Nanomechanical and Adhesive Behavior of Electrophoretically Deposited Hydroxyapatite- and Chitosan-Based Coatings on Ti13Zr13Nb Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Material Preparation
2.2. Electrochemical Oxidation of Ti13Zr13Nb Alloy
2.3. Laser Treatment
2.4. Coatings Deposition
2.4.1. Procedure A—Chitosan–nanoCu Coatings
2.4.2. Procedure B—Chitosan–nanoHAp Coatings
2.4.3. Procedure C—nanoHAp–nanoCu Coatings
2.5. Characterization of Microstructure
2.6. Nanoindentation Studies
2.7. Nanoscratch Test Studies
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in Metals and Alloys for Joint Replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Metallic Biomaterials: Types and Advanced Applications. In New Functional Biomaterials for Medicine and Healthcare; Elsevier: Amsterdam, The Netherlands, 2014; pp. 121–147. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Majkowska-Marzec, B.; Strugala, G. Effects of Solution Composition and Electrophoretic Deposition Voltage on Various Properties of Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy. Ceram. Int. 2018, 44, 19236–19246. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A Review on Properties, Biological Activities and Recent Progress in Biomedical Applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieślik, B. Electrophoretic Deposition and Characteristics of Chitosan-Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings 2020, 10, 245. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Jiang, D.; Jiao, Y.; Wu, Y.; Peng, Z.; Zhou, J.; Wu, J.; Dong, Z. Synthesis and Characterization of a Bi-Functional Hydroxyapatite/Cu-Doped TiO2 Composite Coating. Ceram. Int. 2019, 45, 6693–6701. [Google Scholar] [CrossRef]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial Activity and Biocompatibility of Ag+- and Cu2+-Doped Biphasic Hydroxyapatite/α-Tricalcium Phosphate Obtained from Hydrothermally Synthesized Ag+- and Cu2+-Doped Hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic Deposition: From Traditional Ceramics to Nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Bartmanski, M.; Pawlowski, L.; Mielewczyk-Gryn, A.; Strugala, G.; Rokosz, K.; Gaiaschi, S.; Chapon, P.; Raaen, S.; Zielinski, A. The Influence of Nanometals, Dispersed in the Electrophoretic Nanohydroxyapatite Coatings on the Ti13Zr13Nb Alloy, on Their Morphology and Mechanical Properties. Materials 2021, 14, 1638. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Bartmański, M.; Mielewczyk-Gryń, A.; Zieliński, A. Effects of Surface Pretreatment of Titanium Substrates on Properties of Electrophoretically Deposited Biopolymer Chitosan/Eudragit e 100 Coatings. Coatings 2021, 11, 1120. [Google Scholar] [CrossRef]
- Majkowska-Marzec, B.; Tęczar, P.; Bartmański, M.; Bartosewicz, B.; Jankiewicz, B.J. Mechanical and Corrosion Properties of Laser Surface-Treated Ti13Nb13Zr Alloy with MWCNTs Coatings. Materials 2020, 13, 3991. [Google Scholar] [CrossRef]
- Bartmanski, M.; Zielinski, A.; Jazdzewska, M.; Głodowska, J.; Kalka, P. Effects of Electrophoretic Deposition Times and Nanotubular Oxide Surfaces on Properties of the Nanohydroxyapatite/Nanocopper Coating on the Ti13Zr13Nb Alloy. Ceram. Int. 2019, 45, 20002–20010. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gross, K.A. Nanoindentation on the Surface of Thermally Sprayed Coatings. Surf. Coat. Technol. 2009, 203, 3516–3520. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D.; Bandyopadhyay, N.R. Nanoindentation Study of Microplasma Sprayed Hydroxyapatite Coating. Ceram. Int. 2009, 35, 2295–2304. [Google Scholar] [CrossRef]
- Xu, Y.N.; Liu, M.N.; Wang, M.C.; Oloyede, A.; Bell, J.M.; Yan, C. Nanoindentation Study of the Mechanical Behavior of TiO2 Nanotube Arrays. J. Appl. Phys. 2015, 118, 145301. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Chung, Y.W. Commentary on Using H/E and H3/E2 as Proxies for Fracture Toughness of Hard Coatings. Thin Solid Films 2019, 688, 137265. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Karimzadeh, A.; Ayatollahi, M.R.; Bushroa, A.R.; Herliansyah, M.K. Effect of Sintering Temperature on Mechanical and Tribological Properties of Hydroxyapatite Measured by Nanoindentation and Nanoscratch Experiments. Ceram. Int. 2014, 40, 9159–9164. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kansız, S.; Vurat, M.T.; Parmaksiz, M.; Elçin, A.E.; Elçin, Y.M. Chitosan/PVA Reinforced Boron/Strontium Multi-Substituted Hydroxyapatite-Based Biocomposites: Effects of Synthesis PH and Coating on the Physicochemical, Mechanical, and in Vitro Biological Properties of Scaffolds. Mater. Today Chem. 2024, 35, 101865. [Google Scholar] [CrossRef]
- Apăvăloaiei, I.; Nacu, I.; Cojocaru, F.D.; Balan, V.; Bercea, M.; Niță, L.E.; Vereștiuc, L. Effect of Chitosan on 3D Printed Scaffolds with Gelatin–Hyaluronic Acid, Hydroxyapatite and Magnetic Nanoparticles for Bone Tissues Defects Repair. React. Funct. Polym. 2025, 216, 106422. [Google Scholar] [CrossRef]
- García-Pérez, C.A.; Menchaca-Campos, C.; Sandoval-Espino, J.A.; Antunez, E.E.; Nicolás, A.F.; Santillán-Urquiza, M.A.; Uruchurtu, J. Dip-Coating of Nylon/Chitosan/Hydroxyapatite/TiO2 Biocoatings to Improve Durability of Metallic Prostheses. Heliyon 2025, 11, e43422. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; Zhang, H.; Hao, Y.; Wei, Y.; Liang, Z.; Hu, Y.; Lian, X.; Huang, D. Dual-Functional Black Phosphorus/Hydroxyapatite/Quaternary Chitosan Composite Coating for Antibacterial Activity and Enhanced Osseointegration on Titanium Implants. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136008. [Google Scholar] [CrossRef]
- Nawrotek, K.; Grams, J.; Sobczyk, R.; Kubicka, M.; Czeladzińska, B.; Jóźwiak, P. Effect of Chitosan Structure and Deposition Time on Structural and Mechanical Properties of Chitosan-Hydroxyapatite Tubular-Shaped Electrodeposits for Biomedical Applications. Polym. Test. 2023, 123, 108061. [Google Scholar] [CrossRef]
- Upadhyay, P.; Ullah, A. Enhancement of Mechanical and Barrier Properties of Chitosan-Based Bionanocomposites Films Reinforced with Eggshell-Derived Hydroxyapatite Nanoparticles. Int. J. Biol. Macromol. 2024, 261, 129764. [Google Scholar] [CrossRef] [PubMed]
- Rengasamy, G.; Mahalingam, S. Fabrication and Characterization of Chitosan/Ag@hydroxyapatite Nanocomposite Films for Osteoregeneration: Insights into Mechanical Properties, Biological Performance, and Drug Release. Colloids Surf. A Physicochem. Eng. Asp. 2025, 717, 136790. [Google Scholar] [CrossRef]
- Musil, J. Hard and Superhard Nanocomposite Coatings. Surf. Coat. Technol. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Zykova, A.; Safonov, V.; Dudin, S.; Yakovin, S.; Donkov, N.; Ghaemi, M.H.; Szkodo, M.; Antoszkiewicz, M.; Szyfelbain, M.; Czaban, A. Structural and Mechanical Properties of Hydroxyapatite Coatings Formed by Ion-Beam Assisted Deposition. J. Phys.: Conf. Ser. 2018, 992, 012035. [Google Scholar] [CrossRef]
- Gao, X.; Fraulob, M.; Haïat, G. Biomechanical Behaviour of Bone-Implant Interface: A Review Biomechanical Behaviours of the Bone-Implant Interface: A Review. J. R. Soc. Interface 2019, 16, 20190259. [Google Scholar] [CrossRef]
- Büchter, A.; Joos, U.; Wiesmann, H.-P.; Seper, L.; Meyer, U. Biological and Biomechanical Evaluation of Interface Reaction at Conical Screw-Type Implants. Head. Face Med. 2006, 2, 5. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of Hardness and Elastic Modulus by Instrumented Indentation: Advances in Understanding and Refinements to Methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Djošić, M.; Janković, A.; Stevanović, M.; Stojanović, J.; Vukašinović-Sekulić, M.; Kojić, V.; Mišković-Stanković, V. Hydroxyapatite/Poly(Vinyl Alcohol)/Chitosan Coating with Gentamicin for Orthopedic Implants. Mater. Chem. Phys. 2023, 303, 127766. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Wan, Y. Hydroxyapatite-Reinforced Chitosan Film on PEO-Treated Aluminum Alloys for Enhanced Corrosion Resistance. J. Mater. Res. Technol. 2025, 36, 5911–5924. [Google Scholar] [CrossRef]
- Sun, D.; Sang, Y.; Liao, T.; Huo, Z.; Liu, Y.; Yu, Z.; Ma, Q.; Jiang, W. Silver-Hydroxyapatite Functionalized Chitosan Films: Multifunctional Active Packaging for Enhanced Seafood Preservation against Vibrio Parahaemolyticus and Shewanella Putrefaciens. LWT 2025, 233, 118479. [Google Scholar] [CrossRef]
- Guo, M.; Cao, G.; Zhang, Z.; Guo, J.; Chen, C.; Fu, X.; Hu, J. High-Entropy Strategy Tailoring the Oxidation Resistance of Zr-Si Based ATF Coatings. J. Nucl. Mater. 2025, 615, 155926. [Google Scholar] [CrossRef]
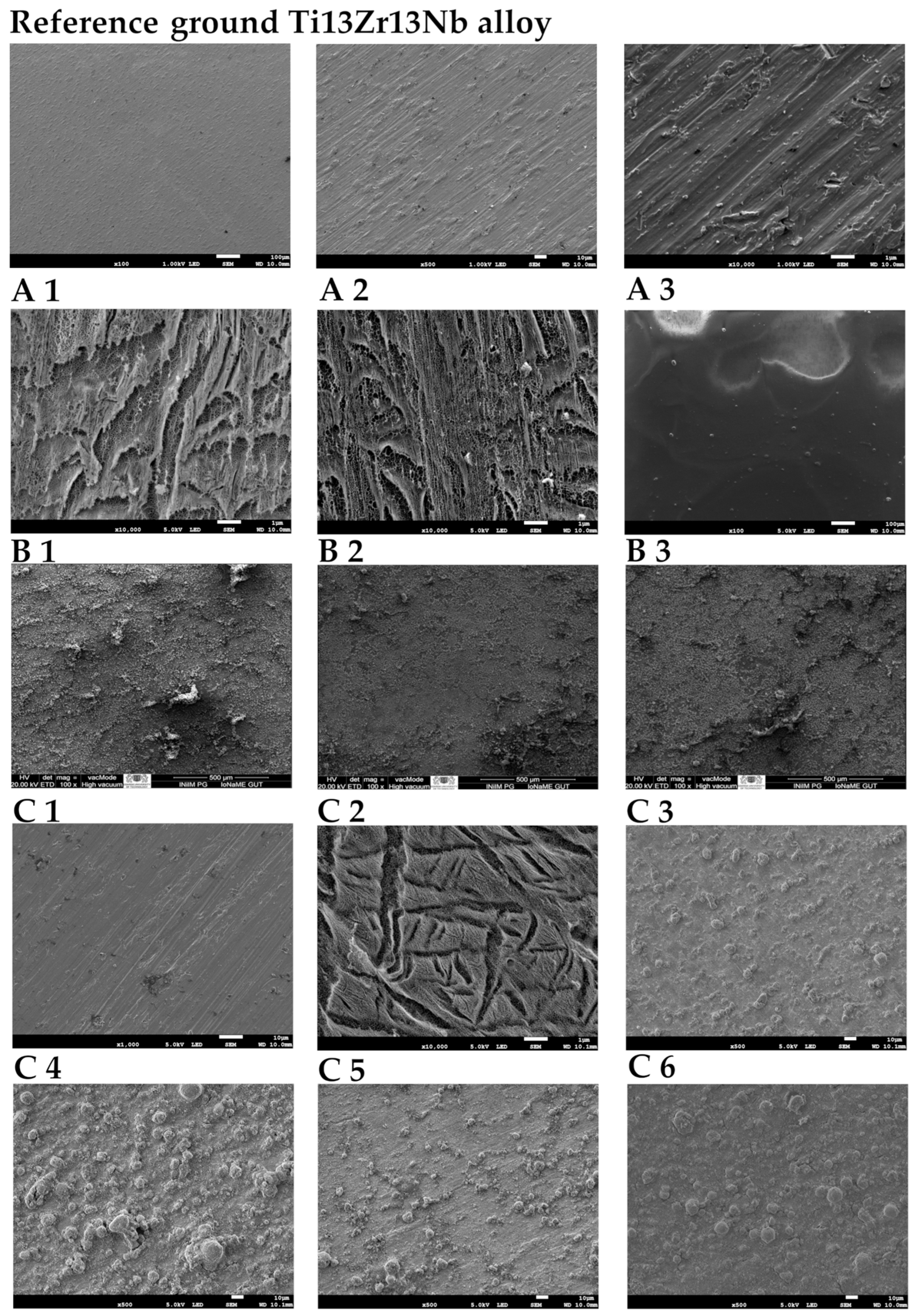

| Element | Nb | Zr | Fe | C | N | O | Ti |
|---|---|---|---|---|---|---|---|
| wt.% | 13.0 | 13.0 | 0.05 | 0.04 | 0.019 | 0.11 | remainder |
| Procedure | Substrate | Electrochemical Oxidation | Laser Treatment | Coating Deposition Parameters |
|---|---|---|---|---|
| A1 | Ti13Zr13Nb | H3PO4 + HF; 20 V; 20 min | none | 1 g/L chitosan + 0.05 g/L CuNPs; 10 V; 1 min |
| A2 | 1 g/L chitosan + 0.05 g/L CuNPs + Tween20; 10 V; 1 min | |||
| A3 | 1 g/L chitosan + 0.05 g/L CuNPs + Tween20; 20 V; 1 min | |||
| B1 | Ti13Zr13Nb | none | none | 1 g/L chitosan + 2.5 g/L HApNPs + Tween20; 30 V; 1 min |
| B2 | 750 W; pulse duration 3.25 ms; frequency 25 Hz | |||
| B3 | 1500 W; pulse duration 3.25 ms; frequency 25 Hz | |||
| C1 | Ti13Zr13Nb | none | none | none |
| C2 | H3PO4 + HF; 20 V; 20 min | none | ||
| C3 | none | 1 g/L HApNPs + 0.05 g/L CuNPs + Tween20; 10 V; 1 min | ||
| C4 | 1 g/L HApNPs + 0.05 g/L CuNPs + Tween20; 20 V; 1 min | |||
| C5 | H3PO4 + HF; 20 V; 20 min | 1 g/L HApNPs + 0.05 g/L CuNPs + Tween20; 10 V; 1 min | ||
| C6 | 1 g/L HApNPs + 0.05 g/L CuNPs + Tween20; 20 V; 1 min |
| Procedure | Number of Indentations | Distance Between Indentation [µm] | Maximum Force [mN] | Loading; Dwell with Maximum Force and Unloading [s] | Poisson’s Ratio for Analyzes [-] |
|---|---|---|---|---|---|
| A1–A3 | 25 | 20 | 50 | 20 s–5 s–15 s | 0.261 |
| B1–B3 | 5 | 10 | 0.3 | ||
| C1–C2 | 25 | 50 | Ti13Zr13Nb—0.3; TiO2—0.25 | ||
| C3–C6 | 15 | 5 | 0.25 |
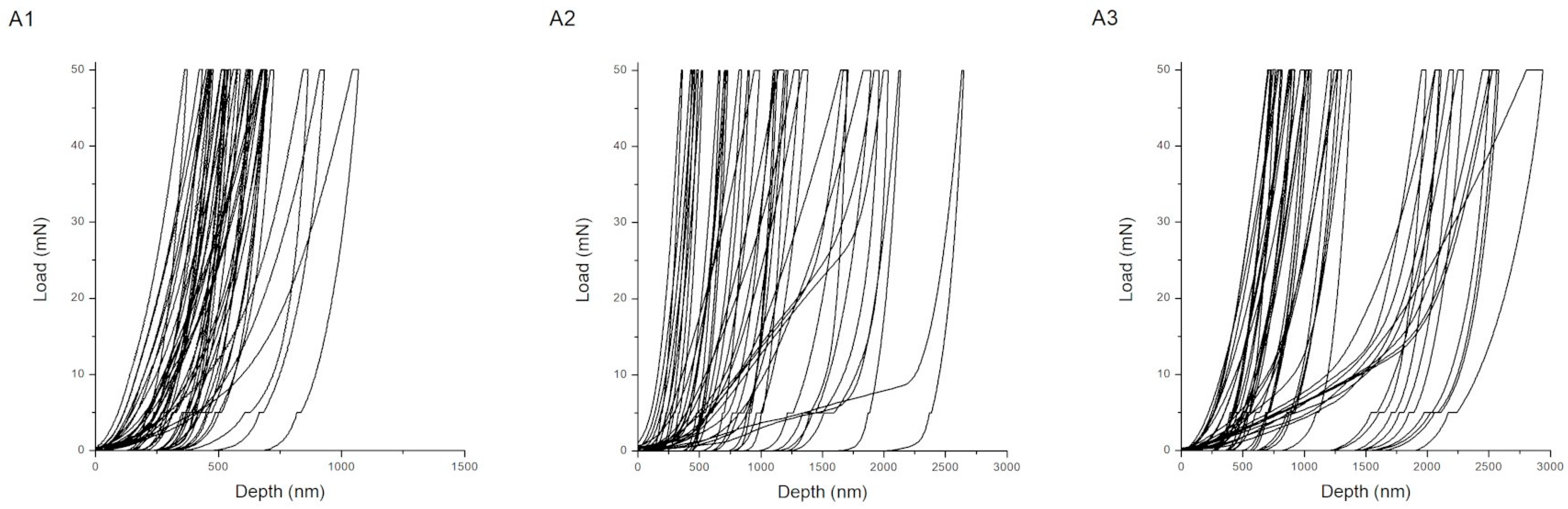
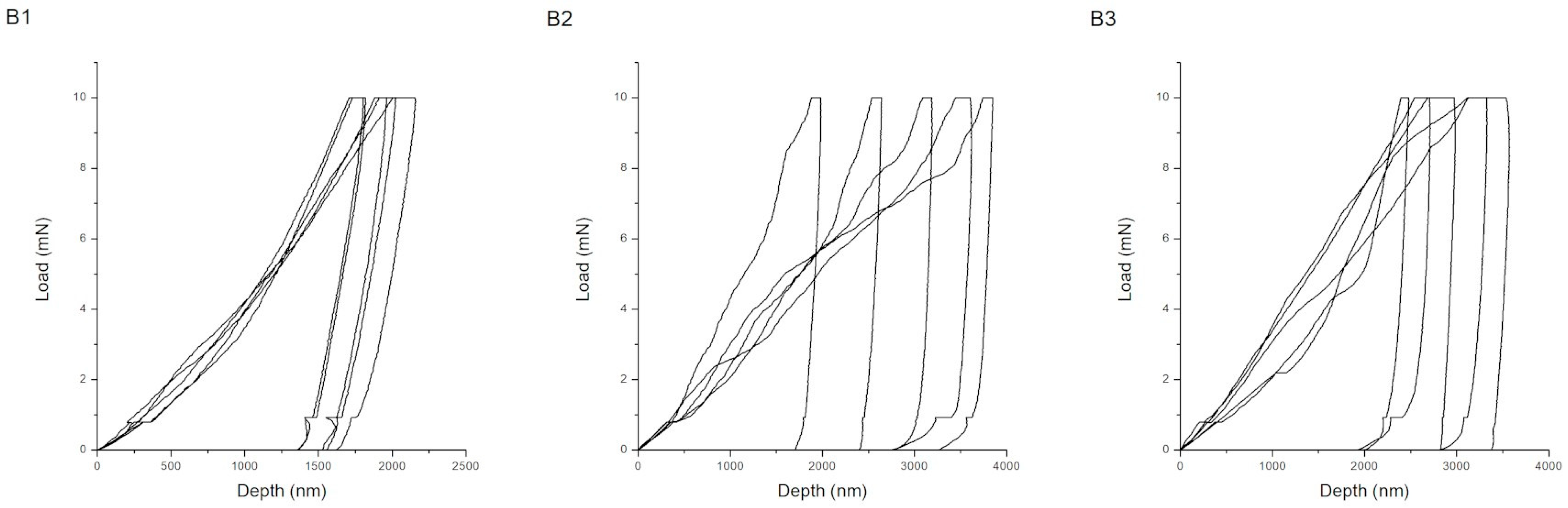
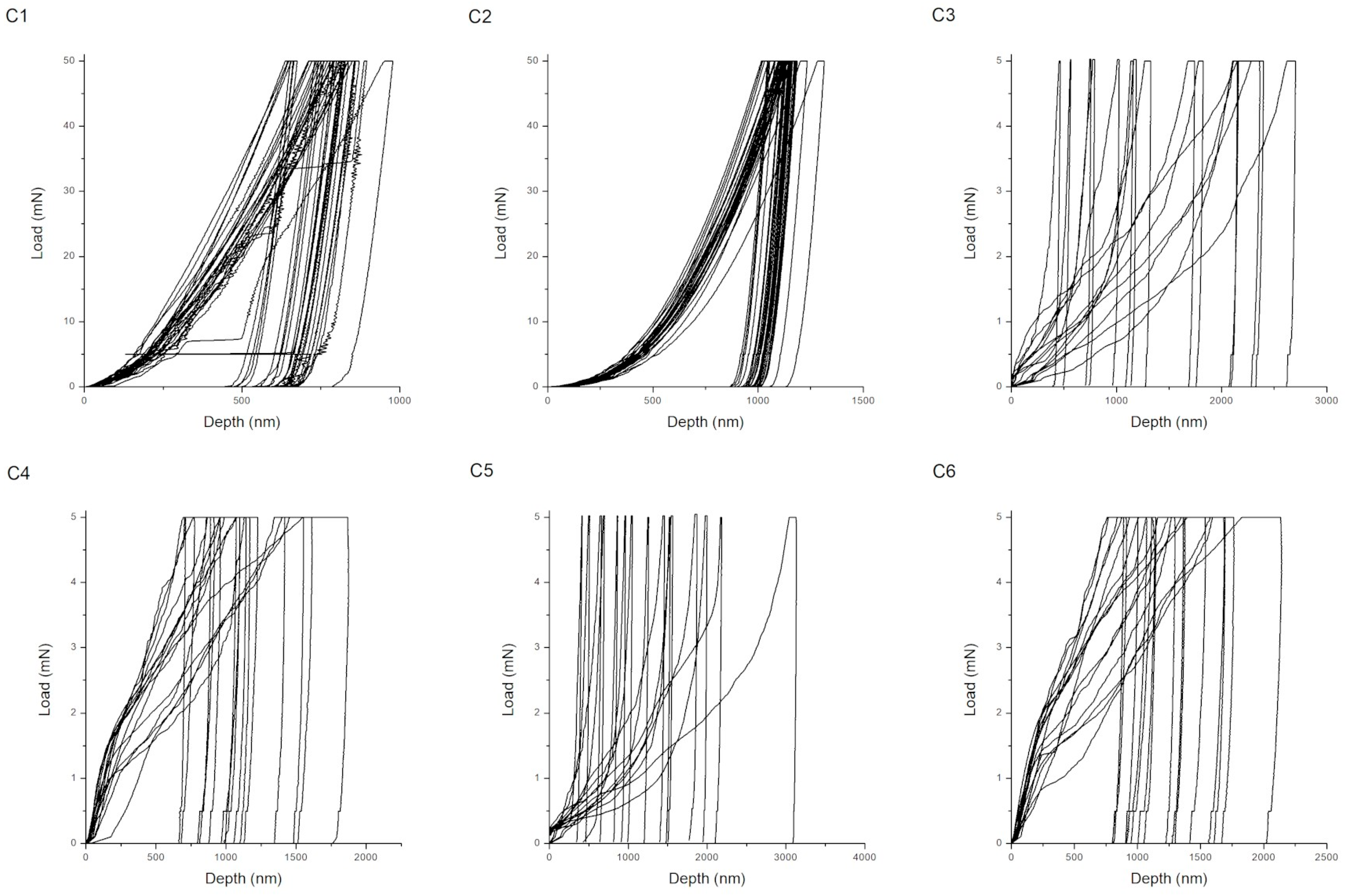
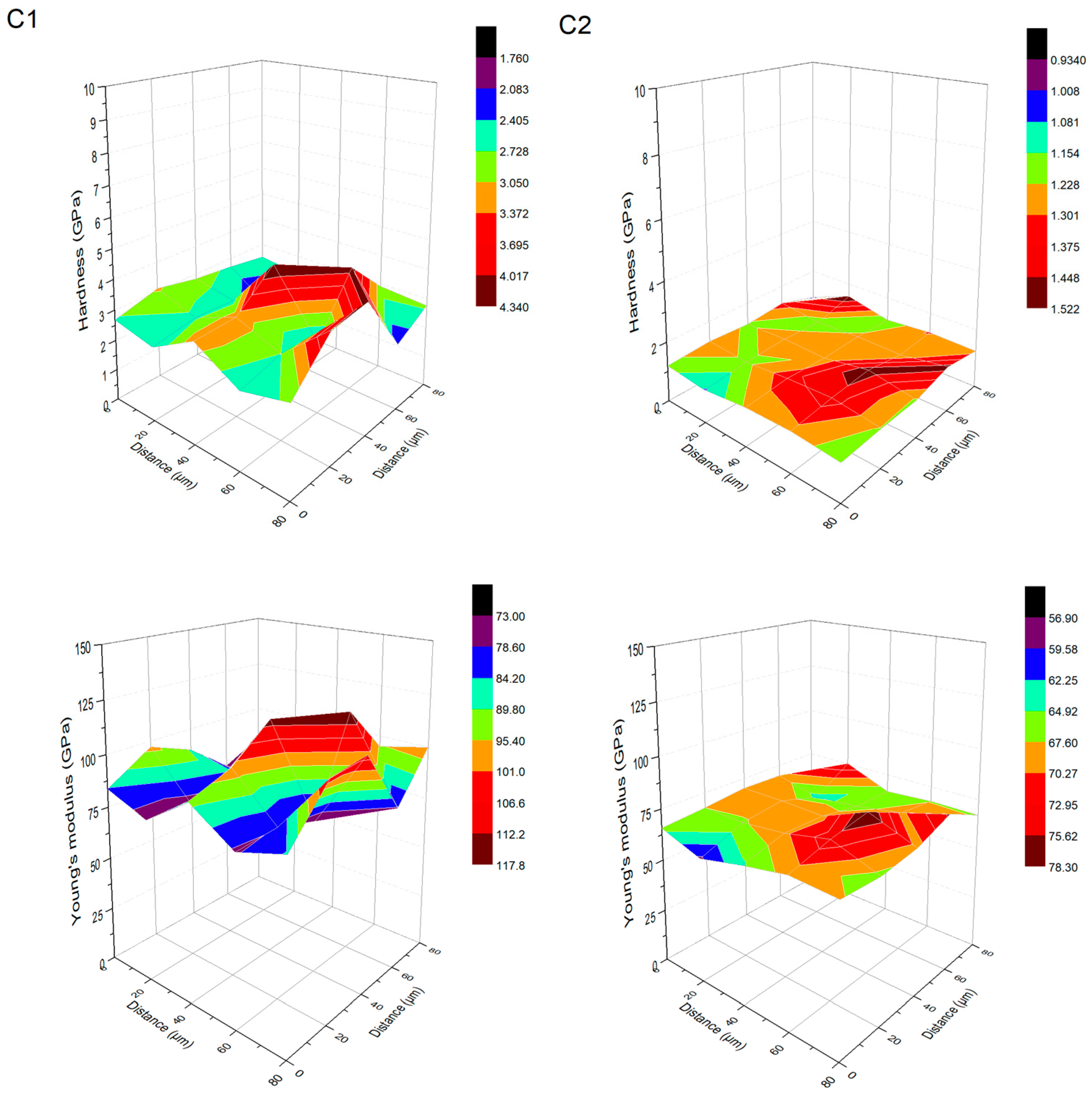
| Procedure | Maximum Depth of Indentations (nm) | Hardness (GPa) | Young’s Modulus (GPa) | H/E Ratio (-) | Wp/We Ratio (-) |
|---|---|---|---|---|---|
| A1 | 619 ± 161 | 8.21 ± 3.83 | 157.49 ± 61.31 | 0.053 ± 0.017 | 1.15 ± 0.26 |
| A2 | 1170 ± 622 | 4.73 ± 5.31 | 85.15 ± 71.77 | 0.048 ± 0.018 | 1.34 ± 0.75 |
| A3 | 1487 ± 714 | 2.43 ± 1.90 | 38.44 ± 20.90 | 0.055 ± 0.023 | 1.34 ± 0.61 |
| B1 | 1954 ± 147 | 0.13 ± 0.02 | 3.16 ± 0.29 | 0.042 ± 0.005 | 4.06 ± 0.61 |
| B2 | 3058 ± 756 | 0.06 ± 0.03 | 5.09 ± 1.09 | 0.010 ± 0.004 | 15.71 ± 4.41 |
| B3 | 3017 ± 44 | 0.05 ± 0.02 | 4.69 ± 0.77 | 0.010 ± 0.002 | 19.04 ± 10.06 |
| C1 | 796 ± 78 | 2.91 ± 0.67 | 89.53 ± 13.27 | 0.032 ± 0.004 | 3.54 ± 0.55 |
| C2 | 1144 ± 57 | 1.26 ± 0.13 | 68.27 ± 4.70 | 0.018 ± 0.001 | 4.75 ± 0.23 |
| C3 | 1499 ± 574 | 0.10 ± 0.08 | 25.81 ±16.38 | 0.004 ± 0.001 | 25.17 ± 13.05 |
| C4 | 1168 ± 325 | 0.14 ± 0.07 | 18.41 ± 11.16 | 0.008 ± 0.003 | 24.64 ± 10.36 |
| C5 | 1397 ± 395 | 0.10 ± 0.06 | 23.95 ± 8.24 | 0.003 ± 0.002 | 12.01 ± 4.70 |
| C6 | 1343 ± 360 | 0.10 ± 0.05 | 12.41 ± 4.77 | 0.008 ± 0.004 | 24.10 ± 8.62 |
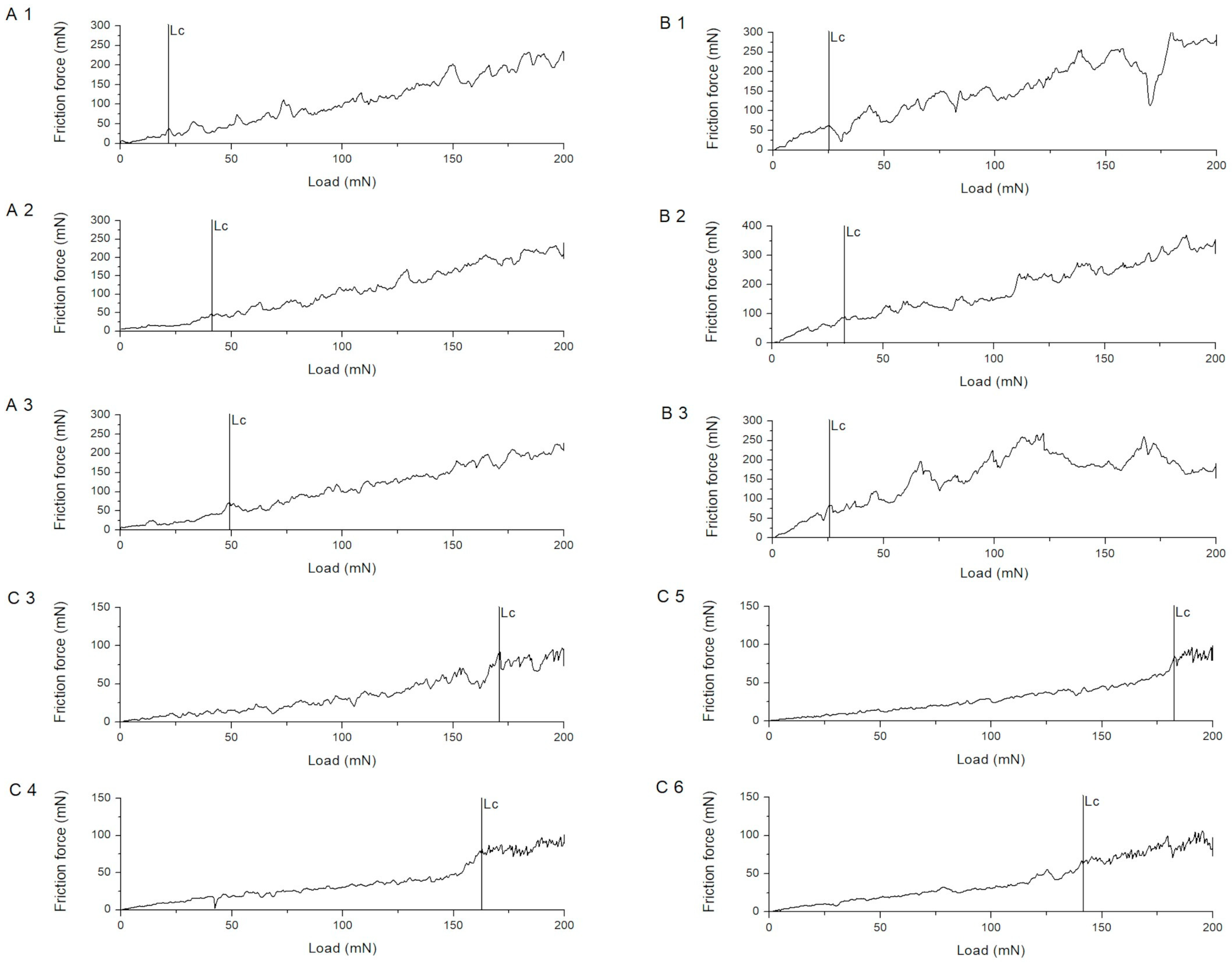
| Procedure | Critical Normal Force (mN) | Critical Friction Force (mN) |
|---|---|---|
| A1 | 24.32 ± 8.08 | 39.23 ± 10.43 |
| A2 | 31.82 ± 8.36 | 40.22 ± 9.64 |
| A3 | 53.67 ± 19.52 | 86.85 ± 20.39 |
| B1 | 27.22 ± 9.67 | 70.89 ± 24.69 |
| B2 | 29.64 ± 4.80 | 83.35 ± 12.90 |
| B3 | 21.68 ± 4.83 | 68.99 ± 14.98 |
| C3 | 165.04 ± 7.87 | 63.10 ± 5.17 |
| C4 | 158.31 ± 11.64 | 77.04 ± 7.35 |
| C5 | 172.95 ± 25.97 | 67.33 ± 30.29 |
| C6 | 149.73 ± 39.32 | 70.89 ± 18.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M. Nanomechanical and Adhesive Behavior of Electrophoretically Deposited Hydroxyapatite- and Chitosan-Based Coatings on Ti13Zr13Nb Alloy. Materials 2025, 18, 5323. https://doi.org/10.3390/ma18235323
Bartmański M. Nanomechanical and Adhesive Behavior of Electrophoretically Deposited Hydroxyapatite- and Chitosan-Based Coatings on Ti13Zr13Nb Alloy. Materials. 2025; 18(23):5323. https://doi.org/10.3390/ma18235323
Chicago/Turabian StyleBartmański, Michał. 2025. "Nanomechanical and Adhesive Behavior of Electrophoretically Deposited Hydroxyapatite- and Chitosan-Based Coatings on Ti13Zr13Nb Alloy" Materials 18, no. 23: 5323. https://doi.org/10.3390/ma18235323
APA StyleBartmański, M. (2025). Nanomechanical and Adhesive Behavior of Electrophoretically Deposited Hydroxyapatite- and Chitosan-Based Coatings on Ti13Zr13Nb Alloy. Materials, 18(23), 5323. https://doi.org/10.3390/ma18235323






