Metal Ions Fortified Tannin-Furanic Rigid Foam: The Impact on the Uniformity and Mechanical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tannin-Furanic Foams
2.3. Foam Characterizations
3. Results and Discussion
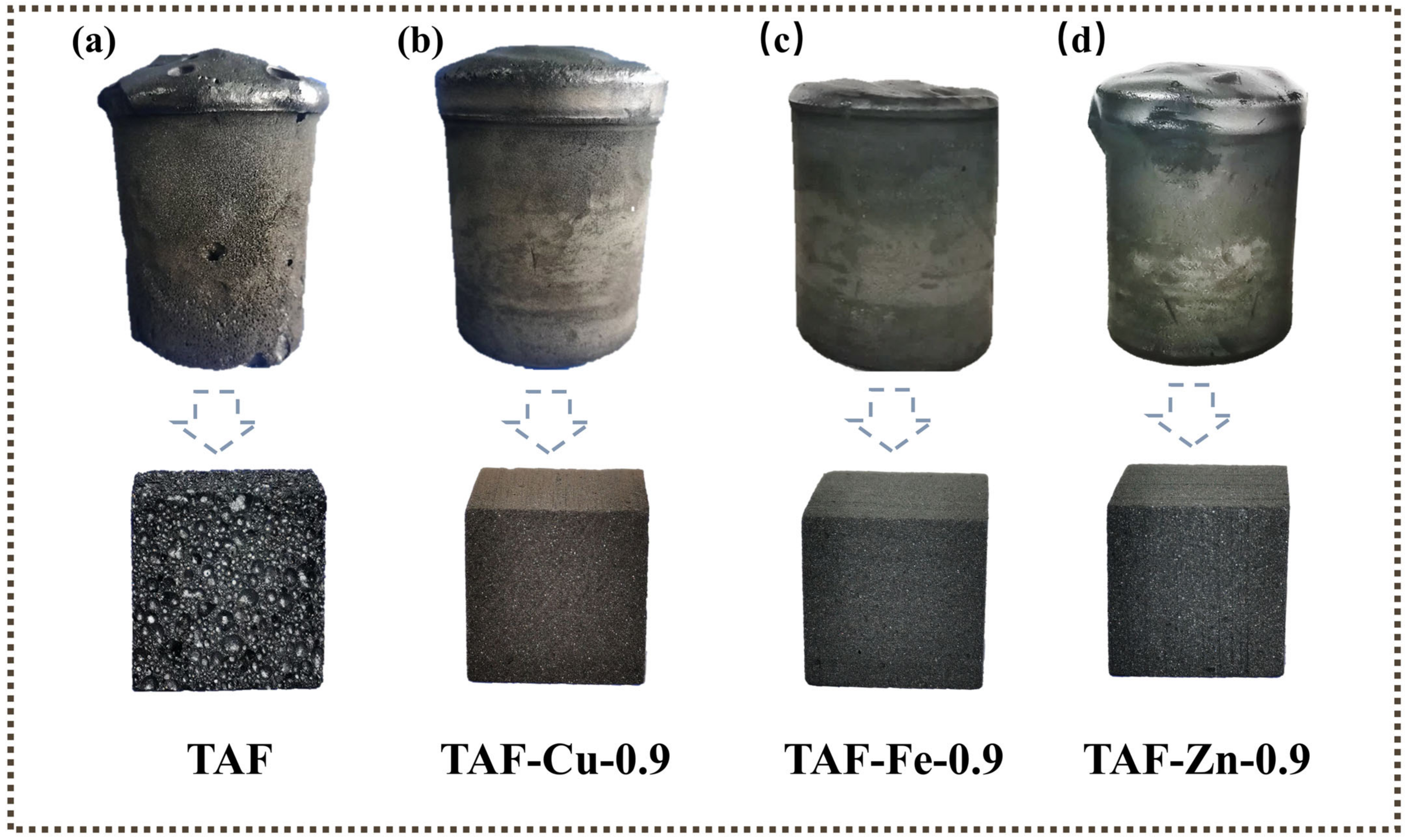
3.1. Digital Photographs of Foams
3.2. The Morphology and Cell Size Distribution of the Foams
3.3. Micro-CT Images of Foams
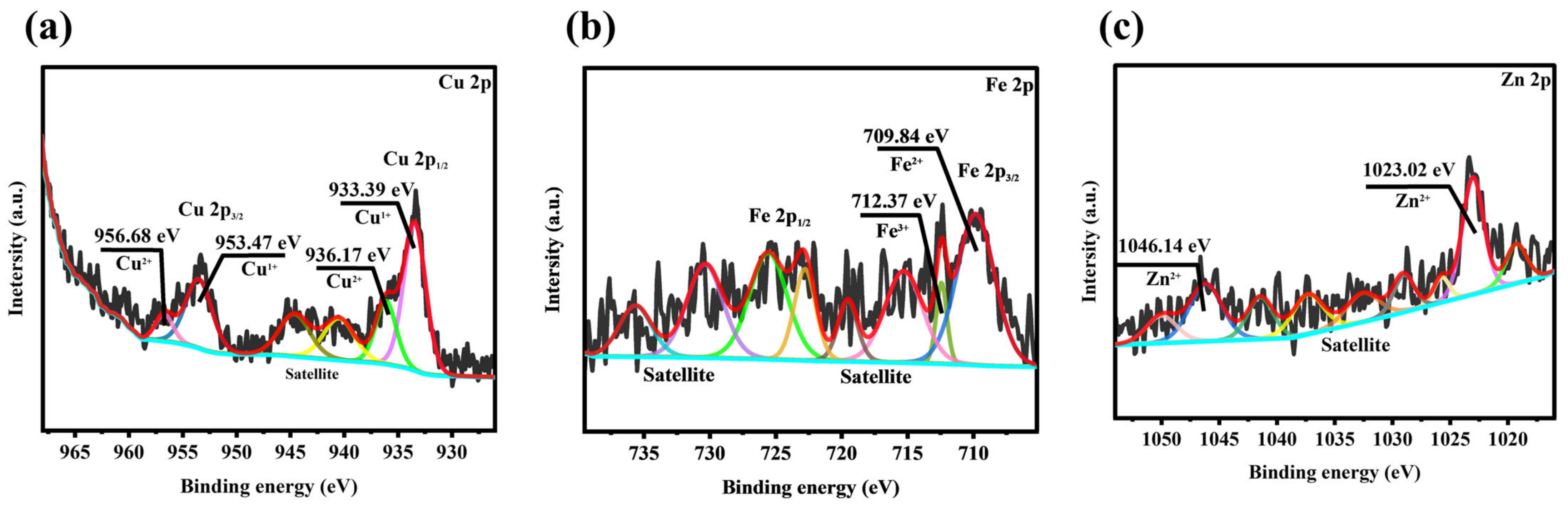
3.4. Analysis of X-Ray Photoelectron Spectroscopy
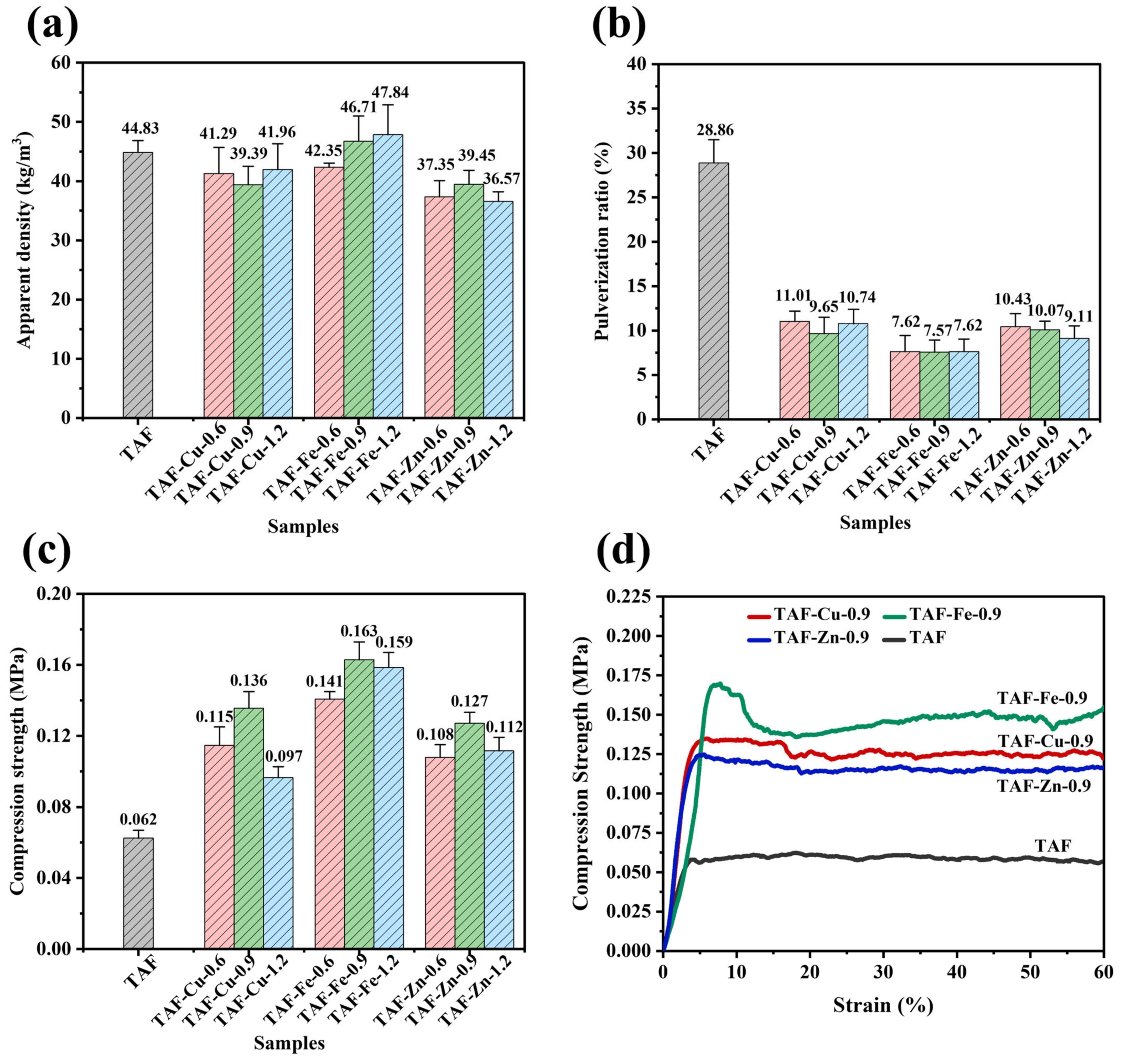
3.5. Physical–Mechanical Properties of Foams
3.6. Thermogravimetric Analysis
3.7. Thermal Conductivity
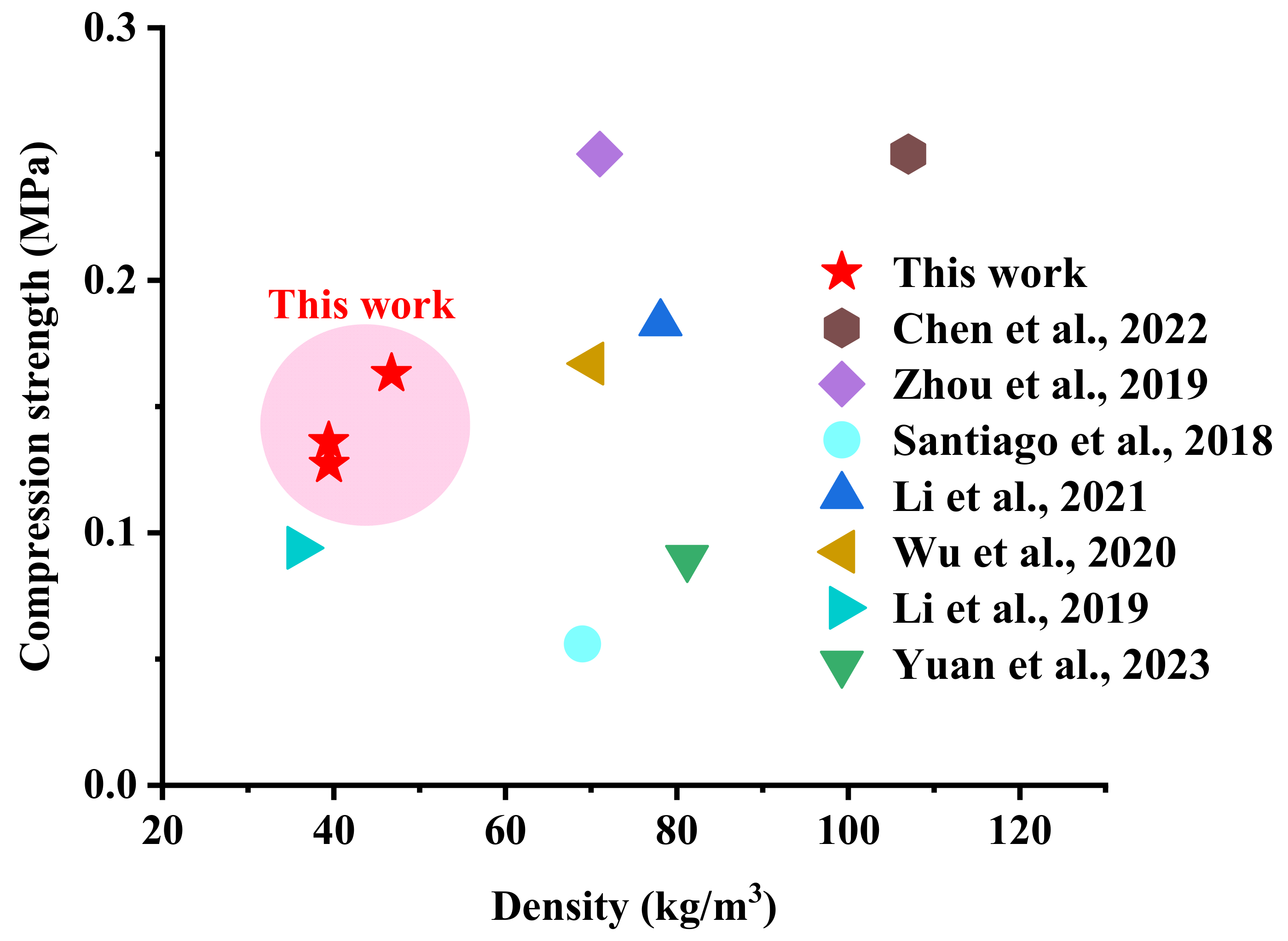
3.8. Comparison with Other Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, S.; Li, H.; Wang, S.; Jiang, R.; Zou, J.; Zhang, X.; Liu, L.; Zhang, G. Experimental Research on an Innovative Sawdust Biomass-Based Insulation Material for Buildings. J. Clean. Prod. 2020, 260, 121029. [Google Scholar] [CrossRef]
- Kumar, D.; Alam, M.; Zou, P.X.W.; Sanjayan, J.G.; Memon, R.A. Comparative Analysis of Building Insulation Material Properties and Performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.M.I.; Rismanchi, B.; Ng, H.M.; Hasan, M.H.; Metselaar, H.S.C.; Muraza, O.; Aditiya, H.B. A Review on Insulation Materials for Energy Conservation in Buildings. Renew. Sustain. Energy Rev. 2017, 73, 1352–1365. [Google Scholar] [CrossRef]
- Azlina Ramlee, N.; Jawaid, M.; Abdul Karim Yamani, S.; Syams Zainudin, E.; Alamery, S. Effect of Surface Treatment on Mechanical, Physical and Morphological Properties of Oil Palm/Bagasse Fiber Reinforced Phenolic Hybrid Composites for Wall Thermal Insulation Application. Constr. Build. Mater. 2021, 276, 122239. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Essawy, H.; Pizzi, A.; Fredon, E.; Gerardin, C.; Du, G.; Zhou, X. Flame-Retardant and Thermally-Insulating Tannin and Soybean Protein Isolate (SPI) Based Foams for Potential Applications in Building Materials. Constr. Build. Mater. 2022, 315, 125711. [Google Scholar] [CrossRef]
- Zemła, M.; Prociak, A.; Michałowski, S. Bio-Based Rigid Polyurethane Foams Modified with Phosphorus Flame Retardants. Polymers 2021, 14, 102. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Y.; Wang, S.; Shaghaleh, H.; Sun, P.; Huang, X.; Xu, X.; Wang, S.; Liu, H. Flame-Retarded Polyurethane Foam Conferred by a Bio-Based Nitrogen-phosphorus-Containing Flame Retardant. React. Funct. Polym. 2021, 168, 105057. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Bao, L.; Lou, C.-W.; Lin, J.-H. Flame-Retardant Agent and Fire-Retardant Fabrics Reinforced the Polyurethane Foam: Combustion Resistance and Mechanical Properties. J. Sandw. Struct. Mater. 2020, 22, 2408–2420. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid Polyurethane Foams with Halogen-free Flame Retardants: Thermal Insulation, Mechanical, and Flame Retardant Properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Quinsaat, J.E.Q.; Feghali, E.; van de Pas, D.J.; Vendamme, R.; Torr, K.M. Preparation of Mechanically Robust Bio-Based Polyurethane Foams Using Depolymerized Native Lignin. ACS Appl. Polym. Mater. 2021, 3, 5845–5856. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, J.; Sepperer, T.; Cesprini, E.; Šket, P.; Tondi, G. Comparing Condensed and Hydrolysable Tannins for Mechanical Foaming of Furanic Foams: Synthesis and Characterization. Molecules 2023, 28, 2799. [Google Scholar] [CrossRef] [PubMed]
- Varila, T.; Romar, H.; Luukkonen, T.; Hilli, T.; Lassi, U. Characterization of Lignin Enforced Tannin/Furanic Foams. Heliyon 2020, 6, e03228. [Google Scholar] [CrossRef]
- Meikleham, N.E.; Pizzi, A. Acid- and Alkali-catalyzed Tannin-based Rigid Foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, J.; Yuan, X.; Yuan, B. Hydrophobic and Fire Safe Polyurethane Foam Coated with Chitosan and Nano-Montmorillonite via Layer-by-Layer Assembly for Emergency Absorption of Oil Spill. Mater. Lett. 2022, 316, 132009. [Google Scholar] [CrossRef]
- Razali, N.I.M.; Ali, F.; Azmi, A.S.; Ismail, T.N.M.T.; Mirghani, M.E.S.; Omar, M.F. Microwave-Assisted Synthesis of Polylactic Acid-Diol for Polyurethane as Biodegradable Packaging Material. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1192, 012015. [Google Scholar] [CrossRef]
- Amirabadi, S.; Ramezani Kakroodi, A.; Dias, O.A.T.; Sain, M.; Park, C.B. Tailoring Nano-Fibrillated Polystyrene Composite with Enhanced Fire Retarding Properties for Foam Applications. Mater. Des. 2022, 214, 110419. [Google Scholar] [CrossRef]
- Liu, C.; Xie, Y.; Gao, D.; Shi, X.; Rao, Z. Fabrication of Fire-Retardant Building Materials via a Hyper-Crosslinking Chemical Conversion Process from Waste Polystyrenes. Energy Built Environ. 2022, 3, 226–232. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Makkawi, Y.; Ibrahim, T.H. Preparation and Characterization of Date Palm Bio-Oil Modified Phenolic Foam. Polymers 2024, 16, 955. [Google Scholar] [CrossRef]
- de Carvalho, G.; Pimenta, J.A.; dos Santos, W.N.; Frollini, E. Phenolic and Lignophenolic Closed Cells Foams: Thermal Conductivity and Other Properties. Polym. Plast. Technol. Eng. 2003, 42, 605–626. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Garcia, D.; Celzard, A. Bioresourced Pine Tannin/Furanic Foams with Glyoxal and Glutaraldehyde. Ind. Crops Prod. 2013, 45, 401–405. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Pizzi, A.; Fredon, E.; Gerardin, C.; Zhou, X.; Du, G. Tannin-Furanic Foams Modified by Soybean Protein Isolate (SPI) and Industrial Lignin Substituting Formaldehyde Addition. Ind. Crops Prod. 2021, 168, 113607. [Google Scholar] [CrossRef]
- Zhou, X.; Li, B.; Xu, Y.; Essawy, H.; Wu, Z.; Du, G. Tannin-Furanic Resin Foam Reinforced with Cellulose Nanofibers (CNF). Ind. Crops Prod. 2019, 134, 107–112. [Google Scholar] [CrossRef]
- Sims, G.L.A.; Jaafar, H.A.S. A Chemical Blowing Agent System (CBAS) Based on Azodicarbonamide. J. Cell. Plast. 1994, 30, 175–188. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Delgado-Sánchez, C.; Basso, M.C.; Pizzi, A.; Fierro, V.; Celzard, A. Mechanically Blown Wall-Projected Tannin-Based Foams. Ind. Crops Prod. 2018, 113, 316–323. [Google Scholar] [CrossRef]
- Li, J.; Liao, J.; Essawy, H.; Zhang, J.; Li, T.; Wu, Z.; Du, G.; Zhou, X. Preparation and Characterization of Novel Cellular/Nonporous Foam Structures Derived from Tannin Furanic Resin. Ind. Crops Prod. 2021, 162, 113264. [Google Scholar] [CrossRef]
- Wu, X.; Yan, W.; Zhou, Y.; Luo, L.; Yu, X.; Luo, L.; Fan, M.; Du, G.; Zhao, W. Thermal, Morphological, and Mechanical Characteristics of Sustainable Tannin Bio-Based Foams Reinforced with Wood Cellulosic Fibers. Ind. Crops Prod. 2020, 158, 113029. [Google Scholar] [CrossRef]
- Karamać, M. Chelation of Cu(II), Zn(II), and Fe(II) by Tannin Constituents of Selected Edible Nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef]
- Yang, W.; Sousa, A.M.M.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.M.; Liu, L. Electrospun Ultra-Fine Cellulose Acetate Fibrous Mats Containing Tannic Acid-Fe3+ Complexes. Carbohydr. Polym. 2017, 157, 1173–1179. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Vancamp, J.; Demeulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Ye, X.; Cui, Y.; Ke, L.; Gao, K.; Huang, X.; Shi, B. Fabrication of 3D Porous Superhydrophobic Sponges Using Plant Polyphenol-Fe3+ Complexes as Adhesive and Their Applications in Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2018, 551, 9–16. [Google Scholar] [CrossRef]
- Li, J.; Zhang, A.; Zhang, S.; Gao, Q.; Zhang, W.; Li, J. Larch Tannin-Based Rigid Phenolic Foam with High Compressive Strength, Low Friability, and Low Thermal Conductivity Reinforced by Cork Powder. Compos. Part. B Eng. 2019, 156, 368–377. [Google Scholar] [CrossRef]
- Yuan, W.; Xi, X.; Zhang, J.; Pizzi, A.; Essawy, H.; Du, G.; Zhou, X.; Chen, X. A Novel Strategy Inspired by Steaming Chinese Steamed Bread for Preparation of Tannin-Furanic Rigid Bio-Foam. Constr. Build. Mater. 2023, 376, 131035. [Google Scholar] [CrossRef]
- Cesano, F.; Scarano, D.; Bertarione, S.; Bonino, F.; Damin, A.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Zecchina, A. Synthesis of ZnO–Carbon Composites and Imprinted Carbon by the Pyrolysis of ZnCl2-Catalyzed Furfuryl Alcohol Polymers. J. Photochchem. Photobio. A 2008, 196, 143–153. [Google Scholar] [CrossRef]
- Cepollaro, E.M.; Caputo, D.; Cimino, S.; Gargiulo, N.; Lisi, L. Synthesis and Characterization of Activated Carbon Foam from Polymerization of Furfuryl Alcohol Activated by Zinc and Copper Chlorides. C 2020, 6, 45. [Google Scholar] [CrossRef]
- ASTM D1622-03; Standard Test Method for Apparent Density of Rigid Cellular Plastics. ASTM: West Conshohocken, PA, USA, 2003.
- GB/T 8813-2020; Rigid Cellular Plastics—Determination of Compression Properties. China National Standardization Administration Committee: Beijing, China, 2020.
- GB/T 12812-2006; Rigid Cellular Plastics—Determination of Friability. China National Standardization Administration Committee: Beijing, China, 2006.
- Hao, B.; Wang, F.; Huang, H.; Wu, Y.; Jia, S.; Liao, Y.; Mao, H. Tannin Foam Immobilized with Ferric Ions for Efficient Removal of Ciprofloxacin at Low Concentrations. J. Hazard. Mater. 2021, 414, 125567. [Google Scholar] [CrossRef]
- Liu, T.; Ma, M.; Ali, A.; Liu, Q.; Bai, R.; Zhang, K.; Guan, Y.; Wang, Y.; Liu, J.; Zhou, H. Self-Assembled Copper Tannic Acid Nanoparticles: A Powerful Nano-Bactericide by Valence Shift of Copper. Nano Today 2024, 54, 102071. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Chen, J.; Li, X. Biocompatible, Functional Spheres Based on Oxidative Coupling Assembly of Green Tea Polyphenols. J. Am. Chem. Soc. 2013, 135, 4179–4182. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, Y.; Zhang, H.; Wang, X.; Zhang, Y.; Zhang, Y.; Miao, L.; Zhao, X.; Wei, H. Copper Tannic Acid Coordination Nanosheet: A Potent Nanozyme for Scavenging ROS from Cigarette Smoke. Small 2020, 16, 1902123. [Google Scholar] [CrossRef]
- Ozawa, H.; Haga, M. Soft Nano-Wrapping on Graphene Oxide by Using Metal–Organic Network Films Composed of Tannic Acid and Fe Ions. Phys. Chem. Chem. Phys. 2015, 17, 8609–8613. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, G.; Qiang, C.; Fu, Y.; Wu, Y.; Li, X.; Han, G. Hollow Ferric-Tannic Acid Nanocapsules with Sustained O2 and ROS Induction for Synergistic Tumor Therapy. Biomater. Sci. 2020, 8, 3844–3855. [Google Scholar] [CrossRef] [PubMed]
- Fullenkamp, D.E.; Barrett, D.G.; Miller, D.R.; Kurutz, J.W.; Messersmith, P.B. PH-Dependent Cross-Linking of Catechols through Oxidation via Fe3+ and Potential Implications for Mussel Adhesion. RSC Adv. 2014, 4, 25127–25134. [Google Scholar] [CrossRef]
- Hillis, W.E.; Urbach, G. The Reaction of (+)—Catechin with Formaldehyde. J. Appl. Chem. 1959, 9, 474–482. [Google Scholar] [CrossRef]
- Hillis, W.E.; Urbach, G. Reaction of Polyphenols with Formaldehyde. J. Appl. Chem. 1959, 9, 665–673. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Bo, C.; Wei, S.; Hu, L.; Jia, P.; Liang, B.; Zhou, J.; Zhou, Y. Synthesis of a Cardanol-Based Phosphorus-Containing Polyurethane Prepolymer and Its Application in Phenolic Foams. RSC Adv. 2016, 6, 62999–63005. [Google Scholar] [CrossRef]
- Zhang, A.; Li, J.; Zhang, S.; Mu, Y.; Zhang, W.; Li, J. Characterization and Acid-Catalysed Depolymerization of Condensed Tannins Derived from Larch Bark. RSC Adv. 2017, 7, 35135–35146. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, D.M.; Cheng, W.M.; Zhou, G. Effect of Polyethylene Glycol on the Mechanical Property, Microstructure, Thermal Stability, and Flame Resistance of Phenol–Urea–Formaldehyde Foams. J. Mater. Sci. 2014, 49, 1556–1565. [Google Scholar] [CrossRef]
- Lacoste, C.; Basso, M.C.; Pizzi, A.; Laborie, M.-P.; Celzard, A.; Fierro, V. Pine Tannin-Based Rigid Foams: Mechanical and Thermal Properties. Ind. Crops Prod. 2013, 43, 245–250. [Google Scholar] [CrossRef]
- Liu, L.; Zou, S.; Li, H.; Deng, L.; Bai, C.; Zhang, X.; Wang, S.; Li, N. Experimental Physical Properties of an Eco-Friendly Bio-Insulation Material Based on Wheat Straw for Buildings. Energy Build. 2019, 201, 19–36. [Google Scholar] [CrossRef]
- Lin, Y.; Li, X.; Huang, Q. Preparation and Characterization of Expanded Perlite/Wood-Magnesium Composites as Building Insulation Materials. Energy Build. 2021, 231, 110637. [Google Scholar] [CrossRef]




| Samples | MT/g | FA/g | Tween-80/g | F/g | 65 wt% p-TSA/g | Water 1/g | Metal Ions Solution 2/g |
|---|---|---|---|---|---|---|---|
| TAF | 24 | 26 | 6 | 14.8 | 24 | 36 | 0 |
| TAF-Cu-0.6 | 35.1 | 1.5 | |||||
| TAF-Cu-0.9 | 34.65 | 2.25 | |||||
| TAF-Cu-1.2 | 34.2 | 3 | |||||
| TAF-Fe-0.6 | 35.1 | 1.5 | |||||
| TAF-Fe-0.9 | 34.65 | 2.25 | |||||
| TAF-Fe-1.2 | 34.2 | 3 | |||||
| TAF-Zn-0.6 | 35.1 | 1.5 | |||||
| TAF-Zn-0.9 | 34.65 | 2.25 | |||||
| TAF-Zn-1.2 | 34.2 | 3 |
| Samples | Tmax (°C) | Residual Mass at 790 °C (%) | ||
|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | ||
| TAF | 49.5 | 282.8 | 456.7 | 43.77 |
| TAF-Cu-0.9 | 50.0 | 293.7 | 461.5 | 41.93 |
| TAF-Fe-0.9 | 42.1 | 241.7 | 457.3 | 42.02 |
| TAF-Zn-0.9 | 57.8 | 238.4 | 460.9 | 41.55 |
| Samples | Thermal Conductivity/(W/m·K) |
|---|---|
| TAF | 0.0329 (0.005) |
| TAF-Cu-0.6 | 0.0443 (0.007) |
| TAF-Cu-0.9 | 0.0486 (0.008) |
| TAF-Cu-1.2 | 0.0496 (0.010) |
| TAF-Fe-0.6 | 0.0492 (0.007) |
| TAF-Fe-0.9 | 0.0531 (0.004) |
| TAF-Fe-1.2 | 0.0552 (0.008) |
| TAF-Zn-0.9 | 0.0473 (0.009) |
| TAF-Zn-0.9 | 0.0503 (0.005) |
| TAF-Zn-0.9 | 0.0526 (0.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, H.; Zhang, J.; Li, F.; Charrier, B.; Essawy, H.; Pizzi, A.; Zhou, X.; Chen, X. Metal Ions Fortified Tannin-Furanic Rigid Foam: The Impact on the Uniformity and Mechanical Performance. Materials 2025, 18, 585. https://doi.org/10.3390/ma18030585
Yang Y, Wu H, Zhang J, Li F, Charrier B, Essawy H, Pizzi A, Zhou X, Chen X. Metal Ions Fortified Tannin-Furanic Rigid Foam: The Impact on the Uniformity and Mechanical Performance. Materials. 2025; 18(3):585. https://doi.org/10.3390/ma18030585
Chicago/Turabian StyleYang, Yang, Haizhu Wu, Jun Zhang, Fajian Li, Bertrand Charrier, Hisham Essawy, Antonio Pizzi, Xiaojian Zhou, and Xinyi Chen. 2025. "Metal Ions Fortified Tannin-Furanic Rigid Foam: The Impact on the Uniformity and Mechanical Performance" Materials 18, no. 3: 585. https://doi.org/10.3390/ma18030585
APA StyleYang, Y., Wu, H., Zhang, J., Li, F., Charrier, B., Essawy, H., Pizzi, A., Zhou, X., & Chen, X. (2025). Metal Ions Fortified Tannin-Furanic Rigid Foam: The Impact on the Uniformity and Mechanical Performance. Materials, 18(3), 585. https://doi.org/10.3390/ma18030585










