IV nuclear reactors: Challenges and opportunities. J. Nucl. Mater. 2008, 383, 189–195. [Google Scholar] [CrossRef]
- Akasaka, N.; Yamashita, S.; Yoshitake, T.; Ukai, S.; Kimura, A. Microstructural changes of neutron irradiated ODS ferritic and martensitic steels. J. Nucl. Mater. 2004, 329–333, 1053–1056. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, L.; Liu, Y.; Huang, Y.; Guo, Q.; Li, H.; Wu, J. Evolution of Al-containing phases in ODS steel by hot pressing and annealing. Powder Technol. 2017, 311, 449–455. [Google Scholar] [CrossRef]
- Balázsi, C.; Gillemot, F.; Horváth, M.; Wéber, F.; Balázsi, K.; Sahin, F.C.; Onüralp, Y.; Horváth, Á. Preparation and structural investigation of nanostructured oxide dispersed strengthened steels. J. Mater. Sci. 2011, 46, 4598–4605. [Google Scholar] [CrossRef]
- Gräning, T.; Rieth, M.; Hoffmann, J.; Möslang, A. Production, microstructure and mechanical properties of two different austenitic ODS steels. J. Nucl. Mater. 2017, 487, 348–361. [Google Scholar] [CrossRef]
- Hilger, I.; Boulnat, X.; Hoffmann, J.; Testani, C.; Bergner, F.; De Carlan, Y.; Ferraro, F.; Ulbricht, A. Fabrication and characterization of oxide dispersion strengthened (ODS) 14Cr steels consolidated by means of hot isostatic pressing, hot extrusion and spark plasma sintering. J. Nucl. Mater. 2016, 472, 206–214. [Google Scholar] [CrossRef]
- Roy, T.; Shivam, V.; Chattopadhyay, K.; Manna, R.; Mukhopadhyay, N.K. Microstructural Evolution and Mechanical Properties of Nano-Yttria Dispersed 316 L Austenitic Stainless Steel by Mechanical Alloying and Sintering. Trans. Indian Inst. Met. 2021, 74, 2093–2104. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, F.; Liu, L.; Li, Q.; Liu, L.; Liu, F.; Huang, C. Effect of grain size and distribution on the corrosion behavior of Y2O3 dispersion-strengthened 304 stainless steel. Mater. Today Commun. 2022, 31, 103723. [Google Scholar] [CrossRef]
- Deng, L.; Luo, J.-R.; Tu, J.; Hu, R.; Guo, N.; Zeng, W.-Y.; Wang, C.-H.; He, P.; Zhang, Y. Achieving excellent mechanical properties of ODS steel by Y2O3 addition. Mater. Sci. Eng. A 2023, 872, 145008. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, X.; Wang, F.; Stockdale, T.; Dzenis, Y.; Nastasi, M.; Cui, B. Fabrication of ODS Austenitic Steels and CoCrFeNi High-Entropy Alloys by Spark Plasma Sintering for Nuclear Energy Applications. Jom 2019, 71, 2856–2867. [Google Scholar] [CrossRef]
- Qiao, Z.; Du, J.; Hu, Z. Simultaneously improve the strength and ductility of additively manufactured Y2O3/316 L composites via optimizing heat treatment. Mater. Charact. 2024, 217, 114347. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Luo, L.; Li, B.; Liu, T.; Zhao, J.; Xu, B.; Wang, L.; Su, Y.; Guo, J.; et al. Laser-based powder bed fusion of pre-alloyed oxide dispersion strengthened steel containing yttrium. Addit. Manuf. 2022, 58, 110574. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, K.; Qian, Z.; Dong, J.; Wu, J.; Ma, Z. Simultaneous enhancement of strength and ductility in selective laser melting manufactured 316L alloy by employing Y2O3 coated spherical powder as precursor. J. Alloys Compd. 2022, 899, 163262. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Sui, Y.; Li, J.-B.; Yue, J.-Y.; Sun, X.-H.; Yang, L.-F.; Liu, C.-S. Dimension effect of Y2O3 nanomaterial on microstructure and tensile properties of laser metal deposited stainless steel coatings. Surf. Coat. Technol. 2021, 419, 127259. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Sui, Y.; Li, J.-B.; Li, Y.-M.; Sun, X.-H.; Liu, C.-S. Laser metal deposited steel alloys with uniform microstructures and improved properties prepared by addition of small amounts of dispersed Y2O3 nanoparticles. Mater. Sci. Eng. A 2021, 806, 140827. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Allahar, K.N.; Burns, J.; Jaques, B.; Charit, I.; Butt, D.P.; Cole, J.I. Fe-Cr-Mo based ODS alloys via spark plasma sintering: A combinational characterization study by TEM and APT. Cryst. Res. Technol. 2014, 49, 645–652. [Google Scholar] [CrossRef]
- Lemonnier, S.; Moitrier, F.; Rossit, J.; Bourré, T.; Roseiro, P.; Guetter, G.; Boehmler, J. Multimodal particle size distribution by mixing nanopowders for full densification of spark plasma sintered SiC ceramics. Open Ceram. 2021, 7, 100164. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Z.; Sun, H.; Hu, H.; Li, S. Microstructural observation and tensile properties of ODS-304 austenitic steel. Mater. Sci. Eng. A 2013, 559, 287–292. [Google Scholar] [CrossRef]
- Jang, K.-N.; Kim, T.-K.; Kim, K.-T. The effect of cooling rates on carbide precipitate and microstructure of 9CR-1MO oxide dispersion strengthened(ODS) steel. Nucl. Eng. Technol. 2019, 51, 249–256. [Google Scholar] [CrossRef]
- Sovizi, S.; Seraji, M. The Densification Behavior of Metals and Alloys During Spark Plasma Sintering: A Mini-Review. Sci. Sinter. 2019, 51, 135–152. [Google Scholar] [CrossRef]
- Ninawe, P.S.; Ganesh, S.; Sai Karthik, P.; Chandrasekhar, S.B.; Vijay, R. Microstructure and mechanical properties of spark plasma sintered austenitic ODS steel. Adv. Powder Technol. 2022, 33, 103584. [Google Scholar] [CrossRef]
- Mao, X.; Oh, K.H.; Jang, J. Evolution of ultrafine grained microstructure and nano-sized semi-coherent oxide particles in austenitic oxide dispersion strengthened steel. Mater. Charact. 2016, 117, 91–98. [Google Scholar] [CrossRef]
- Koul, S.; Shivam, V.; Chattopadhyay, K.; Manna, R.; Biswas, K.; Mukhopadhyay, N.K. Development of Oxide Dispersed Austenitic Stainless Steel through Mechanical Alloying and Spark Plasma Sintering. J. Mater. Eng. Perform. 2022, 31, 9522–9533. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behaviour of austenitic stainless steels—combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Molin, S.; Persson, Å.H.; Skafte, T.L.; Smitshuysen, A.L.; Jensen, S.H.; Andersen, K.B.; Xu, H.; Chen, M.; Hendriksen, P.V. Effective yttrium based coating for steel interconnects of solid oxide cells: Corrosion evaluation in steam-hydrogen atmosphere. J. Power Sources 2019, 440, 226814. [Google Scholar] [CrossRef]
- Rashid, M.W.A.; Gakim, M.; Rosli, Z.M.; Azam, M.A. Formation of Cr23C6 during the Sensitization of AISI 304 Stainless Steel and its Effect to Pitting Corrosion. Int. J. Electrochem. Sci. 2012, 7, 9465–9477. [Google Scholar] [CrossRef]
- Aydoğdu, G.H.; Aydinol, M.K. Determination of susceptibility to intergranular corrosion and electrochemical reactivation behaviour of AISI 316L type stainless steel. Corros. Sci. 2006, 48, 3565–3583. [Google Scholar] [CrossRef]
- Matula, M.; Hyspecka, L.; Svoboda, M.; Vodarek, V.; Dagbert, C.; Galland, J.; Stonawska, Z.; Tuma, L. Intergranular corrosion of AISI 316L steel. Mater. Charact. 2001, 46, 203–210. [Google Scholar] [CrossRef]
| Initial Powders | Purity (%) | Size (μm) |
|---|---|---|
| Pre-alloyed 316L steel | 99.99 | 30–60 |
| Y2O3 | 99.9 | 1–2 |
| Alloy | D90 (μm) | D10 (μm) | D50 (μm) | Span |
|---|---|---|---|---|
| 316L-1Y2O3 | 486.8 | 126.9 | 267.7 | 1.4 |
| 316L-3Y2O3 | 336.3 | 48.0 | 121.6 | 2.4 |
| 316L-5Y2O3 | 209.7 | 35.6 | 80.3 | 2.2 |
| Material | CYS0.2 (MPa) | CYS0.2 600 °C (MPa) | HV1 |
|---|---|---|---|
| 316L (SPS) | 313 ± 9 | 296 ± 7 | 227 ± 8 |
| 316L-1Y2O3 | 775 ± 21 | 550 ± 32 | 352 ± 9 |
| 316L-3Y2O3 | 1022 ± 15 | 803 ± 9 | 410 ± 9 |
| 316L-5 Y2O3 | 1042 ± 7 | 835 ± 6 | 435 ± 12 |
| Material | Ecorr (mV/ACLE) | βc mV/Decade | Eb (mV/ACLE) | ∆E (mV) |
|---|---|---|---|---|
| 316L (ref) | −220 | 166.8 | 472 | 252 |
| 316L (SPS) | −225 | 177.7 | 116 | 109 |
| 316L-1Y2O3 | −200 | 180.5 | 395 | 195 |
| 316L-3Y2O3 | −235 | 178.9 | 328 | 93 |
| Material | Q (C) | X (cm2) | QPa (C/cm2) |
|---|---|---|---|
| 316L (SPS) | 0.068 | 0.029 | 20.7 |
| 316L-1Y2O3 | 0.025 | 0.043 | 09.0 |
| 316L-3Y2O3 | 0.007 | 0.043 | 03.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokorný, J.; Kubásek, J.; Donik, Č.; Nečas, D.; Hybášek, V.; Fojt, J.; Dobkowska, A.; Paulin, I.; Čapek, J.; Godec, M. Enhanced Performance of Austenitic Oxide Dispersion-Strengthened 316L Steel: A Study on Y2O3 Reinforcement and Corrosion Behaviour. Materials 2025, 18, 641. https://doi.org/10.3390/ma18030641
Pokorný J, Kubásek J, Donik Č, Nečas D, Hybášek V, Fojt J, Dobkowska A, Paulin I, Čapek J, Godec M. Enhanced Performance of Austenitic Oxide Dispersion-Strengthened 316L Steel: A Study on Y2O3 Reinforcement and Corrosion Behaviour. Materials. 2025; 18(3):641. https://doi.org/10.3390/ma18030641
Chicago/Turabian StylePokorný, Jan, Jiří Kubásek, Črtomir Donik, David Nečas, Vojtěch Hybášek, Jaroslav Fojt, Anna Dobkowska, Irena Paulin, Jaroslav Čapek, and Matjaž Godec. 2025. "Enhanced Performance of Austenitic Oxide Dispersion-Strengthened 316L Steel: A Study on Y2O3 Reinforcement and Corrosion Behaviour" Materials 18, no. 3: 641. https://doi.org/10.3390/ma18030641
APA StylePokorný, J., Kubásek, J., Donik, Č., Nečas, D., Hybášek, V., Fojt, J., Dobkowska, A., Paulin, I., Čapek, J., & Godec, M. (2025). Enhanced Performance of Austenitic Oxide Dispersion-Strengthened 316L Steel: A Study on Y2O3 Reinforcement and Corrosion Behaviour. Materials, 18(3), 641. https://doi.org/10.3390/ma18030641








