Preliminary Study of Microbial Corrosion of Stainless Steel AISI 304 Under Conditions Simulating Deep Radioactive Waste Disposal
Abstract
1. Introduction
2. Materials and Methods
2.1. Stainless Steel Biocorrosion by Microbial Community
2.2. The Effect of Microbial Components on Stainless Steel Corrosion
2.3. Methods
3. Results
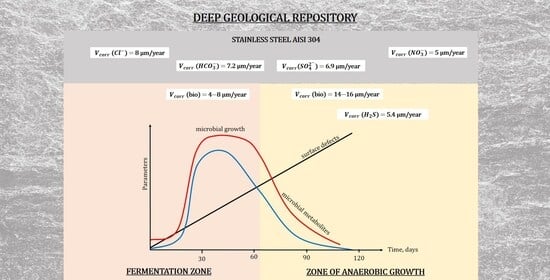
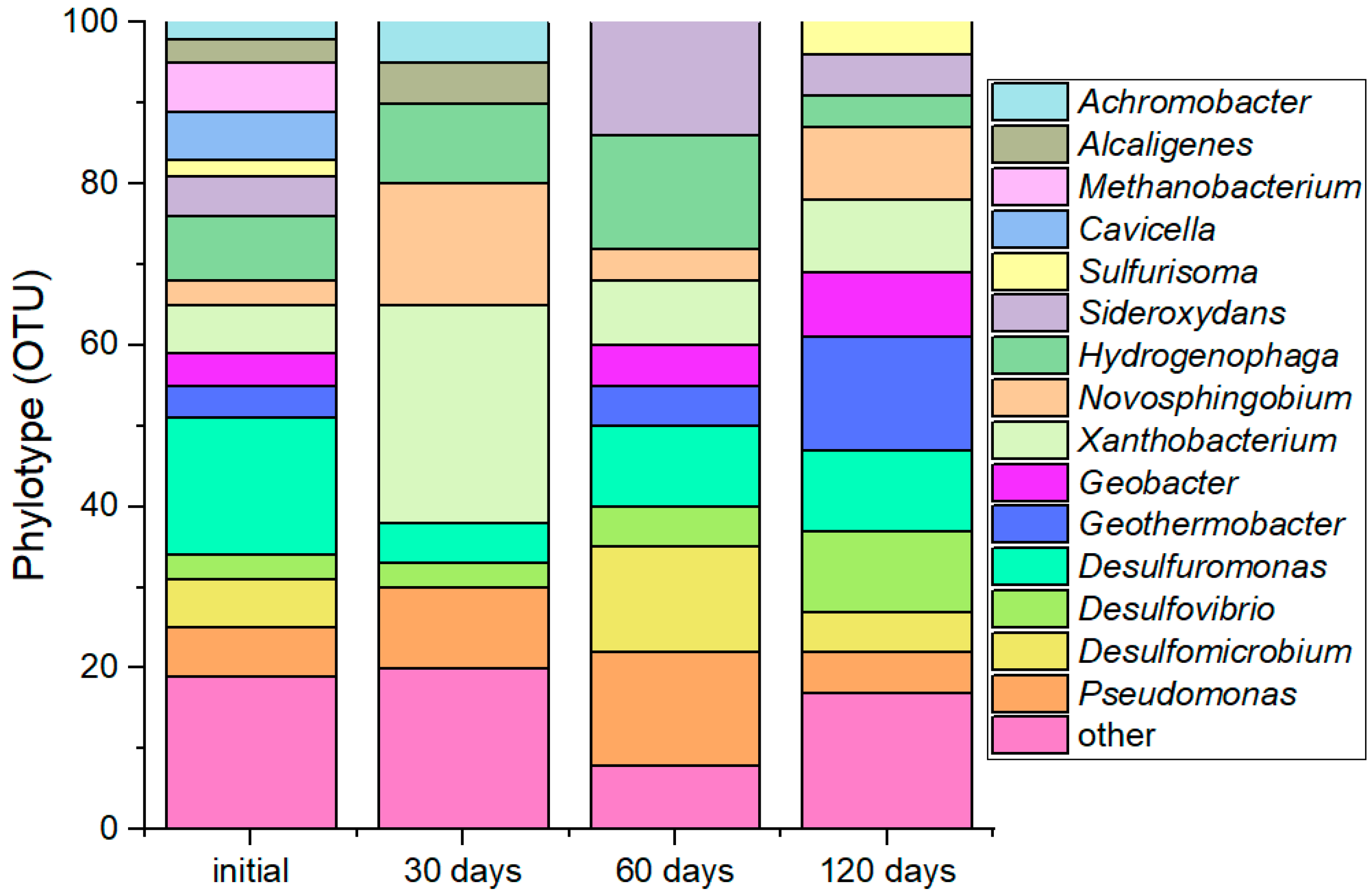
3.1. Dynamics in Microbial Community Structure
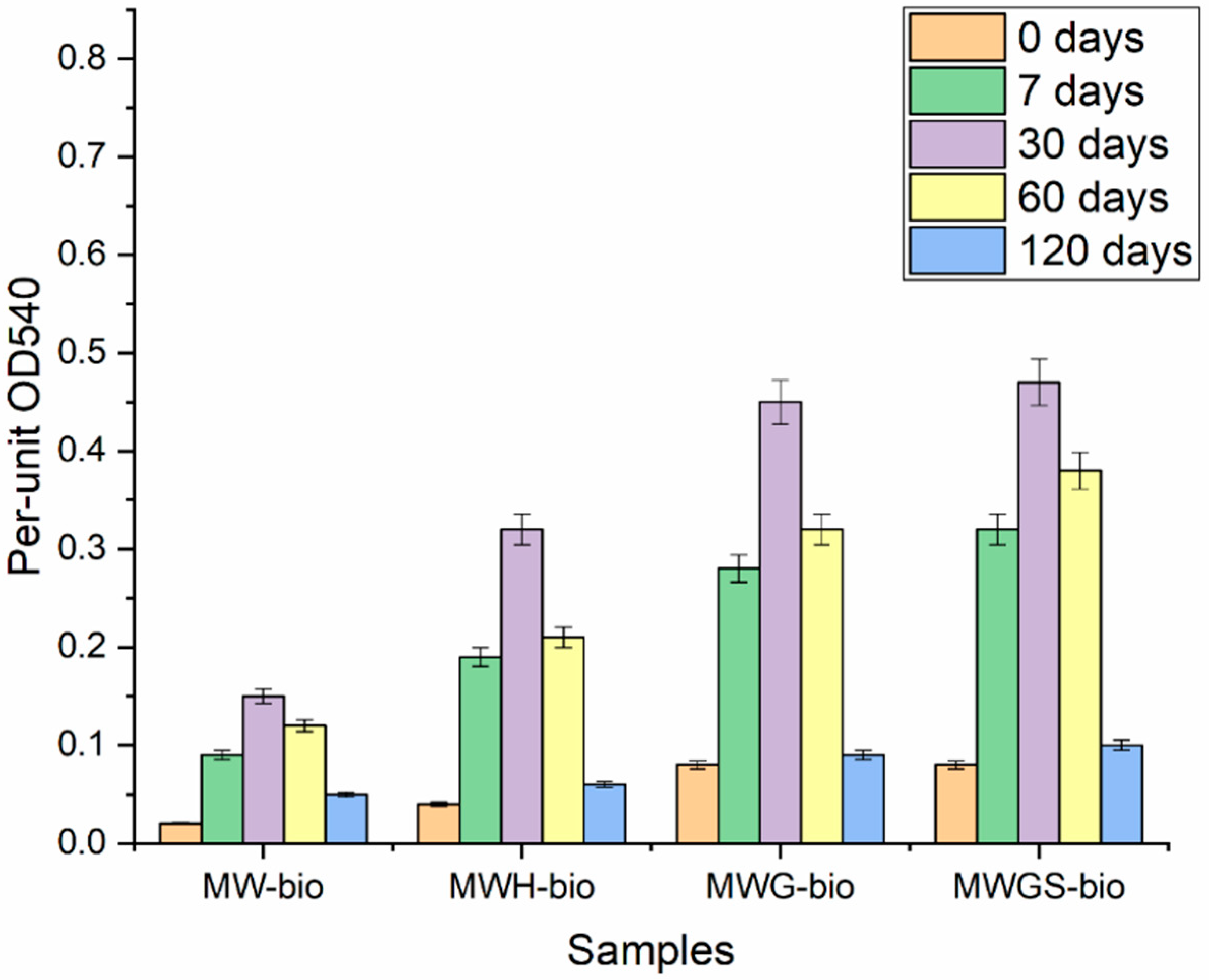
3.2. Microbial Fouling of Samples
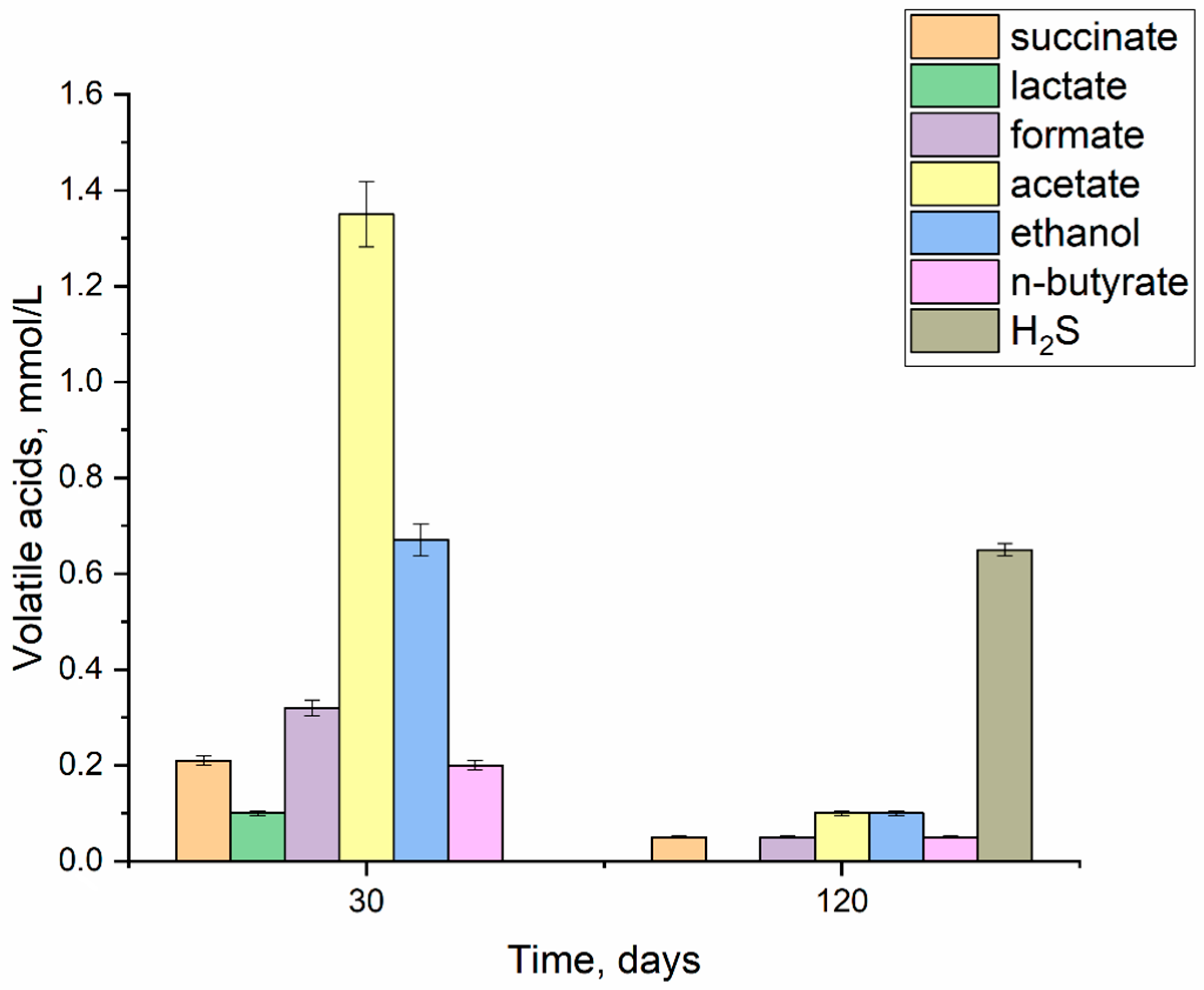
3.3. Volatile Acids and H2S Formation
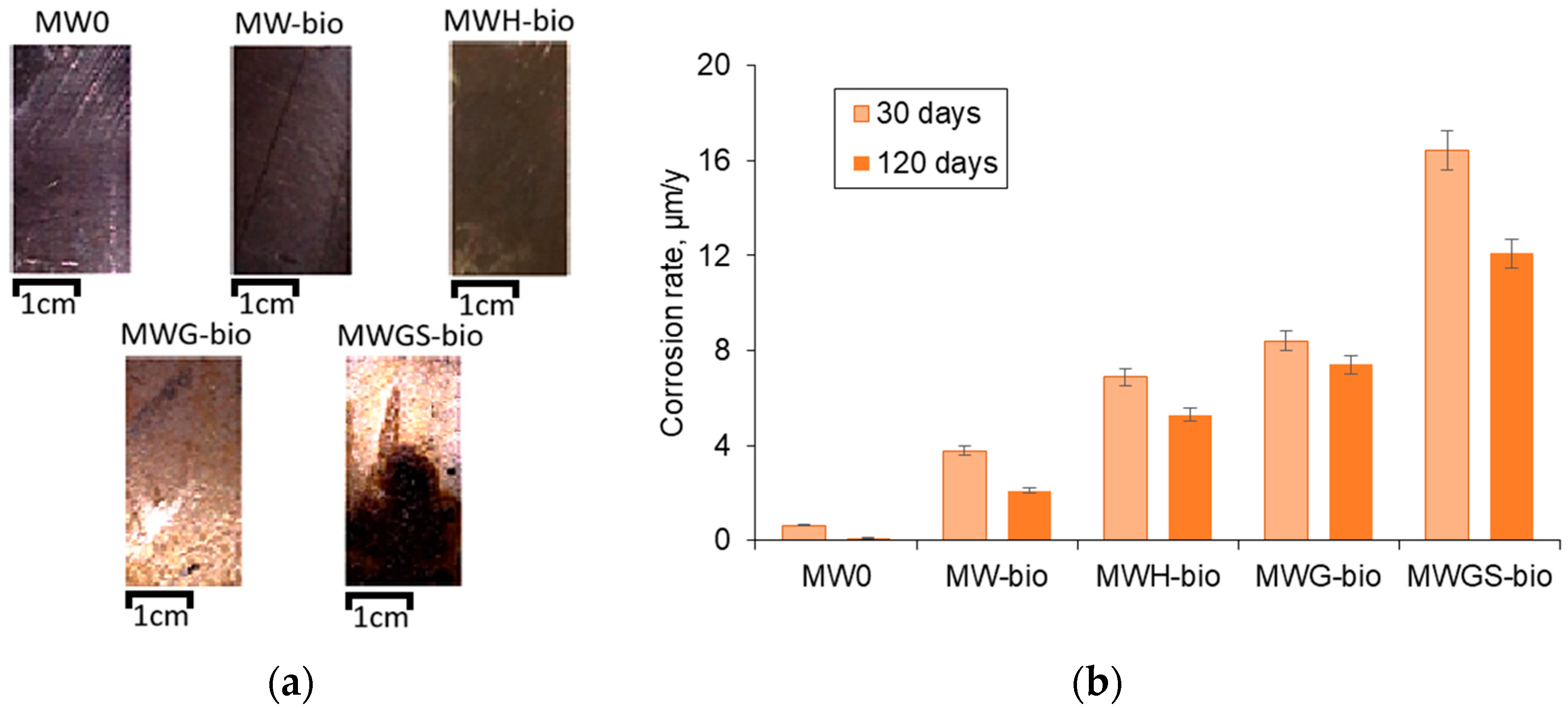
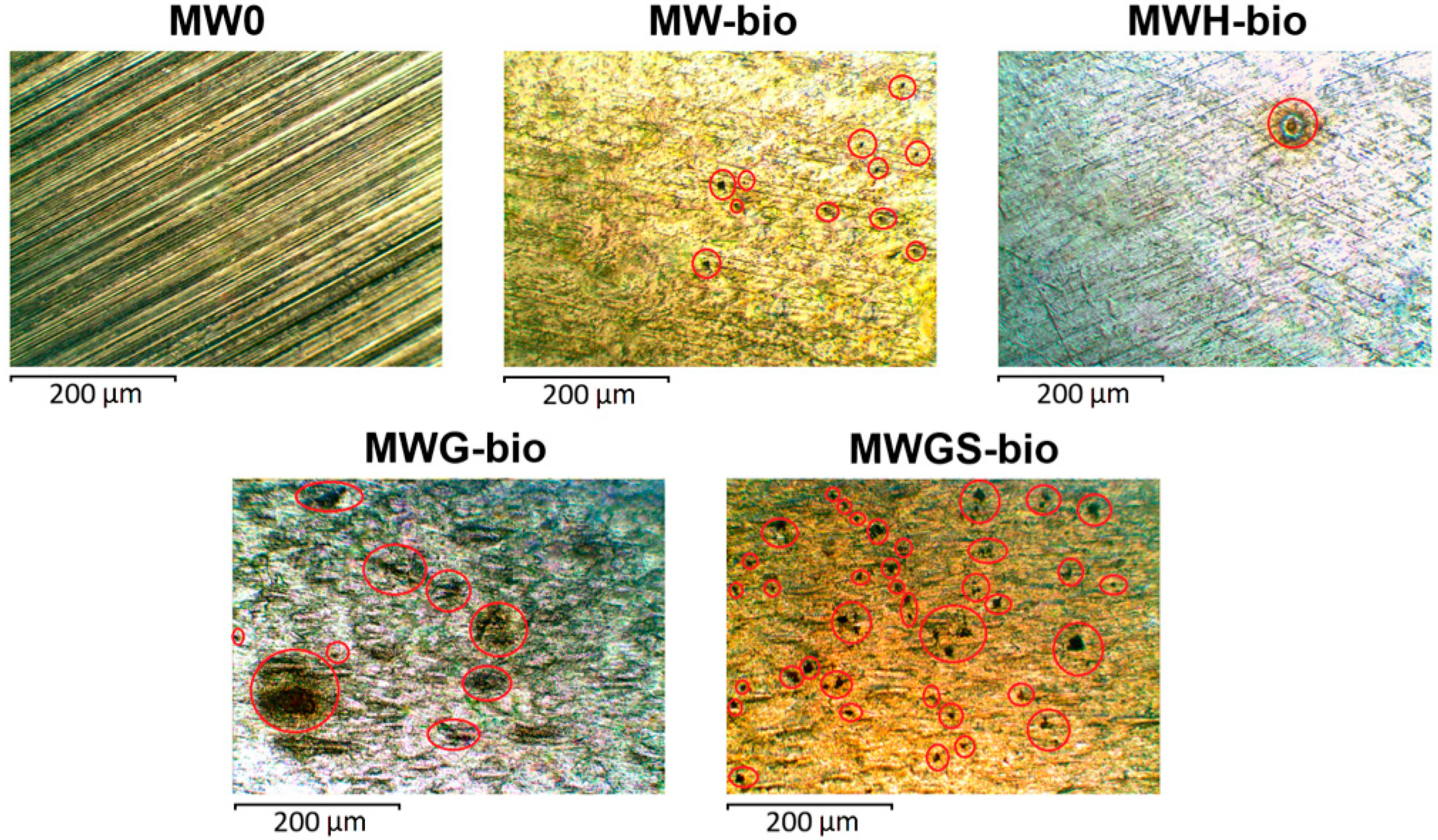
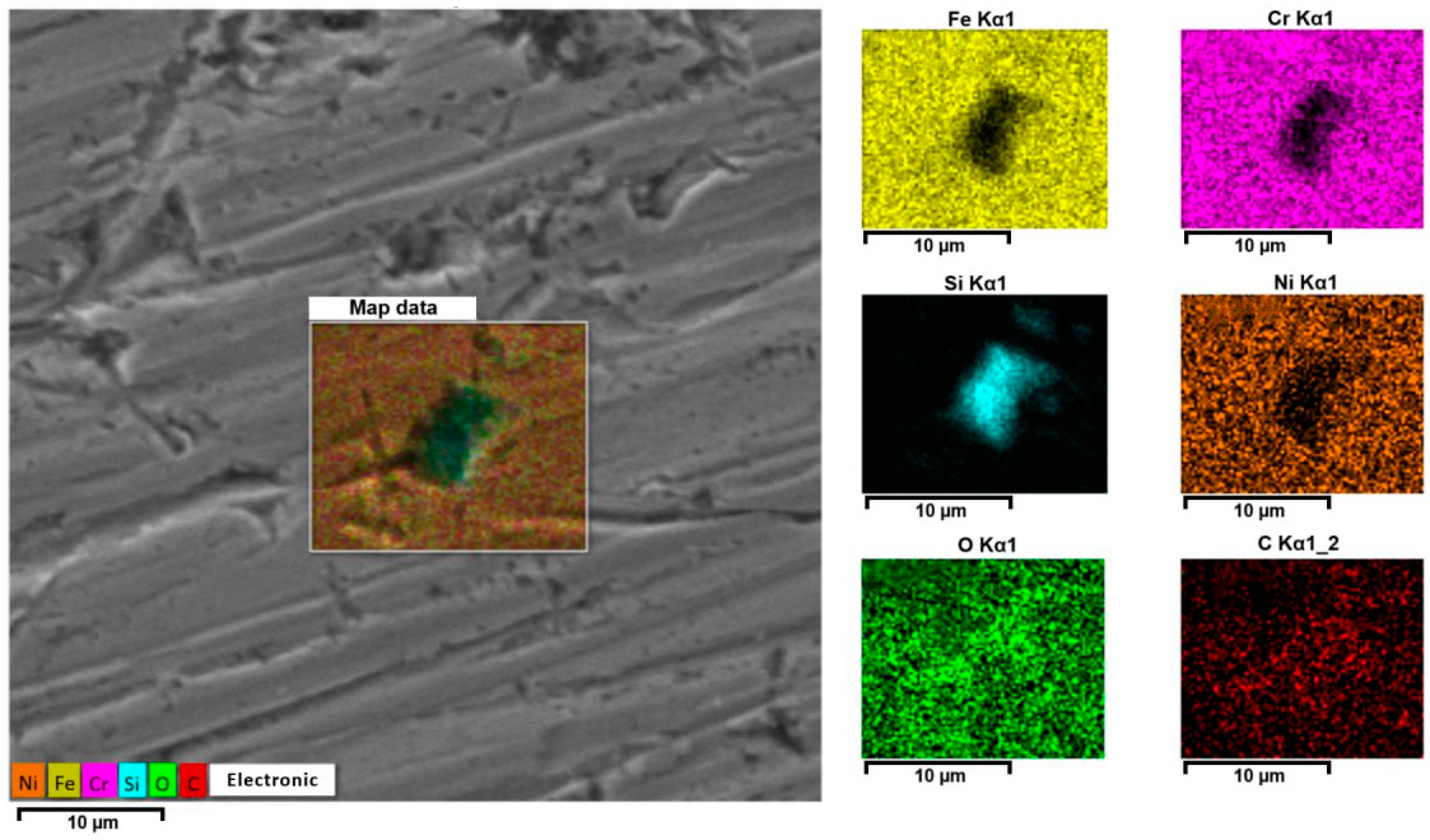
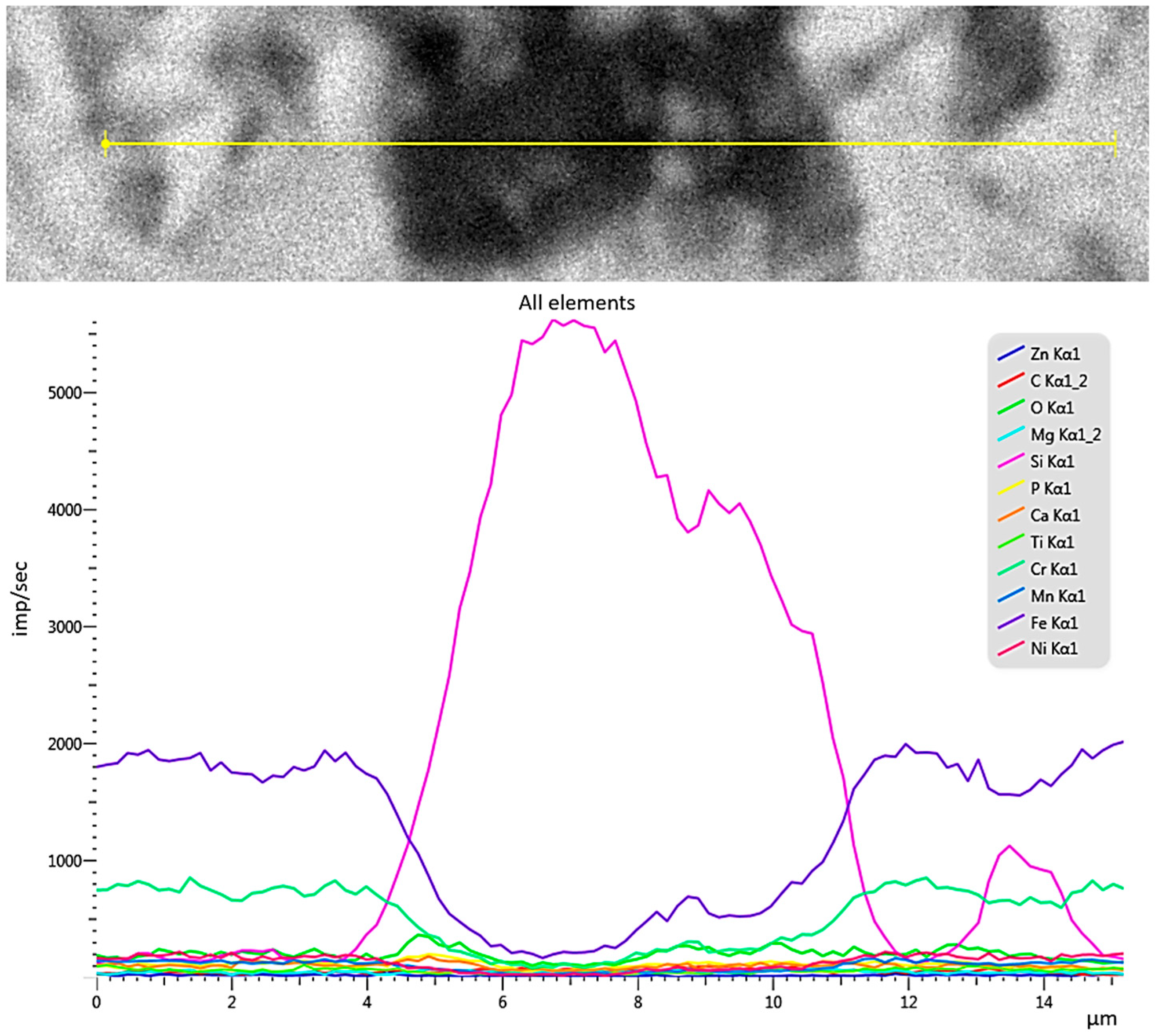
3.4. Description of Defects on the Surface of Stainless Steel
4. Discussion
5. Conclusions
- It has been established that the local dissolution of stainless steel AISI 304 occurs under the influence of abiotic factors and the formation of bacterial biofilm. In the given conditions, the formation of a biological film on the surface of AISI 304 occurs much faster (within 30 days) than is usually observed in nature [66,67].
- The maximum accumulation of microbial metabolites was experimentally recorded on day 30, including acetate, ethanol, formate, succinate and n-butyrate, and lactate. It was found that after 120 days, the concentration of organic metabolites decreases by 90%, which leads to a slowdown in the local dissolution of the stainless steel.
- It was found that the presence of glucose and sulfate ions stimulates the growth of sulfate-reducing bacteria, which promotes the initiation of pitting corrosion and the formation of colonies of pits on the surface of stainless steel.
- It has been shown that in anoxic environments, the passive film is unable to recover quickly after microbial exposure, and the metabolites enhance the locative destructive effect of chloride ions.
- Analysis of the data from gravimetric measurements and electrochemical studies has shown the consistency of the results in assessing the corrosion behavior of steel under various conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RW | Radioactive waste |
| SNF | Spent nuclear fuel |
| ESB | Engineered safety barrier |
| DGR | Deep geological repository |
| MIC | Microbial-induced corrosion |
| MLW | Medium-level waste |
| HLW | High-level waste |
References
- Olszewska, W.; Miśkiewicz, A.; Zakrzewska-Kołtuniewicz, G.; Lankof, L.; Pająk, L. Multibarrier System Preventing Migration of Radionuclides from Radioactive Waste Repository. Nukleonika 2015, 60, 557–563. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Steinmetz, H.J. Approaches to Disposal of Nuclear Waste. Energies 2022, 15, 7804. [Google Scholar] [CrossRef]
- Wersin, P.; Johnson, L.H.; McKinle, I.G. Performance of the Bentonite Barrier at Temperatures beyond 100 °C: A Critical Review. Phys. Chem. Earth Parts A/B/C 2007, 32, 780–788. [Google Scholar] [CrossRef]
- King, F. Canister Materials for the Disposal of Nuclear Waste. In Comprehensive Nuclear Materials, 2nd ed.; Konings, R.J.M., Stoller, R.E., Eds.; Elsevier: Oxford, UK, 2020; pp. 387–413. [Google Scholar] [CrossRef]
- Rebak, R.B. Environmentally Assisted Cracking Research of Engineering Alloys for Nuclear Waste Repository Containers. MRS Online Proc. Libr. 2012, 1475, 449–458. [Google Scholar] [CrossRef]
- Bennett, D.G.; Gens, R. Overview of European Concepts for High-Level Waste and Spent Fuel Disposal with Special Reference Waste Container Corrosion. J. Nucl. Mater. 2008, 379, 1–8. [Google Scholar] [CrossRef]
- McGuire, M.F. Stainless Steels for Design Engineers; ASM International: Materials Park, OH, USA, 2008. [Google Scholar]
- Revie, R.W. Uhlig’s Corrosion Handbook, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dastgerdi, A.A.; Brenna, A.; Ormellese, M.; Pedeferri, M.; Bolzoni, F. Experimental Design to Study the Influence of Temperature, pH, and Chloride Concentration on the Pitting and Crevice Corrosion of UNS S30403 Stainless Steel. Corros. Sci. 2019, 159, 108160. [Google Scholar] [CrossRef]
- Zatkalíková, V.; Uhríčik, M.; Markovičová, L.; Kuchariková, L. Corrosion Behavior of Sensitized AISI 304 Stainless Steel in Acid Chloride Solution. Materials 2022, 15, 8543. [Google Scholar] [CrossRef] [PubMed]
- Sanusi, M.S.; Shamsudin, S.R.; Rahmat, A.; Wardan, R. Electrochemical corrosion behaviours of AISI 304 austenitic stainless steel in NaCl solutions at different pH. AIP Conf. Proc. 2018, 2030, 020116. [Google Scholar] [CrossRef]
- Frankel, G.S.; Sridhar, N. Understanding Localized Corrosion. Mater. Today 2008, 11, 38–44. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science; Springer: New York, NY, USA, 2010. [Google Scholar]
- Alar, V.; Žmak, I.; Runje, B.; Horvatić, A. Development of Models for Prediction of Corrosion and Pitting Potential on AISI 304 Stainless Steel in Different Environmental Conditions. Int. J. Electrochem. Sci. 2016, 11, 7674–7689. [Google Scholar] [CrossRef]
- Ress, J.; Monrrabal, G.; Díaz, A.; Pérez-Pérez, J.; Bastidas, J.M.; Bastidas, D.M. Microbiologically Influenced Corrosion of Welded AISI 304 Stainless Steel Pipe in Well Water. Eng. Fail. Anal. 2020, 116, 104734. [Google Scholar] [CrossRef]
- Sharma, P.; Roy, H. Pitting Corrosion Failure of an AISI Stainless Steel Pointer Rod. Eng. Fail. Anal. 2014, 44, 400–407. [Google Scholar] [CrossRef]
- Wei, X.; Ling, X.; Zhang, M. Influence of Surface Modifications by Laser Shock Processing on the Acid Chloride Stress Corrosion Cracking Susceptibility of AISI 304 Stainless Steel. Eng. Fail. Anal. 2018, 91, 165–171. [Google Scholar] [CrossRef]
- Bellezze, T.; Viceré, A.; Giuliani, G.; Sorrentino, E.; Roventi, G. Study of Localized Corrosion of AISI 430 and AISI 304 Batches Having Different Roughness. Metals 2018, 8, 244. [Google Scholar] [CrossRef]
- Von Molkte, T.; Pistorius, P.C.; Sandenbergh, R.F. The Influence of Heat-Tinted Surface Layers on the Corrosion Resistance of Stainless Steels. In Proceedings of the INFACON 6: 6th International Ferroalloys Congress, Cape Town, South Africa, 8–11 March 1992; Volume 1, pp. 185–195. [Google Scholar]
- Prošek, T.; Le Gac, A.; Thierry, D.; Le Manchet, S.; Lojewski, C.; Fanica, A.; Johansson, E.; Canderyd, C.; Dupoiron, F.; Snauwaert, T.; et al. Low-Temperature Stress Corrosion Cracking of Austenitic and Duplex Stainless Steels Under Chloride Deposits. Corrosion 2014, 70, 1052–1063. [Google Scholar] [CrossRef]
- Rajala, P.; Nuppunen-Puputti, M.; Wheat, C.G.; Carpen, L. Fluctuation in Deep Groundwater Chemistry and Microbial Community and Their Impact on Corrosion of Stainless Steels. Sci. Total Environ. 2022, 824, 153965. [Google Scholar] [CrossRef]
- Ziadi, I.; Akrout, H.; Hassairi, H.; El-Bassi, L.; Bousselmi, L. Investigating the biocorrosion mechanism of 304L stainless steel in raw and treated urban wastewaters. Eng. Fail. Anal. 2019, 101, 342–356. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Olsson, C.-O.A.; Landolt, D. Passive Films on Stainless Steels—Chemistry, Structure and Growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Ibars, J.R.; Moreno, D.A.; Ranninger, C. MIC of Stainless Steels: A Technical Review on the Influence of Microstructure. Int. Biodeterior. Biodegrad. 1992, 29, 343–355. [Google Scholar] [CrossRef]
- Enning, D.; Garrelfs, J. Corrosion of Iron by Sulfate-Reducing Bacteria: New Views of an Old Problem. Appl. Environ. Microbiol. 2014, 80, 1226–1236. [Google Scholar] [CrossRef]
- Wadood, H.Z.; Rajasekar, A.; Farooq, A.; Deen, K.M. Effect of Bacillus and Pseudomonas biofilms on the corrosion behavior of AISI 304 stainless steel. Int. J. Mater. Res. 2023, 114, 118–126. [Google Scholar] [CrossRef]
- Pedersen, K. Metabolic Activity of Subterranean Microbial Communities in Deep Granitic Groundwater Supplemented with Methane and H2. ISME J. 2013, 7, 839–849. [Google Scholar] [CrossRef]
- Huang, L.; Chang, W.; Zhang, D.; Huang, Y.; Li, Z.; Lou, Y.; Qian, H.; Jiang, C.; Li, X.; Mol, A. Acceleration of Corrosion of 304 Stainless Steel by Outward Extracellular Electron Transfer of Pseudomonas aeruginosa Biofilm. Corros. Sci. 2022, 199, 110159. [Google Scholar] [CrossRef]
- Kalnaowakul, P.; Xu, D.; Rodchanarowan, A. Accelerated Corrosion of 316L Stainless Steel Caused by Shewanella algae Biofilms. ACS Appl. Bio Mater. 2020, 3, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.Y.; Yang, C.; Ueki, T.; Pittman, C.C.; Xu, D.; Woodard, T.L.; Holmes, D.E.; Gu, T.; Wang, F.; Lovley, D.R. Stainless Steel Corrosion via Direct Iron-to-Microbe Electron Transfer by Geobacter Species. ISME J. 2021, 15, 3084–3093. [Google Scholar] [CrossRef]
- Wakai, S.; Eno, N.; Mizukami, H.; Sunaba, T.; Miyanaga, K.; Miyano, Y. Microbiologically Influenced Corrosion of Stainless Steel Independent of Sulfate-Reducing Bacteria. Front. Microbiol. 2022, 13, 982047. [Google Scholar] [CrossRef]
- Kato, S.; Itoh, T.; Yuki, M.; Nagamori, M.; Aoki, M.; Kikuchi, S.; Kawasaki, M.; Takashima, C.; Ioka, S. Isolation and Characterization of a Novel Sulfuriferula Species from a Radioactive Wastewater Treatment Plant, and Emended Description of the Genus Sulfuriferula. R. Soc. Open Sci. 2021, 8, 201577. [Google Scholar] [CrossRef]
- Dou, W.; Jin, Y.; Zhou, W.; Jia, Y.; Du, P.; Chen, S. Biocorrosion of Copper Metal by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Bioelectrochemistry 2016, 109, 43–48. [Google Scholar] [CrossRef]
- Wu, T.; Yan, M.; Xu, J.; Liu, Y.; Sun, C.; Yu, C. Microbiologically Influenced Corrosion of 2707 Hyper-Duplex Stainless Steel by Marine Pseudomonas aeruginosa Biofilm. Mater. Corros. 2018, 69, 330–336. [Google Scholar] [CrossRef]
- Small, J.; Nykyri, M.; Helin, M.; Hovi, U.; Sarlin, T.; Itävaara, M. Experimental and Modelling Investigations of the Biogeochemistry of Gas Production from Low and Intermediate Level Radioactive Waste. Appl. Geochem. 2008, 23, 1383–1418. [Google Scholar] [CrossRef]
- Kamnev, E.N.; Morozov, V.N.; Tatarinov, V.N.; Kaftan, V.I. Geodynamics Aspects of Investigations in Underground Research Laboratory (Niznekansk Massif). Eurasian Min. 2018, 2, 11–14. [Google Scholar] [CrossRef]
- Linge, I.I.; Utkin, S.S.; Kulagina, T.A.; Trokhov, N.N. Underground Research Laboratory in “the Yenisei” Section of the Nizhnekansky Massif of the Krasnoyarsk Region. J. Sib. Fed. Univ. Eng. Technol. 2019, 12, 830–841. [Google Scholar] [CrossRef]
- Abramova, E.; Popova, N.; Artemiev, G.; Boldyrev, K.; Kazakov, K.; Kryuchkov, D.; Safonov, A. Biological Factors Affecting the Evolution of Safety Barrier Materials in the Yeniseisky Deep Geological Repository. Eng. Geol. 2023, 312, 106931. [Google Scholar] [CrossRef]
- Abramova, E.; Shapagina, N.; Artemiev, G.; Safonov, A. Microbial Corrosion of Copper Under Conditions Simulating Deep Radioactive Waste Disposal. Biology 2024, 13, 1086. [Google Scholar] [CrossRef]
- Laverov, N.P.; Yudintsev, S.V.; Kochkin, B.T.; Malkovsky, V.I. The Russian Strategy of Using Crystalline Rock as a Repository for Nuclear Waste. Elements 2016, 12, 253–256. [Google Scholar] [CrossRef]
- Rozov, K.B.; Rumynin, V.G.; Nikulenkov, A.M.; Leskova, P.G. Sorption of 137Cs, 90Sr, Se, 99Tc, 152(154)Eu, 239(240)Pu on Fractured Rocks of the Yeniseysky Site (Nizhne-Kansky Massif, Krasnoyarsk Region, Russia). J. Environ. Radioact. 2018, 192, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Marcelin, S.; Pébère, N.; Régnier, S. Electrochemical Characterisation of a Martensitic Stainless Steel in a Neutral Chloride Solution. Electrochim. Acta 2013, 87, 32–40. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The Electrochemical Behaviour of Stainless Steel AISI 304 in Alkaline Solutions with Different pH in the Presence of Chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an Aqueous Soluble Tetrazolium/Formazan Assay for Cell Growth Assays in Culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef]
- Rybkina, A.; Gladkikh, N.; Marshakov, A.; Petrunin, M.; Nazarov, A. Effect of Sign—Alternating Cyclic Polarisation and Hy-drogen Uptake on the Localised Corrosion of X70 Pipeline Steel in Near—Neutral Solutions. Metals 2020, 10, 245. [Google Scholar] [CrossRef]
- GOST 9.908-85; Unified System of Corrosion and Ageing Protection. Metals and Alloys. Methods for Determination of Corrosion and Corrosion Resistance Indices. RussianGost: Moscow, Russia, 1989; p. 17.
- Shakhalov, A.A. Autoclave Technology for Processing Substandard Copper Concentrates using Hydrothermal Treatment (In Russ). Ph.D. Thesis, Ural Federal University named after the First President of Russia B. N. Yeltsin, Yekaterinburg, Russia, 19 February 2021. [Google Scholar]
- Telegdi, J.; Shaban, A.; Trif, L. Review on the Microbiologically Influenced Corrosion and the Function of Biofilms. Int. J. Corros. Scale Inhib. 2020, 9, 1–33. [Google Scholar] [CrossRef]
- Kocijan, A.; Donik, Č.; Jenko, M. Electrochemical and XPS Studies of the Passive Film Formed on Stainless Steels in Borate Buffer and Chloride Solutions. Corros. Sci. 2007, 49, 2083–2098. [Google Scholar] [CrossRef]
- Detriche, S.; Vivegnis, S.; Vanhumbeeck, J.-F.; Felten, A.; Louette, P.; Renner, F.U.; Delhalle, J.; Mekhalif, Z. XPS Fast Depth Profile of the Native Oxide Layers on AISI 304, 316 and 430 Commercial Stainless Steels and Their Evolution with Time. J. Electron Spectrosc. Relat. Phenom. 2020, 243, 146970. [Google Scholar] [CrossRef]
- Swayne, M.; Perumal, G.; Padmanaban, D.B.; Mariotti, D.; Brabazon, D. Exploring the Impact of Laser Surface Oxidation Pa-rameters on Surface Chemistry and Corrosion Behaviour of AISI 316L Stainless Steel. Appl. Surf. Sci. Adv. 2024, 22, 100622. [Google Scholar] [CrossRef]
- Martínez-Baltodano, F.; Pech-Rodríguez, W.; Vargas-Guti’errez, G. Effects of Ag Nanoparticles Electrodeposited on Oxy-Nitrocarburized AISI 304 SS into the Corrosive and Antibacterial Activity. Electrochim. Acta 2025, 533, 146548. [Google Scholar] [CrossRef]
- Kwak, J.; Jung, S.-M.; Kim, K.-S.; Choe, G.; Son, S.; Kim, H.S.; Kim, Y.-T. Fe–N–C Surface Treatment as Method for Enhancing Spontaneous Passivation of low Ni and Cr Stainless Steel. Appl. Surf. Sci. Adv. 2025, 30, 100871. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Chang, Y.-T.; Hung, C.H.; Lee, J.-W.; Liao, H.M.; Chou, H.L. Microbial community analysis of anaerobic bio-corrosionin different ORP profiles. Int. Biodeterior. Biodegrad. 2014, 95, 93–101. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, D.; Li, Y.; Yang, K.; Gu, T. Electron Mediators Accelerate the Microbiologically Influenced Corrosion of 304 Stainless Steel by the Desulfovibrio vulgaris Biofilm. Bioelectrochemistry 2015, 101, 14–21. [Google Scholar] [CrossRef]
- Mehanna, M.; Basseguy, R.; Delia, M.-L.; Bergel, A. Role of direct microbial electron transfer in corrosion of steels. Electrochem. Commun. 2009, 11, 568–571. [Google Scholar] [CrossRef]
- Maji, K.; Lavanya, M. Microbiologically Influenced Corrosion in Stainless Steel by Pseudomonas aeruginosa: An Overview. J. Bio Tribo Corros. 2024, 10, 16. [Google Scholar] [CrossRef]
- Little, B.J.; Blackwood, D.J.; Hinks, J.; Lauro, F.M.; Marsili, E.; Okamoto, A.; Rice, S.A.; Wade, S.A.; Flemming, H.-C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Javed, M.A.; Stoddart, P.R.; Palombo, E.A.; McArthur, S.L.; Wade, S.A. Inhibition or acceleration: Bacterial test media can determine the course of microbiologically influenced corrosion. Corros. Sci. 2014, 86, 149–158. [Google Scholar] [CrossRef]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards Understanding Interactions between Biofilms and Metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Jia, R.; Unsal, T.; Xu, D. Toward a Better Understanding of Microbiologically Influenced Corrosion Caused by Sulfate-Reducing Bacteria. J. Mater. Sci. Technol. 2019, 35, 631–636. [Google Scholar] [CrossRef]
- Little, B.J.; Hinks, J.; Blackwood, D.J. Microbially influenced corrosion: Towards an interdisciplinary perspective on mechanisms. Int. Biodeterior. Biodegrad. 2020, 154, 105062. [Google Scholar] [CrossRef]
- Jia, R.; Unsal, T.; Xu, D.; Lekbach, Y.; Gu, T. Microbiologically Influenced Corrosion and Current Mitigation Strategies: A State of the Art Review. Front. Microbiol. 2019, 10, 2335. [Google Scholar] [CrossRef]
- Nuppunen-Puputti, M.; Kietäväinen, R.; Purkamo, L.; Rajala, P.; Itävaara, M.; Kukkonen, I.; Bomberg, M. Rock Surface Fungi in Deep Continental Biosphere—Exploration of Microbial Community Formation with Subsurface in Situ Biofilm Trap. Microorganisms 2021, 9, 64. [Google Scholar] [CrossRef]
- Lopes, F.A.; Morin, P.; Oliveira, R.; Melo, L.F. Interaction of Desulfovibrio desulfuricans Biofilms with Stainless Steel Surface and Its Impact on Bacterial Metabolism. J. Appl. Microbiol. 2006, 101, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
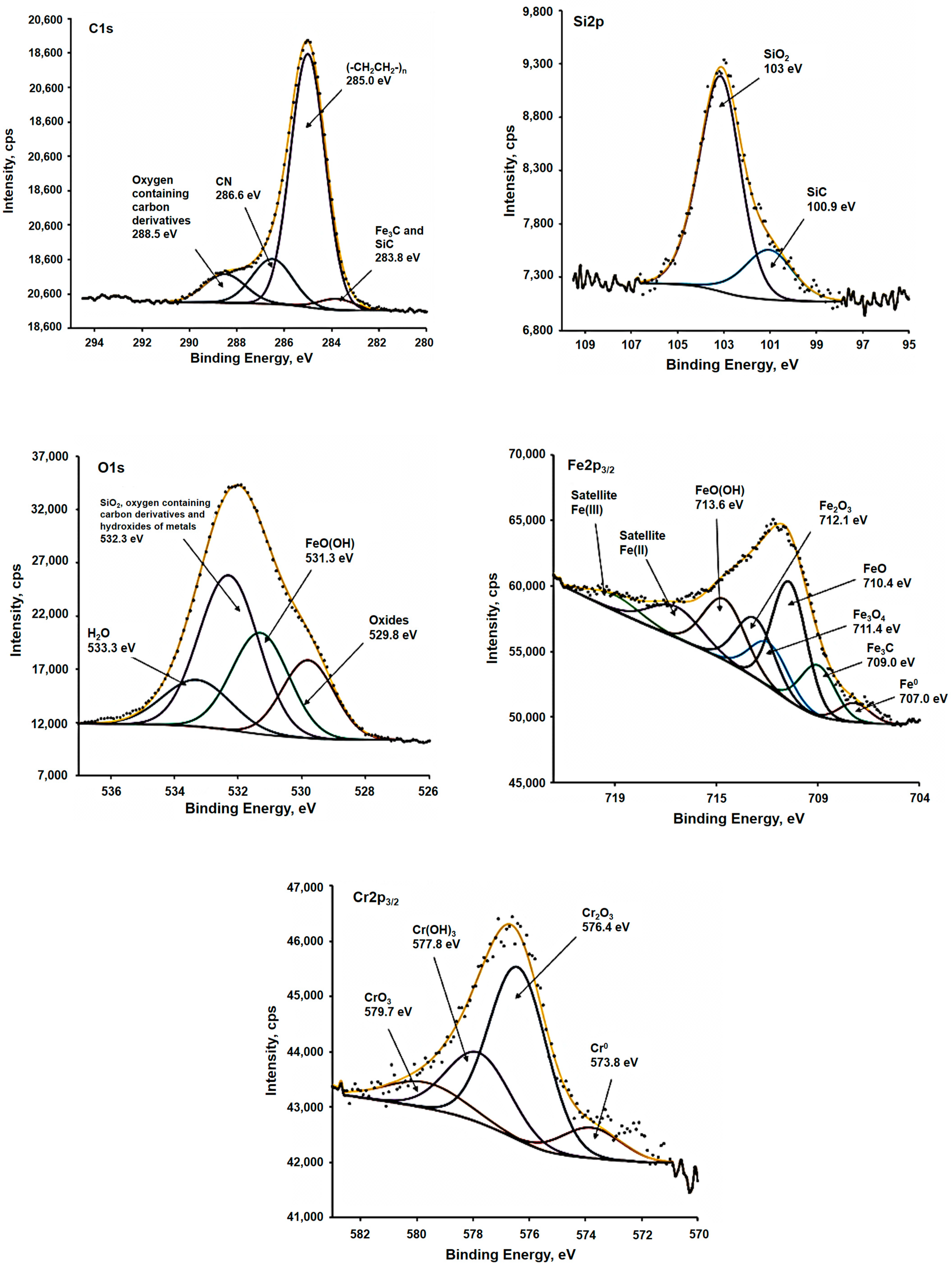
| 1. Stainless Steel Biocorrosion by Microbial Community | |||
| Conditions | Samples | Time, Days | Analyses |
|
| 7, 30, 60, 120 |
|
| 2. The Effect of Microbial Components on Stainless Steel Corrosion | |||
| Conditions | Samples | Time, Days | Analyses |
|
| 3, 10, 20, 45, 90, 120 |
|
| Sample | Average Number of Pittings per 1 mm2 | Average Diameter of Pitting, μ | Pitting Depth, µm |
|---|---|---|---|
| MW-bio | 6 | 13.8 | 83 |
| MWH-bio | 1 | 26.7 | 88 |
| MWG-bio | 13 | 18.8 | 46 |
| MWGS-bio | 63 | 16.1 | 25 |
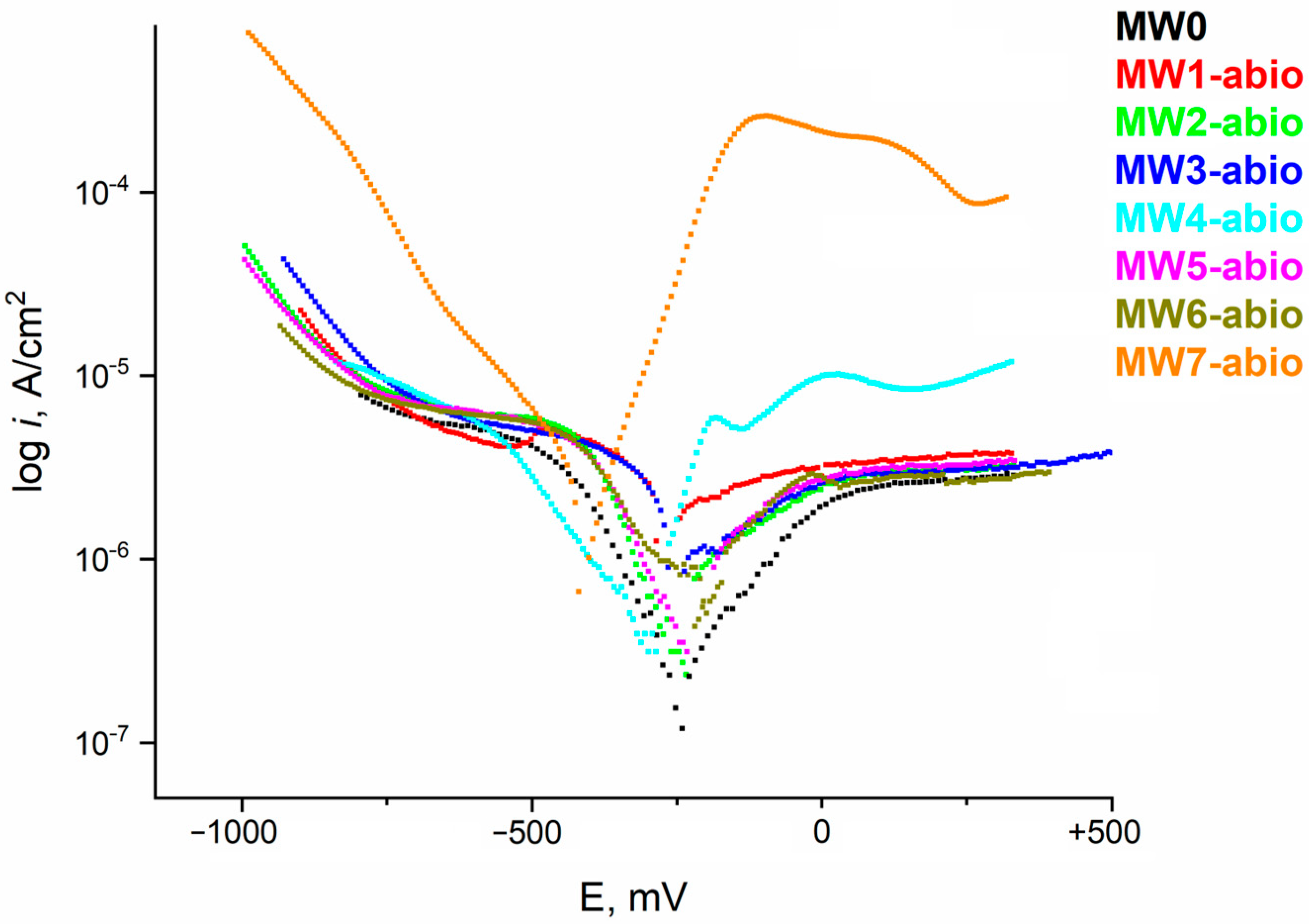
| Sample | Ecorr, mV (s.c.e.) | icorr, µA/cm2 | Vcorr, µm/Year |
|---|---|---|---|
| MW0 | −263 | 0.2334 | 2.7 |
| MW1-abio (Cl−) | −285 | 0.6930 | 8.0 |
| MW2-abio (HCO3−) | −291 | 0.6268 | 7.2 |
| MW3-abio (NO3−) | −272 | 0.4311 | 5.0 |
| MW4-abio (SO42−) | −320 | 0.5962 | 6.9 |
| MW5-abio (S2−) | −325 | 0.4698 | 5.4 |
| MW6-abio (microbial metabolites) | −236 | 0.5860 | 6.8 |
| MW7-abio (S2− + HCO3− + microbial metabolites) | −337 | 1.2127 | 14.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abramova, E.; Tripachev, O.; Shapagina, N.; Safonov, A. Preliminary Study of Microbial Corrosion of Stainless Steel AISI 304 Under Conditions Simulating Deep Radioactive Waste Disposal. Materials 2025, 18, 5329. https://doi.org/10.3390/ma18235329
Abramova E, Tripachev O, Shapagina N, Safonov A. Preliminary Study of Microbial Corrosion of Stainless Steel AISI 304 Under Conditions Simulating Deep Radioactive Waste Disposal. Materials. 2025; 18(23):5329. https://doi.org/10.3390/ma18235329
Chicago/Turabian StyleAbramova, Elena, Oleg Tripachev, Natalia Shapagina, and Alexey Safonov. 2025. "Preliminary Study of Microbial Corrosion of Stainless Steel AISI 304 Under Conditions Simulating Deep Radioactive Waste Disposal" Materials 18, no. 23: 5329. https://doi.org/10.3390/ma18235329
APA StyleAbramova, E., Tripachev, O., Shapagina, N., & Safonov, A. (2025). Preliminary Study of Microbial Corrosion of Stainless Steel AISI 304 Under Conditions Simulating Deep Radioactive Waste Disposal. Materials, 18(23), 5329. https://doi.org/10.3390/ma18235329