Effect of Hyaluronic Acid Content on Functional Properties, Antioxidant Activity, and In Vitro Digestion of Food-Grade Furcellaran Hydrogels and Emulgels
Highlights
- HA addition increased stability and modified swelling in gels
- HA boosted antioxidant activity before and after digestion
- Gels showed elastic-solid behavior across rheological tests
- HA enhances bioactive delivery potential of composite gels
- Materials suitable for hydrophilic and lipophilic compound carriers
- Systems show promise for food, cosmetic and pharma uses
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Hydrogel and Emulgel Preparation
2.3. Zeta Potential
2.4. Swelling Ratio
2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.6. Scanning Electron Microscopy (SEM)
2.7. X-Ray Diffraction
2.8. Color Measurement
2.9. Texture Profile Analysis (TPA)
2.10. Large Deformation Mechanical Analysis
2.11. Rheological Evaluation
2.12. In Vitro Digestion
2.13. Simulation of the Intestinal Absorption Process Using Caco-2 Cells
2.14. Cell Culture and Treatment
2.15. Analysis of Mitochondrial Membrane Potential
2.16. DPPH Free Radical Scavenging Capacity Assay
2.17. Statistical Analysis
3. Results and Discussion
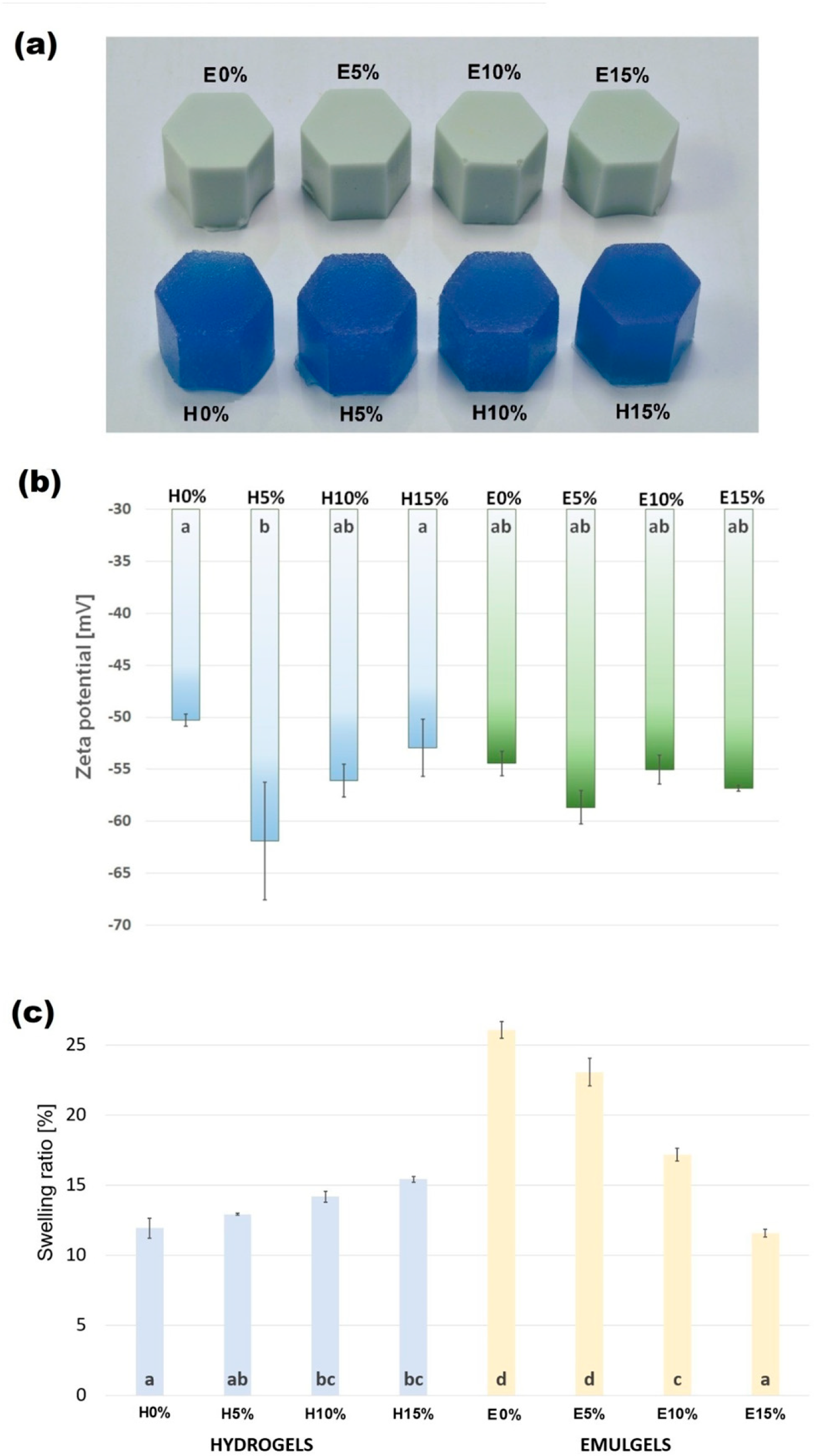
3.1. Physical Evaluation
3.1.1. Zeta Potential
3.1.2. Swelling Properties
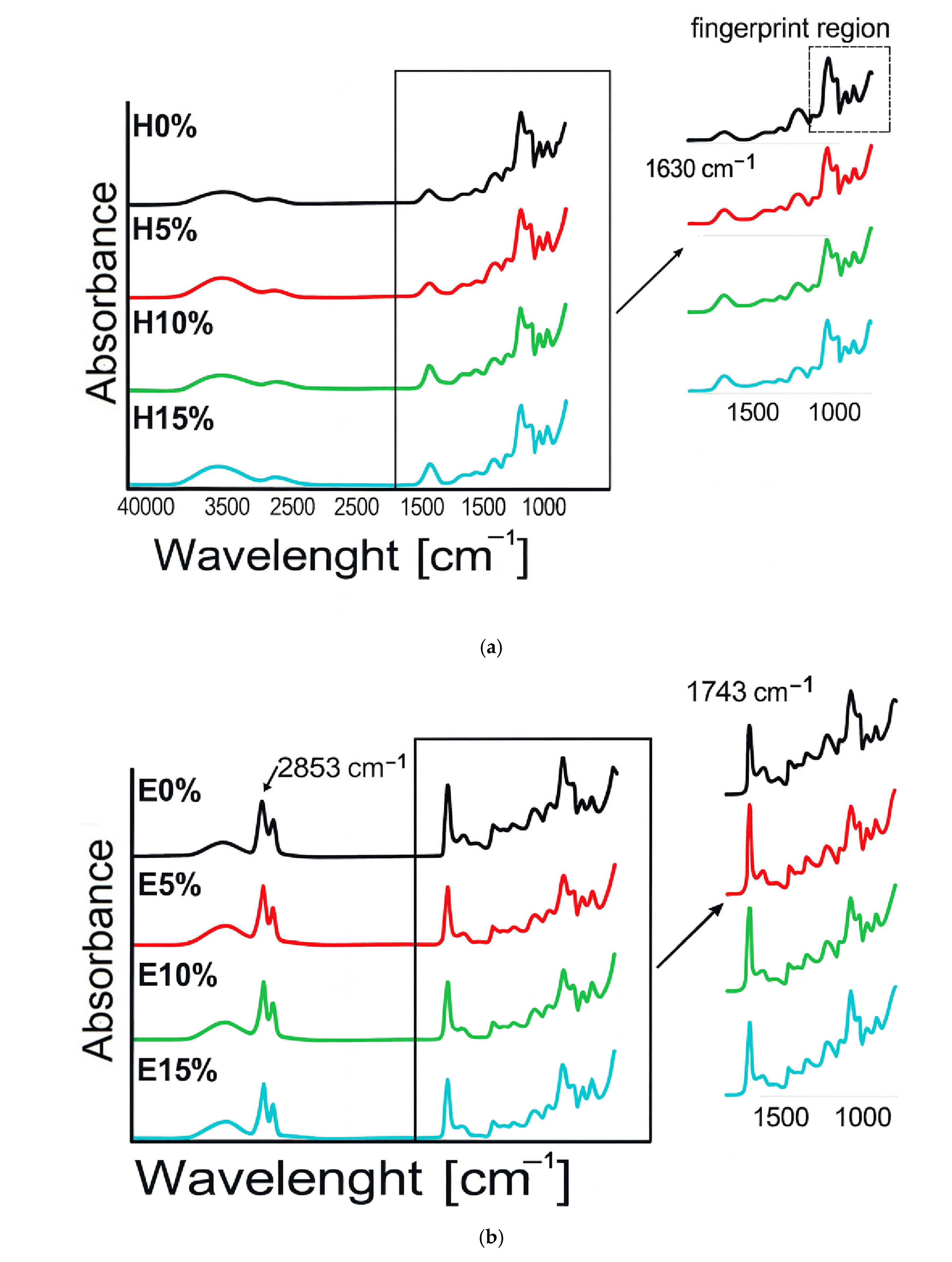
3.2. FT-IR Analysis
3.3. X-Ray Diffraction Analysis
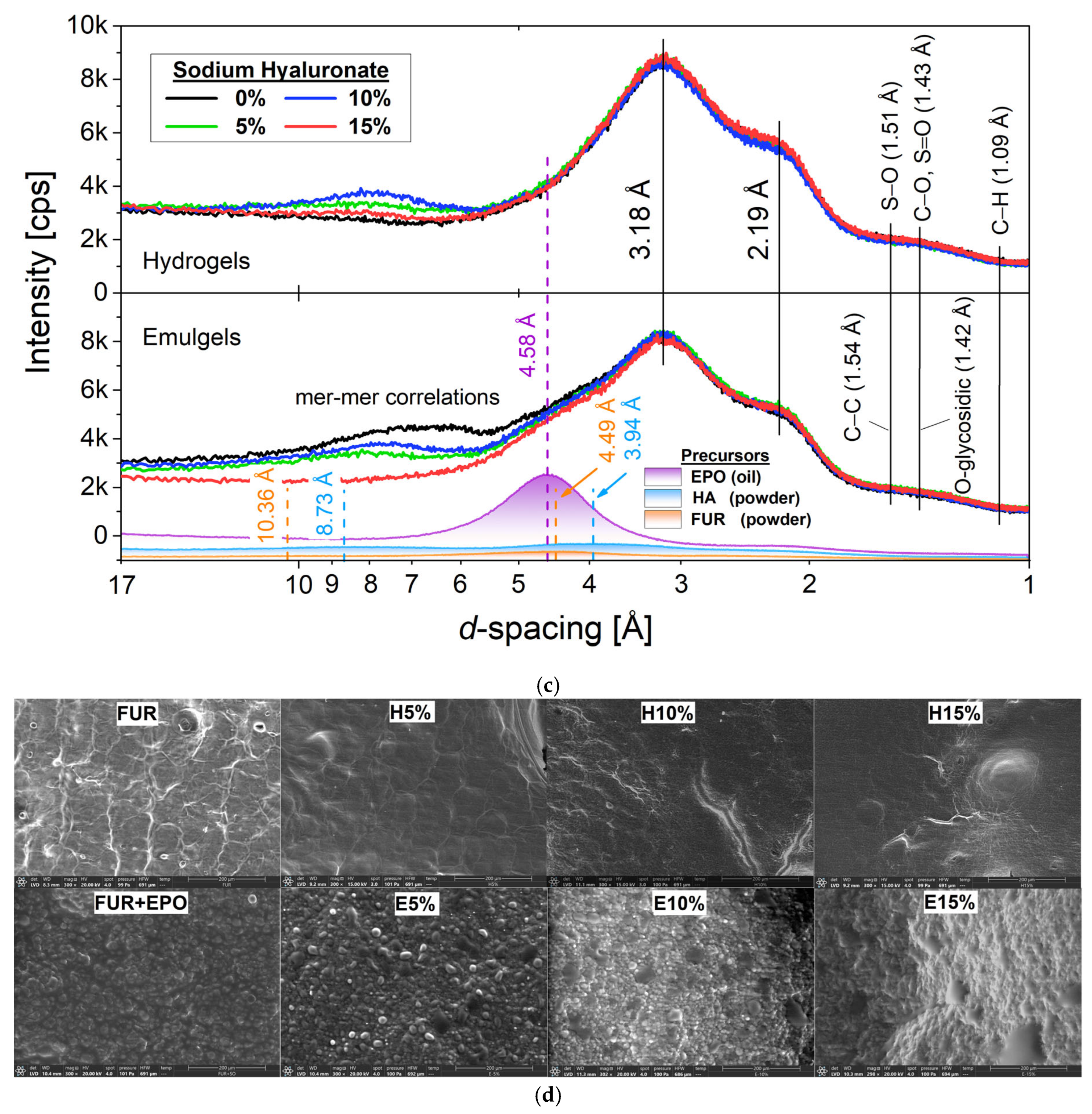
3.4. Morphological Properties by ESEM
3.5. Color Analysis of Emulgels
3.6. Texture of Hydrogels and Emulgels
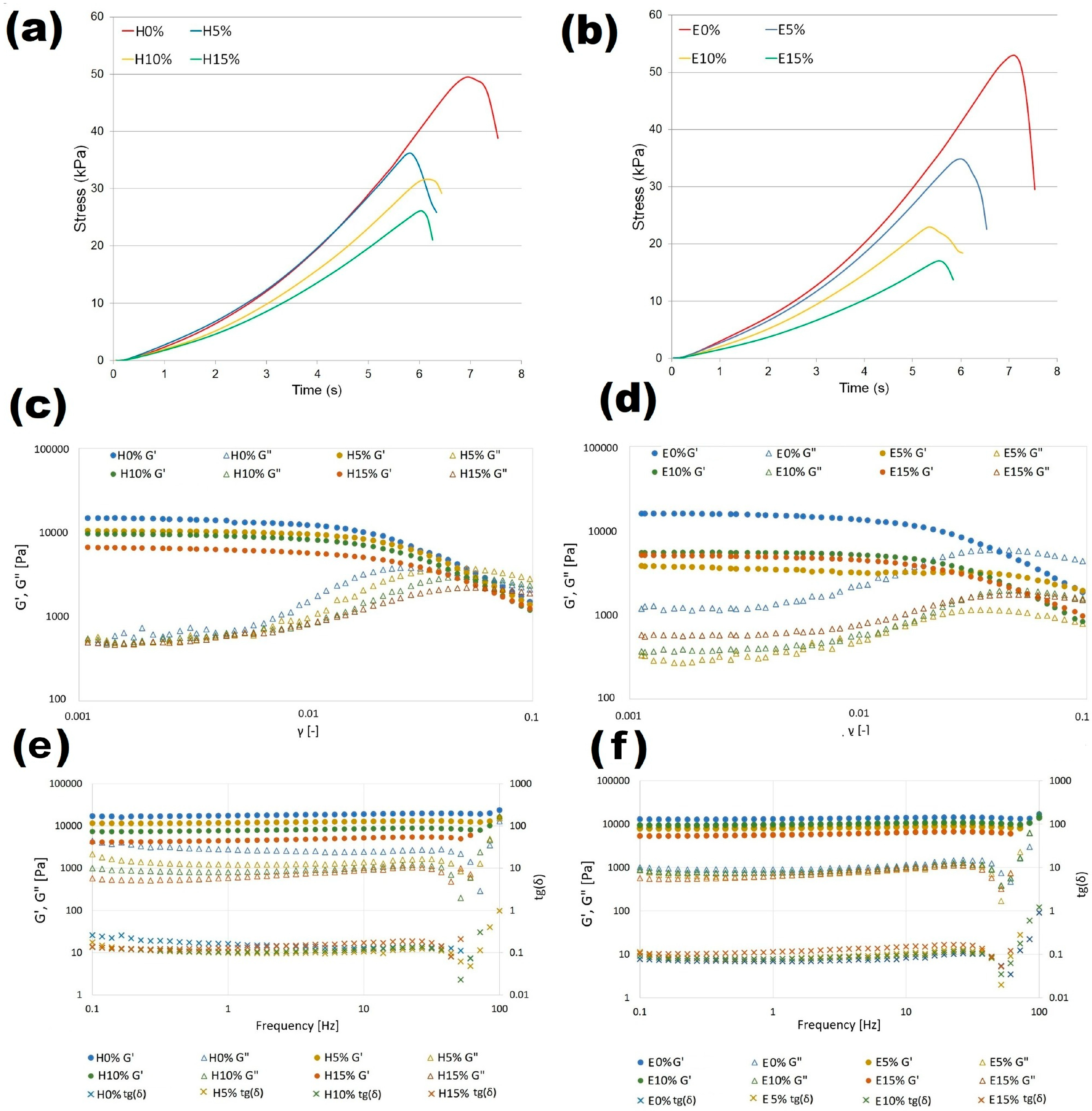
3.7. Large Deformation Properties
3.8. Rheological Characterization of Emulgels
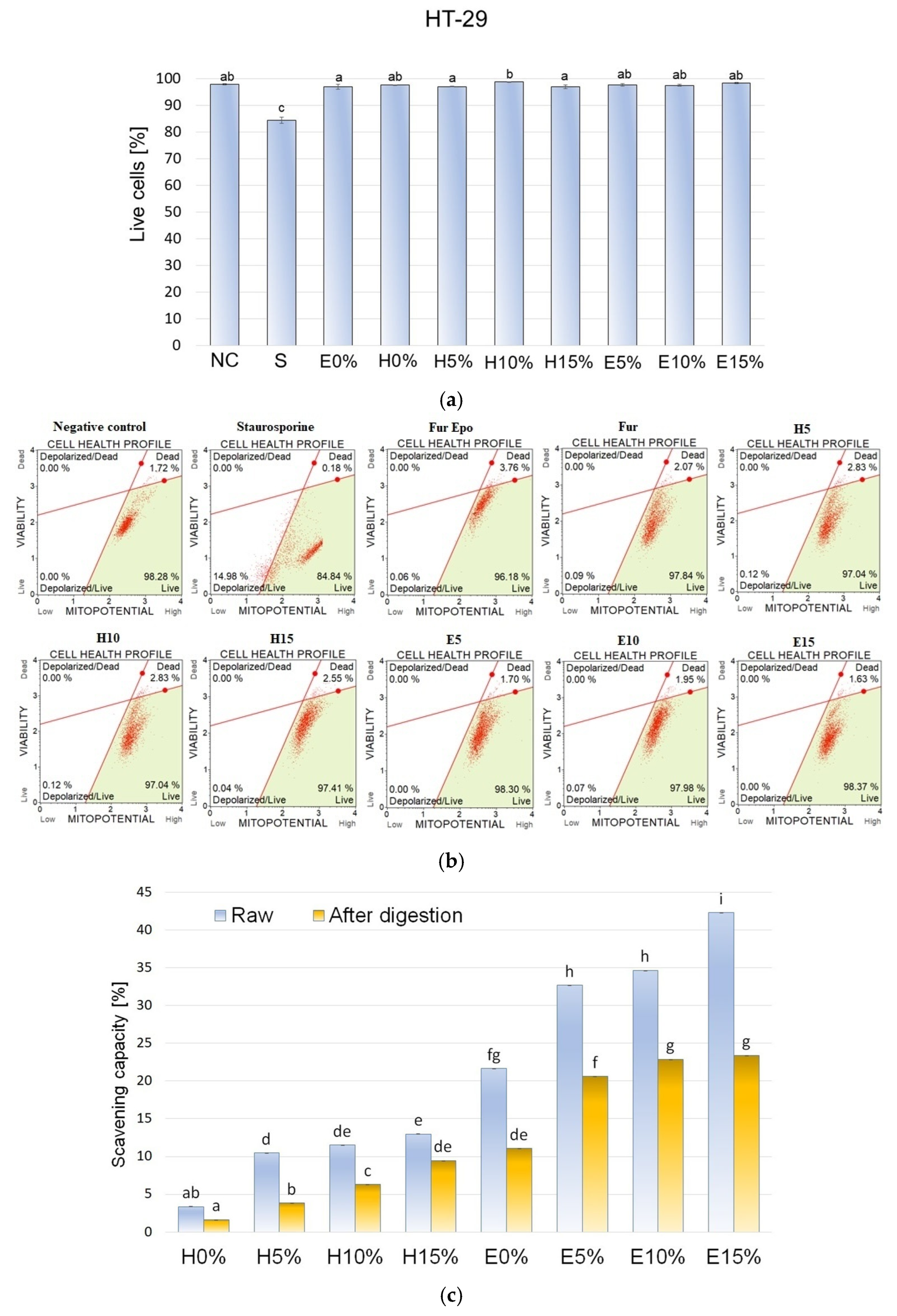
3.9. Mitochondrial Membrane Potential
3.10. DPPH Radical Scavenging Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-Based Functional Films for Packaging Applications: A Review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef] [PubMed]
- Sabalingam, S.; Siriwardhene, M.A. A Review on Emerging Applications of Emulgel as Topical Drug Delivery System. World J. Adv. Res. Rev. 2022, 13, 452–463. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, L.; Zhuang, Y.; Gu, Y.; Cheng, G.; Fan, X.; Ding, Y.; Liu, H. Protein-Stabilized Emulsion Gels with Improved Emulsifying and Gelling Properties for the Delivery of Bioactive Ingredients: A Review. Foods 2023, 12, 2703. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, Structure–Property Relationships and Applications of Different Emulsion Gels: Bulk Emulsion Gels, Emulsion Gel Particles, and Fluid Emulsion Gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Laos, K.; Ring, S.G. Characterisation of Furcellaran Samples from Estonian Furcellaria lumbricalis (Rhodophyta). J. Appl. Phycol. 2005, 17, 461–464. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Vieira, R.P.; Jamróz, E.; Anjos, C.A.R. Furcellaran: An Innovative Biopolymer in the Production of Films and Coatings. Carbohydr. Polym. 2021, 252, 117221. [Google Scholar] [CrossRef]
- Jamróz, E.; Janik, M.; Juszczak, L.; Kruk, T.; Kulawik, P.; Szuwarzyński, M.; Kawecka, A.; Khachatryan, K. Composite Biopolymer Films Based on a Polyelectrolyte Complex of Furcellaran and Chitosan. Carbohydr. Polym. 2021, 274, 118627. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active Edible Furcellaran/Whey Protein Films with Yerba Mate and White Tea Extracts: Preparation, Characterization and Application to Fresh Soft Rennet-Curd Cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef]
- Laos, K.; Lõugas, T.; Mändmets, A.; Vokk, R. Encapsulation of β-Carotene from Sea Buckthorn (Hippophaë rhamnoides L.) Juice in Furcellaran Beads. Innov. Food Sci. Emerg. Technol. 2007, 8, 395–398. [Google Scholar] [CrossRef]
- Mazzucco, A. Hyaluronic Acid: Evaluation of Efficacy with Different Molecular Weights. Int. J. Chem. Res. 2019, 1, 13–18. [Google Scholar] [CrossRef]
- Cheng, Q.; Liu, C.; Zhao, J.; Li, W.; Guo, F.; Qin, J.; Wang, Y. Unlocking the Potential of Hyaluronic Acid: Physicochemical Properties, Modification, and Role in Food Applications. Trends Food Sci. Technol. 2023, 142, 104218. [Google Scholar] [CrossRef]
- Wang, N.; Hu, J.; Zhang, K.; Zhang, Y.; Jiang, Y.; Wang, X.; Ban, Q. Development and Characterization of a Casein–Hyaluronic Acid Emulsion Gel with High Water-Holding Capacity for 3D Printing. Food Hydrocoll. 2023, 140, 108632. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, K.; Chen, Y.; Hu, J.; Jiang, Y.; Wang, X.; Ban, Q. Tuning Whey Protein Isolate/Hyaluronic Acid Emulsion Gel Structure to Enhance Quercetin Bioaccessibility. Food Chem. 2023, 429, 136910. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, W.; Zheng, M.; Huo, M.; Zhong, C. Bacterial Cellulose/Hyaluronic Acid Composite Hydrogels with Improved Viscoelastic Properties. Plast. Rubber Compos. 2018, 47, 165–175. [Google Scholar] [CrossRef]
- Gaidash, A.A.; Krut’ko, V.K.; Musskaya, O.N.; Sycheva, O.A.; Kul’bitskaya, L.V.; Mel’nikova, G.B.; Skrotskaya, K.V.; Blinova, M.I.; Kulak, A.I. Structure and Physicochemical Properties of Collagen Gels Treated with Hyaluronic Acid. Russ. J. Appl. Chem. 2022, 95, 1701–1714. [Google Scholar] [CrossRef]
- Bayles, B.; Usatine, R. Evening Primrose Oil. Public Health 2009, 80, 1405–1408. [Google Scholar]
- Mahboubi, M. Evening Primrose (Oenothera biennis) Oil in the Management of Female Ailments. J. Menopausal Med. 2019, 25, 74. [Google Scholar] [CrossRef]
- Stępień, A.; Juszczak, L.; Kowalski, G.; Synkiewicz-Musialska, B.; Zachariasz, P.; Jamróz, E. Technological Properties of Furcellaran–Whey Protein Isolate Emulgels with Various Evening Primrose Oil Concentrations. Int. J. Biol. Macromol. 2025, 293, 139140. [Google Scholar] [CrossRef]
- Stępień, A.; Juszczak, L.; Synkiewicz-Musialska, B.; Zachariasz, P.; Jamróz, E. Influence of Furcellaran and Safflower Oil Concentration on the Properties of Model Emulgel Systems. Int. J. Biol. Macromol. 2024, 278, 134751. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Corredig, M.; Dupont, D.; Dufour, C.; et al. A Standardised Static In Vitro Digestion Method for Food—International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Luo, A.; Xie, M.; Li, X.; Zhou, C.; Liu, Q. Preparation and Antioxidant Properties of Quercetin-Loaded Hyaluronic Acid Complexes via pH-Induced Co-Assembly. Food Biosci. 2024, 62, 105399. [Google Scholar] [CrossRef]
- Tirgarian, B.; Farmani, J.; Milani, J.M. Edible Oleofilms with High Vegetable Oil Content from Novel SPI/Gelatin/Chitosan Nanofiber Emulgels. Food Hydrocoll. 2023, 134, 108082. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Hao, Y.; Qi, J.; Dong, N.; Wu, C.; Wang, Q. Composite Hydrogels from Crosslinked Hyaluronic Acid and Sodium Alginate. J. Appl. Polym. Sci. 2015, 132, 41898. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Krzyściak, P.; Talaga-Ćwiertnia, K.; Juszczak, L. Intelligent and Active Furcellaran–Gelatin Films Containing Green or Pu-Erh Tea Extracts. Int. J. Biol. Macromol. 2019, 122, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; El Fawal, G.F.; Kamoun, E.A.; Fouda, M.M.G. Controlled Drug Release from Crosslinked κ-Carrageenan/Hyaluronic Acid Membranes. Int. J. Biol. Macromol. 2015, 77, 322–329. [Google Scholar] [CrossRef]
- Nasirpour-Tabrizi, P.; Azadmard-Damirchi, S.; Hesari, J.; Heshmati, M.K.; Savage, G.P. Physicochemical and Rheological Properties of Low-Fat Emulgels Containing Flaxseed Oil. LWT 2020, 133, 110107. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; McClements, D.J.; Shi, M.; Shang, Q.; Liu, X.; Liu, F. Physicochemical and Functional Properties of Lactoferrin–Hyaluronic Acid Complexes. LWT 2021, 151, 112121. [Google Scholar] [CrossRef]
- Sharma, R.; Jain, H.; Pratibha; Godugu, C.; Chella, N. Aceclofenac-Loaded Hyaluronic–Oleic Acid Micellar Gel for Osteoarthritis Management. J. Drug Deliv. Sci. Technol. 2023, 84, 104560. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S.; Nuthong, P. Effect of Virgin Coconut Oil on Surimi Gel Properties. J. Food Sci. Technol. 2018, 55, 496–505. [Google Scholar] [CrossRef]
- Ewurum, A.; Alur, A.A.; Glenn, M.; Schnepf, A.; Borchman, D. Hyaluronic Acid–Lipid Binding. BMC Chem. 2021, 15, 36. [Google Scholar] [CrossRef]
- Yan, S.; Han, G.; Wang, Q.; Zhang, S.; You, R.; Luo, Z.; Xu, A.; Li, X.; Li, M.; Zhang, Q.; et al. Directed Assembly of Silk Fibroin/Hyaluronic Acid Composite Hydrogels. Compos. Part B Eng. 2019, 176, 107204. [Google Scholar] [CrossRef]
- Ouasti, S.; Donno, R.; Cellesi, F.; Sherratt, M.J.; Terenghi, G.; Tirelli, N. Network Connectivity and Mechanical Properties of Hyaluronic Acid/PEG Hydrogels. Biomaterials 2011, 32, 6456–6470. [Google Scholar] [CrossRef]
- Xin, X.; Borzacchiello, A.; Netti, P.A.; Ambrosio, L.; Nicolais, L. Hyaluronic-Acid-Based Semi-Interpenetrating Materials. J. Biomater. Sci. Polym. Ed. 2004, 15, 1223–1236. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Liu, X.; Zhao, G.; Yang, L.; Zhu, L.; Liu, H. Improvement in Texture and Color of Soy Protein Isolate Gel Containing Capsorubin and Carotenoid Emulsions Following Microwave Heating. Food Chem. 2023, 428, 136743. [Google Scholar] [CrossRef]
- Grishko, V.; Xu, M.; Ho, R.; Mates, A.; Watson, S.; Kim, J.T.; Wilson, G.L.; Pearsall, A.W. Effects of Hyaluronic Acid on Mitochondrial Function under Oxidative Stress. J. Biol. Chem. 2009, 284, 9132–9139. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, Y.; Luo, X.; Wang, X.; Wang, C.; Wen, B.; Guan, X.; Xu, Y.; Liu, B. Effect of Chemical Refining on Composition and Oxidative Stability of Evening Primrose Oil. J. Food Process. Preserv. 2020, 44, e14800. [Google Scholar] [CrossRef]
- Ke, C.; Qiao, D.; Gan, D.; Sun, Y.; Ye, H.; Zeng, X. Antioxidant Activity of Capsule Polysaccharides from Streptococcus equi subsp. zooepidemicus. Carbohydr. Polym. 2009, 75, 677–682. [Google Scholar] [CrossRef]
| Sample | Furcellaran Content [g/100 mL] | Sodium Hyaluronate 1.5% Gel (g/100 mL) | Evening PrimRose Oil Content [ml/100 mL] | Spirulina [g/100 mL] |
|---|---|---|---|---|
| H0% | 3.0 | 0.0 | 0 | 0.6 |
| H5% | 3.0 | 5.0 | 0 | 0.6 |
| H10% | 3.0 | 10.0 | 0 | 0.6 |
| H15% | 3.0 | 15.0 | 0 | 0.6 |
| E0% | 3.0 | 0.0 | 10 | 0.6 |
| E5% | 3.0 | 5.0 | 10 | 0.6 |
| E10% | 3.0 | 10.0 | 10 | 0.6 |
| E15% | 3.0 | 15.0 | 10 | 0.6 |
| L* | a* | b* | H° | C* | WI | YI | |
|---|---|---|---|---|---|---|---|
| H0% | 26.18 ± 0.97 a | −1.4 ± 0.11 a | −15.39 ± 0.75 a | 84.79 ± 0.56 a | 119.61 ± 11.67 a | 24.58 ± 1.06 a | −84.15 ± 6.51 a |
| H5% | 23.41 ± 0.58 b | −1.8 ± 0.23 b | −18.01 ± 0.34 b | 84.29 ± 0.79 a | 163.94 ± 5.95 b | 21.3 ± 0.51 b | −109.95 ± 1.68 b |
| H10% | 19.94 ± 0.34 c | −0.61 ± 0.07 c | −18.55 ± 0.79 bc | 88.10 ± 0.20 b | 172.57 ± 14.56 bc | 17.81 ± 0.24 c | −132.91 ± 4.39 c |
| H15% | 18.65 ± 0.15 d | −0.23 ± 0.05 d | −19.34 ± 0.19 c | 89.33 ± 0.16 c | 187.02 ± 3.60 c | 16.38 ± 0.15 d | −148.15 ± 1.93 d |
| E0% | 72.25 ± 0.10 e | −7.51 ± 0.05 e | −0.45 ± 0.02 d | 3.44 ± 0.17 d | 28.33 ± 0.40 d | 71.24 ± 0.09 e | −0.89 ± 0.04 e |
| E5% | 74.89 ± 2.07 f | −6.51 ± 0.19 f | −0.86 ± 0.08 d | 7.54 ± 0.75 e | 21.55 ± 1.20 d | 75.65 ± 0.09 f | −1.60 ± 0.14 e |
| E10% | 77.23 ± 0.48 f | −6.21 ± 0.05 g | −1.85 ± 0.03 e | 16.62 ± 0.18 f | 21.00 ± 0.36 d | 76.32 ± 0.47 f | −3.43 ± 0.07 e |
| E15% | 77.24 ± 0.15 f | −5.62 ± 0.05 h | −2.36 ± 0.04 e | 22.8 ± 0.23 g | 18.61 ± 0.32 d | 76.44 ± 0.13 f | −4.37 ± 0.06 e |
| Texture | ||||
|---|---|---|---|---|
| Hardness [N] | Springiness | Cohesiveness | Gumminess [N] | |
| H0% | 18.77 ± 1.16 a | 0.850 ± 0.079 a | 0.045 ± 0.013 a | 0.868 ± 0.063 a |
| H5% | 9.01 ± 0.81 b | 0.922 ± 0.067 abc | 0.047 ± 0.002 ab | 0.587 ± 0.098 b |
| H10% | 8.12 ± 0.76 bc | 0.938 ± 0.039 abc | 0.059 ± 0.005 abc | 0.545 ± 0.065 b |
| H15% | 5.92 ± 0.56 d | 0.980 ± 0.068 abc | 0.087 ± 0.007 de | 0.254 ± 0.019 c |
| E0% | 11.97 ± 0.41 e | 0.902 ± 0.073 ab | 0.070 ± 0.015 abcd | 0.823 ± 0.093 ad |
| E5% | 11.59 ± 0.86 e | 0.933 ± 0.075 abc | 0.072 ± 0.008 bcd | 0.721 ± 0.015 d |
| E10% | 6.80 ± 0.87 dc | 1.056 ± 0.195 bc | 0.080 ± 0.004 cd | 0.460 ± 0.057 be |
| E15% | 4.05 ± 0.25 f | 1.120 ± 0.115 c | 0.110 ± 0.020 e | 0.362 ± 0.045 ce |
| FractureMechanic | ||||
| Fracture Stress [kPa] | Fracture Work [mJ] | ElasticModulus [kPa] | FractureDisplacement [mm] | |
| H0% | 53.20 ± 4.65 a | 53.95 ± 5.78 a | 76.62 ± 3.43 a | 7.24 ± 0.30 a |
| H5% | 37.72 ± 2.87 b | 32.73 ± 1.66 b | 76.46 ± 4.26 a | 6.00 ± 0.24 bc |
| H10% | 33.73 ± 3.27 b | 30.85 ± 3.25 b | 63.50 ± 1.33 b | 6.36 ± 0.29 c |
| H15% | 26.34 ± 0.40 c | 23.68 ± 1.31 c | 57.71 ± 1.42 c | 6.12 ± 0.19 bc |
| E0% | 53.34 ± 1.54 a | 53.74 ± 1.11 a | 81.76 ± 1.96 d | 7.16 ± 0.08 a |
| E5% | 35.59 ± 2.56 b | 32.60 ± 3.95 b | 73.11 ± 2.15 a | 6.16 ± 0.24 bc |
| E10% | 25.90 ± 2.43 c | 22.47 ± 3.61 c | 60.66 ± 0.84 cb | 5.84 ± 0.42 bc |
| E15% | 17.33 ± 1.25 d | 14.90 ± 1.14 d | 47.65 ± 2.54 e | 5.70 ± 0.13 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, A.; Juszczak, L.; Koronowicz, A.; Such, A.; Kowalski, G.; Synkiewicz-Musialska, B.; Zachariasz, P.; Jamróz, E. Effect of Hyaluronic Acid Content on Functional Properties, Antioxidant Activity, and In Vitro Digestion of Food-Grade Furcellaran Hydrogels and Emulgels. Materials 2025, 18, 5581. https://doi.org/10.3390/ma18245581
Stępień A, Juszczak L, Koronowicz A, Such A, Kowalski G, Synkiewicz-Musialska B, Zachariasz P, Jamróz E. Effect of Hyaluronic Acid Content on Functional Properties, Antioxidant Activity, and In Vitro Digestion of Food-Grade Furcellaran Hydrogels and Emulgels. Materials. 2025; 18(24):5581. https://doi.org/10.3390/ma18245581
Chicago/Turabian StyleStępień, Anna, Lesław Juszczak, Aneta Koronowicz, Aleksandra Such, Grzegorz Kowalski, Beata Synkiewicz-Musialska, Piotr Zachariasz, and Ewelina Jamróz. 2025. "Effect of Hyaluronic Acid Content on Functional Properties, Antioxidant Activity, and In Vitro Digestion of Food-Grade Furcellaran Hydrogels and Emulgels" Materials 18, no. 24: 5581. https://doi.org/10.3390/ma18245581
APA StyleStępień, A., Juszczak, L., Koronowicz, A., Such, A., Kowalski, G., Synkiewicz-Musialska, B., Zachariasz, P., & Jamróz, E. (2025). Effect of Hyaluronic Acid Content on Functional Properties, Antioxidant Activity, and In Vitro Digestion of Food-Grade Furcellaran Hydrogels and Emulgels. Materials, 18(24), 5581. https://doi.org/10.3390/ma18245581









