Composite Oxidation Mechanism of Cu/Cu Contact Pairs During Current-Carrying Rolling in O2-N2-H2O Vapor Mixture
Highlights
- Composite oxidation mechanism.
- The ways in which surface oxidation affects the friction performance of current-carrying components.
- These results provided an in-depth understanding of the oxidation mechanisms of friction pairs in complex atmospheric environments.
Abstract
1. Introduction
2. Experimental Details
2.1. Test Method of Current-Carrying Rolling Contact
2.2. Test Sample
2.3. Test Conditions and Parameters
2.4. Analysis of Worn Surfaces
3. Results
3.1. Current-Carrying Tribological Performance of Cu/Cu Pairs in Mixed Atmosphere
3.2. Surface Characterization
4. Discussion
4.1. Composite Oxidation Mechanism
4.2. Pathways of Oxidation Effects on Current-Carrying Tribological Performance
5. Conclusions
- Based on the experimental results obtained under dry N2/O2 mixtures, humid N2, and humid N2/O2 atmospheres, thermal oxidation, tribo-oxidation, and anodic oxidation collectively contributed to the composite oxidation of Cu/Cu contact pairs during current-carrying rolling. The highest degree of oxidation occurred under humid N2/O2, with XPS analysis confirming CuO as the primary surface oxidation product.
- C-AFM results revealed that surface oxidation caused a significant reduction in conductive α-spots, thereby increasing the macroscopic contact resistance. Contact resistance exhibited a quasi-linear relationship with the surface CuO content.
- The oxidized surface exhibited enhanced hydrophilicity and greater adhesion, resulting in an elevated friction coefficient. The increase in the friction coefficient subsequently promoted the initiation of surface fatigue wear.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Yang, X.; Kang, Y.; Li, Z.; Li, H. Progress on Current-Carry Friction and Wear: An Overview from Measurements to Mechanism. Coatings 2022, 12, 1345. [Google Scholar] [CrossRef]
- Deng, S.; Zhao, K.; Kang, C. Design of A New Power Slip-ring. Shipboard Electron. Countermeas. 2020, 43, 123–125. [Google Scholar] [CrossRef]
- Yin, N.; Xing, Z.; He, K.; Zhang, Z. Tribo-informatics approaches in tribology research: A review. Friction 2023, 11, 1–22. [Google Scholar] [CrossRef]
- Liu, Z.; Jia, H.; Wang, L.; Hou, X. The key technologies of novel roll-ring for space applications. Spacecr. Environ. Eng. 2016, 33, 72–76. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, N.; Chen, S.; Liu, C. Tribo-informatics: Concept, architecture, and case study. Friction 2021, 9, 642–655. [Google Scholar] [CrossRef]
- Huang, Q.; Song, C.; Liu, Z.; Hou, X.; Pang, X.; Sun, C.; Lu, H.; Wang, S.; Zhang, Y. Research progress on the characteristics of current-carrying tribology in electrical transmission. Space Sol. Power Wirel. Transm. 2024, 1, 37–47. [Google Scholar] [CrossRef]
- Fukuda, K.; Kurono, Y.; Izumi, N.; Sugimura, J. Influence of Trace Water and Oxygen in a Hydrogen Environment on Pure Fe Friction and Wear. Tribol. Online 2010, 5, 80–86. [Google Scholar] [CrossRef]
- Gharam, A.A.; Lukitsch, M.J.; Qi, Y.; Alpas, A.T. Role of oxygen and humidity on the tribo-chemical behaviour of non-hydrogenated diamond-like carbon coatings. Wear 2011, 271, 2157–2163. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, X.; Li, H.; Wei, H.; Xu, X.; Sheng, L.; Zhu, M. Investigation on the fretting wear behavior of titanium alloy under different atmospheres by an in situ XPS spectrometry. Int. J. Mod. Phys. B 2022, 36, 2250109. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Li, J.; Zhang, Y.; Zhang, Y. Effect of Rotating Speed on Surface Damage of Rolling Current-Carrying Pairs in a Water Environment. Tribology 2021, 41, 365–372. [Google Scholar] [CrossRef]
- Wu, R.; Song, C.; Wu, H.; Lv, B.; Zhang, Y.; Zhang, Y. Effect of relative humidity on the current-carrying tribological properties of Cu–C sliding contact pairs. Wear 2022, 492–493, 204219. [Google Scholar] [CrossRef]
- Li, H.; Ji, D.; Shen, M.; Xiao, Y.; Zhao, H.; Liu, X.; Xiong, G. Effect of Environmental Humidity on Tribological Behavior ofCarbon/Copper Current-Carrying Sliding Contact Pairs. Tribology 2022, 42, 709–718. [Google Scholar] [CrossRef]
- Luo, X.; Gao, J.; Xie, W.; Hasan, R.M.M.; Qin, Y. Flexible single-step fabrication of programmable 3D nanostructures by pulse-modulated local anodic oxidation. CIRP Ann. 2023, 72, 177–180. [Google Scholar] [CrossRef]
- Nanjing Shenyuan Sheng Intelligent Technology Co., Ltd.; Henan University of Science and Technology. A Rolling Current-Carrying Friction Wear Tester. 201710172189.6. 28 February 2020. [Google Scholar]
- Dreano, A.; Fouvry, S.; Guillonneau, G. A combined friction energy and tribo-oxidation formulation to describe the high temperature fretting wear response of a cobalt-based alloy. Wear 2019, 426–427, 712–724. [Google Scholar] [CrossRef]
- BH GSO OIML R121: 2024; The Scale of Relative Humidity of Air Certified Against Saturated Salt Solutions. Bahrain Standards and Metrology Directorate: Manama, Kingdom of Bahrain, 2024.
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89–96. [Google Scholar] [CrossRef]
- Jia, H.; Hou, X.; Wang, L.; Zhang, X.; Cheng, Z. Application of Magnetically-Coupled Wireless PowerTransmission Technology on Space RotaryPower Transmission Joint. Space Electron. Technol. 2016, 13, 38–43. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1985; ISBN 9781139171731. [Google Scholar]
- Sun, Y.; Song, C.; Zhang, Y.; Li, M.; Zhang, Y. Oxidation on the current-carrying rolling surface and its subsequent impact on the damage of Cu contact pairs in O2/N2 mixture. Mater. Lett. 2021, 288, 129349. [Google Scholar] [CrossRef]
- Ding, Y.; Lu, Z.; Hu, Y.; Cao, J.; Guo, T. Oxidation behavior of pure copper and its influencing factors. J. Lanzhou Univ. Technol. 2010, 36, 1–4. [Google Scholar] [CrossRef]
- Luo, Y. Investigation for the Transport Behavior and Mechanism of Oxidation Process of Nano-Scale. Master’s Thesis, Fudan University, Shanghai, China, 2008. [Google Scholar] [CrossRef]
- Xiao, Y.; Shen, L.; Xiong, K.; Yao, P.; Xu, Y.; Shen, M. Effect of High-Temperature Oxidation on Tribological Propertiesof Cu-Based Friction Materials ContainingDifferent Forms of Graphite. Tribology 2025, 45, 124–139. [Google Scholar] [CrossRef]
- Ort, F.; Rutjes, F. Science of Synthesis: Click Chemistry. Thieme Chem. 2021, 1, 22. [Google Scholar]
- Katsuki, F. Single asperity tribochemical wear of silicon by atomic force microscopy. J. Mater. Res. 2009, 24, 173–178. [Google Scholar] [CrossRef]
- Bardin, T.T.; Pronko, J.G.; Kozak, D.A. Influence of hydroxyls on the adhesion of Au films to GaAs. Appl. Phys. Lett. 1989, 54, 173–175. [Google Scholar] [CrossRef]
- Lichtenberger, O.; Woltersdorf, J. On the atomic mechanisms of water-enhanced silicon wafer direct bonding. Mater. Chem. Phys. 1996, 44, 222–232. [Google Scholar] [CrossRef]
- Yeo, C.Y.; Xu, D.W.; Yoon, S.F.; Fitzgerald, E.A. Low temperature direct wafer bonding of GaAs to Si via plasma activation. Appl. Phys. Lett. 2013, 102, 054107. [Google Scholar] [CrossRef]
- Sun, Y.; Song, C.; Liu, Z.; Li, J.; Wang, L.; Sun, C.; Zhang, Y. Tribological and conductive behavior of Cu/Cu rolling current-carrying pairs in a water environment. Tribol. Int. 2020, 143, 106055. [Google Scholar] [CrossRef]
- Garg, V.; Zanna, S.; Seyeux, A.; Wiame, F.; Maurice, V.; Marcus, P. Inhibition of the initial stages of corrosion by 2-mercaptobenzothiazole adsorption and the effects of interfacial oxides on copper in neutral chloride conditions. Corros. Sci. 2023, 225, 111596. [Google Scholar] [CrossRef]
- Fukuda, M.; Koga, N. Kinetics and Mechanisms of the Thermal Decomposition of Copper(II) Hydroxide: A Consecutive Process Comprising Induction Period, Surface Reaction, and Phase Boundary-Controlled Reaction. J. Phys. Chem. C 2018, 122, 12869–12879. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ahn, H.S.; Bard, A.J. Surface Interrogation Scanning Electrochemical Microscopy for a Photoelectrochemical Reaction: Water Oxidation on a Hematite Surface. Anal. Chem. 2018, 90, 3045–3049. [Google Scholar] [CrossRef]
- Sharma, D.; Nicoara, N.; Jackson, P.; Witte, W.; Hariskos, D.; Sadewasser, S. Charge-carrier-concentration inhomogeneities in alkali-treated Cu(In,Ga)Se2 revealed by conductive atomic force microscopy tomography. Nat. Energy 2024, 9, 163–171. [Google Scholar] [CrossRef]
- Shin, M.W.; Rhee, T.H.; Jang, H. Nanoscale Friction Characteristics of a Contact Junction with a Field-Induced Water Meniscus. Tribol. Lett. 2016, 62, 31. [Google Scholar] [CrossRef]
- Newbury, E.; Ritchie, M. Is Scanning Electron Microscopy/Energy Dispersive X-ray Spectrometry (SEM/EDS) Quantitative? Scanning 2013, 35, 141–168. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Handoko, A.D.; Du, Y.; Xi, S.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Chen, S.; Li, W.H.; Jiang, W.; Yang, J.; Zhu, J.; Wang, L.; Ou, H.; Zhuang, Z.; Chen, M.; Sun, X.; et al. MOF Encapsulating N-Heterocyclic Carbene-Ligated Copper Single-Atom Site Catalyst towards Efficient Methane Electrosynthesis. Angew. Chem. 2022, 134, e202114450. [Google Scholar] [CrossRef]
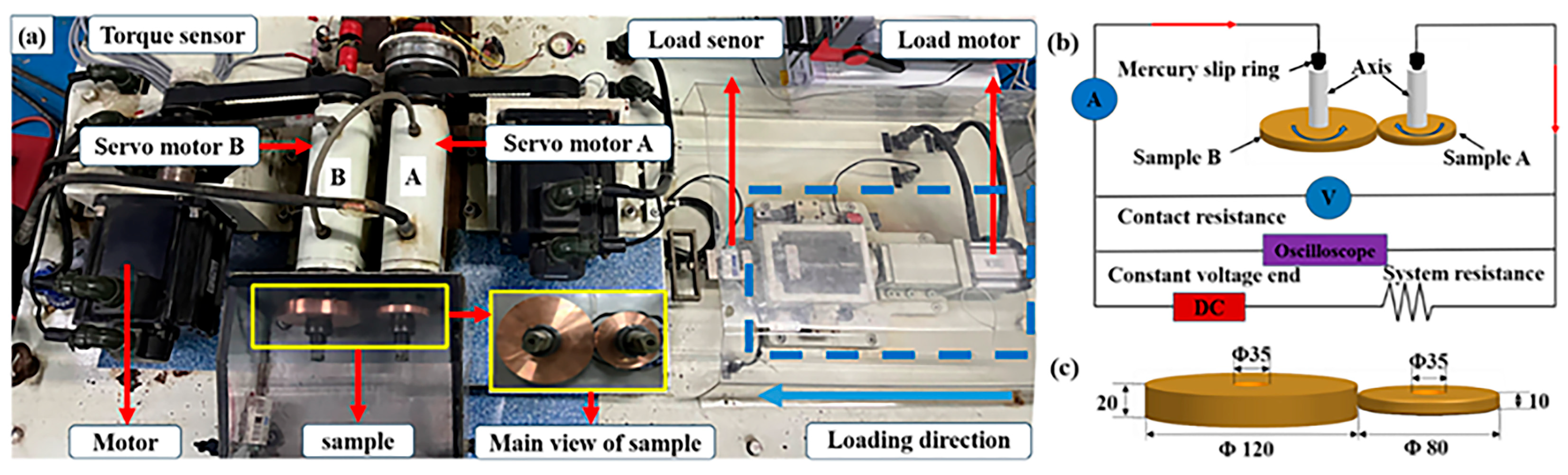
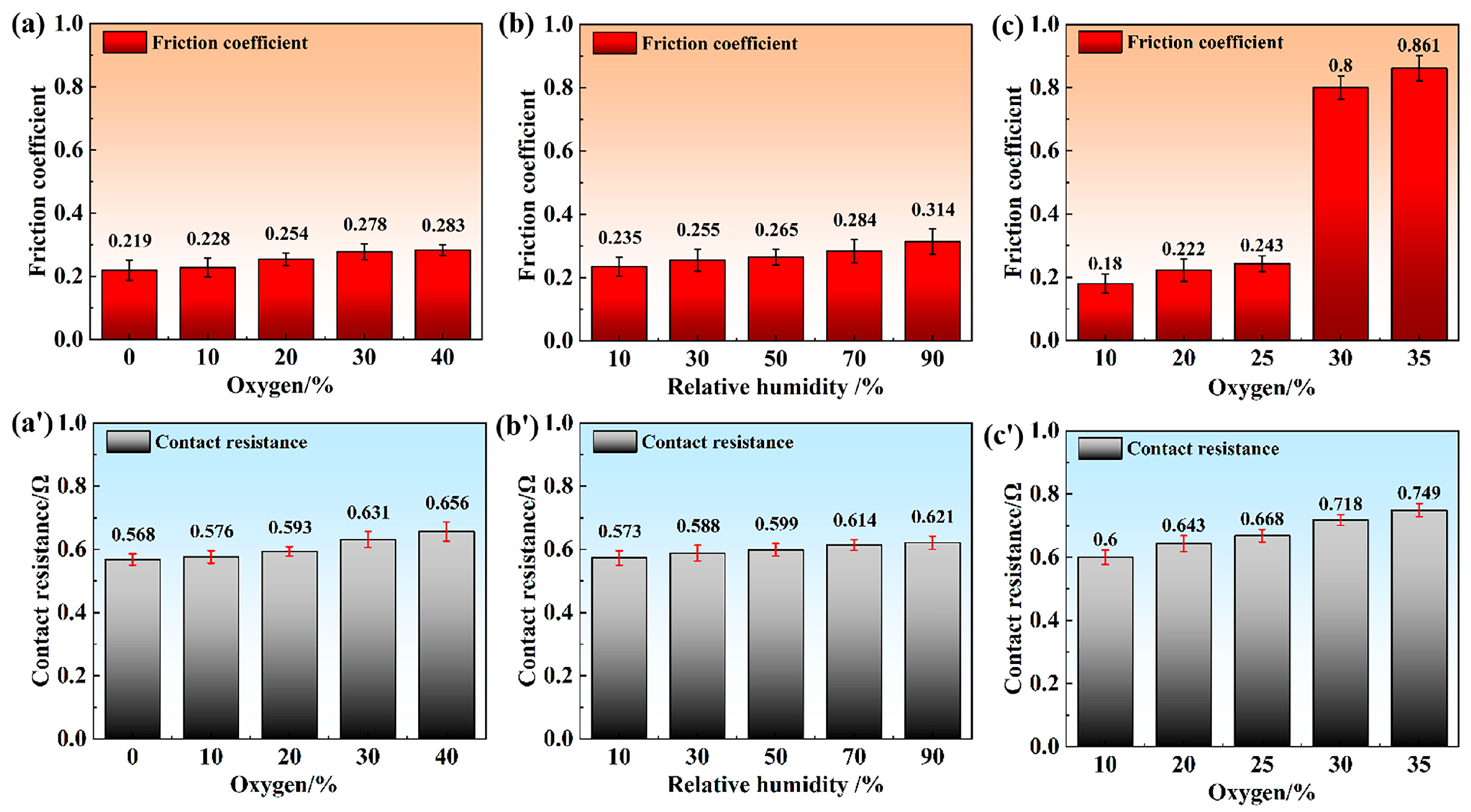


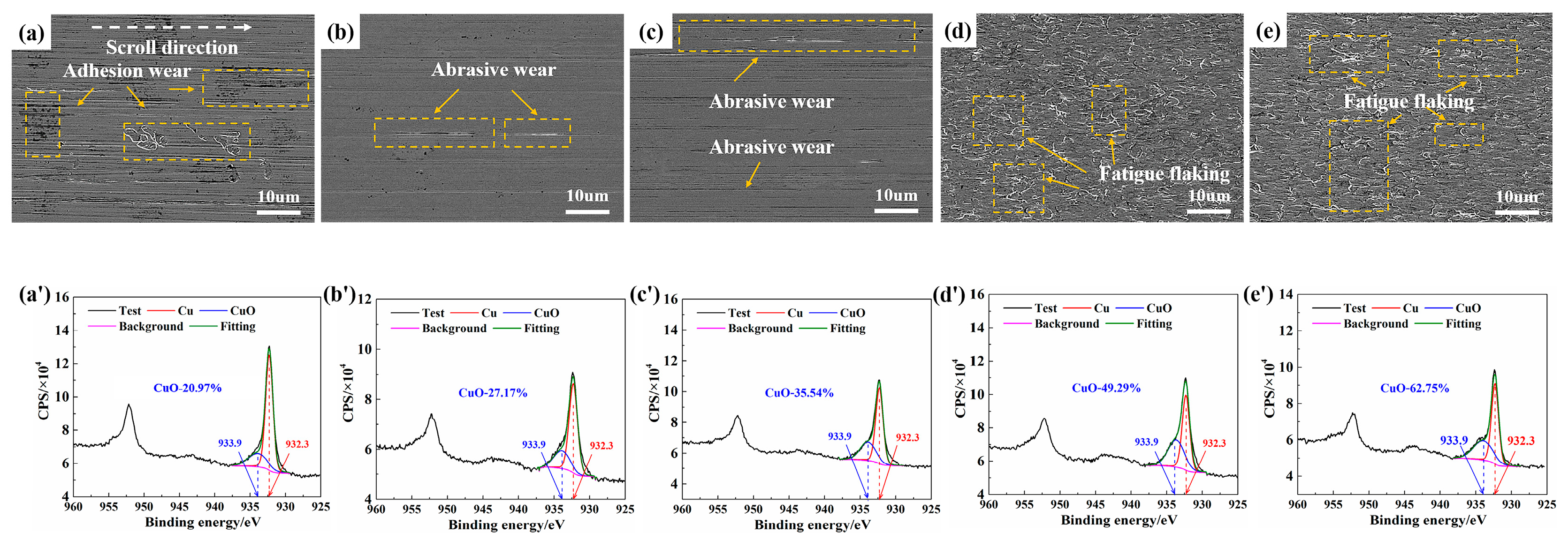

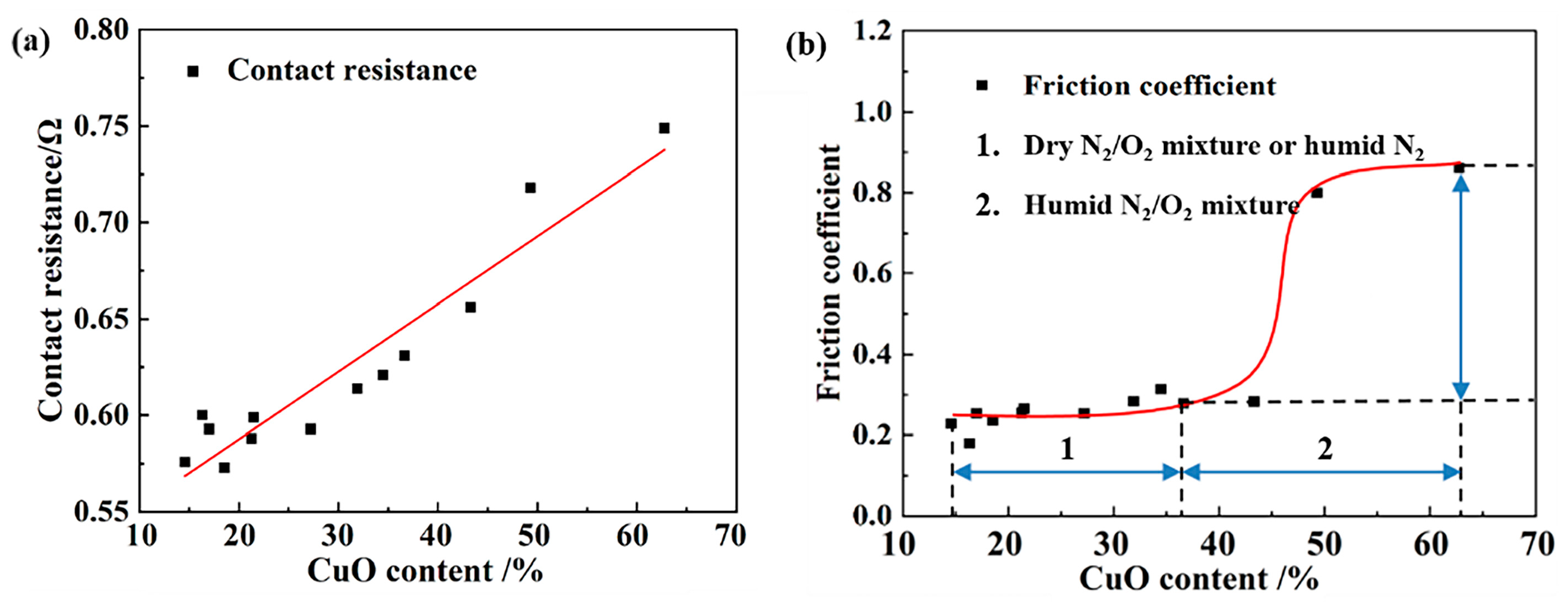



| Atmosphere | Preparation Method | Ingredients/Content |
|---|---|---|
| Dry N2/O2 mixture | The preparation was carried out by mixing dry gases, with O2 content controlled through flow rate regulation. | O2 content: 0%, 10%, 20%, 30%, 40%/vol.% |
| humid N2 | Dry gas was introduced into the saturated electrolyte solution to achieve different humidity levels [16]. | humidity level: 10%, 30%, 50%, 70%, 90%. |
| 50% humidified N2/O2 mixture | Dry N2/O2 mixture gas was introduced into the saturated electrolyte solution to achieve 50% humidity [17]. | O2 content:10%, 20%, 25%, 30%, 35%/vol.% |
| Parameters | Value |
|---|---|
| Rotational speed of sample A | 6 rpm |
| Rotational speed of sample B | 4 rpm |
| Slip-to-roll ratio | 0% |
| Linear speed | 0.025 m/s |
| Normal contact load | 40 N |
| Contact pressure | 240 MPa |
| Current intensity | 1.5 A |
| Test time | 100 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Li, F.; Li, Y.; Wu, H.; Li, B.; Song, C.; Fu, Z.; Zhang, Y. Composite Oxidation Mechanism of Cu/Cu Contact Pairs During Current-Carrying Rolling in O2-N2-H2O Vapor Mixture. Materials 2025, 18, 5693. https://doi.org/10.3390/ma18245693
Cheng J, Li F, Li Y, Wu H, Li B, Song C, Fu Z, Zhang Y. Composite Oxidation Mechanism of Cu/Cu Contact Pairs During Current-Carrying Rolling in O2-N2-H2O Vapor Mixture. Materials. 2025; 18(24):5693. https://doi.org/10.3390/ma18245693
Chicago/Turabian StyleCheng, Jianhua, Fei Li, Yuhang Li, Haihong Wu, Bohan Li, Chenfei Song, Zhibin Fu, and Yongzhen Zhang. 2025. "Composite Oxidation Mechanism of Cu/Cu Contact Pairs During Current-Carrying Rolling in O2-N2-H2O Vapor Mixture" Materials 18, no. 24: 5693. https://doi.org/10.3390/ma18245693
APA StyleCheng, J., Li, F., Li, Y., Wu, H., Li, B., Song, C., Fu, Z., & Zhang, Y. (2025). Composite Oxidation Mechanism of Cu/Cu Contact Pairs During Current-Carrying Rolling in O2-N2-H2O Vapor Mixture. Materials, 18(24), 5693. https://doi.org/10.3390/ma18245693






