An Assessment of Fabrication, Properties, and Medical Applications of Chitosan–Nanometal Coatings
Abstract
1. Characteristics, Properties, and Applications of Chitosan
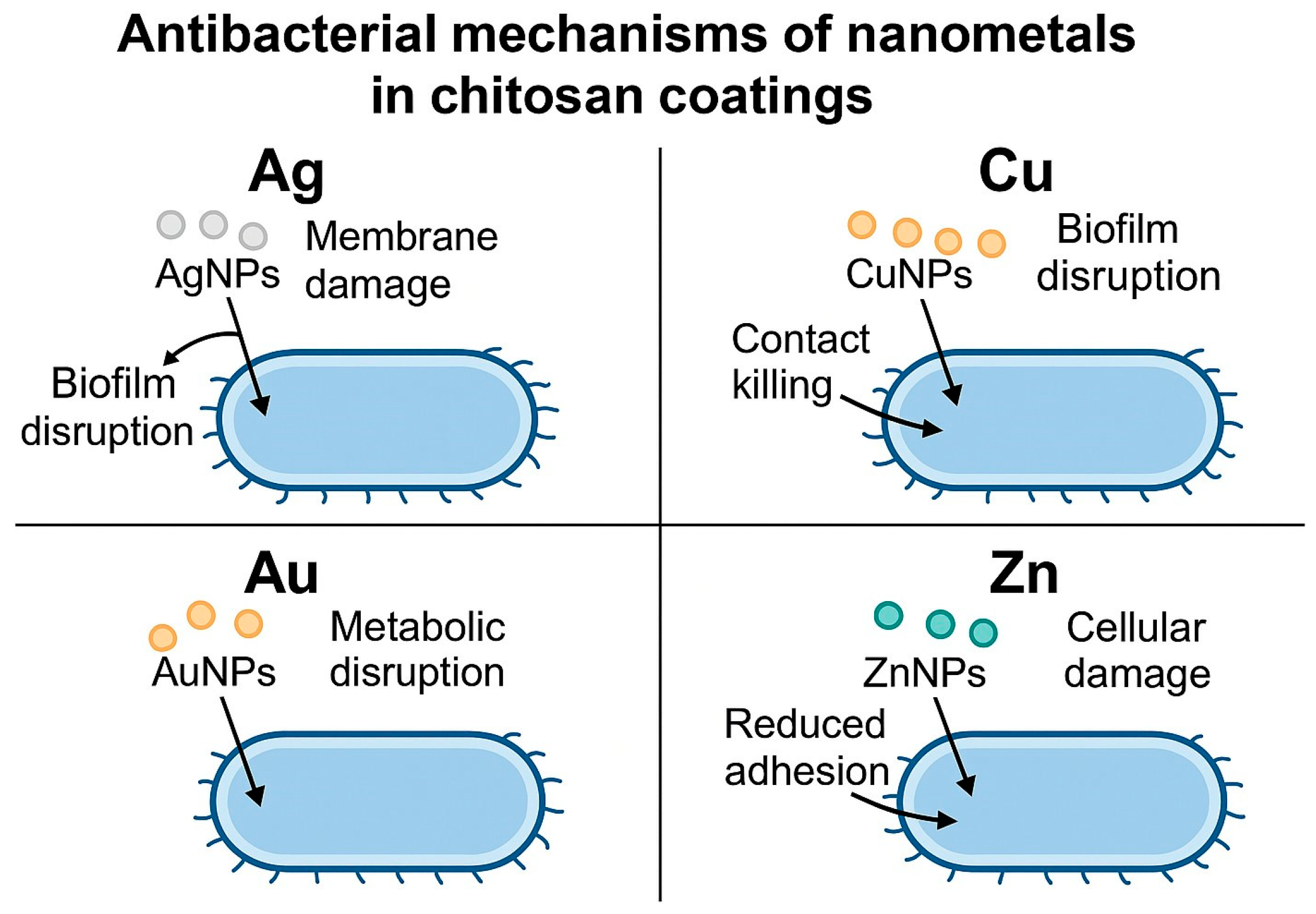
2. Chitosan–Nanosilver Coatings and Composite Materials
3. Chitosan–Nanocopper Coatings and Composite Materials
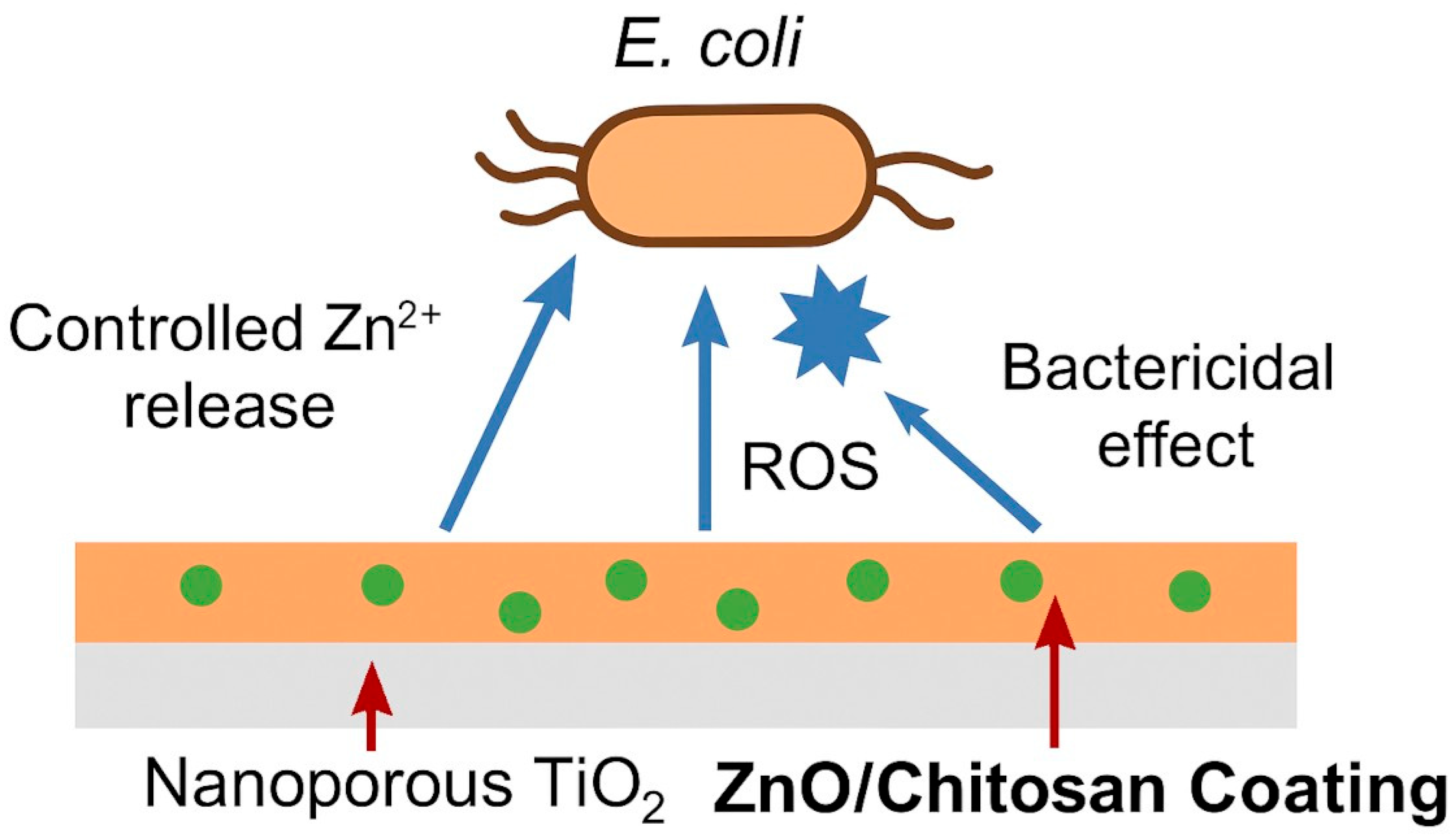
4. Chitosan–Nanozinc Coatings and Composites
5. Other Chitosan–Nanometal Coatings and Composite Materials
6. Assessment of Properties and Applications of Chitosan–Nanometals
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Tiyyagura, H.R.; Pottathara, Y.B.; Sadasivuni, K.K.; Ponnamma, D.; Douglas, T.E.L.; Skirtach, A.G.; Mohan, M.K. Surface Functionalization of Chitosan as a Coating Material for Orthopaedic Applications: A Comprehensive Review. Carbohydr. Polym. 2021, 255, 117487. [Google Scholar] [CrossRef]
- Ładniak, A.; Jurak, M.; Wiącek, A.E. Physicochemical Characteristics of Chitosan-TiO2 Biomaterial. 2. Wettability and Biocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127546. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; Schiavon, M.A.; dos Reis, A.C. Chitosan Coatings on Titanium-Based Implants—From Development to Characterization and Behavior: A Systematic Review. Carbohydr. Polym. 2024, 344, 122496. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, H.; Xing, R.; Li, R.; Gao, K.; Li, G.; Liu, S. Fungal and Microalgal Chitin: Structural Differences, Functional Properties, and Biomedical Applications. Polymers 2025, 17, 2722. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Nia, M.H.; Wilson, L.D. Recent Progress on the Application of Chitosan, Starch and Chitosan–Starch Composites for Meat Preservation—A Mini Review. J. Compos. Sci. 2024, 8, 302. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Park, K.H.; Song, H.-J.; Park, Y.-J. Calcium Phosphate-Chitosan Coatings Deposited on Titanium Surfaces via Pulse Galvanostatic Electrodeposition for Dental Implants. Int. J. Electrochem. Sci. 2020, 15, 9611–9621. [Google Scholar] [CrossRef]
- Hassan, M.K.; Abdelrehim, S.A.A.; Elkhooly, T.A.; Elmezayyen, A.S.; Mansour-Gabr, M.M.; Abdel Ghany, N.A. Investigation of Structure, Morphology, and Corrosion Behavior of Carboxylic Acids/Hydroxyapatite/Chitosan Coatings on Ti Discs for Implants. Thin Solid. Films 2024, 798, 140378. [Google Scholar] [CrossRef]
- Pawłowski, Ł.; Akhtar, M.A.; Zieliński, A.; Boccaccini, A.R. Electrophoretic Deposition and Characterization of Composite Chitosan/Eudragit E 100 or Poly(4-Vinylpyridine)/Mesoporous Bioactive Glass Nanoparticles Coatings on Pre-Treated Titanium for Implant Applications. Surf. Coat. Technol. 2024, 479, 130542. [Google Scholar] [CrossRef]
- Bozkurt, Y.B.; Kavasoğlu, Y.S.; Atik, B.; Kovacı, H.; Uzun, Y.; Çelik, A. Comparison Study of Corrosion Behavior for Chitosan Coated Ti6Al4V Alloy Produced by Selective Laser Melting and Forging. Prog. Org. Coat. 2023, 182, 107655. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-K.; Lee, M.-H.; Jang, Y.-S. Anti-Corrosion and Stable Osteointegration of a Biodegradable Magnesium Implant by Chitosan-Combined Calcium Phosphate Coating. Surf. Interfaces 2025, 56, 105636. [Google Scholar] [CrossRef]
- Murugesan, S.; Scheibel, T. Chitosan-Based Nanocomposites for Medical Applications. J. Polym. Sci. 2021, 59, 1610–1642. [Google Scholar] [CrossRef]
- Chernikova, E.E.; Zagvozkin, M.D.; Buzaev, A.A.; Kurzina, I.A.; Ulasevitch, S.A. Research and Development of PH-Sensitive Delivery Systems for Protein Molecule Delivery Based on Chitosan and Hydroxyapatite. J. Compos. Sci. 2025, 9, 525. [Google Scholar] [CrossRef]
- Rahimi, M.; Mir, S.M.; Baghban, R.; Charmi, G.; Plummer, C.M.; Shafiei-Irannejad, V.; Soleymani, J.; Pietrasik, J. Chitosan-Based Biomaterials for the Treatment of Bone Disorders. Int. J. Biol. Macromol. 2022, 215, 346–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cao, Z.; Wang, Y.; Feng, Y.; Liu, G.; Zhou, F.; Liu, W. Polymer Brush Enhanced Self-Lubricating Antibacterial Hydrogel Coatings on Titanium Alloys for Joint Implants. Tribol. Int. 2026, 214, 111173. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A Study of Chitosan Hydrogel with Embedded Mesoporous Silica Nanoparticles Loaded by Ibuprofen as a Dual Stimuli-Responsive Drug Release System for Surface Coating of Titanium Implants. Colloids Surf. B Biointerfaces 2014, 123, 657–663. [Google Scholar] [CrossRef]
- Kiryk, J.; Michalak, M.; Majchrzak, Z.; Laszczyńska, M.; Kiryk, S.; Szotek, S.; Gerber, H.; Nawrot-Hadzik, I.; Matys, J.; Dobrzyński, M. Functionalization Strategies of Chitosan-Based Scaffolds with Growth Factors for Bone Regeneration: A Systematic Review. Mar. Drugs 2025, 23, 396. [Google Scholar] [CrossRef]
- Beltrán-Novelo, L.G.; Aguilar-Pérez, F.J.; De La Garza-Ramos, M.A.; Cienfuegos-Sarmiento, A.A.; Herrera-Atoche, J.R.; Chuc-Gamboa, M.G.; Rodríguez-Chávez, J.A.; Cauich-Rodríguez, J.V. Mechanical and Antimicrobial Evaluation of Chitosan-Coated Elastomeric Orthodontic Modules. Dent. J. 2025, 13, 447. [Google Scholar] [CrossRef]
- Ko, S. Multifunctional Surface Coating Using Chitosan and Its Chemical Functionalization. Bull. Korean Chem. Soc. 2022, 43, 1207–1211. [Google Scholar] [CrossRef]
- Chen, Z.; Weng, J.; Du, X.; Ji, R.; Yang, X.; Yang, Y.; Ma, M. Quaternized Chitosan/Glycyrrhizic Acid Co-Decorated Titanium with Enhanced Antimicrobial, Immunomodulatory, and Osteogenic Properties for Dental Implant Applications. Carbohydr. Polym. 2025, 367, 123984. [Google Scholar] [CrossRef]
- Ballarre, J.; Aydemir, T.; Liverani, L.; Roether, J.A.; Goldmann, W.H.; Boccaccini, A.R. Versatile Bioactive and Antibacterial Coating System Based on Silica, Gentamicin, and Chitosan: Improving Early Stage Performance of Titanium Implants. Surf. Coat. Technol. 2020, 381, 125138. [Google Scholar] [CrossRef]
- Yang, D.; Luo, Q.; Li, C.; He, Y.; Li, D.; Xu, Z.; Ge, L.; Mu, C.; Li, D. Antibacterial Collagen-Based Heterogeneous Bilayer Barrier Membrane for Guiding Bacteria-Infected Bone Defects Regeneration. Int. J. Biol. Macromol. 2025, 321, 146539. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional PH Sensitive 3D Scaffolds for Treatment and Prevention of Bone Infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Früh, A.; Rutkowski, S.; Goreninskii, S.I.; Verzunova, K.N.; Soldatova, E.A.; Dorozhko, E.V.; Frueh, J.; Bakina, O.V.; Buldakov, M.A.; et al. Antibacterial Double-Layer Calcium Phosphate/Chitosan Composite Coating on Metal Implants for Tissue Engineering. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135652. [Google Scholar] [CrossRef]
- Wang, X.; He, X.; Zhang, H.; Hao, Y.; Wei, Y.; Liang, Z.; Hu, Y.; Lian, X.; Huang, D. Dual-Functional Black Phosphorus/Hydroxyapatite/Quaternary Chitosan Composite Coating for Antibacterial Activity and Enhanced Osseointegration on Titanium Implants. Colloids Surf. A Physicochem. Eng. Asp. 2025, 708, 136008. [Google Scholar] [CrossRef]
- Pan, T.; Li, L.; Wang, S.; Xie, S.; Wei, J.; Guo, L. Biocomposite Containing Polyetherketoneketone and Heterojunction of NaNbOX@CeO2 with Improved Piezoelectricity and Nanozyme Activity for Killing Bacteria and Enhancing Osteoblastic Differentiation. Biomater. Adv. 2026, 179, 214491. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Muñoz-Piña, S.; González, M.U.; Izquierdo-Barba, I.; Fernández-Martínez, I.; Rico, V.; Arcos, D.; García-Valenzuela, A.; Palmero, A.; Vallet-Regi, M.; et al. Antibacterial Nanostructured Ti Coatings by Magnetron Sputtering: From Laboratory Scales to Industrial Reactors. Nanomaterials 2019, 9, 1217. [Google Scholar] [CrossRef]
- Pal, A.; Sable, H.; Uniyal, A.; Chaudhary, V. Green Nano-Semiconductors and Nanometals to Avert Complex Phenomena of Antimicrobial Resistance (AMR). Curr. Opin. Biomed. Eng. 2025, 36, 100626. [Google Scholar] [CrossRef]
- Silva, A.O.; Cunha, R.S.; Hotza, D.; Machado, R.A.F. Chitosan as a Matrix of Nanocomposites: A Review on Nanostructures, Processes, Properties, and Applications. Carbohydr. Polym. 2021, 272, 118472. [Google Scholar] [CrossRef]
- Santosa, S.J.; Hadi, M.; Hatmanto, A.D.; Darmanastri, S.M.; Kusrini, E.; Nugrahaningtyas, K.D.; Usman, A. A Novel Eco-Friendly Method for Synthesizing Silver Nanoparticles (AgNPs)-Decorated Chitosan Film Having High Antibacterial Efficacy. JCIS Open 2025, 20, 100155. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Al Omari, R.H.; Younes, M.K.; Algburi, S. Carboxylated Chitosan-Phthalate/ZrO2 Nanocomposite for Removal of Methylene Blue Dye: Characterization and Adsorption Modeling via Response Surface Methodology. J. Mol. Struct. 2025, 1339, 142386. [Google Scholar] [CrossRef]
- Alhemadan, A.H.; Bakhsh, E.M.; Akhtar, K.; Homdi, T.A.; Khan, S.B. Date Palm Endocarp Film and Chitosan Coated Date Palm Endocarp Film Stabilized Silver Nanoparticles for the Catalytic Reduction of P-Nitrophenol. Environ. Technol. Innov. 2025, 40, 104485. [Google Scholar] [CrossRef]
- Chandrababu, V.; Parameswaranpillai, J.; Gopi, J.A.; Pathak, C.; Midhun Dominic, C.D.; Feng, N.L.; Krishnasamy, S.; Muthukumar, C.; Hameed, N.; Ganguly, S. Progress in Food Packaging Applications of Biopolymer-Nanometal Composites—A Comprehensive Review. Biomater. Adv. 2024, 162, 213921. [Google Scholar] [CrossRef]
- Tayel, A.A.; Ebaid, A.M.; Otian, A.M.; Mahrous, H.; El Rabey, H.A.; Salem, M.F. Application of Edible Nanocomposites from Chitosan/Fenugreek Seed Mucilage/Selenium Nanoparticles for Protecting Lemon from Green Mold. Int. J. Biol. Macromol. 2024, 273, 133109. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Zeng, X.; Zhang, X.; Ren, B.; Yang, X. Bentonite and Gallotannin-Mediated Silver Nanoparticle-Modified Chitosan Biofilms for Food Preservation. Int. J. Biol. Macromol. 2025, 320, 146100. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Jin, W.; Xu, Y.; Mei, F.; Cheng, D.; Cai, G.; Wang, X. Biomass-Based Food Packaging Film of Chitosan/Flavonoids/Silver Nanoparticle for Shelf Life Extension of Food. Colloids Surf. A Physicochem. Eng. Asp. 2025, 727, 138154. [Google Scholar] [CrossRef]
- Zhuo, M.; Liu, C.; Wang, Q.; Wang, Z.; Wang, Y.; Yu, F.; Zhang, Y. Catharanthus Roseus Extract-Assisted Silver Nanoparticles Chitosan Films with High Antioxidant and Antimicrobial Properties for Fresh Food Preservation. Int. J. Biol. Macromol. 2025, 309, 142771. [Google Scholar] [CrossRef]
- Baziyani, G.I.; Diab, M.A.; Ghori, S.W.; Singh, P.K.; Muniyandy, E.; Abdullaev, A.; Latipova, M.; Madaminov, B.; Khalaf Issa, S.; Smerat, A.; et al. Silver Nanoparticles Supported over Chitosan-Tannic Acid Composite-Modified Magnetic Nanoparticles as Recyclable Catalyst for Creation of Propargylamines. Inorg. Chem. Commun. 2025, 182, 115547. [Google Scholar] [CrossRef]
- Nogueira, B.R.; Backx, B.P.; Delazare, T. Starch, Pectin and Chitosan-Based Bioplastics with Silver Nanoparticles: An Eco-Friendly Alternative for the Food Industry. Sustain. Chem. Environ. 2025, 10, 100246. [Google Scholar] [CrossRef]
- Lieu, M.D.; Nguyen, H.H.; Huynh, N.A.T.; Tuyen Ha, P.K.; Dang, T.K.T.; Nguyen, T.H. Green Synthesized Silver Nanoparticles by Avocado Leaves Incorporating Chitosan Edible Coating for Avocado Preservation under Ambient Conditions. Biocatal. Agric. Biotechnol. 2025, 67, 103650. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł.; Zieliński, A.; Mielewczyk-Gryń, A.; Strugała, G.; Cieślik, B. Electrophoretic Deposition and Characteristics of Chitosan-Nanosilver Composite Coatings on a Nanotubular TiO2 Layer. Coatings 2020, 10, 245. [Google Scholar] [CrossRef]
- Bartmański, M.; Ronowska, A.; Mania, S.; Banach-Kopeć, A.; Kozłowska, J. Biological and Antibacterial Properties of Chitosan-Based Coatings with AgNPs and CuNPs Obtained on Oxidized Ti13Zr13Nb Titanium Alloy. Mater. Lett. 2024, 360, 135997. [Google Scholar] [CrossRef]
- Clifford, A.; Pang, X.; Zhitomirsky, I. Biomimetically Modified Chitosan for Electrophoretic Deposition of Composites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 544, 28–34. [Google Scholar] [CrossRef]
- Hileuskaya, K.; Kraskouski, A.; Ihnatsyeu-Kachan, A.; Saichuk, A.; Pinchuk, S.; Nikalaichuk, V.; Ladutska, A.; Kulikouskaya, V.; Neves, M.C.; Freire, M.G.; et al. New Insights into Chitosan-Ag Nanocomposites Synthesis: Physicochemical Aspects of Formation, Structure-Bioactivity Relationship and Mechanism of Antioxidant Activity. Int. J. Biol. Macromol. 2025, 300, 140077. [Google Scholar] [CrossRef]
- Devi, N.; Pandey, S.K.; Wangoo, N. Cationic Carboxymethyl Chitosan Nanofibers Embedded with Silver Nanoparticles for Enhanced Antibacterial Applications. Biophys. Chem. 2026, 328, 107537. [Google Scholar] [CrossRef] [PubMed]
- Önal, E. Hybrid Zn (II)/Pd (II) Porphyrin-Immobilized Chitosan Hydrogels with Silver Nanoparticles for Enhanced Photophysical and Photochemical Properties. Dye. Pigment. 2026, 245, 113289. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired Anchoring AgNPs onto Micro-Nanoporous TiO2 Orthopedic Coatings: Trap-Killing of Bacteria, Surface-Regulated Osteoblast Functions and Host Responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]
- Piñera-Avellaneda, D.; Buxadera-Palomero, J.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Surface Competition between Osteoblasts and Bacteria on Silver-Doped Bioactive Titanium Implant. Biomater. Adv. 2023, 146, 213311. [Google Scholar] [CrossRef]
- Mohamed, A.S.A.; Abdelaziz, A.A.; Abo-Kamar, A.M.; Al-Madboly, L.A.; El-Maradny, Y.A.; El-Fakharany, E.M. Multifunctional Efficacy of the Fabricated Chitosan-Coated Silver Nanoparticles as an Antiviral Agent against SARS-CoV-2: Potent and Safe Mechanistic Insights. Int. J. Biol. Macromol. 2025, 320, 145623. [Google Scholar] [CrossRef]
- Rengasamy, G.; Chinnalagu, D.K.; Chinniah, K.; Mahalingam, S. Biodegradable Multifunctional Fabrication of Silver-Doped Zinc Oxide Nanoparticle on Chitosan/Polyvinyl Alcohol Flexible Film for Effective Antibacterial Potential and Attenuation of Human Liver Carcinoma Cells. Int. J. Biol. Macromol. 2025, 321, 146309. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Ferreira, J.M.F.; Kannan, S. Mechanically Stable Antimicrobial Chitosan-PVA-Silver Nanocomposite Coatings Deposited on Titanium Implants. Carbohydr. Polym. 2015, 121, 37–48. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Xu, R.; Wang, W.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Electrochemically Deposited Chitosan/Ag Complex Coatings on Biomedical NiTi Alloy for Antibacterial Application. Surf. Coat. Technol. 2013, 232, 370–375. [Google Scholar] [CrossRef]
- Say, Y.; Aksakal, B.; Sinirlioglu, Z.A. Silver/Selenium/Chitosan Co-Substituted Bioceramic Coatings of Ni–Ti Alloy: Antibacterial Efficiency and Cell Viability. Int. J. Appl. Ceram. Technol. 2022, 19, 2701–2712. [Google Scholar] [CrossRef]
- Marsico, M.; Guarnieri, A.; Triunfo, M.; Curcio, M.; Galasso, A.; Scieuzo, C.; Salvia, R.; Falabella, P.; Teghil, R.; De Bonis, A. Alternative Source of Chitosan for the Direct Laser Synthesis of Ag@chitosan Composites with Antibacterial and Photocatalytic Properties. Next Mater. 2025, 9, 100952. [Google Scholar] [CrossRef]
- Yan, P.; Chen, D.; Muhammad, S.; Guo, Y.; Gao, Q.; Niu, B.; Liu, Y.; Liu, C. Carboxymethyl Chitosan-Immobilized Silver Nanoparticles for Intravesical Instillation: A Strategy to Prevent Catheter-Associated Urinary Tract Infections. Int. J. Biol. Macromol. 2025, 320, 146001. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Ur Rehman, M.A. Chitosan/Gelatin-Based Bioactive and Antibacterial Coatings Deposited via Electrophoretic Deposition. J. Appl. Polym. Sci. 2021, 138, 50220. [Google Scholar] [CrossRef]
- Alemi, P.S.; Mohamadali, M.; Arabahmadi, S.; Irani, S.; Sharifi, F. Carboxymethyl Chitosan and Chitosan as a Bioactive Delivery System: A Review. Biotechnol. Appl. Biochem. 2025, e2758. [Google Scholar] [CrossRef]
- El-Sheikh, M.A. Synthesis of a Novel Carboxymethyl Chitosan-Silver—Ginger Nanocomposite, Characterization, and Antimicrobial Efficacy. Carbohydr. Polym. Technol. Appl. 2024, 8, 100561. [Google Scholar] [CrossRef]
- Anitha, J.; Muthusankar, A.; Sangeetha, M.; Kishore, V.L.; Sherin, P.J.; Selvakumar, R.; Chandraprakash, K.; Premkumar, T. Chitosan-Coated Silver Nanoparticles Synthesized Using Moringa Oleifera Flower Extract: A Potential Therapeutic Approach against Triple-Negative Breast Cancer. Int. J. Biol. Macromol. 2025, 320, 145995. [Google Scholar] [CrossRef]
- Ijaz, F.; Ali, S.; Pervaiz, A.; Khan, K.; Afsar, T.; Aldisi, D.; Amor, H.; Razak, S. Sericin-Chitosan Conjugated Silver Nanoparticles Protect against 1,2-Dimethylhydrazine-Induced Colon Cancer in Mice by Regulating Metastatic Biomarkers, Prohibiting Dysplasia and Enhancing Antioxidant Potential. Int. J. Biol. Macromol. 2025, 321, 146478. [Google Scholar] [CrossRef]
- Bambaeero, A.; Bazargan-Lari, R.; Vafa, E.; Memarzadeh, R. Electrophoretic Deposition of a Schiff Base Natural Chitosan, Reinforced with Polyvinyl Alcohol (PVA) and Silver Nanoparticles/Silver-Doped Bioactive Glass Nanocomposite, onto Anodized Pure Titanium Implants. Mater. Chem. Phys. 2025, 345, 131294. [Google Scholar] [CrossRef]
- Castillejo, A.; Martínez, G.; Delgado-Pujol, E.J.; Villalobo, E.; Carrillo, F.; Casado-Jurado, D.; Pérez-Bernal, J.L.; Begines, B.; Torres, Y.; Alcudia, A. Enhanced Porous Titanium Biofunctionalization Based on Novel Silver Nanoparticles and Nanohydroxyapatite Chitosan Coatings. Int. J. Biol. Macromol. 2025, 299, 139846. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Abdollahi, S.; Gohil, T.; Obiefuna, A.C.; Omoniyi, T.; Omosebi, T.O.; Phillips, T.S.; Elhabashi, N. Influence of Non-Staining Chitosan-Based Nano-Silver Fluoride on Shear Bond Strengths of Dental Restorations. J. Compos. Sci. 2025, 9, 518. [Google Scholar] [CrossRef]
- Singh, R.; Sana, S.S.; Bansal, S.; Chaudhary, R.; Gupta, S.; Kim, S.C.; Kumar, A. Lignin-Wrapped Silver Nanoparticles-Based Chitosan/Polyvinylpyrrolidone Nano-Composite Hydrogel Films for Infected Wounds. Inorg. Chem. Commun. 2025, 182, 115652. [Google Scholar] [CrossRef]
- Karataş, M.; Erzen, B.; Aydoğmuş, E.; Orhan, R. PVA/Chitosan Biofilms Enriched with Biosynthesized Silver Nanoparticles and Tea Tree Oil: Towards Multifunctional and Environmentally Friendly Materials. Int. J. Biol. Macromol. 2025, 312, 144164. [Google Scholar] [CrossRef]
- Ngo, P.K.T.; Luu, C.H.; Nguyen, H.P.; Nguyen, D.N.; Nguyen, K.D.; Van Luu, T.; Le, P.K.; Pan, Z.; Phan, V.H.G.; Li, Y.; et al. Multifunctional Haemostatic and Antibacterial Wound Dressing: Chitosan-Silk Fibroin Composite with Green-Synthesised Silver Nanoparticles and Deferoxamine. Surf. Interfaces 2025, 72, 107112. [Google Scholar] [CrossRef]
- Gholizadeh, R.; Mosleh-Shirazi, S.; Amani, A.M. Investigation of Electrospun Chitosan Nanofibers Reinforced with Mesoporous Silicon Oxide Decorated with Silver Nanoparticles in Diabetic Wound Treatment. Int. J. Biol. Macromol. 2025, 322, 146432. [Google Scholar] [CrossRef] [PubMed]
- Thinakaran, S.; Loordhuswamy, A.M.; Venkateshwapuram Rengaswami, G.D. Electrophoretic Deposition of Chitosan/Nano Silver Embedded Micro Sphere on Centrifugal Spun Fibrous Matrices—A Facile Biofilm Resistant Biocompatible Material. Int. J. Biol. Macromol. 2020, 148, 68–78. [Google Scholar] [CrossRef]
- Parchen, G.P.; Volpe, J.; Hospinal-Santiani, M.; Souto, D.E.P.; Hillaireau, H.; Soccol, V.T.; de Freitas, R.A. Antibody Functionalized Thiolated Chitosan Stabilized Silver Nanoparticles as Theranostics toward Visceral Leishmaniasis. Int. J. Biol. Macromol. 2025, 320, 145817. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.S.; Ahmed, A.Y.; Ghori, S.W.; M, R.M.; Ray, S.; Chennakesavulu, K.; Sharma, R.; Matyakubov, B.; Sabirov, S.; Diab, M.A.; et al. Silver Nanoparticles Supported on Chitosan-Glutaraldehyde Polymers as an Efficient and Robust Heterogeneous Catalyst for Direct Synthesis of Propargylamines. J. Organomet. Chem. 2025, 1040, 123808. [Google Scholar] [CrossRef]
- Khan, Z.; AL-Thabaiti, S.A. Chitosan Capped Silver Nanoparticles: Adsorption and Photochemical Activities. Arab. J. Chem. 2022, 15, 104154. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Wu, C.; Sun, T.; Li, X. Preparation, Characterization and Antibacterial Mechanism of the Chitosan Coatings Modified by Ag/ZnO Microspheres. J. Sci. Food Agric. 2020, 100, 5527–5538. [Google Scholar] [CrossRef]
- Hua, P.; Pan, X.; Liu, J.; Chen, X.; Li, M.; Guo, Y.; Li, X.; Leng, P.; Fan, G.; Zheng, M.; et al. Development of a Novel LBL@DHBA/Cu-MOF Coating on Titanium Implants to Accelerate Early Osseointegration by Promoting Neural-Vascular-Bone Coupling Regeneration. Chem. Eng. J. 2025, 514, 163246. [Google Scholar] [CrossRef]
- Bartmański, M.; Pawłowski, Ł. Properties of Chitosan/CuNPs Coatings Electrophoretically Deposited on TiO2 Nanotubular Oxide Layer of Ti13Zr13Nb Alloy. Mater. Lett. 2022, 308, 130982. [Google Scholar] [CrossRef]
- Liu, H.; Xu, D.; Ma, Y.; Qian, J.; Yang, Y.; Yu, B.; Ren, L.; Yang, K. Mechanisms of Hierarchical Topographies Tuning Bacteria and Cell Biological Responses to the Surfaces of Pure Titanium and Cu-Bearing Titanium Alloy. ACS Appl. Mater. Interfaces 2022, 14, 19226–19240. [Google Scholar] [CrossRef]
- Wu, P.; Lu, Q.; Liu, T.; Nie, J.; Liu, Z.; Rao, X. In-Situ Grown Multi-Layered Porous TiO2 Composited Copper Doped Tannic Acid Microflora on Titanium Surface for NIR Responsive Enhanced Anti-Bacteria. Mater. Lett. 2025, 398, 138936. [Google Scholar] [CrossRef]
- Ayala-Peña, V.B.; Martin, M.J.; Otarola, J.; Favatela, F.; Gonzalez, J.S.; Conesa, A.L.; García, C.C.; Sepúlveda, C.S.; Alvarez, V.A.; Lassalle, V.L. Virucidal and Antibacterial Chitosan–NanoCu Film-Coating-Based Technology: Complete Analysis of Its Performance on Various Surfaces. Viruses 2025, 17, 1347. [Google Scholar] [CrossRef]
- El-Wahab, G.M.M.A.; Khedr, Y.I.; Masoud, S.A.; Nassar, A.M.K. Carbendazim-Chitosan and Copper- and Cobalt-Fusarium Nanoparticles Biological Activity against Potato Root Rot Disease Caused by Rhizoctonia Solani. Plant Nano Biol. 2025, 11, 100136. [Google Scholar] [CrossRef]
- Bian, J.; Wu, T.; Zhou, Q.; Xie, H.; Chen, C. Silane-Coupled Chitosan-Cyclodextrin/Rosmarinic Acid-Zinc Complex Coating Improves the Osseointegration of Titanium Implants in High-Glucose Environments. Appl. Surf. Sci. 2023, 638, 158015. [Google Scholar] [CrossRef]
- Li, K.; Xie, E.; Liu, C.; Hu, J.; Chen, Q.; Li, J.; Wang, H.; Meng, Q.; Liu, D.; Meng, B.; et al. “Disguise Strategy” to Bacteria: A Multifunctional Hydrogel with Bacteria-Targeting and Photothermal Conversion Properties for the Repair of Infectious Bone Defects. Bioact. Mater. 2025, 47, 343–360. [Google Scholar] [CrossRef]
- Blinov, A.; Rekhman, Z.; Yasnaya, M.; Gvozdenko, A.; Golik, A.; Kravtsov, A.; Shevchenko, I.; Askerova, A.; Prasolova, A.; Pirogov, M.; et al. Enhancement of Stability and Activity of Zinc Carbonate Nanoparticles Using Chitosan, Hydroxyethyl Cellulose, Methyl Cellulose and Hyaluronic Acid for Multifaceted Applications in Medicine. Int. J. Biol. Macromol. 2025, 298, 139768. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Okba, E.A.; Ibrahim, M.M.; Elshami, F.I.; Shaban, S.Y. A Kinetic and Mechanistic Study of Chitosan-Functionalized Lanthanum Zinc Ferrite Nanoparticles: Balancing Biomolecular Affinity with Anticancer, Antibacterial, and Antioxidant Functions. Inorg. Chem. Commun. 2025, 181, 115230. [Google Scholar] [CrossRef]
- Bartmański, M.; Wekwejt, M.; Urbanowicz, K.; Mielewczyk-Gryń, A.; Gajowiec, G.; Pałubicka, A.; Michno, A.; Serafin, P.K.; Koszałka, P. Chitosan-Nanogold and Chitosan-Nanozinc Electrodeposited Coatings for Biomedical Applications. J. Biomed. Mater. Res. B Appl. Biomater. 2025, 113, e35571. [Google Scholar] [CrossRef]
- Bartmanski, M.; Pawłowski, Ł.; Knabe, A.; Mania, S.; Banach-Kopeć, A.; Sakowicz-Burkiewicz, M.; Ronowska, A. The Effect of Marginal Zn2+ Excess Released from Titanium Coating on Differentiation of Human Osteoblastic Cells. ACS Appl. Mater. Interfaces 2024, 16, 48412–48427. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Wang, Y.H.; Kuo, C.H.; Ou, S.F.; Huang, P.Z.; Song, T.Y.; Chen, Y.C.; Chen, S.T.; Wu, C.H.; Hsueh, Y.H.; et al. Hybrid ZnO/Chitosan Antimicrobial Coatings with Enhanced Mechanical and Bioactive Properties for Titanium Implants. Carbohydr. Polym. 2021, 257, 117639. [Google Scholar] [CrossRef]
- Herin, R.F.; Sebastiammal, S.; Judit, A.S.S.; Thirupathi, K.; Asaithambi, P.; Alanazi, A.K.; Santhamoorthy, M.; Phan, T.T.V. Green Synthesis of Chitosan-Modified Zinc Oxide Nanoparticles Using Nigella Sativa Seed Extract and Its Antimicrobial, Antidiabetic, and Antioxidant Applications. Inorg. Chem. Commun. 2025, 182, 115477. [Google Scholar] [CrossRef]
- Hidayat, M.I.; Hardiansyah, A.; Khoiriah, K.; Yulianti, E.; Wardhani, R.A.K.; Fahrialdi, F.; Yusuf, M.R.I. Composite Films Based on Chitosan Incorporating Molybdenum Disulfide Nanosheets and Zinc Oxide Nanoparticles with Potential Antibacterial Application. Food Chem. 2025, 477, 143480. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Heras, C.; Lozano, D.; Vallet-Regí, M.; Salinas, A.J. Nanodevices Based on Mesoporous Glass Nanoparticles Enhanced with Zinc and Curcumin to Fight Infection and Regenerate Bone. Acta Biomater. 2023, 166, 655–669. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; El-Aziz, E.A.; Elmaaty, T.M.A.; Ramadan, S.M. Loading of Chitosan—Nano Metal Oxide Hybrids onto Cotton/Polyester Fabrics to Impart Permanent and Effective Multifunctions. Int. J. Biol. Macromol. 2017, 105, 769–776. [Google Scholar] [CrossRef]
- Tamizhselvan, A.; Muthumanickam, D.; Murugesan, A.K.; Malathi, G.; Liu, B.; Sengottuvelan, B. Water-Soluble as Well as Bio-Compatible Acryloyl Chitosan-Grafted Piperazinium Polymers Stabilized Zinc Oxide Nanoparticles and Their Anticancer Study. Surf. Interfaces 2025, 59, 105906. [Google Scholar] [CrossRef]
- Amirafshari, H.; Khazaal, R.M.; Marzban, A.; Cheraghipour, K.; Masoori, L.; Sepahvand, A.; Mahmoudvand, H. Therapeutic Effects of Zinc Oxide Nanoparticles Encapsulated within Chitosan-Camphor against Hydatid Cysts through Suppressing Oxidative Stress, Inflammation, and DNA Damage. Biomed. Pharmacother. 2025, 188, 118128. [Google Scholar] [CrossRef]
- Mathew, D.; Thomas, B.; Sudheep, N.M.; Muhammed Shamil, K.V.; Anjana, R.; Krishna, A.; Thomas, J.B.; Krishnankutty, R.E. Development of Garlic Extract-Loaded Zinc Oxide Nanoparticle/Chitosan/Polyvinyl Alcohol Nanocomposite-Based Active Eco-Friendly Packaging Films for Extending Shelf-Life of Fish Fillets. Int. J. Biol. Macromol. 2025, 327, 147273. [Google Scholar] [CrossRef]
- Adhikary, P.; Chetia, J.; Sharma, M.; Badwaik, L.S. Shelf Life Extension of Grapes through Chitosan Coating Reinforced Zinc Oxide Nanoparticles Containing Phytocompounds from Lemon Pomace. Sci. Hortic. 2025, 342, 114018. [Google Scholar] [CrossRef]
- Wei, X.; Li, Q.; Hao, H.; Yang, H.; Li, Y.; Sun, T.; Li, X. Preparation, Physicochemical and Preservation Properties of Ti/ZnO/in Situ SiOx Chitosan Composite Coatings. J. Sci. Food Agric. 2020, 100, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Rangraz, Y. ZnSe Nanoparticles Anchored on N-Doped Mesoporous Carbon Prepared from Zinc-Based Bio-MOF and Chitosan as an Efficient Catalyst for Selective Hydrogenation of Nitroarenes under Mild Conditions. Int. J. Biol. Macromol. 2025, 320, 145794. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Shuang, Q.; Wu, K.; Nan, Y.; Liu, W.; Lu, A.; Kong, L.; Wu, J.; Zhang, B.; Hou, X.; et al. Decorated Gold Nanoparticles on Baicalein/Chitosan-Modified Zinc Oxide Nanoparticles for One-Pot Preparation of Pyrano[2,3-d]Pyrimidines and Inhibiting the Gastrointestinal Stromal Tumor Progression by Controlling the Gene Expression of the Cell Cycle. Int. J. Biol. Macromol. 2025, 322, 146612. [Google Scholar] [CrossRef]
- Hosseini, M.; Khalil-Allafi, J.; Safavi, M.S.; Ghalandarzadeh, A. Fascinating Osteoblast Compatibility and Antibacterial Activity of HA-Ta2O5 Composite Coating Deposited by Plasma Electrolytic Oxidation. J. Alloys Compd. 2025, 1023, 180193. [Google Scholar] [CrossRef]
- Elgamal, A.M.; Ali, E.A.B.; Saad, G.R.; Abdelhamid, I.A.; Elsabee, M.Z.; Hamed, A.A. Biologically Active Ionic Chitosan Schiff Base Nanocomposites: Synthesis, Characterization and Antimicrobial Activity against Helicobacter Pylori. Int. J. Biol. Macromol. 2024, 282, 137321. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; De Giglio, E. Gallium-Modified Chitosan/Poly(Acrylic Acid) Bilayer Coatings for Improved Titanium Implant Performances. Carbohydr. Polym. 2017, 166, 348–357. [Google Scholar] [CrossRef]
- Min, H.; Wu, Y.; Wen, J.; Guo, J.; Jiang, S.; Cheng, J. Sol-Gel Synthesized TiO2-Chitosan Nanocomposite as Antibacterial Coating for Orthopedic Implants: Investigation of Properties and Antimicrobial Mechanisms. Alex. Eng. J. 2025, 129, 572–581. [Google Scholar] [CrossRef]
- Montaser, A.S.; Wassel, A.R.; Al-Shaye’a, O.N. Synthesis, Characterization and Antimicrobial Activity of Schiff Bases from Chitosan and Salicylaldehyde/TiO2 Nanocomposite Membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef]
- Cisternas, M.A.; Retamal, M.J.; Saikia, P.; Casanova, N.; Moraga, N.; Chandia, A.; Alvarez, A.; Díaz Droguett, D.E.; Guzman, F.; Maendl, S.; et al. Study of Phospholipid Bilayers Supported on Chitosan-Titanium Nitride Coatings Produced by Plasma Immersion Ion Implantation (PIII). Biophys. J. 2018, 114, 105a. [Google Scholar] [CrossRef]
- Rengasamy, G.; Mahalingam, S. Multifunctional Composite Film of Chitosan/Reduced Graphene Oxide Infused Silver Nanoparticle for Antibacterial and Cytotoxic Effects on HeLa Cells. Int. J. Biol. Macromol. 2025, 321, 146356. [Google Scholar] [CrossRef]
- Kannan, P.R.; Sangkert, S.; Jiang, C.; Li, Y.; Zhao, R.; Iqbal, M.Z.; Kong, X. Ultrasmall Zinc Oxide Nanoparticle-Reinforced Chitosan-Fucoidan Scaffolds for Enhanced Antibacterial Activity and Accelerated Osteogenesis. Int. J. Biol. Macromol. 2025, 310, 143390. [Google Scholar] [CrossRef]
- Hia, E.M.; Park, J.; Suh, I.W.; Park, C.H. Synergistic Effects of Modified Zinc Oxide Nanoparticle in a Hybrid Chitosan-Gelatin Hydrogel for Bone Regeneration. Int. J. Biol. Macromol. 2025, 315, 144490. [Google Scholar] [CrossRef]
- Predoi, D.; Ciobanu, C.S.; Iconaru, S.L.; Petre, R.A.; Rokosz, K.; Raaen, S.; Predoi, M.V. Development and Physico-Chemical and Antibacterial Characterization of Chromium-Doped Hydroxyapatite in a Chitosan Matrix Coating. Polymers 2025, 17, 2633. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yuan, W.; Lu, Z.; Li, C.M. Polymer/Nanosilver Composite Coatings for Antibacterial Applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 69–83. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications—A Systematic Review. Biol. Trace Elem. Res. 2020, 197, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Zaimoglu, M.; Secinti, K.D.; Altinoz, M.A.; Bozkurt, M.; Eroglu, U.; Ozpiskin, O.; Mammadkhanli, O.; Bayatli, E.; Caglar, Y.S.; Attar, A. Organelle-Level Toxicity of Nanometals Relevant to Titanium Implants. Original Research and Comprehensive Literature Overview. Tissue Cell 2024, 91, 102612. [Google Scholar] [CrossRef]
- Hadrup, N.; Lam, H.R. Oral Toxicity of Silver Ions, Silver Nanoparticles and Colloidal Silver—A Review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Hadrup, N.; Vogel, U.; Jacobsen, N.R. Biokinetics of Inhaled Silver, Gold, Copper Oxide, and Zinc Oxide Nanoparticles: A Review. Nanotoxicology 2025, 19, 259–289. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of Nanoparticle Toxicity on Their Physical and Chemical Properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, A.; Esmaeil Nejad, A.; Rezai Rad, M.; Namdari, M.; Sabetmoghaddam, T. In Vitro Release of Silver Ions and Expression of Osteogenic Genes by MC3T3-E1 Cell Line Cultured on Nano-Hydroxyapatite and Silver/Strontium-Coated Titanium Plates. Odontology 2023, 111, 33–40. [Google Scholar] [CrossRef]
- Gulati, K.; Scimeca, J.-C.; Ivanovski, S.; Verron, E. Double-Edged Sword: Therapeutic Efficacy versus Toxicity Evaluations of Doped Titanium Implants. Drug Discov. Today 2021, 26, 2734–2742. [Google Scholar] [CrossRef] [PubMed]
- Nagime, P.V.; Chandak, V.S.; Kotnala, S.; Pandey, M.; Jayeoye, T.J.; Shafi, S.; Chandra, S.; Singh, S.; Chidrawar, V.R.; Singh, S. Articulating the Multifaceted Application of Bioactive Compound Decorated Silver/Gold Nanoparticles: Current and Future Prospective. Biocatal. Agric. Biotechnol. 2025, 69, 103776. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Khalid, T.; Irfan, A.; Nasim, I.; Rubab, L.; Al-Hussain, S.A.; Samad, N.; Aslam, S.; Zaki, M.E.A. Recent Insights into Biogenic Silver, Gold, Iron, and Copper Nanoparticles for Antimicrobial, Cytotoxic, and Drug Delivery Applications. Inorg. Chem. Commun. 2025, 182, 115615. [Google Scholar] [CrossRef]
- Mbanga, O.; Cukrowska, E.; Gulumian, M. Dissolution Kinetics of Silver Nanoparticles: Behaviour in Simulated Biological Fluids and Synthetic Environmental Media. Toxicol. Rep. 2022, 9, 788–796. [Google Scholar] [CrossRef]
- Tomić, S.L.; Vuković, J.S. Antimicrobial Activity of Silver, Copper, and Zinc Ions/Poly(Acrylate/Itaconic Acid) Hydrogel Matrices. Inorganics 2022, 10, 38. [Google Scholar] [CrossRef]
- Movva, H.; Karmakar, A.; Hariom, S.K.; S, R.; Hasan, M.U.; Das, R.K.; Nelson, E.J.R.; Srivastava, P. Cellular Interactions of Colloidal Nanosilver and Role of Alginate Capping in Prevention of Soluble Ag+ Leaching. Chem. Phys. Impact 2025, 11, 100915. [Google Scholar] [CrossRef]
- Foss, B.L.; Ghimire, N.; Tang, R.; Sun, Y.; Deng, Y. Bacteria and Osteoblast Adhesion to Chitosan Immobilized Titanium Surface: A Race for the Surface. Colloids Surf. B Biointerfaces 2015, 134, 370–376. [Google Scholar] [CrossRef]
- Covato, C.; Pilipenco, A.; Scheberl, A.; Reimhult, E.; Subbiahdoss, G. Osteoblasts Win the Race for the Surface on DNA Polyelectrolyte Multilayer Coatings against S. Epidermidis but Not against S. Aureus. Colloids Surf. B Biointerfaces 2025, 245, 114336. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.; Xu, D.; Cai, K. Surface Functionalization of Titanium Substrates with Chitosan-Lauric Acid Conjugate to Enhance Osteoblasts Functions and Inhibit Bacteria Adhesion. Colloids Surf. B Biointerfaces 2014, 119, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhao, S.; Zhang, H.; Liu, B.; She, P.; Yue, X. Development of High-Strength Porous Polyetheretherketone Foam/Nanosilver Antibacterial Composites for the Prevention of Postoperative Infections in Bone Repair. Compos. Commun. 2022, 31, 101127. [Google Scholar] [CrossRef]
- Zein, M.A.; Asghar, B.H.; Almohyawi, A.M.; Alqahtani, N.F.; Alharbi, A.; Alkabli, J.; Elshaarawy, R.F.M.; Ismail, L.A. Multifunctional Nanocomposites Integrated Green Synthesized Amphiphilic Chitosan/Thyme Extract/Nanosilver for Antimicrobial and Anti-Biofilm Applications. React. Funct. Polym. 2024, 194, 105791. [Google Scholar] [CrossRef]
- Guo, T.; Wang, D.; Gao, S.S. Incorporating Nanosilver with Glass Ionomer Cement—A Literature Review. J. Dent. 2024, 149, 105288. [Google Scholar] [CrossRef]
- El-Fakharany, E.M.; Abu-Serie, M.M.; Habashy, N.H.; Eltarahony, M. Augmenting Apoptosis-Mediated Anticancer Activity of Lactoperoxidase and Lactoferrin by Nanocombination with Copper and Iron Hybrid Nanometals. Sci. Rep. 2022, 12, 13153. [Google Scholar] [CrossRef]
- Geissel, F.J.; Platania, V.; Gogos, A.; Herrmann, I.K.; Belibasakis, G.N.; Chatzinikolaidou, M.; Sotiriou, G.A. Antibiofilm Activity of Nanosilver Coatings against Staphylococcus Aureus. J. Colloid. Interface Sci. 2022, 608, 3141–3150. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.; Cave, G.W.V.; Loughlin, M.; Puddu, V.; Volodkin, D. Vaterite-Nanosilver Hybrids with Antibacterial Properties and PH-Triggered Release. Mater. Today Chem. 2023, 30, 101586. [Google Scholar] [CrossRef]
- Natarajan, P.; Kumar, S.M.; Natarajan, S.; Sridharan, D.K.S.; Narayana Kalkura, D.S. Nano-Particle Coated or Impregnated Acrylic Resins in Dental Applications: A Systematic Review of in Vivo Evidence on Mechanical Properties, Biocompatibility and Clinical Performance. J. Oral Biol. Craniofac. Res. 2025, 15, 1190–1199. [Google Scholar] [CrossRef]
- Zhuang, H.-Z.; Chen, Y.-F.; Yu, Y.-C.; Huang, C.-R.; Jiang, Y.-S.; Chen, C.-S.; Jan, J.-S. Gelatin Composite Gel Particles Comprised of In-Situ Formed Zinc Oxide and Silver Nanoparticles with Enhanced Antibacterial Activities via Enzymatic Degradation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132509. [Google Scholar] [CrossRef]
- Rajendran, R.; Mani, A. Photocatalytic, Antibacterial and Anticancer Activity of Silver-Doped Zinc Oxide Nanoparticles. J. Saudi Chem. Soc. 2020, 24, 1010–1024. [Google Scholar] [CrossRef]
- Behzadnia, A.; Montazer, M.; Mahmoudi Rad, M.; Rastgoo, M. Fabrication of Multifunctional Wool Textile Using the Synthesis of Silver-Modified N-Doped ZnO/TiO2 Photocatalysts. Heliyon 2024, 10, e36522. [Google Scholar] [CrossRef]
- Shahalaei, M.; Azad, A.K.; Sulaiman, W.M.A.W.; Derakhshani, A.; Mofakham, E.B.; Mallandrich, M.; Kumarasamy, V.; Subramaniyan, V. A Review of Metallic Nanoparticles: Present Issues and Prospects Focused on the Preparation Methods, Characterization Techniques, and Their Theranostic Applications. Front. Chem. 2024, 12, 1398979. [Google Scholar] [CrossRef] [PubMed]
- Ulucan-Karnak, F.; Kuru, C.I.; Saǧin, F.G. Critical Evaluation of Publications and Patents in Nanobiotechnology-Based Research in the Last Decade. Turk. J. Biochem. 2023, 48, 606–619. [Google Scholar] [CrossRef]
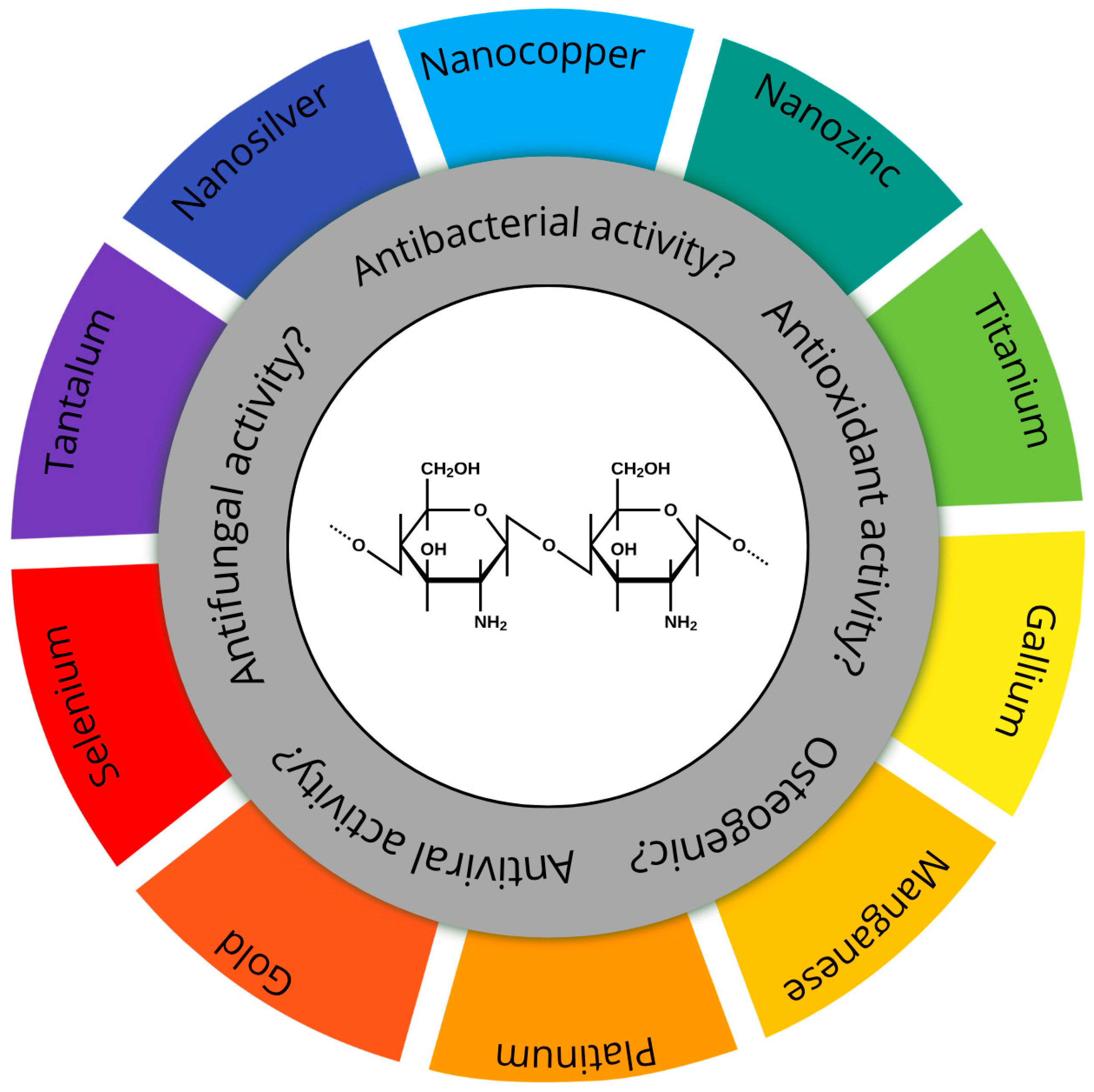
| Base Composition | Metal Nanoparticles | Physical Form | Fabrication | Reference |
|---|---|---|---|---|
| Chitosan–DHBA | Ag | Coating | EPD | [43] |
| Chitosan | Ag | Composite | Hydrothermal synthesis | [44] |
| Carboxyle chitosan | Ag | Composite | Synthesis | [45] |
| Chitosan | Ag | Composite | Sonochemical synthesis | [49] |
| Chitosan–PVA | Ag | Coating | Spread casting | [51] |
| Chitosan | Ag | Coating | Electrodeposition | [52] |
| Chitosan | Ag | Composite | Synthesis | [54] |
| Chitosan | Ag | Coating | Drying | [30] |
| Carboxyle chitosan | Ag | Composite | Freeze drying | [55] |
| Carboxyle chitosan–ginger oil | Ag | Composite | Synthesis | [58] |
| Chitosan | Ag | Composite | Synthesis | [59] |
| Chitosan–rGO | Ag | Coating | Solution casting | [103] |
| Chitosan–PVA–BG | Ag | Coating | EPD | [61] |
| Chitosan–TPP–nHAp | Ag | Hydrogel | Synthesis | [62] |
| Chitosan–fluor | Ag | Solution | Synthesis | [63] |
| Chitosan–PPy–lignin | Ag | Hydrogel | Synthesis | [64] |
| Chitosan–PVA | Ag | Coating | Drying | [65] |
| Chitosan–silk deferoxamine | Ag | Composite | Freeze drying | [66] |
| Chitosan–SiO2 | Ag | Coating | Electrospinning | [67] |
| Chitosan | Ag | Coating | EDP | [41,42] |
| Chitosan–PEG | Ag | Coating | EPD | [68] |
| Chitosan–PVA | Ag–ZnO | Coating | Drying | [50] |
| Chitosan | Ag–Se | Coating | Sol-gel | [53] |
| Chitosan–gelatine–BG | Ag, Mn | Coating | EPD | [56] |
| Chitosan | Cu | Coating | Layer-by-layer | [73] |
| Chitosan | Cu | Coating | EDP | [42,74] |
| Chitosan | CuO | Coating | Spraying | [77] |
| Si–chitosan–cyclodextrin–rosmarinic acid | Zn | Coating | Immersion | [79] |
| CaP–chitosan | Zn | Coating | MAO | [24] |
| Chitosan | Zn | Coating | EDP | [83,84] |
| Porphyrin–chit chitosan | Zn(Pd)–Ag | Hydrogel | Synthesis | [46] |
| Chitosan–cellulose–HIA | Zn (carbonate NPs) | Composite | Synthesis | [81] |
| Chitosan | Zn (La-Zn ferrite) | Composite | Synthesis | [82] |
| Chitosan | Zn (oxide) | Composite | Synthesis | [86] |
| Chitosan | Zn (oxide), Mo (sulfite) | Coating | Mixing | [87] |
| Chitosan | Zn (oxide) | Solution | Synthesis | [89] |
| Acryloyl chitosan–grafted piperazinium | Zn (oxide) | Composite | Synthesis | [90] |
| Chitosan–camphor | Zn (oxide) | Gel | Synthesis | [91] |
| Chitosan–fudoidan | Zn (oxide) | Scaffold | Synthesis | [104] |
| Chitosan–gelatin | Zn (oxide) | Composite | Freeze drying | [105] |
| Chitosan | Au | Coating | EDP | [83] |
| Baicalein–chitosan | Au, Zn (oxide) | Composite | Synthesis | [96] |
| Chitosan–poly(acrylic acid) | Ga | Coating | Electrodeposition | [99] |
| Chitosan | Ti (oxide) | Coating | Sol-gel | [100] |
| Chitosan–phospholipid | Ti (nitride) | Coating | Plasma immersion/PVD | [102] |
| Chitosan–salicylaldehyde | Ti (oxide) | Membrane | Casting | [101] |
| Chitosan–HAp | Cr (in HAp) | Coating | Dip coating | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartmański, M. An Assessment of Fabrication, Properties, and Medical Applications of Chitosan–Nanometal Coatings. Materials 2025, 18, 5322. https://doi.org/10.3390/ma18235322
Bartmański M. An Assessment of Fabrication, Properties, and Medical Applications of Chitosan–Nanometal Coatings. Materials. 2025; 18(23):5322. https://doi.org/10.3390/ma18235322
Chicago/Turabian StyleBartmański, Michał. 2025. "An Assessment of Fabrication, Properties, and Medical Applications of Chitosan–Nanometal Coatings" Materials 18, no. 23: 5322. https://doi.org/10.3390/ma18235322
APA StyleBartmański, M. (2025). An Assessment of Fabrication, Properties, and Medical Applications of Chitosan–Nanometal Coatings. Materials, 18(23), 5322. https://doi.org/10.3390/ma18235322






