A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Chitosan Nanoparticles (CH-NPs)
2.2. Characterization Techniques
2.3. Inhibition of Growth
2.3.1. Agar Diffusion Assay
2.3.2. Microdilution Assay
2.3.3. Bacterial Viability Quantification from the Microdilution Assay
2.4. Cell Cultures
MTT Cytotoxicity Assay
2.5. Statistical Analysis
3. Results
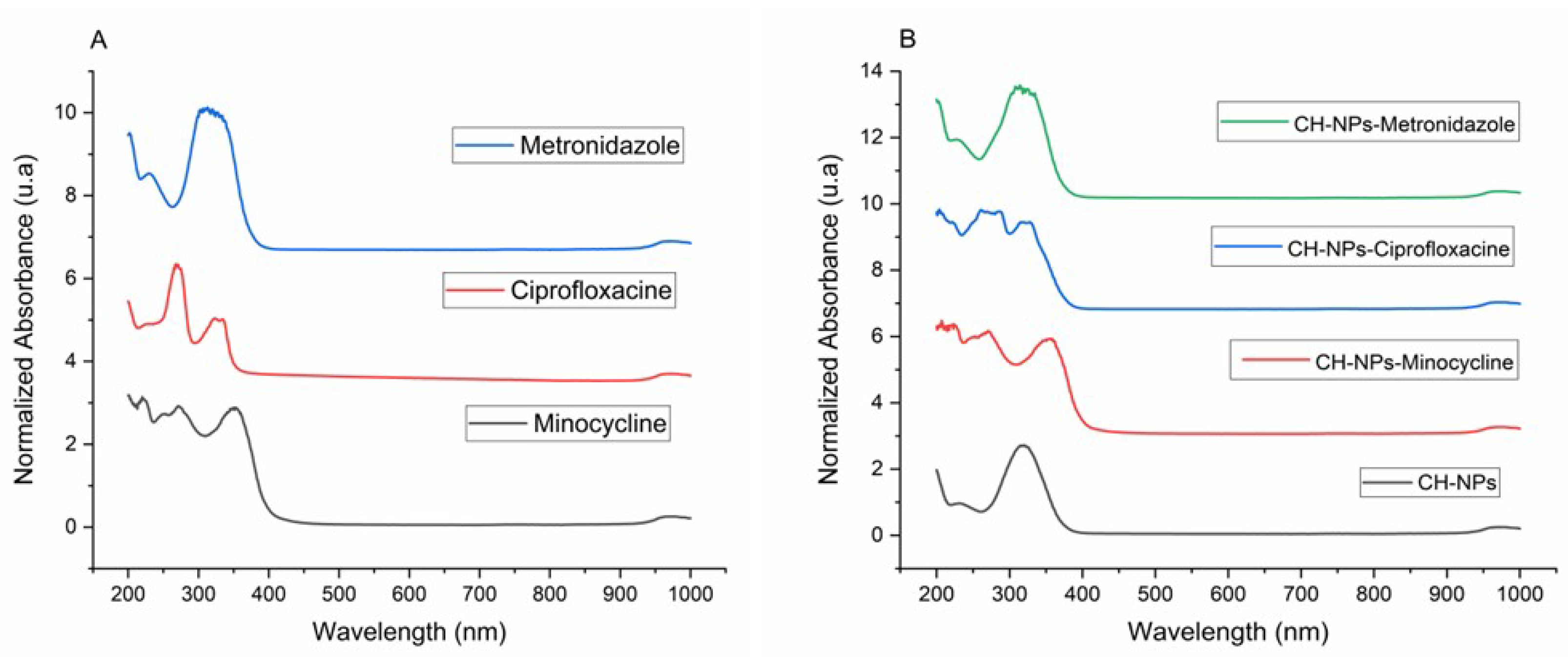
3.1. Characterization of the CH-NPs with FTIR and UV-Vis
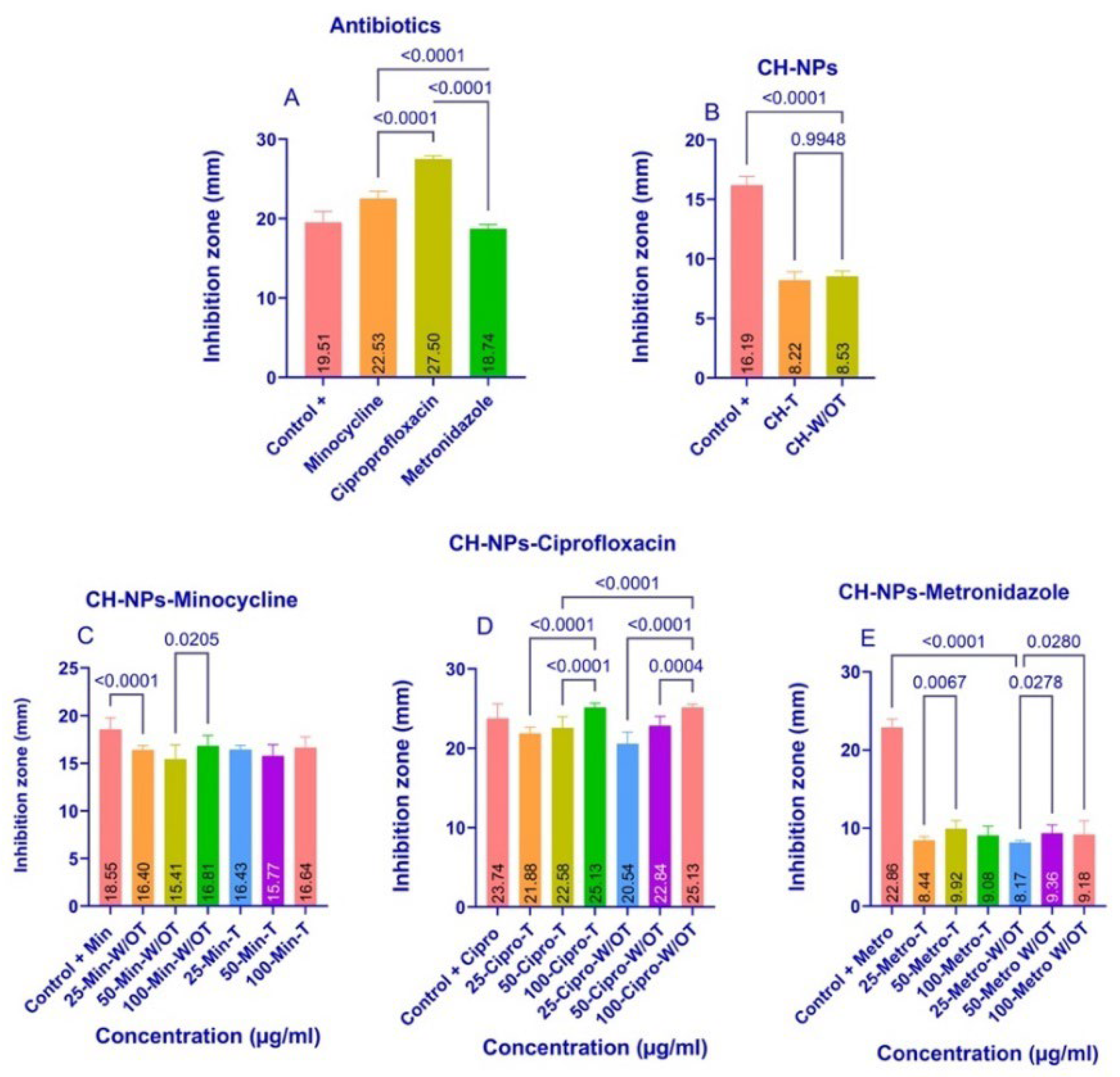
3.2. Agar Diffusion Assay
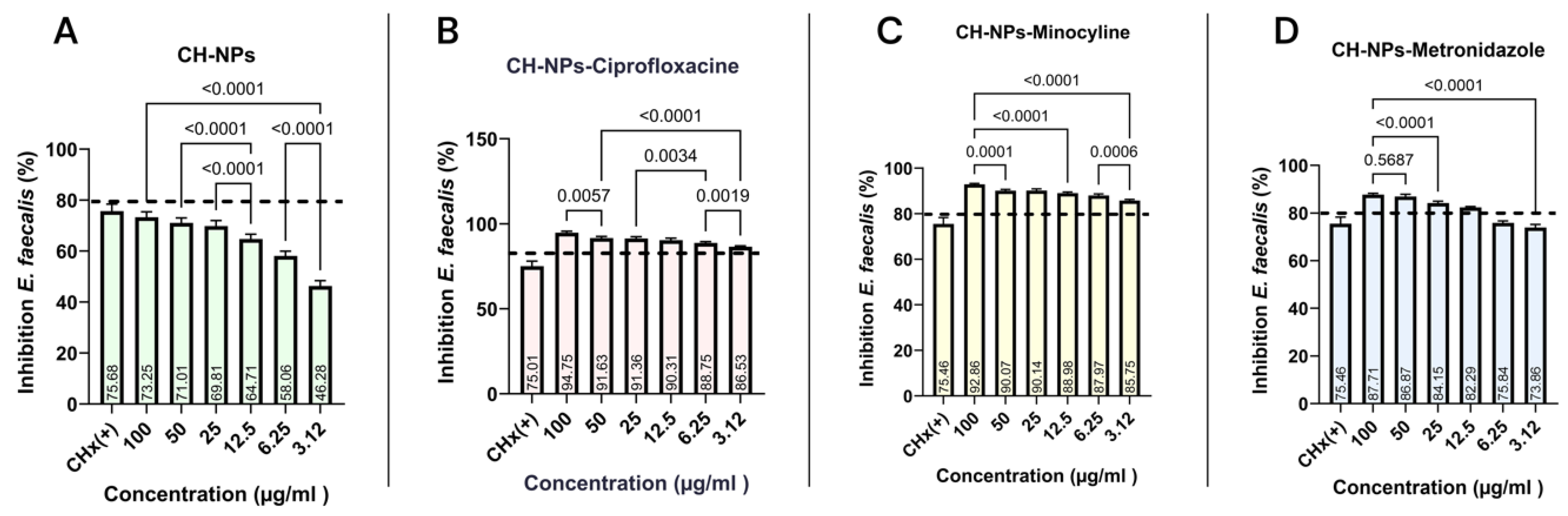
3.3. Microdilution Assay
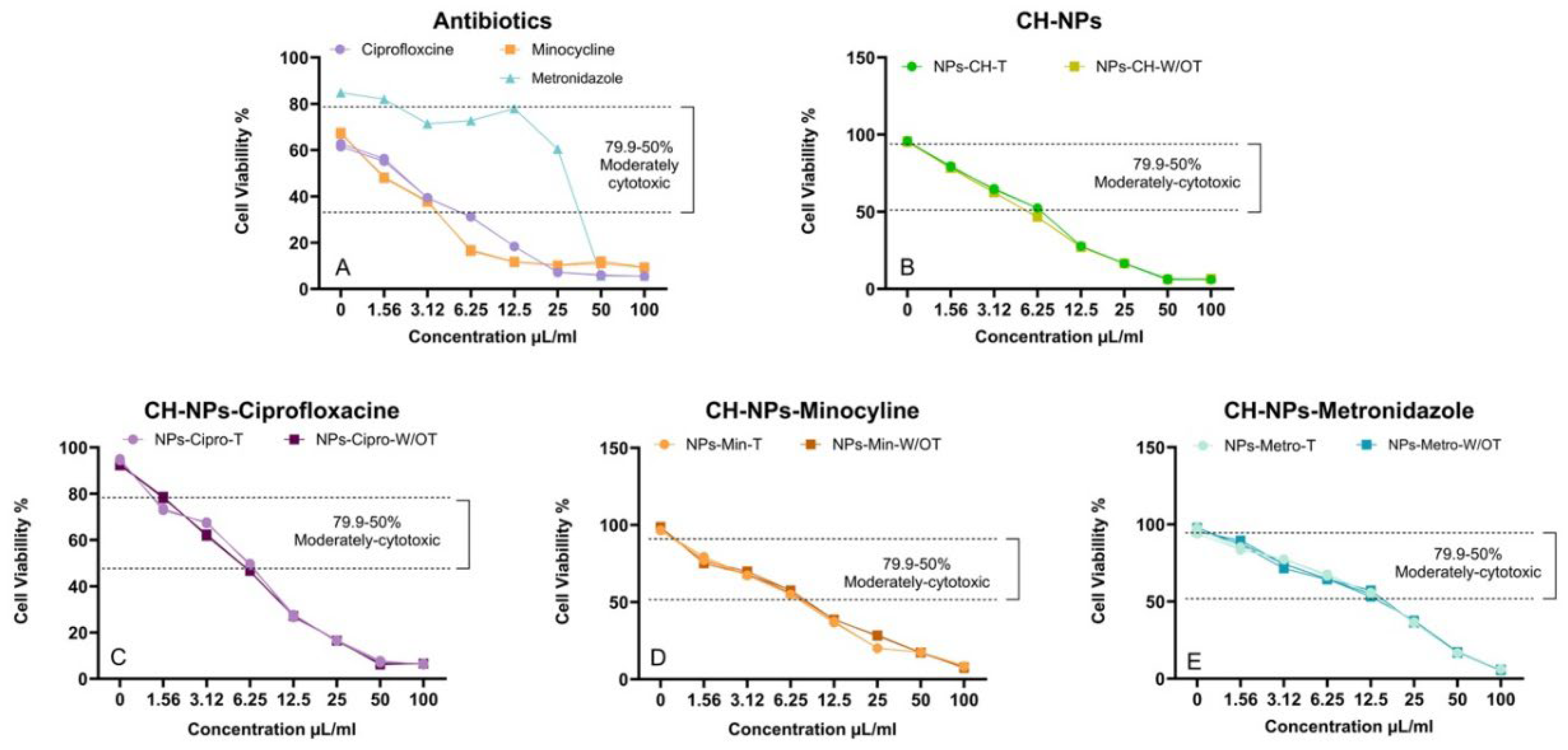
3.4. Cytotoxicity Assay
4. Discussion
4.1. Characterization of the CH-NPs
4.2. Inhibition of Growth
4.3. Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH-NPs | Chitosan nanoparticles |
| FTIR | Fourier Transform Infrared Spectroscopy |
| CHx | Chlorhexidine |
| E. faecalis | Enteroccocus faecalis |
| hDPSC | Human dental pulp stem cells |
| ENES | National School of Higher Studies |
| W/OT | Room temperature |
| W/T | Temperature |
| MIC | Minimum inhibitory concentration |
| MTT | Thiazolyl blue tetrazolium bromide |
| DMSO | Dimethyl sulfoxide |
| MEM | Minimum essential medium |
| CC50 | Average cell cytotoxicity value |
| CH-NPs-Min-T | Chitosan nanoparticles with minocycline with temperature |
| CH-NPs-Min-W/OT | Chitosan nanoparticles with minocyclin, room temperature |
| CH-NPs-Cipro-T | Chitosan nanoparticles with ciprofloxacine with temperature |
| CH-NPs-Cipro-W/OT | Chitosan nanoparticles with ciprofloxacine, room temperature |
| CH-NPs-Metro-T | Chitosan nanoparticles with metronidazole with temperature |
| CH-NPs-Metro-W/OT | Chitosan nanoparticles with metronidazole, room temperature |
References
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Micó-Muñoz, P.; Giner-Lluesma, T.; Micó-Martínez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Jhajharia, K.; Parolia, A.; Shetty, K.V.; Mehta, L.K. Biofilm in endodontics: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Macià, M.D.; Del Pozo, J.L.; Díez-Aguilar, M.; Guinea, J. Microbiological diagnosis of biofilm-related infections. Enferm. Infecc. Microbiol. Clin. (Engl. Ed.) 2018, 36, 375–381. [Google Scholar] [CrossRef]
- Abusrewil, S.; Alshanta, O.A.; Albashaireh, K.; Alqahtani, S.; Nile, C.J.; Scott, J.A.; McLean, W. Detection, treatment and prevention of endodontic biofilm infections: What’s new in 2020? Crit. Rev. Microbiol. 2020, 46, 194–212. [Google Scholar] [CrossRef]
- Siqueira Junior, J.F.; Rôças Idas, N.; Marceliano-Alves, M.F.; Pérez, A.R.; Ricucci, D. Unprepared root canal surface areas: Causes, clinical implications, and therapeutic strategies. Braz. Oral Res. 2018, 32, e65. [Google Scholar] [CrossRef]
- Ahmed, H.M.A.; Rossi-Fedele, G.; Dummer, P.M.H. Critical analysis of a new system to classify root and canal morphology—A systematic review. Aust. Endod. J. 2023, 49, 750–768. [Google Scholar] [CrossRef]
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef]
- Reffuveille, F.; Leneveu, C.; Chevalier, S.; Auffray, Y.; Rincé, A. Lipoproteins of Enterococcus faecalis: Bioinformatic identification, expression analysis and relation to virulence. Microbiology 2011, 157 Pt 11, 3001–3013. [Google Scholar] [CrossRef][Green Version]
- Kim, M.A.; Rosa, V.; Min, K.S. Characterization of Enterococcus faecalis in different culture conditions. Sci. Rep. 2020, 10, 21867. [Google Scholar] [CrossRef]
- Blancas, B.; Lanzagorta, M.D.L.; Jiménez-Garcia, L.F.; Lara, R.; Molinari, J.L.; Fernandez, A.M. Estudio de la Ultraestructura de Enterococcus faecalis y Streptococcus mutans Incubados con Péptidos Antimicrobianos Salivales. Investig. Dent. Clínica Exp. 2021, 7, 365–375. [Google Scholar] [CrossRef]
- Rayos-Verdugo, J.Y.; Rivera-Chaparro, F.; Castro-Salazar, G.Y.; Ramírez-Álvarez, M.; Romero-Quintana, J.G.; Loyola-Rodríguez, J.P.; Zavala-Alonso, N.V.; Avendaño-Félix, M.; Soto-Sainz, J.E.; Silva-Benítez, E.d.L. Propylene Glycol Potentiates the Inhibitory Action of CTZ Paste on Antibiotic-Resistant Enterococcus faecalis Isolated from the Root Canal: An In Vitro Study. Microorganisms 2023, 11, 2208. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.F.A.; Vianna, M.E.; Zaia, A.A.; Almeida, J.F.A.; Souza-Filho, F.J.; Ferraz, C.C.R. Chlorhexidine in endodontics. Braz. Dent. J. 2013, 24, 89–102. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Santos-Junior, A.O.; De Castro Pinto, L.; Mateo-Castillo, J.F.; Pinheiro, C.R. Success or failure of endodontic treatments: A retrospective study. J. Conserv. Dent. 2019, 22, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Carbajal Mejía, J.B.; Aguilar Arrieta, A. Reduction of viable Enterococcus faecalis in human radicular dentin treated with 1% cetrimide and conventional intracanal medicaments. Dent. Traumatol. 2016, 32, 321–327. [Google Scholar] [CrossRef]
- Raura, N.; Garg, A.; Arora, A.; Roma, M. Nanoparticle technology and its implications in endodontics: A review. Biomater. Res. 2020, 24, 21. [Google Scholar] [CrossRef]
- Meire, M.A.; van der Waal, S.V. A critical analysis of research methods and experimental models to study intracanal medicaments. Int. Endod. J. 2022, 55, 330–345. [Google Scholar] [CrossRef]
- Afkhami, F.; Elahy, S.; Nahavandi, A.M.; Kharazifard, M.J.; Sooratgar, A. Discoloration of teeth due to different intracanal medicaments. Restor. Dent. Endod. 2019, 44, e10. [Google Scholar] [CrossRef]
- Liu, H.; Lu, J.; Jiang, Q.; Haapasalo, M.; Qian, J.; Tay, F.R.; Shen, Y. Biomaterial scaffolds for clinical procedures in endodontic regeneration. Bioact. Mater. 2022, 12, 257–277. [Google Scholar] [CrossRef]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.S.; Cai, X.; Guo, J.M.; Pashley, D.H.; Breschi, L.; Xu, H.H.K.; Wang, X.Y.; Tay, F.R.; Niu, L.N. Chitosan-Based Extrafibrillar Demineralization for Dentin Bonding. J. Dent. Res. 2019, 98, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Alghofaily, M.; Almana, A.; Alrayes, J.; Lambarte, R.; Weir, M.D.; Alsalleeh, F. Chitosan–Gelatin Scaffolds Loaded with Different Antibiotic Formulations for Regenerative Endodontic Procedures Promote Biocompatibility and Antibacterial Activity. J. Funct. Biomater. 2024, 15, 186. [Google Scholar] [CrossRef]
- Ryu, S.; Park, S.; Lee, H.Y.; Lee, H.; Cho, C.W.; Baek, J.S. Biodegradable Nanoparticles-Loaded PLGA Microcapsule for the Enhanced Encapsulation Efficiency and Controlled Release of Hydrophilic Drug. Int. J. Mol. Sci. 2021, 22, 2792. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Zhang, Y.; Yu, Z.; Qi, C. Preparation of a Novel Chitosan Based Biopolymer Dye and Application in Wood Dyeing. Polymers 2016, 8, 338. [Google Scholar] [CrossRef]
- Jose, J.; Teja, K.V.; Janani, K.; Alam, M.K.; Khattak, O.; Salloum, M.G.; Magar, S.S.; Magar, S.P.; Rajeshkumar, S.; Palanivelu, A.; et al. Preparation of a Novel Nanocomposite and Its Antibacterial Effectiveness against Enterococcus faecalis—An In Vitro Evaluation. Polymers 2022, 14, 1499. [Google Scholar] [CrossRef]
- ISO 9001:2015(es); Sistemas de Gestión de la Calidad—Requisitos. ISO: Geneva, Switzerland, 2015.
- Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021.
- Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; CLSI Supplement M02; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 12th ed.; CLSI Supplement M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024.
- NOM-210-SSA1-2014; Productos y Servicios. Métodos de Prueba Microbiológicos. Determinación de Microorganismos Indicadores. Determinación de Microorganismos Patógenos. Norma Oficial Mexicana: Mexico City, Mexico, 2014.
- NOM-010-STPS-2014; Contaminating Chemical Agents in the Work Environment—Inspection, Evaluation and Control. Norma Oficial Mexicana: Mexico City, Mexico, 2014.
- Qi, J.; Gong, M.; Zhang, R.; Song, Y.; Liu, Q.; Zhou, H.; Wang, J.; Mei, Y. Evaluation of the antibacterial effect of tea tree oil on Enterococcus faecalis and biofilm in vitro. J. Ethnopharmacol. 2021, 281, 114566. [Google Scholar] [CrossRef]
- Ibrahim, A.; Moodley, D.; Uche, C.; Maboza, E.; Olivier, A.; Petrik, L. Antimicrobial and cytotoxic activity of electrosprayed chitosan nanoparticles against endodontic pathogens and Balb/c 3T3 fibroblast cells. Sci. Rep. 2021, 11, 24487. [Google Scholar] [CrossRef]
- Sokolonski, A.R.; Amorim, C.F.; Almeida, S.R.; Lacerda, L.E.; Araújo, D.B.; Meyer, R.; Portela, R.D. Comparative antimicrobial activity of four different endodontic sealers. Braz. J. Microbiol. 2023, 54, 1717–1721. [Google Scholar] [CrossRef]
- Athanassiadis, B.; Abbott, P.V.; George, N.; Walsh, L.J. Estudio in vitro de la actividad antimicrobiana de algunos medicamentos endodóncicos y sus bases mediante un ensayo de difusión en pocillos de agar. Aust. Dent. J. 2009, 54, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.P.; Kidd, J.M.; Jenkins, S.G.; Nicolau, D.P.; Housman, S.T. In vitro activity of ampicillin and ceftriaxone against ampicillin-susceptible Enterococcus faecium. J. Antimicrob. Chemother. 2019, 74, 2269–2273. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Bueno, I.; Garcia-Contreras, R.; Aranda-Herrera, B.; Sakagami, H.; Lopez-Ayuso, C.A.; Nakajima, H.; Jurado, C.A.; Nurrohman, H. Cytotoxicity, Differentiation, and Biocompatibility of Root-End Filling: A Comprehensive Study. Biomimetics 2023, 8, 514. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Garcia-Contreras, R.; Chavez-Granados, P.A.; Jurado, C.A.; Aranda-Herrera, B.; Afrashtehfar, K.I.; Nurrohman, H. Natural Bioactive Epigallocatechin-Gallate Promote Bond Strength and Differentiation of Odontoblast-like Cells. Biomimetics 2023, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Cytotoxicity Enhancement of α-Mangostin with Folate-Conjugated Chitosan Nanoparticles in MCF-7 Breast Cancer Cells. Molecules 2023, 28, 7585. [Google Scholar] [CrossRef]
- Lopez-Ayuso, C.A.; Garcia-Contreras, R.; Manisekaran, R.; Figueroa, M.; Rangel-Grimaldo, M.; Jacome, M.; Dominguez-Perez, R.A.; Lopez-Morales, S.; Cristians, S.; Acosta-Torres, L.S. Biological and mechanical properties of a self-curing acrylic resin enriched with AgNPs as a proposal for orthopedic aparatology. Nanoscale Adv. 2025, 7, 2068–2082. [Google Scholar] [CrossRef]
- Bautista-Martinez, D.; Scougall-Vilchis, R.J.; Contreras-Bulnes, R.; Garcia-Contreras, R. Assessment of cytotoxicity, odontoblast-like differentiation, shear bond strength, and microhardness of four orthodontic adhesive composites. J. Oral Sci. 2024, 66, 220–225. [Google Scholar] [CrossRef]
- Tkachenko, Y.; Niedzielski, P. FTIR as a Method for Qualitative Assessment of Solid Samples in Geochemical Research: A Review. Molecules 2022, 27, 8846. [Google Scholar] [CrossRef]
- Korniienko, V.; Husak, Y.; Diedkova, K.; Varava, Y.; Grebnevs, V.; Pogorielova, O.; Bērtiņš, M.; Korniienko, V.; Zandersone, B.; Ramanaviciene, A.; et al. Antibacterial Potential and Biocompatibility of Chitosan/Polycaprolactone Nanofibrous Membranes Incorporated with Silver Nanoparticles. Polymers 2024, 16, 1729. [Google Scholar] [CrossRef]
- Wu, L.; Chen, W.; Li, F.; Morrow, B.R.; Garcia-Godoy, F.; Hong, L. Sustained Release of Minocycline From Minocycline-Calcium-Dextran Sulfate Complex Microparticles for Periodontitis Treatment. J. Pharm. Sci. 2018, 107, 3134–3142. [Google Scholar] [CrossRef]
- Hibbard, T.; Nyambura, B.; Scholes, P.; Totolici, M.; Shankland, K.; Al-Obaidi, H. Preparation and Physiochemical Analysis of Novel Ciprofloxacin / Dicarboxylic Acid Salts. J. Pharm. Sci. 2023, 112, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Golj, D.R.; Dinka, M.O.; Sherefedin, U.; Belay, A.; Gelanu, D.; Megersa, G.D. Solvent polarity effects on the FTIR spectrum, and thermodynamic and electronic properties of metronidazole and its binding with antibacterial drugs: A DFT and molecular docking study. RSC Adv. 2025, 15, 28538–28554. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Gulani, V.; Seiberlich, N.; Liu, K.; Sunshine, J.L.; Duerk, J.L.; Griswold, M.A. Magnetic resonance fingerprinting. Nature 2013, 495, 187–192. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.; Cechella, B.C.; Bernardi, A.V.; de Lima Pimenta, A.; Felippe, W.T. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against Enterococcus faecalis biofilm. Indian J. Dent. Res. 2018, 29, 347–351. [Google Scholar] [CrossRef]
- Hung, W.K.; Mahyuddin, A.; Sockalingam, S.N.M.P.; Shafiei, Z.; Abdul Rahman, M.; Mahamad Apandi, N.I.; Ghani, Z.D.F.A.; Zakaria, A.S.I. Cytotoxic Evaluation of Effective Ecoproduce (EEP) as a Potential Root Canal Irrigant: A Preliminary In Vitro Study. Appl. Sci. 2023, 13, 10125. [Google Scholar] [CrossRef]
- Palasuk, J.; Kamocki, K.; Hippenmeyer, L.; Platt, J.A.; Spolnik, K.J.; Gregory, R.L.; Bottino, M.C. Bimix antimicrobial scaffolds for regenerative endodontics. J. Endod. 2014, 40, 1879–1884. [Google Scholar] [CrossRef]
- Chamorro-Petronacci, C.M.; Torres, B.S.; Guerrero-Nieves, R.; Pérez-Sayáns, M.; Carvalho-de Abreu Fantini, M.; Cides-da-Silva, L.C.; Magariños, B.; Rivas-Mundiña, B. Efficacy of Ciprofloxacin, Metronidazole and Minocycline in Ordered Mesoporous Silica against Enterococcus faecalis for Dental Pulp Revascularization: An In-Vitro Study. Materials 2022, 15, 2266. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Amin, M.Y.; Pawar, J.S.; Akhter, N.; Lucy, I.B. Conventional methods and future trends in antimicrobial susceptibility testing. Saudi J. Biol. Sci. 2023, 30, 103582. [Google Scholar] [CrossRef]
- Khan, Z.A.; Siddiqui, M.F.; Park, S. Current and Emerging Methods of Antibiotic Susceptibility Testing. Diagnostics 2019, 9, 49. [Google Scholar] [CrossRef]
- Pascale, C.; Geaman, J.; Mendoza, C.; Gao, F.; Kaminski, A.; Cuevas-Nunez, M.; Darvishan, B.; Mitchell, J.C.; Carrilho, M.R.; Sigar, I. In vitro assessment of antimicrobial potential of low molecular weight chitosan and its ability to mechanically reinforce and control endogenous proteolytic activity of dentine. Int. Endod. J. 2023, 56, 1337–1349. [Google Scholar] [CrossRef]
- Belkadi, R.; Sanz-Serrano, D.; Ventura, F.; Mercade, M. Chitosan-based endodontic irrigation solutions and TGF-β1 treatment: Creating the most favourable environment for the survival and proliferation of stem cells of the apical papilla in vitro. Int. Endod. J. 2024, 57, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [PubMed]
- Grierosu, C.; Calin, G.; Damir, D.; Marcu, C.; Cernei, R.; Zegan, G.; Anistoroaei, D.; Moscu, M.; Carausu, E.M.; Duceac, L.D.; et al. Development and Functionalization of a Novel Chitosan-Based Nanosystem for Enhanced Drug Delivery. J. Funct. Biomater. 2023, 14, 538. [Google Scholar] [CrossRef] [PubMed]
- Vouzara, T.; Koulaouzidou, E.; Ziouti, F.; Economides, N. Combined and independent cytotoxicity of sodium hypochlorite, ethylenediaminetetraacetic acid and chlorhexidine. Int. Endod. J. 2016, 49, 764–773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Flores, A.I.; Velazquez-Enriquez, U.; Scougall-Vilchis, R.J.; Acosta-Torres, L.S.; Rodriguez-Vilchis, L.E.; Contreras-Bulnes, R.; Serrano-Diaz, P.N.; Garcia-Contreras, R. A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant. Materials 2025, 18, 5552. https://doi.org/10.3390/ma18245552
Lopez-Flores AI, Velazquez-Enriquez U, Scougall-Vilchis RJ, Acosta-Torres LS, Rodriguez-Vilchis LE, Contreras-Bulnes R, Serrano-Diaz PN, Garcia-Contreras R. A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant. Materials. 2025; 18(24):5552. https://doi.org/10.3390/ma18245552
Chicago/Turabian StyleLopez-Flores, Alejandra Itzel, Ulises Velazquez-Enriquez, Rogelio Jose Scougall-Vilchis, Laura Susana Acosta-Torres, Laura Emma Rodriguez-Vilchis, Rosalía Contreras-Bulnes, Paloma Netzayeli Serrano-Diaz, and Rene Garcia-Contreras. 2025. "A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant" Materials 18, no. 24: 5552. https://doi.org/10.3390/ma18245552
APA StyleLopez-Flores, A. I., Velazquez-Enriquez, U., Scougall-Vilchis, R. J., Acosta-Torres, L. S., Rodriguez-Vilchis, L. E., Contreras-Bulnes, R., Serrano-Diaz, P. N., & Garcia-Contreras, R. (2025). A New Disinfection Approach Using a Chitosan-Based Endodontic Irrigant. Materials, 18(24), 5552. https://doi.org/10.3390/ma18245552







