Corticotomy Depth as a Modulator of Orthodontic Tooth Movement and PDL Stress—A Finite Element Study
Abstract
1. Introduction
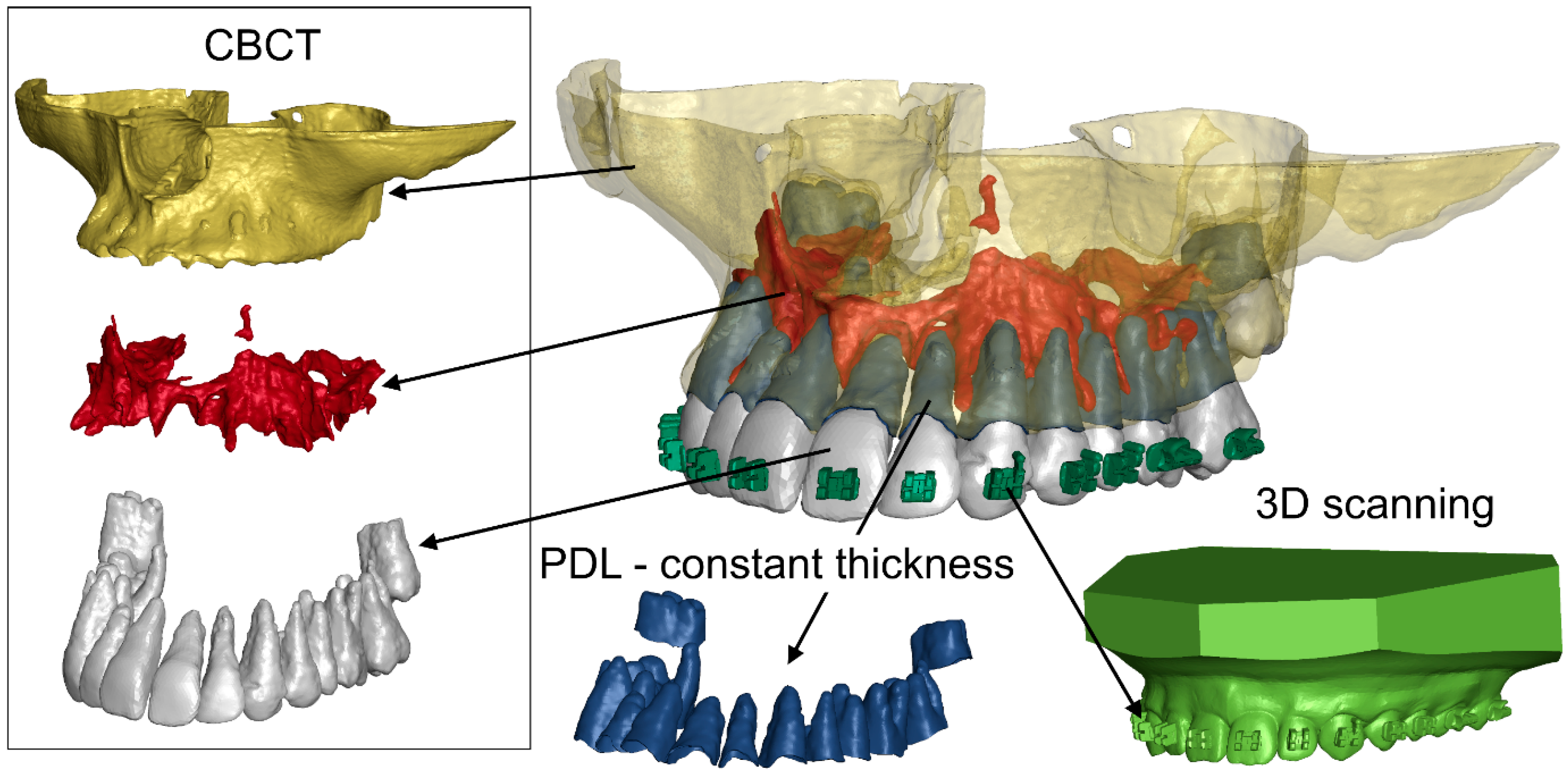
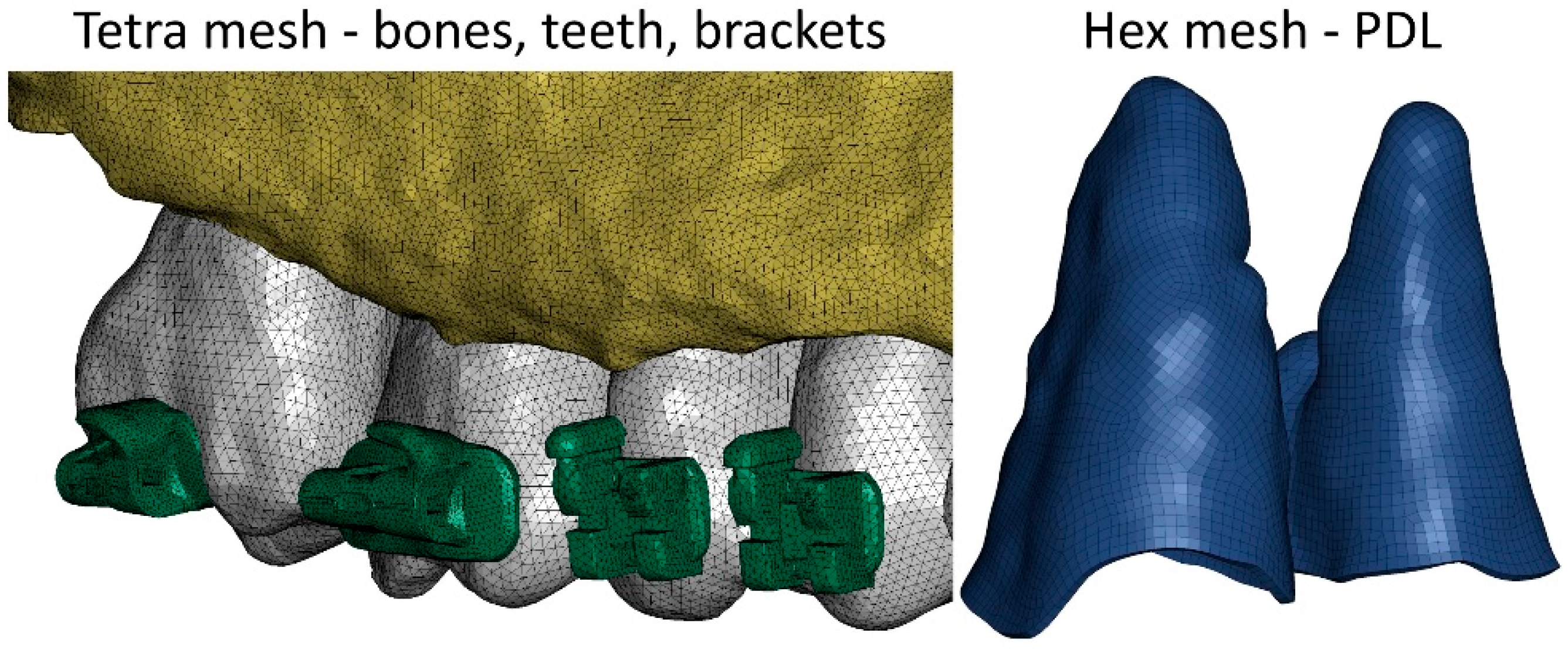
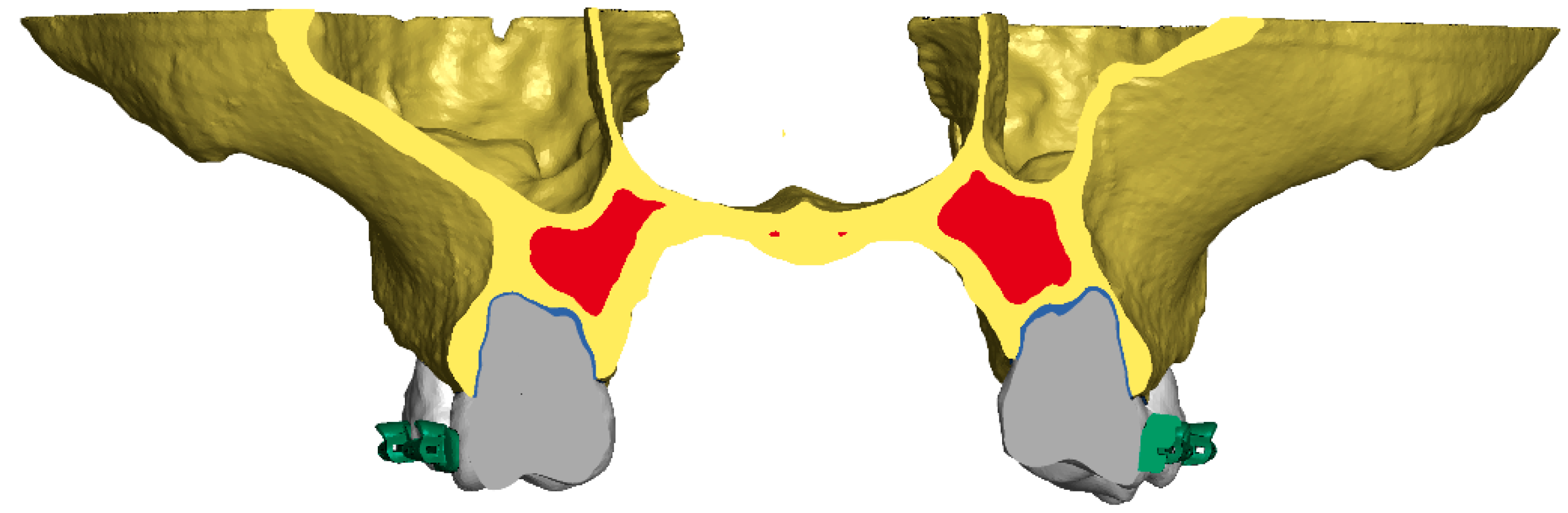
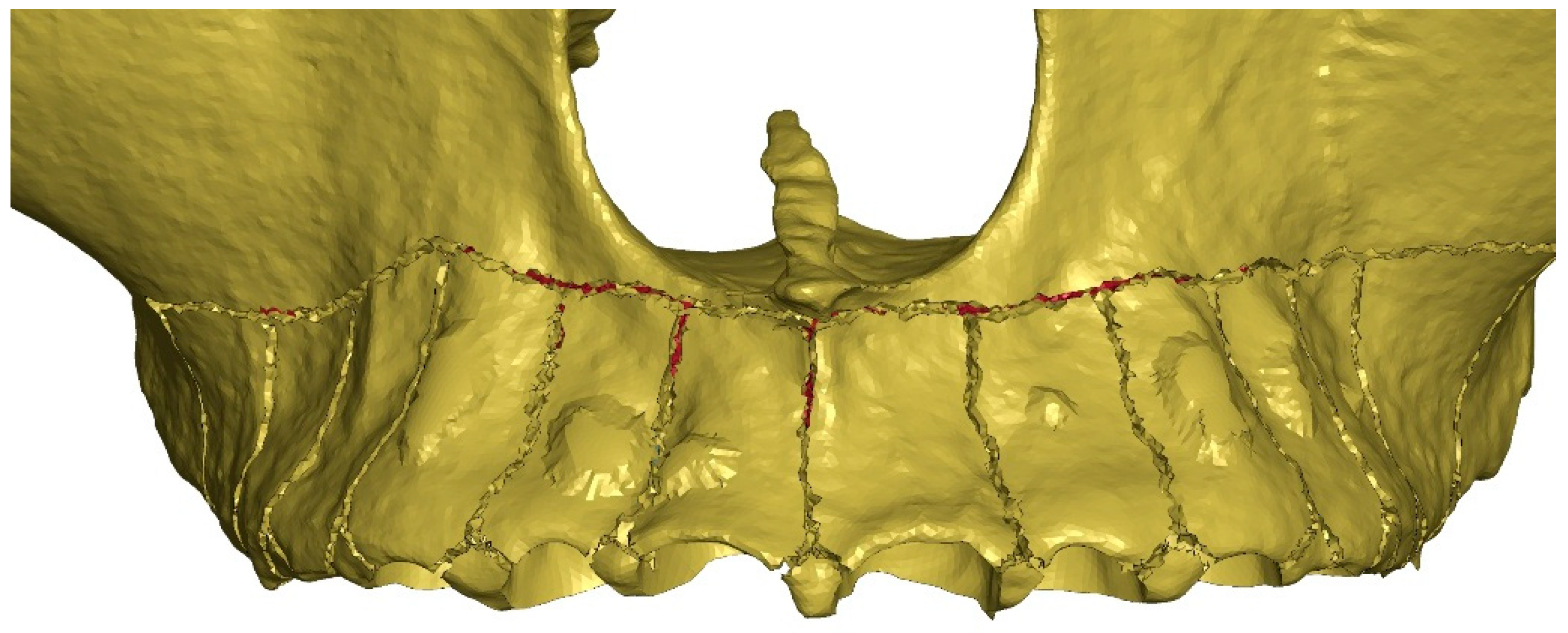
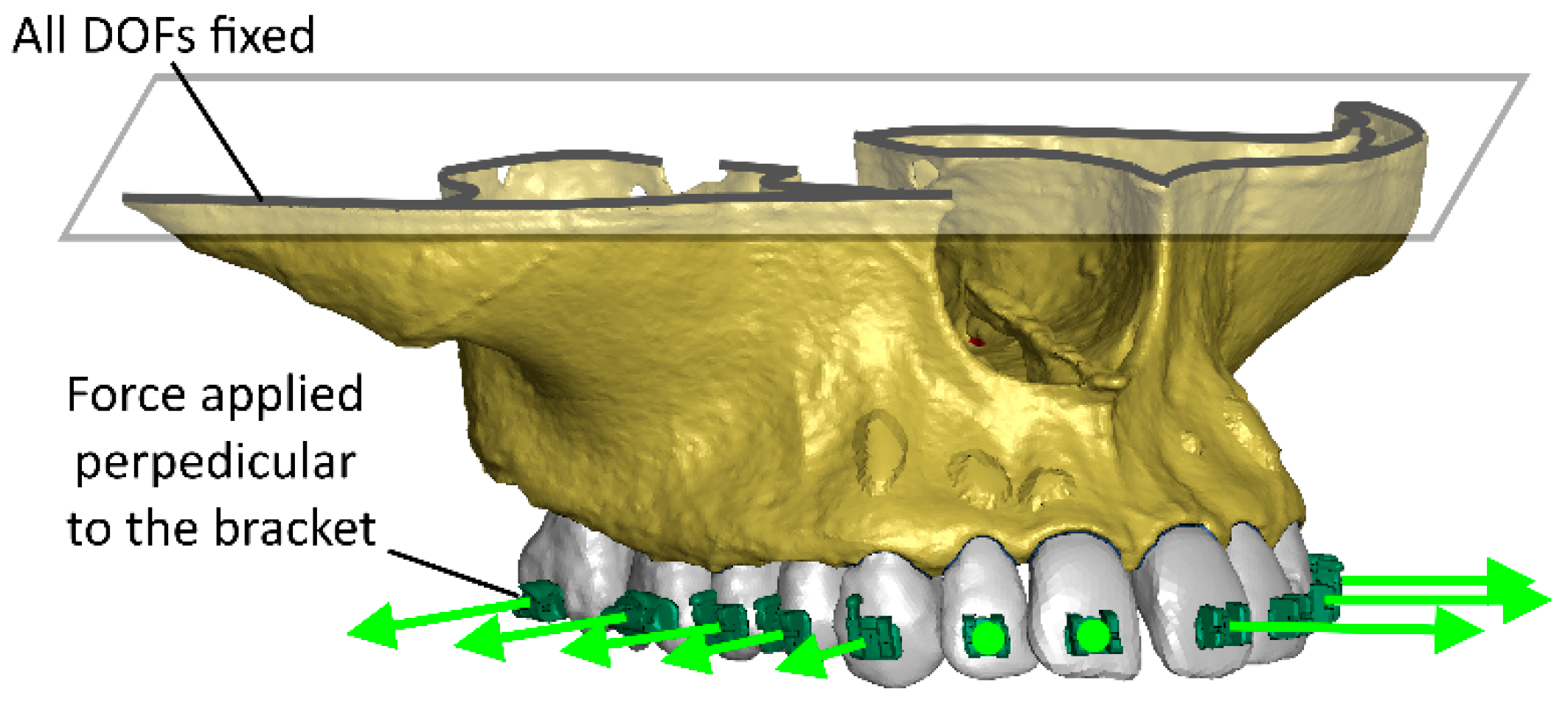
2. Materials and Methods
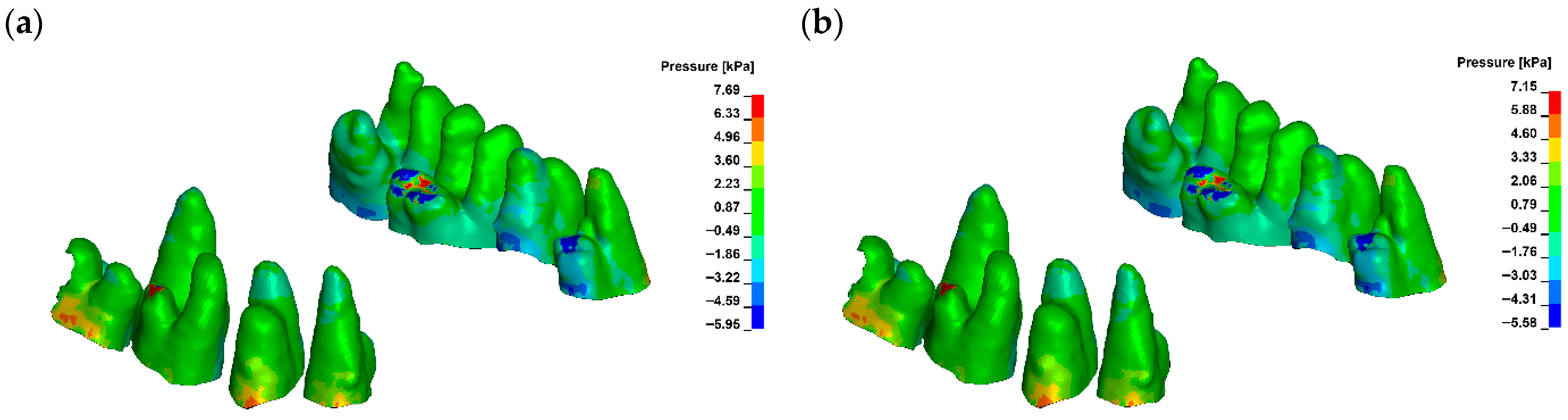
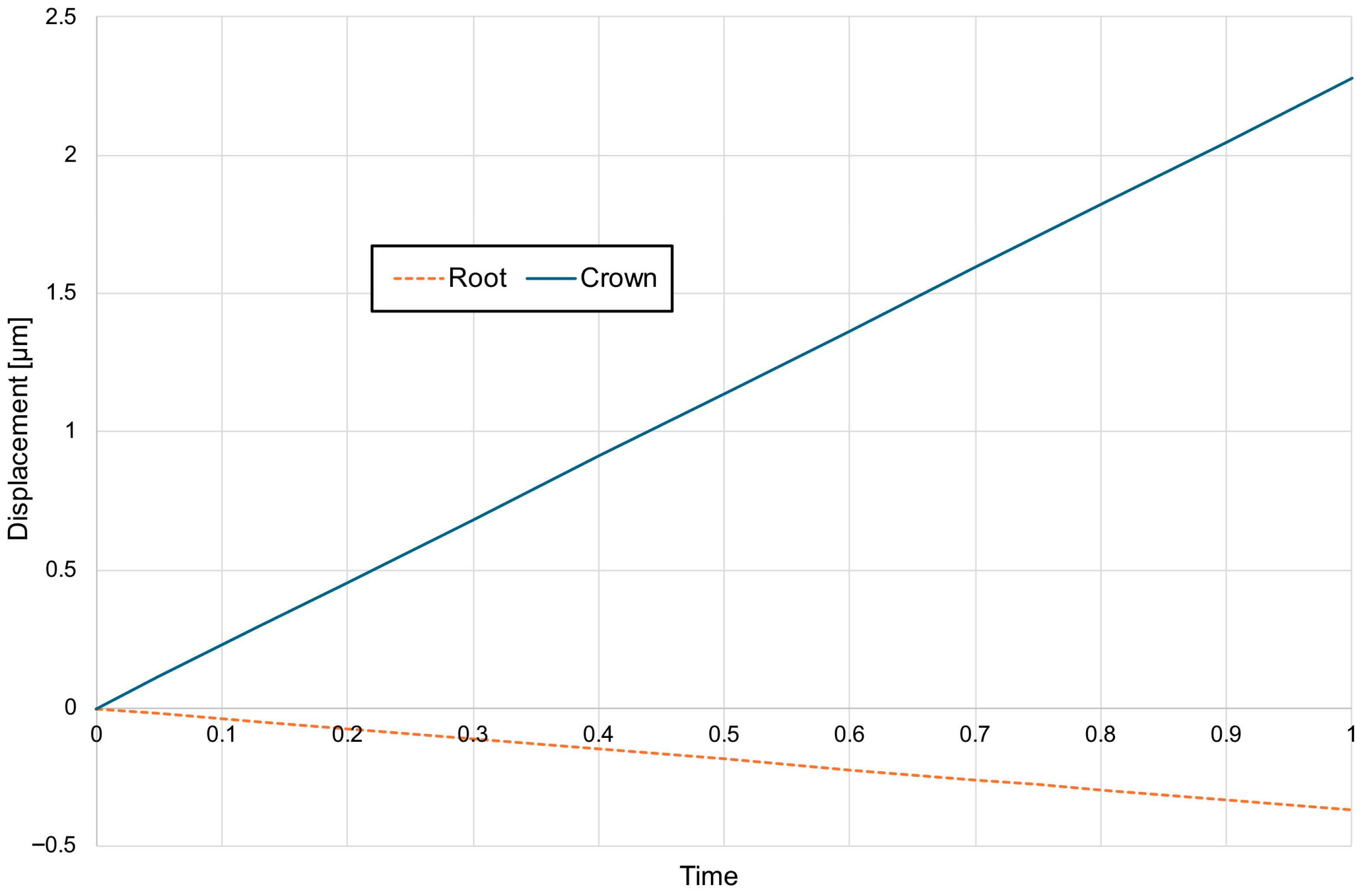
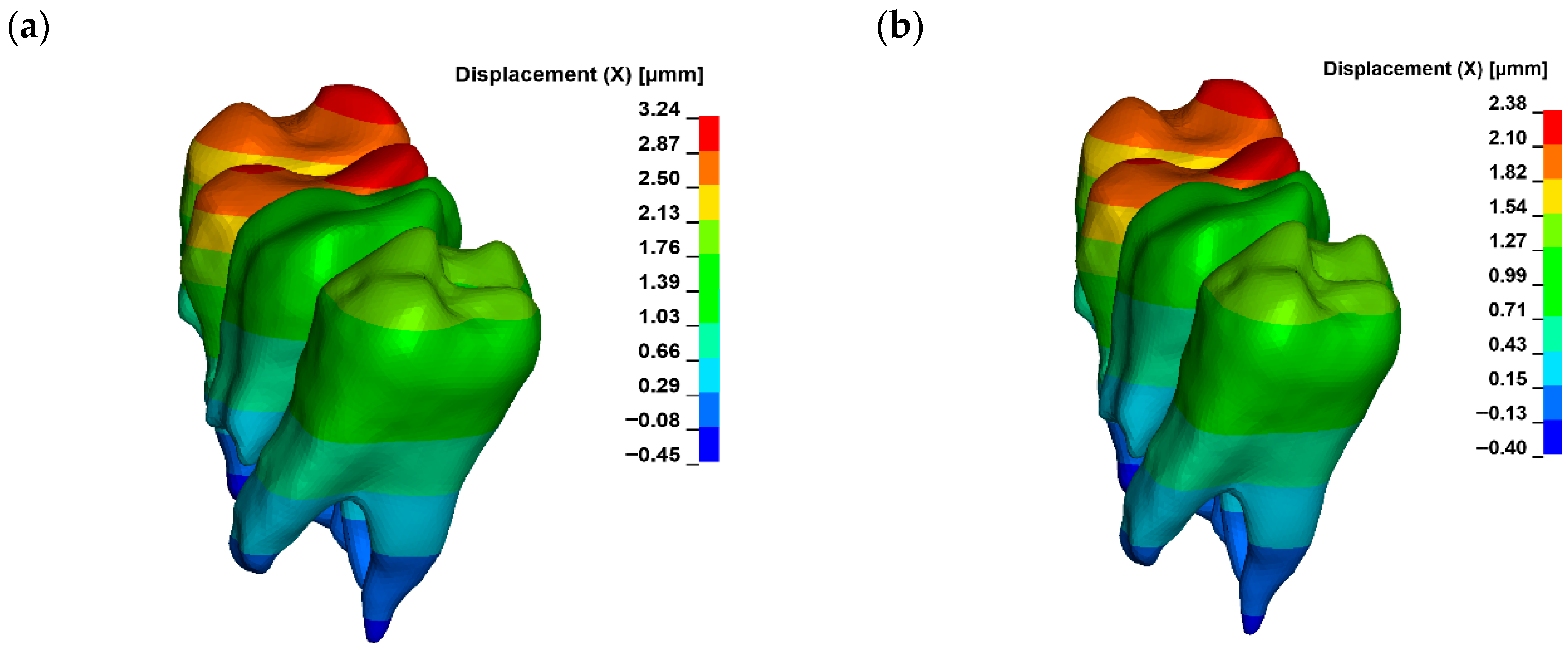
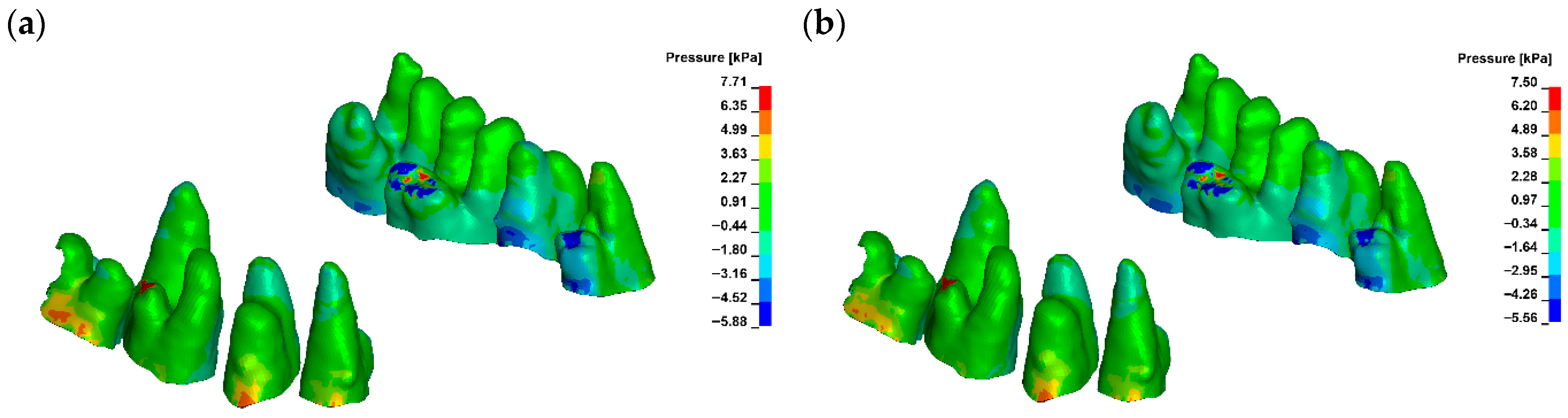
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iared, W.; da Silva, E.M.K.; Iared, W.; Macedo, C.R. Esthetic perception of changes in facial profile resulting from orthodontic treatment with extraction of premolars: A systematic review. J. Am. Dent. Assoc. 2017, 148, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.V.; Rodrigues, V.P.; Rodrigues, M.F.; de Melo, H.V.F.; Dos Santos, P.C.F. Soft tissue facial profile changes after orthodontic treatment with or without tooth extractions in Class I malocclusion patients: A comparative study. J. Oral Biol. Craniofac. Res. 2019, 9, 172–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allgayer, S.; Mezomo, M.B. Do premolar extractions necessarily result in a flat face? No, when properly indicated. Dent. Press J. Orthod. 2018, 23, 82–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elias, K.G.; Sivamurthy, G.; Bearn, D.R. Extraction vs nonextraction orthodontic treatment: A systematic review and meta-analysis. Angle Orthod. 2024, 94, 83–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moon, S.; Mohamed, A.M.A.; He, Y.; Dong, W.; Yaosen, C.; Yang, Y. Extraction vs. Nonextraction on Soft-Tissue Profile Change in Patients with Malocclusion: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2021, 2021, 7751516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drobocky Oles, B.; Smith Richard, J. Changes in facial profile during orthodontic treatment with extraction of four first premolars. Am. J. Orthod. Dentofac. Orthop. 1989, 95, 220–230. [Google Scholar] [CrossRef]
- Young, T.M.; Smith, R.J. Effects of orthodontics on the facial profile: A comparison of changes during nonextraction and four premolar extraction treatment. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.L.; Sharma, V.P.; Tandon, P.; Singh, G.P.; Sachan, K. Comparison of esthetic outcome after extraction or non-extraction orthodontic treatment in class II division 1 malocclusion patients. Contemp. Clin. Dent. 2013, 4, 206–212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cross, J.J. The Tweed philosophy: The Tweed years. Semin. Orthod. 1996, 2, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Altamash, S.; Sakrani, H.; Ahmed, N.; Marya, A.; Heboyan, A. Non-extraction orthodontic treatment for severe dental crowding using miniscrew-assisted rapid maxillary expansion. J. Surg. Case Rep. 2022, 2022, rjac509. [Google Scholar] [CrossRef] [PubMed]
- Raghis, T.R.; Alsulaiman, T.M.A.; Mahmoud, G.; Youssef, M. Efficiency of maxillary total arch distalization using temporary anchorage devices (TADs) for treatment of Class II-malocclusions: A systematic review and meta-analysis. Int. Orthod. 2022, 20, 100666. [Google Scholar] [CrossRef] [PubMed]
- Oğuz, F.; Özden, S.; Cicek, O. Distalization Methods for Maxillary Molars Utilizing Temporary Anchorage Devices (TADs): A Narrative Review. Appl. Sci. 2024, 14, 11333. [Google Scholar] [CrossRef]
- Wishney, M. Potential risks of orthodontic therapy: A critical review and conceptual framework. Aust. Dent. J. 2017, 62 (Suppl. S1), 86–96. [Google Scholar] [CrossRef] [PubMed]
- Johal, A.; Katsaros, C.; Kiliaridis, S.; Letao, P. State of the science on controversial topics: Orthodontic therapy and gingival recession (a report of the Angle Society of Europe 2013 meeting). Prog. Orthod. 2013, 14, 16. [Google Scholar] [CrossRef]
- Jati, A.S.; Furquim, L.Z.; Consolaro, A. Gingival recession: Its causes and types, and the importance of orthodontic treatment. Dent. Press J. Orthod. 2016, 21, 18–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sözen, Ö.; Erolu, E.G.; Ünal, B.K. Gingival Recession Due to Orthodontic Treatment: Prevention and Treatment Methods. J. Oral Med. Dent. Res. 2025, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yagci, A.; Veli, I.; Uysal, T.; Ucar, F.I.; Ozer, T.; Enhos, S. Dehiscence and fenestration in skeletal Class I, II, and III malocclusions assessed with cone-beam computed tomography. Angle Orthod. 2012, 82, 67–74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sawan, N.M.; Ghoneima, A.; Stewart, K.; Liu, S. Risk factors contributing to gingival recession among patients undergoing different orthodontic treatment modalities. Interv. Med. Appl. Sci. 2018, 10, 19–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalina, E.; Grzebyta, A.; Zadurska, M. Bone Remodeling during Orthodontic Movement of Lower Incisors-Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 15002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.-W.; Baik, H.-S.; Mo, S.-S.; Giap, H.-V.; Lee, K.-J. Age-related osteogenesis on lateral force application to rat incisor—Part II: Bony recession and cortical remodeling. APOS Trends Orthod. 2021, 11, 174–182. [Google Scholar] [CrossRef]
- Sendyk, M.; Linhares, D.S.; Pannuti, C.M.; Paiva, J.B.; Neto, J.R. Effect of orthodontic treatment on alveolar bone thickness in adults: A systematic review. Dent. Press J. Orthod. 2019, 24, 34–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorbunkova, A.; Pagni, G.; Brizhak, A.; Farronato, G.; Rasperini, G. Impact of Orthodontic Treatment on Periodontal Tissues: A Narrative Review of Multidisciplinary Literature. Int. J. Dent. 2016, 2016, 4723589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamaguchi, M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod. Craniofac. Res. 2009, 12, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Praneetpong, N.; Ono, W.; Ono, N. Mechanisms of Osteoclastogenesis in Orthodontic Tooth Movement and Orthodontically Induced Tooth Root Resorption. J. Bone Metab. 2023, 30, 297–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsolakis, I.A.; Christopoulou, I.; Sitaras, S.; Lyros, I.; Rontogianni, A.; Dalampira, M.; Tsolakis, A.I. Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review. Bioengineering 2023, 10, 1275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otero, L.; García, D.A.; Wilches-Buitrago, L. Expression and Presence of OPG and RANKL mRNA and Protein in Human Periodontal Ligament with Orthodontic Force. Gene Regul. Syst. Biol. 2016, 10, 15–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meeran, N.A. Biological response at the cellular level within the periodontal ligament on application of orthodontic force—An update. J. Orthod. Sci. 2012, 1, 2–10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, J.; Guo, L.; Yang, Y.; Liu, Y.; Zhang, C. Mechanical force regulates root resorption in rats through RANKL and OPG. BMC Oral. Health 2022, 22, 290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karaduman, B.; Uraz, A.; Altan, G.; Tuncer, B.B.; Alkan, Ö.; Gönen, S.; Pehlivan, S.; Çetiner, D. Changes of tumor necrosis factor-α, interleukin-10, and tartrate-resistant acid phosphatase5b in the crevicular fluid in relation to orthodontic movement. Eur. J. Inflamm. 2015, 13, 3–13. [Google Scholar] [CrossRef]
- Ogawa, S.; Kitaura, H.; Kishikawa, A.; Qi, J.; Shen, W.R.; Ohori, F.; Noguchi, T.; Marahleh, A.; Nara, Y.; Ochi, Y.; et al. TNF-α is responsible for the contribution of stromal cells to osteoclast and odontoclast formation during orthodontic tooth movement. PLoS ONE 2019, 14, e0223989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanzaki, H.; Chiba, M.; Shimizu, Y.; Mitani, H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J. Bone Miner. Res. 2002, 17, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kawatsu, M.; Takeshita, N.; Takimoto, A.; Yoshimoto, Y.; Seiryu, M.; Ito, A.; Kimura, S.; Kawamoto, T.; Hiraki, Y.; Shukunami, C.; et al. Scleraxis upregulated by transforming growth factor-β1 signaling inhibits tension-induced osteoblast differentiation of priodontal ligament cells via ephrin A2. Bone 2021, 149, 115969. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, B.; Jeon, H.H.; Ni, J.; Qiu, D.; Liu, A.; Hong, J.J.; Ali, M.; Wang, A.; Troka, M.; Graves, D.T. Osteoimmunology in Periodontitis and Orthodontic Tooth Movement. Curr. Osteoporos. Rep. 2023, 21, 128–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakao, K.; Goto, T.; Gunjigake, K.K.; Konoo, T.; Kobayashi, S.; Yamaguchi, K. Intermittent force induces high RANKL expression in human periodontal ligament cells. J. Dent. Res. 2007, 86, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Park, Y.G.; Kim, J.W.; Kyung, H.M. Assessment of alveolar bone remodeling after rapid maxillary expansion using cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 24–35. [Google Scholar] [CrossRef]
- Garrett, B.J.; Caruso, J.M.; Rungcharassaeng, K.; Farrage, J.R.; Kim, J.S.; Taylor, G.D. Skeletal effects to the maxilla after rapid maxillary expansion assessed with cone-beam computed tomography. Am. J. Orthod. Dentofac. Orthop. 2008, 134, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Wilcko, M.T.; Wilcko, W.M.; Pulver, J.J.; Bissada, N.F.; Bouquot, J.E. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J. Oral Maxillofac. Surg. 2009, 67, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Wilcko, M.T.; Wilcko, W.M.; Ferguson, D.J. Periodontal accelerated osteogenic orthodontics: A description of the surgical technique. J. Oral Maxillofac. Surg. 2009, 67, 2160–2166. [Google Scholar] [CrossRef]
- Ren, A.; Maltha, J.C.; Kuijpers-Jagtman, A.M. Effectiveness of surgical methods for accelerating orthodontic tooth movement: A systematic review. Orthod. Craniofacial Res. 2010, 13, 221–231. [Google Scholar] [CrossRef]
- Firat, E.; Yuksel, S.; Yagci, A.; Can, M. Effects of corticotomy and piezocision on the rate of orthodontic tooth movement: A systematic review and meta-analysis. Eur. J. Orthod. 2019, 41, 544–558. [Google Scholar] [CrossRef]
- Kuc, A.E.; Kulgawczyk, M.; Sulewska, M.E.; Kuc, N.; Kawala, B.; Lis, J.; Sarul, M.; Kotuła, J. The Effect of Corticotomy-Assisted Orthodontic Therapy (CAOT) or Periodontally Accelerated Osteogenic Orthodontics (PAOO) on Bone Remodeling and the Health of Periodontium: A Systematic Review of Systematic Reviews. J. Clin. Med. 2024, 13, 5726. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, P.M.; Dalstra, M.; Melsen, B. Orthodontic tooth movement and mechanical stress—A finite element analysis. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 280–287. [Google Scholar] [CrossRef]
- Rajgopal, N. Finite Element Analysis in Orthodontics, Dentistry. In Current Trends in Orthodontics; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Kuc, A.E.; Sybilski, K.; Kotuła, J.; Piątkowski, G.; Kowala, B.; Lis, J.; Saternus, S.; Sarul, M. The Hydrostatic Pressure Distribution in the Periodontal Ligament and the Risk of Root Resorption—A Finite Element Method (FEM) Study on the Nonlinear Innovative Model. Materials 2024, 17, 1661. [Google Scholar] [CrossRef] [PubMed]
- Kuc, A.E.; Sybilski, K.; Stankiewicz, M.; Kotuła, J.; Kuc, N.; Hajduk, G.; Małachowski, J.; Sarul, M. The Influence of Bone Density on Stresses in the Periodontal Ligament During Orthodontic Movement-Finite Element Study on Innovative Model. Materials 2025, 18, 776. [Google Scholar] [CrossRef]
- Pietron, K.; Mazurkiewicz, Ł.; Sybilski, K.; Małachowski, J. Correlation of bone material model using voxel mesh and parametric optimization. Materials 2022, 15, 5163. [Google Scholar] [CrossRef]
- Martinez, S. A variable finite element model of the human masticatory system for different loading conditions, AIF—Zebris cooperation project. In Proceedings of the 5th GACM Colloquium on Computational Mechanics, Hamburg, Germany, 30 September–2 October 2010. [Google Scholar]
- Liu, X.; Liu, M.; Tang, W. A visco-hyperelastic constitutive model of human periodontal ligament and the verification with finite element method. J. Phys. Conf. Ser. 2022, 2321, 012001. [Google Scholar] [CrossRef]
- Choy, M.; Ernesto, S. A Comprehensive Finite Element Model of the Human Masticatory System. Master’s Thesis, Karlsruher Institut für Technologie (KIT), Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Simon, M.; Lenz, J.; Karl, S.; Hans, S. A Variable Finite Element Model of the Overall Human Masticatory System for Evaluation of Stress Distributions During Biting and Bruxism. In Proceedings of the 10th European LS-DYNA Conference, Würzburg, Germany, 15–17 June 2015. [Google Scholar]
- Mani, A.; Raptis, M.; Zoldan, B.; Sangsuwon, C.; Lee, Y.B.; Alyami, B.; Corpodian, C.; Barrera, L.M.; Alansari, S.; Khoo, E.; et al. Effect of micro-osteoperforations on the rate of tooth movement. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 639–648. [Google Scholar] [CrossRef]
- Patatou, A.; Iacovou, N.; Zaxaria, P.; Vasoglou, M.; Vasoglou, G. Corticotomy-Assisted Orthodontic Treatment: A Literature Review. Oral 2023, 3, 389–401. [Google Scholar] [CrossRef]
- Uysal, C.; Baloş Tuncer, B.; Tuncer, C. Maxillary posterior intrusion with corticotomy-assisted approaches with zygomatic anchorage—A finite element stress analysis. Prog. Orthod. 2019, 20, 8. [Google Scholar] [CrossRef]
- Pacheco, A.A.; Saga, A.Y.; de Lima, K.F.; Paese, V.N.; Tanaka, O.M. Stress Distribution Evaluation of the Periodontal Ligament in the Maxillary Canine for Retraction by Different Alveolar Corticotomy Techniques: A Three-dimensional Finite Element Analysis. J. Contemp. Dent. Pract. 2016, 17, 32–37. [Google Scholar] [CrossRef]
- Pathomkulmai, T.; Chanmanee, P.; Samruajbenjakun, B. Effect of Extending Corticotomy Depth to Trabecular Bone on Accelerating Orthodontic Tooth Movement in Rats. Dent. J. 2022, 10, 158. [Google Scholar] [CrossRef]
- Lee, W. Corticotomy for orthodontic tooth movement. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 251–258. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Yang, C. Finite Element Analysis on Corticotomy-facilitated Orthodontic Tooth Movement in Rat. Yiyong Shengwu Lixue/J. Med. Biomech. 2018, 33, 48–54. [Google Scholar] [CrossRef]
- DE Stefani, A.; Bruno, G.; Irlandese, G.; Gracco, A. Is the corticotomy assisted orthodontic treatment efficient in the expansion of narrow arches in adult patients? Minerva Dent. Oral Sci. 2021, 70, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Dab, S.; Chen, K.; Flores-Mir, C. Short- and long-term potential effects of accelerated osteogenic orthodontic treatment: A systematic review and meta-analysis. Orthod. Craniofac. Res. 2019, 22, 61–68. [Google Scholar] [CrossRef]
- Gao, J.; Nguyen, T.; Oberoi, S.; Oh, H.; Kapila, S.; Kao, R.T.; Lin, G.H. The Significance of Utilizing a Corticotomy on Periodontal and Orthodontic Outcomes: A Systematic Review and Meta-Analysis. Biology 2021, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Apalimova, A.; Roselló, À.; Jané-Salas, E.; Arranz-Obispo, C.; Marí-Roig, A.; López-López, J. Corticotomy in orthodontic treatment: Systematic review. Heliyon 2020, 6, e04013. [Google Scholar] [CrossRef] [PubMed]
- Al-Ibrahim, H.M.; Hajeer, M.Y.; Burhan, A.S.; Sultan, K.; Ajaj, M.A.; Mahaini, L. The Efficacy of Accelerating Orthodontic Tooth Movement by Combining Self-Ligating Brackets With One or More Acceleration Methods: A Systematic Review. Cureus 2022, 14, e32879. [Google Scholar] [CrossRef]
- Kamal, A.T.; Malik, D.E.S.; Fida, M.; Sukhia, R.H. Does periodontally accelerated osteogenic orthodontics improve orthodontic treatment outcome? A systematic review and meta-analysis. Int. Orthod. 2019, 17, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Alsino, H.I.; Hajeer, M.Y.; Burhan, A.S.; Alkhouri, I.; Darwich, K. The Effectiveness of Periodontally Accelerated Osteogenic Orthodontics (PAOO) in Accelerating Tooth Movement and Supporting Alveolar Bone Thickness During Orthodontic Treatment: A Systematic Review. Cureus 2022, 14, e24985. [Google Scholar] [CrossRef]
- Wang, C.W.; Yu, S.H.; Mandelaris, G.A.; Wang, H.L. Is periodontal phenotype modification therapy beneficial for patients receiving orthodontic treatment? An American Academy of Periodontology best evidence review. J. Periodontol. 2020, 91, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Pietruska, M.J.; Waszkiewicz, E.; Skurska, A.; Sajewicz, E.; Dolińska, E.; Pietruska, M. The Cone Beam Computed Tomography Evaluation of Cortical Bone Plate after Piezocision-Assisted Orthodontic Upper Arch Expansion: A Case Series. Materials 2021, 14, 6967. [Google Scholar] [CrossRef]
- Sulewska, M.; Duraj, E.; Bugała-Musiatowicz, B.; Waszkiewicz-Sewastianik, E.; Milewski, R.; Pietruski, J.K.; Sajewicz, E.; Pietruska, M. Assessment of the effect of the corticotomy-assisted orthodontic treatment on the maxillary periodontal tissue in patients with malocclusions with transverse maxillary deficiency: A case series. BMC Oral Health 2018, 18, 162. [Google Scholar] [CrossRef]
- Sulewska, M.E.; Baczewska, A.; Bugała-Musiatowicz, B.; Waszkiewicz-Sewastianik, E.; Pietruski, J.K.; Pietruska, M. Long-Term Assessment of Periodontal Tissues after Corticotomy-Assisted Orthodontic Arch Expansion. J. Clin. Med. 2021, 10, 5588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, A.; Prasantha, G.S.; Mathew, S.; Sabrish, S. Analysis of stress in periodontium associated with orthodontic tooth movement: A three dimensional finite element analysis. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1841–1853. [Google Scholar] [CrossRef]
- Moga, R.A.; Buru, S.M.; Olteanu, C.D.; Botez, M.; Cosgarea, R. Assessment of the Maximum Amount of Orthodontic Force for Dental Pulp and Apical Neuro-Vascular Bundle in Intact and Reduced Periodontium on Bicuspids (Part II). Int. J. Environ. Res. Public Health 2023, 20, 1179. [Google Scholar] [CrossRef]
- Hohmann, A.; Wolfram, U.; Geiger, M.; Boryor, A.; Faltin, R.; Faltin, K.; Sander, F.G. Periodontal ligament hydrostatic pressure with areas of root resorption after application of a continuous torque moment. Angle Orthod. 2007, 77, 653–659. [Google Scholar] [CrossRef]
- Hohmann, A.; Wolfram, U.; Geiger, M.; Boryor, A.; Kober, C.; Sander, C.; Sander, F.G. Correspondences of hydrostatic pressure in periodontal ligament with regions of root resorption: A clinical and a finite element study of the same human teeth. Comput. Biol. Med. 2009, 93, 155–161. [Google Scholar] [CrossRef]
- Nazeri, A.; Castillo, J.A., Jr.; Ghaffari-Rafi, A. Impact of Molar Distalization with Clear Aligners on Periodontal Ligament Stress and Root Resorption Risk: A Systematic Review of 3D Finite Element Analysis Studies. Dent. J. 2025, 13, 65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montevecchi, M.; Parrilli, A.; Fini, M.; Gatto, M.R.; Muttini, A.; Checchi, L. The influence of root surface distance to alveolar bone and periodontal ligament on periodontal wound healing. J. Periodontal Implant. Sci. 2016, 46, 303–319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, C.; Wang, C.; Deng, F.; Fan, Y. Biomechanical effects of corticotomy approaches on dentoalveolar structures during canine retraction: A 3-dimensional finite element analysis. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Alqerban, A.; Willems, G.; Bernaerts, C.; Vangastel, J.; Politis, C.; Jacobs, R. Orthodontic treatment planning for impacted maxillary canines using conventional records versus 3D CBCT. Eur. J. Orthod. 2014, 36, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Wilcko, M.T.; Wilcko, W.M.; Bouquot, J.E. Periodontally accelerated osteogenic orthodontics: A novel surgical approach for accelerated tooth movement. Int. J. Periodontics Restor. Dent. 2001, 21, 423–433. [Google Scholar]
- Kim, Y.; Park, J.; Kim, S. Combining virtual model and cone beam computed tomography to assess periodontal changes after anterior tooth movement. BMC Oral Health 2011, 18, 180. [Google Scholar] [CrossRef]
- Melsen, B. Biological reactions of alveolar bone to orthodontic loading of oral implants. Clin. Oral Implant. Res. 2001, 12, 144–152. [Google Scholar] [CrossRef]
- Lyu, M.; Xu, D.; Zhang, X.; Yuan, Q. Maxillary sinus floor augmentation: A review of current evidence on anatomical factors and a decision tree. Int. J. Oral Sci. 2023, 15, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tadinada, A.; Fung, K.; Thacker, S.; Mahdian, M.; Jadhav, A.; Schincaglia, G.P. Radiographic evaluation of the maxillary sinus prior to dental implant therapy: A comparison between two-dimensional and three-dimensional radiographic imaging. Imaging Sci. Dent. 2015, 45, 169–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rios, H.F.; Tyndall, D.A.; Ludlow, J.B. The use of cone-beam computed tomography in management of patients requiring dental implants: An American Academy of Periodontology best evidence review. J. Periodontol. 2017, 88, 803–815. [Google Scholar] [CrossRef]
- Rahpeyma, A.; Khajehahmadi, S. Open sinus lift surgery and the importance of preoperative cone-beam computed tomography scan: A review. Int. J. Oral Health 2015, 7, 127–133. [Google Scholar] [PubMed] [PubMed Central]
- European Parliament and Council. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 17 November 2025).


| Component | Young’s Modulus [MPa] | Poisson’s Ratio [ ] |
|---|---|---|
| Steel | 210,000 | 0.30 |
| Tooth [48] | 18,600 | 0.31 |
| Cancellous [49] | 2000 | 0.30 |
| µ1 [MPa] | α1 [MPa] | Poisson’s Ratio [ ] |
|---|---|---|
| 2.5 × 10−3 | 150 | 0.46 |
| Displacement [µm] | ||||||
|---|---|---|---|---|---|---|
| Young Modulus [MPa] | ||||||
| 12,500 | 15,500 | 18,500 | 21,500 | 24,500 | 27,500 | |
| G0 | 2.57 | 2.37 | 2.23 | 2.13 | 2.04 | 1.98 |
| G1 | 2.63 | 2.42 | 2.27 | 2.16 | 2.08 | 2.01 |
| G2 | 2.89 | 2.64 | 2.47 | 2.34 | 2.24 | 2.15 |
| G3 | 3.24 | 2.95 | 2.75 | 2.59 | 2.48 | 2.38 |
| Hydrostatic Pressure Min [kPa] | ||||||
| Young Modulus [MPa] | ||||||
| 12,500 | 15,500 | 18,500 | 21,500 | 24,500 | 27,500 | |
| G0 | −5.95 | −5.84 | −5.76 | −5.69 | −5.63 | −5.58 |
| G1 | −5.95 | −5.85 | −5.76 | −5.69 | −5.63 | −5.58 |
| G2 | −5.95 | −5.84 | −5.76 | −5.69 | −5.63 | −5.58 |
| G3 | −5.88 | −5.79 | −5.72 | −5.66 | −5.61 | −5.56 |
| Hydrostatic Pressure Max [kPa] | ||||||
| Young Modulus [MPa] | ||||||
| 12,500 | 15,500 | 18,500 | 21,500 | 24,500 | 27,500 | |
| G0 | 7.69 | 7.53 | 7.4 | 7.3 | 7.22 | 7.15 |
| G1 | 7.69 | 7.53 | 7.41 | 7.3 | 7.22 | 7.15 |
| G2 | 7.94 | 7.77 | 7.64 | 7.54 | 7.45 | 7.38 |
| G3 | 7.71 | 7.66 | 7.62 | 7.58 | 7.54 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuc, A.E.; Sybilski, K.; Kotuła, J.; Hajduk, G.; Sulewska, M.; Saternus, S.; Kulikowska-Kulesza, J.E.; Kotarska, M.; Kawala, B.; Małachowski, J.; et al. Corticotomy Depth as a Modulator of Orthodontic Tooth Movement and PDL Stress—A Finite Element Study. Materials 2025, 18, 5290. https://doi.org/10.3390/ma18235290
Kuc AE, Sybilski K, Kotuła J, Hajduk G, Sulewska M, Saternus S, Kulikowska-Kulesza JE, Kotarska M, Kawala B, Małachowski J, et al. Corticotomy Depth as a Modulator of Orthodontic Tooth Movement and PDL Stress—A Finite Element Study. Materials. 2025; 18(23):5290. https://doi.org/10.3390/ma18235290
Chicago/Turabian StyleKuc, Anna Ewa, Kamil Sybilski, Jacek Kotuła, Grzegorz Hajduk, Magdalena Sulewska, Szymon Saternus, Justyna Ewa Kulikowska-Kulesza, Małgorzata Kotarska, Beata Kawala, Jerzy Małachowski, and et al. 2025. "Corticotomy Depth as a Modulator of Orthodontic Tooth Movement and PDL Stress—A Finite Element Study" Materials 18, no. 23: 5290. https://doi.org/10.3390/ma18235290
APA StyleKuc, A. E., Sybilski, K., Kotuła, J., Hajduk, G., Sulewska, M., Saternus, S., Kulikowska-Kulesza, J. E., Kotarska, M., Kawala, B., Małachowski, J., & Sarul, M. (2025). Corticotomy Depth as a Modulator of Orthodontic Tooth Movement and PDL Stress—A Finite Element Study. Materials, 18(23), 5290. https://doi.org/10.3390/ma18235290









