1. Introduction
Gray cast iron holds an irreplaceable position in critical components such as automotive engine blocks and machine tool guideways due to its excellent casting properties, cost-effectiveness, and damping characteristics [
1]. Hypoeutectic gray cast iron, a key subclass of gray cast iron, features a carbon content below the carbon equivalent corresponding to the eutectic point [
2]. During solidification, primary austenite precipitates first, growing in dendritic morphology; as temperature decreases further, the remaining liquid undergoes eutectic transformation to form a eutectic structure consisting of graphite and austenite [
3]. Following primary crystallization, austenite undergoes eutectoid transformation, yielding a matrix of pearlite or ferrite, within which lamellar graphite is distributed. While graphite imposes some fragmentation on the matrix, it endows the material with unique properties. Hypoeutectic gray cast iron inherits the advantages of gray cast iron, including excellent vibration damping, superior machinability, and low cost, while exhibiting higher strength than hypereutectic gray cast iron due to the presence of primary austenite dendrites, making it suitable for moderate load conditions [
4]. Its performance is highly dependent on the morphology of primary austenite dendrites, graphite morphology, and eutectic cell structure formed during solidification. However, conventional hypoeutectic gray cast iron often suffers from coarse primary austenite dendrites and non-uniform distribution of graphite flakes during solidification, leading to insufficient material strength and significant fluctuations in mechanical properties [
5,
6,
7].
Modifying the microstructure of hypoeutectic gray cast iron through carbon equivalent (CE) adjustment or inoculant addition (e.g., ferrosilicon, FeSi) represents an effective strategy, which fundamentally addresses issues such as undesirable graphite morphology, inhomogeneous matrix structure, and excessive harmful phases through systematic regulation of both macroscale compositional optimization and microscale nucleation intervention [
8,
9,
10]. Adjusting CE—a critical parameter integrating the combined effects of carbon, silicon, and other elements on graphite formation in cast iron—alters the graphitization driving force of molten iron [
11]. When CE approaches the eutectic composition, the undercooling required for graphite precipitation decreases, curbing the excessive growth of primary austenite dendrites and facilitating the formation of uniform graphite-austenite eutectic structures during the eutectic transformation [
12]. This process concurrently suppresses cementite formation and prevents chill structures. Additionally, CE indirectly influences subsequent eutectoid transformations by modulating the synergistic effects of carbon and silicon to balance the pearlite-to-ferrite ratio in the matrix, thereby optimizing the trade-off between strength and toughness while mitigating structural defects caused by compositional segregation [
13]. Inoculation, exemplified by FeSi addition, primarily acts through microscale nucleation control. FeSi introduces heterogeneous nucleation sites with high lattice matching to graphite, activating dormant nucleation substrates in the molten iron and significantly increasing graphite nucleation density [
14]. This leads to refined graphite size and eutectic cell structure, avoiding coarse or abnormal graphite morphologies. However, the development of efficient and controllable external nucleation technologies to further optimize solidification processes remains a focal point in both academic research and industrial practice.
In recent years, silicon carbide (SiC) has been regarded as a potential inoculant for cast iron due to its unique physicochemical properties, such as a high melting point and low lattice mismatch with graphite lattice. Experimental evidence indicates that SiC undergoes decomposition in molten iron (SiC → Si + C), which not only replenishes carbon sources but also promotes heterogeneous nucleation through the surfaces of undissolved particles [
15,
16]. Krzysztof Janerka et al. [
17] found that in eutectic ductile cast iron systems, increasing SiC addition decreases the liquidus temperature while maintaining a stable solidus temperature, narrowing the crystallization temperature range and thereby improving the quantity and quality of graphite precipitation. Additionally, the graphite nodules exhibit an “irregular morphology with protruding lamellae” and increased internal microcracks after SiC addition. B. Domeij et al. [
18] further demonstrated that SiC promotes early uniform dendrite growth by providing heterogeneous nucleation sites, reducing the undercooling required for austenite nucleation. However, existing studies still exhibit multiple gaps that hinder the efficient regulation of hypoeutectic gray cast iron. First, for the ultrafine and highly active SiC particles (SiCp) subjected to high-energy activation treatment, their dispersion behavior, absorption rate, and particle-size-dependent dissolution kinetics in the hypoeutectic gray cast iron melt remain unclear. Second, the quantitative influence laws of such activated particles on the thermodynamic and kinetic parameters of the solidification process—such as undercooling and crystallization latent heat—as well as the structure–property correlations governing the resultant microstructural refinement, have not yet been systematically elucidated. Finally, the microscopic mechanism by which high-energy activated SiC particles (EASiCp) regulate the primary austenite dendrites and eutectic clusters through the synergistic effects of “direct nucleation” and “reaction-induced carbon-rich zone formation” remains to be deeply clarified. In particular, the quantitative correlation model between this microstructural evolution and the resulting mechanical properties (e.g., tensile strength and hardness) has not yet been established, which hinders the transformation of this technology from a laboratory phenomenon into industrial application.
To address the aforementioned research gaps, this study employs high-energy mechanical activation technology to prepare SiCp, and systematically investigates their role in the solidification process of hypoeutectic gray cast iron through a combination of experimental research and theoretical analysis. The focus of this work includes: (1) Accurate characterization of the dispersion behavior and reaction mechanisms of EASiCp within the molten alloy; (2) Quantitative analysis of their influence on key solidification kinetic parameters, such as undercooling and latent heat of crystallization; (3) In-depth elucidation of the microscopic mechanism by which EASiCp achieve simultaneous refinement of primary austenite dendrites, graphite, and eutectic clusters through a “nucleation promotion–growth inhibition” synergistic mechanism. Ultimately, this study aims to establish a comprehensive quantitative correlation model linking process parameters → solidification kinetics → microstructure → tensile strength, thereby providing both the theoretical foundation and technical support for the development of high-efficiency and controllable nucleation technologies for high-performance cast iron.
4. Conclusions
This study systematically investigates the regulatory role and mechanisms of high-energy mechanically activated silicon carbide particles (EASiCp) on the solidification process, microstructure, and mechanical properties of hypoeutectic gray cast iron. The main conclusions are as follows:
- (1)
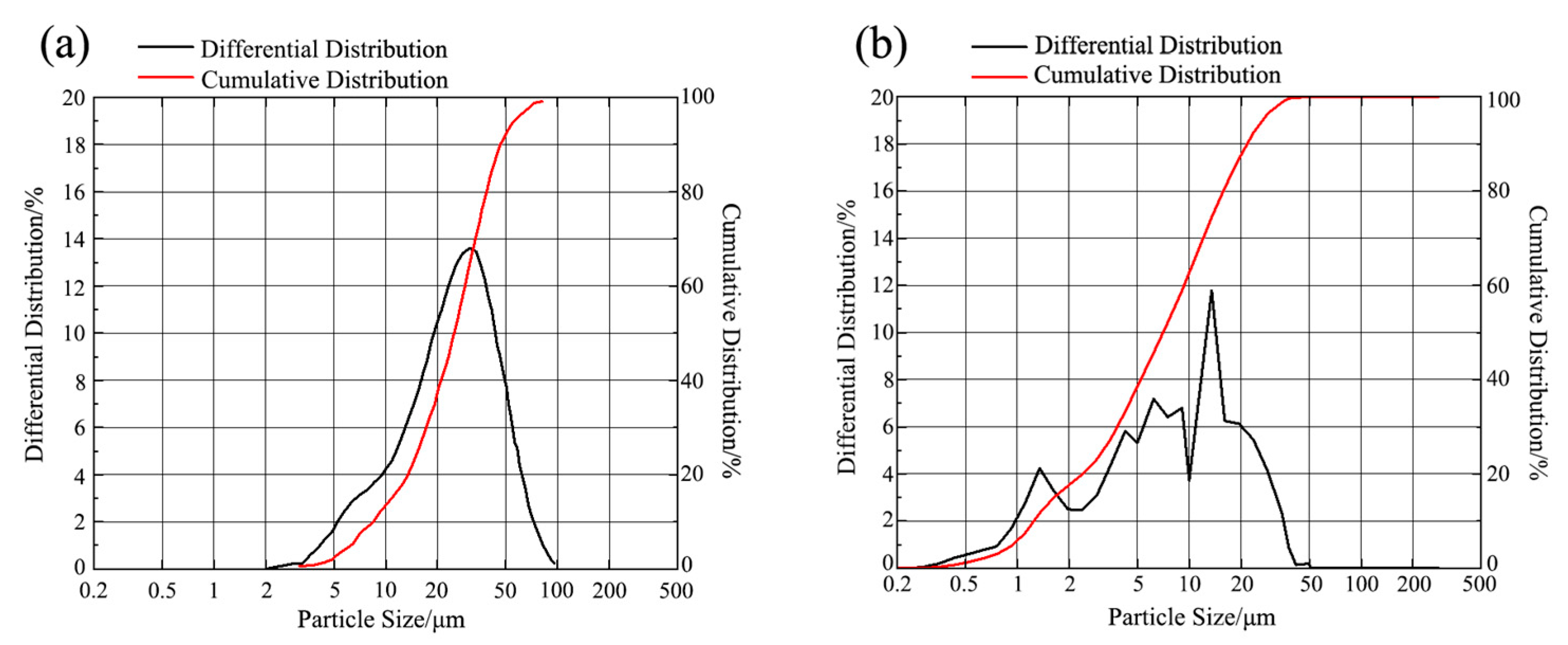
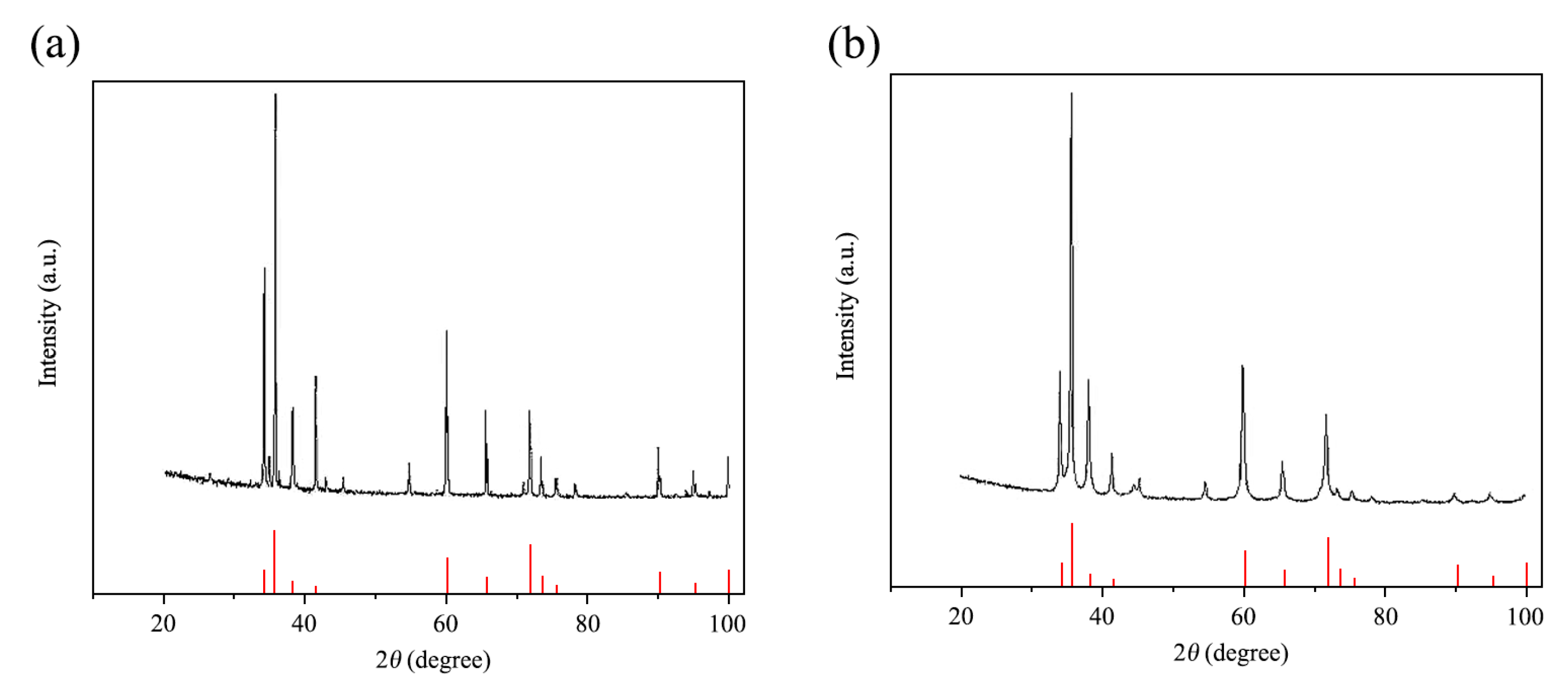
Particle characteristics successfully optimized: The high-energy activation treatment significantly refined the SiCp, reducing the average particle size from 26.53 μm to 9.51 μm, and increasing the specific surface area from 0.35 m2/g to 1.78 m2/g. XRD analysis confirmed that after activation, the particle grain size was refined to about 17.4 nm, with significant lattice distortion, placing them in a high-activity state.
- (2)
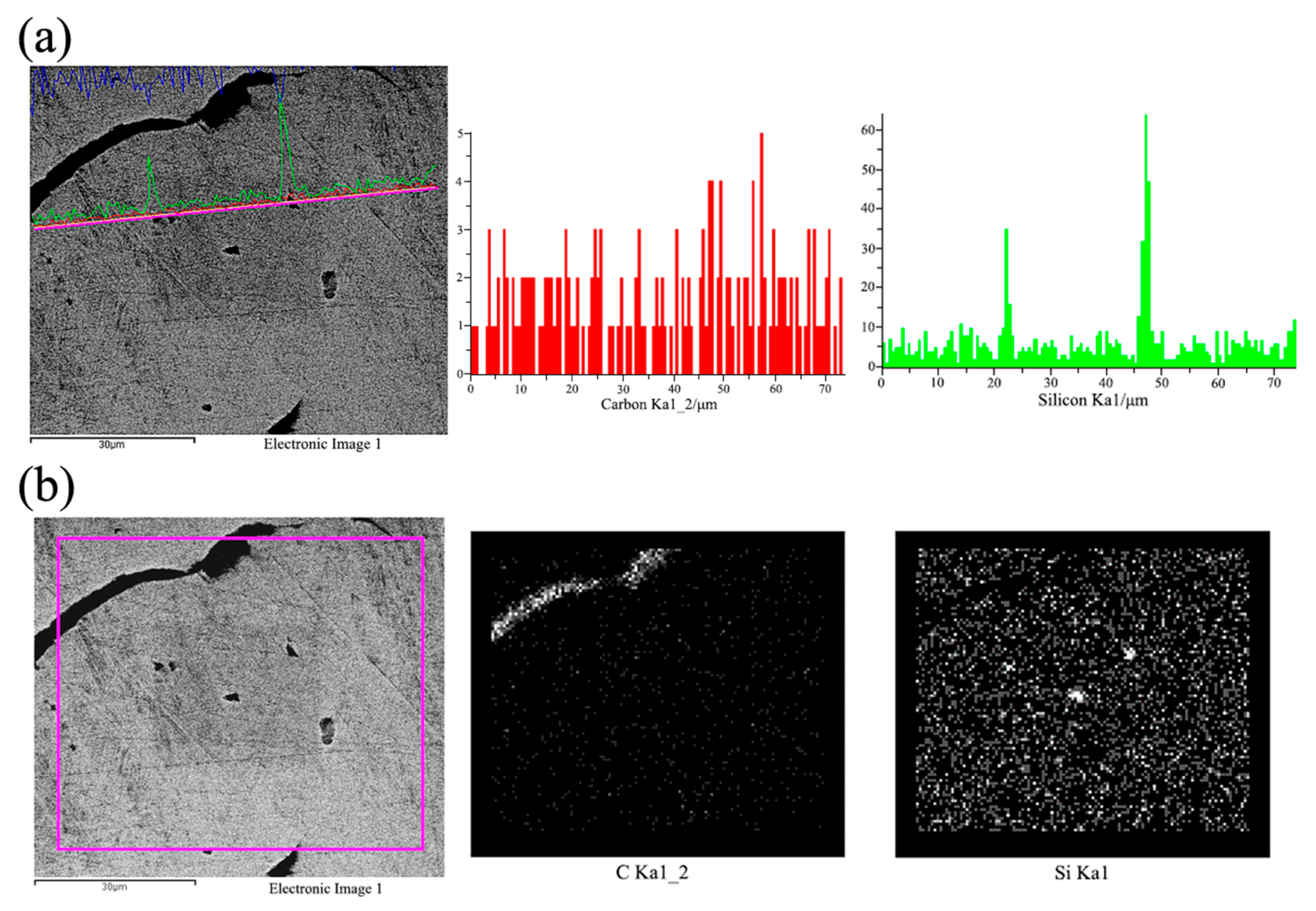
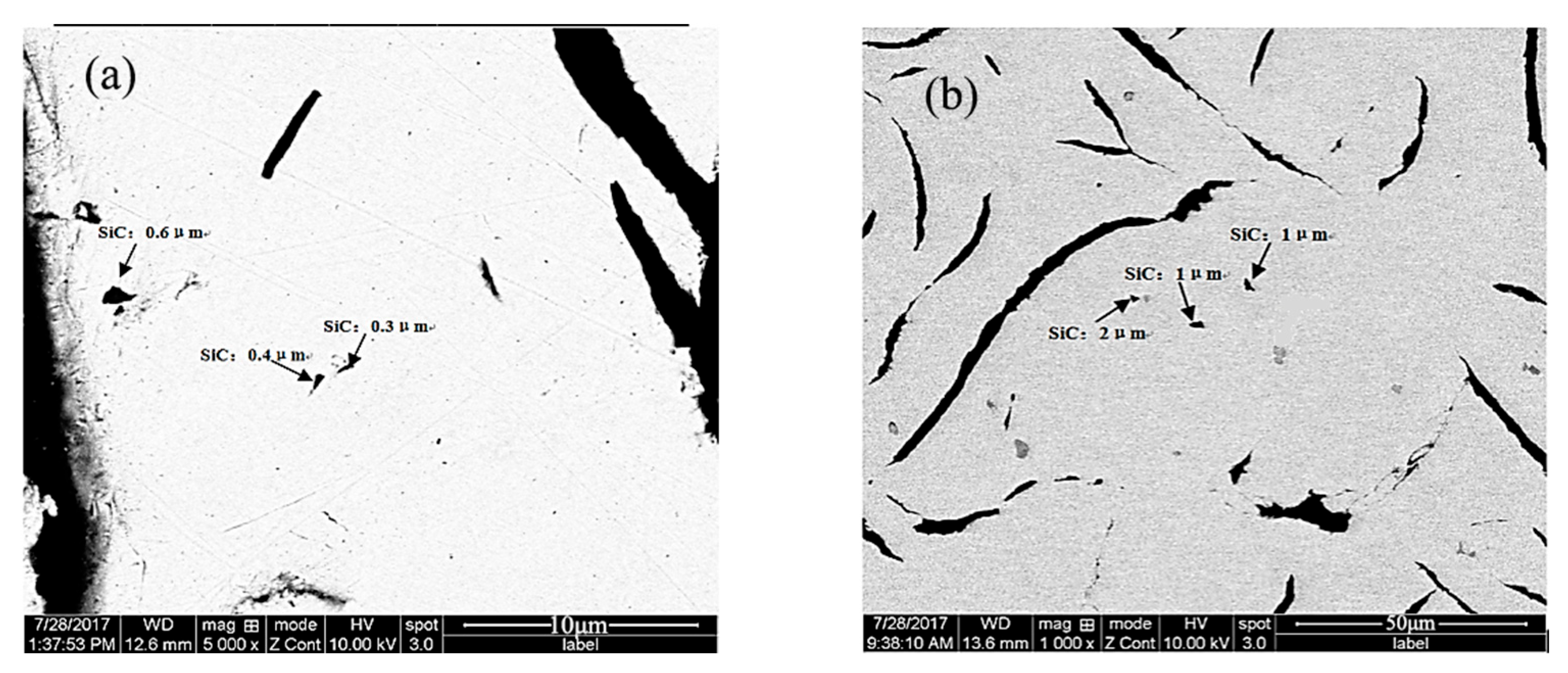
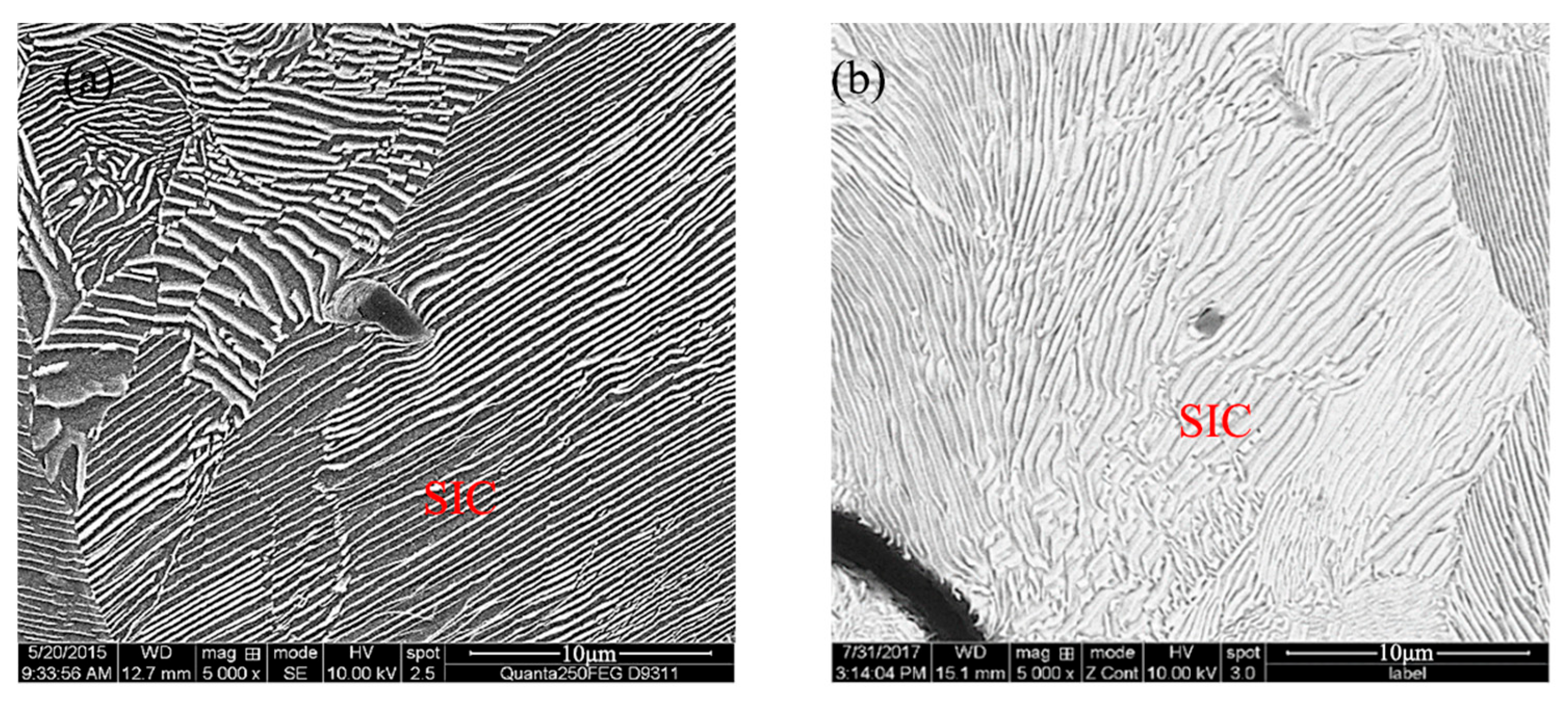
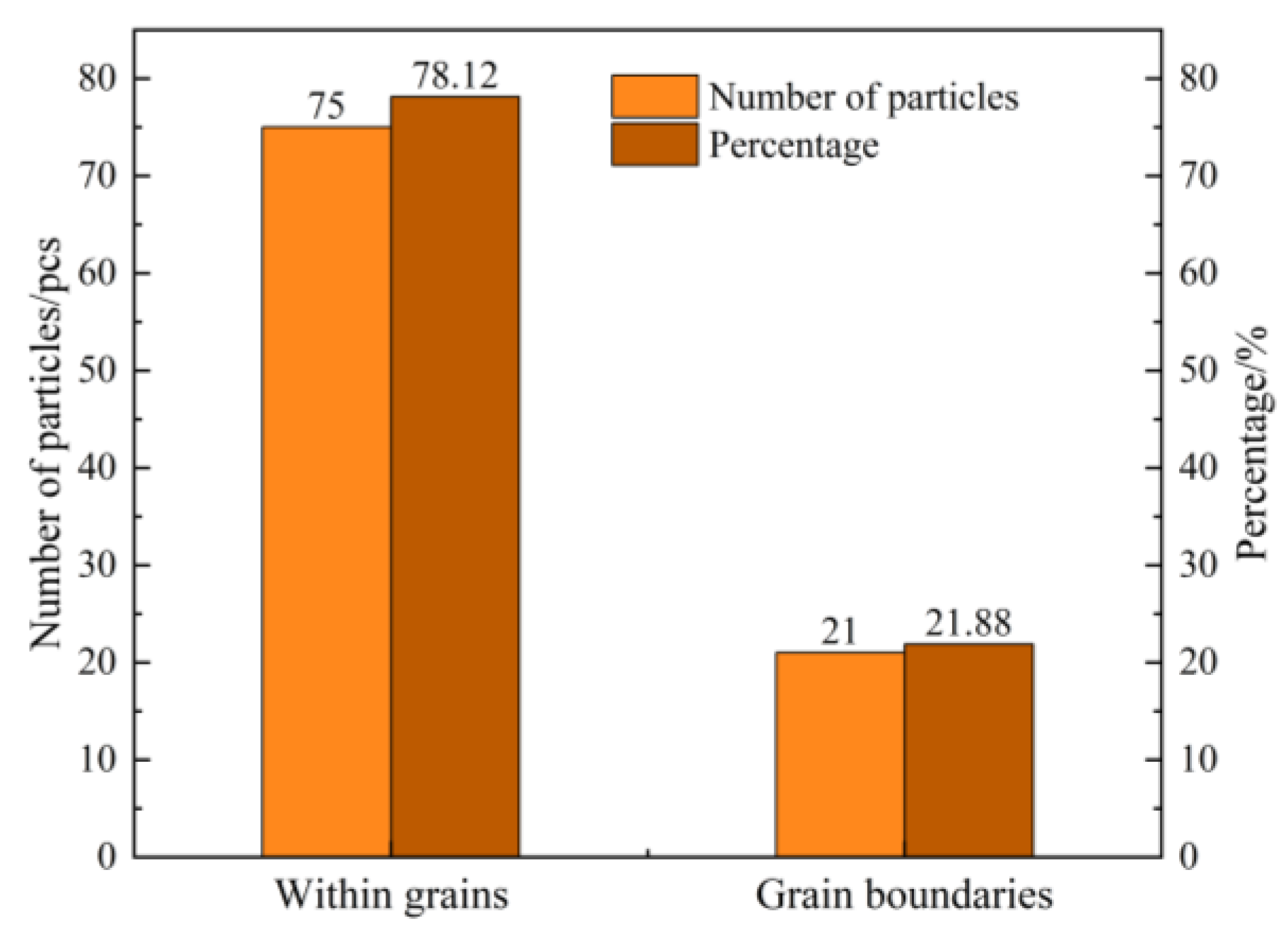
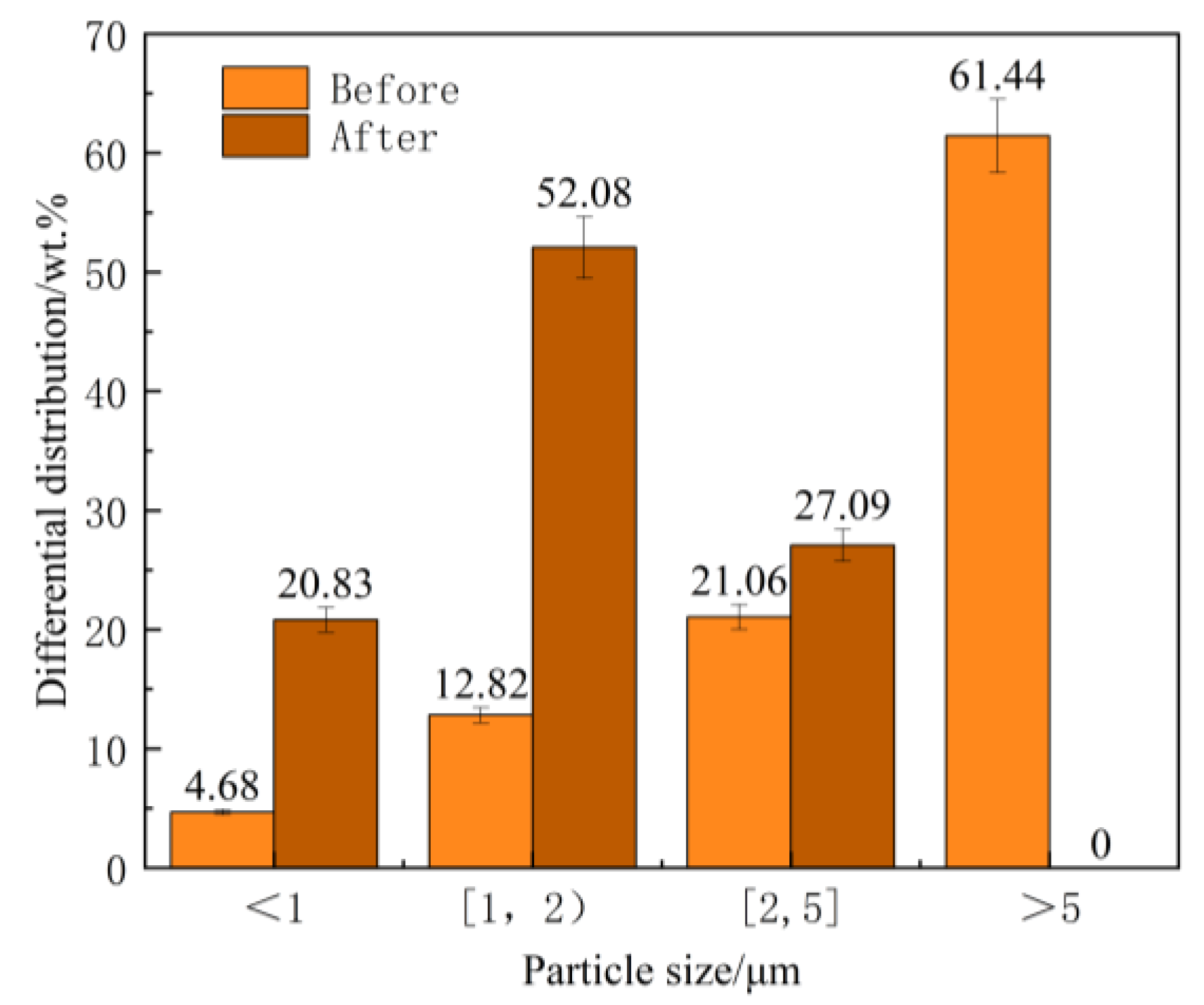
Stable and controllable behavior in the melt: The EASiCp particles exhibited a stable absorption rate (68–72%) in the iron melt, with a particle-size-dependent dissolution behavior. Ultimately, the submicron particles (0.3–2 μm) were dispersed in the matrix, mainly located within the grains (78.12%) and at the grain boundaries (21.88%).
- (3)
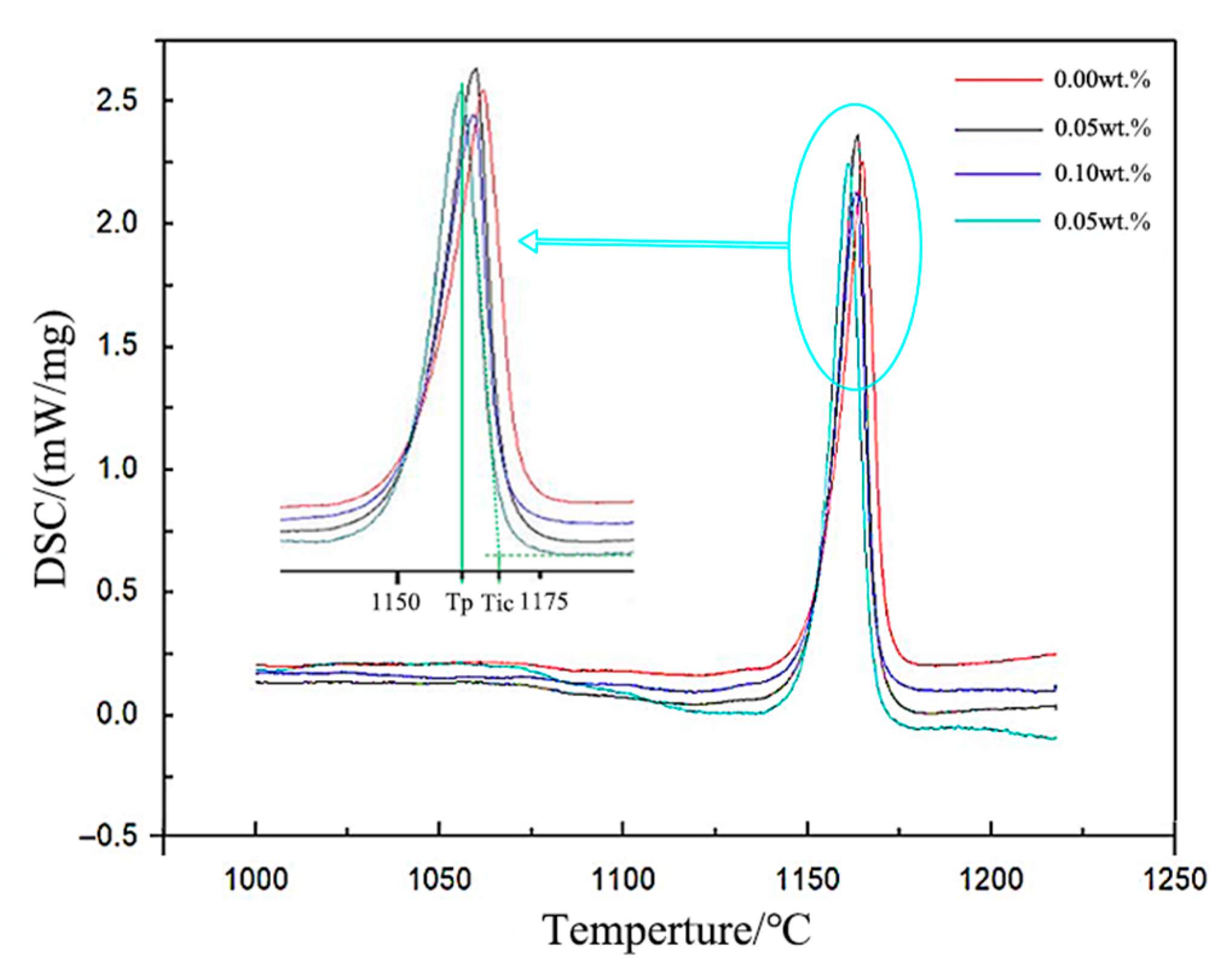
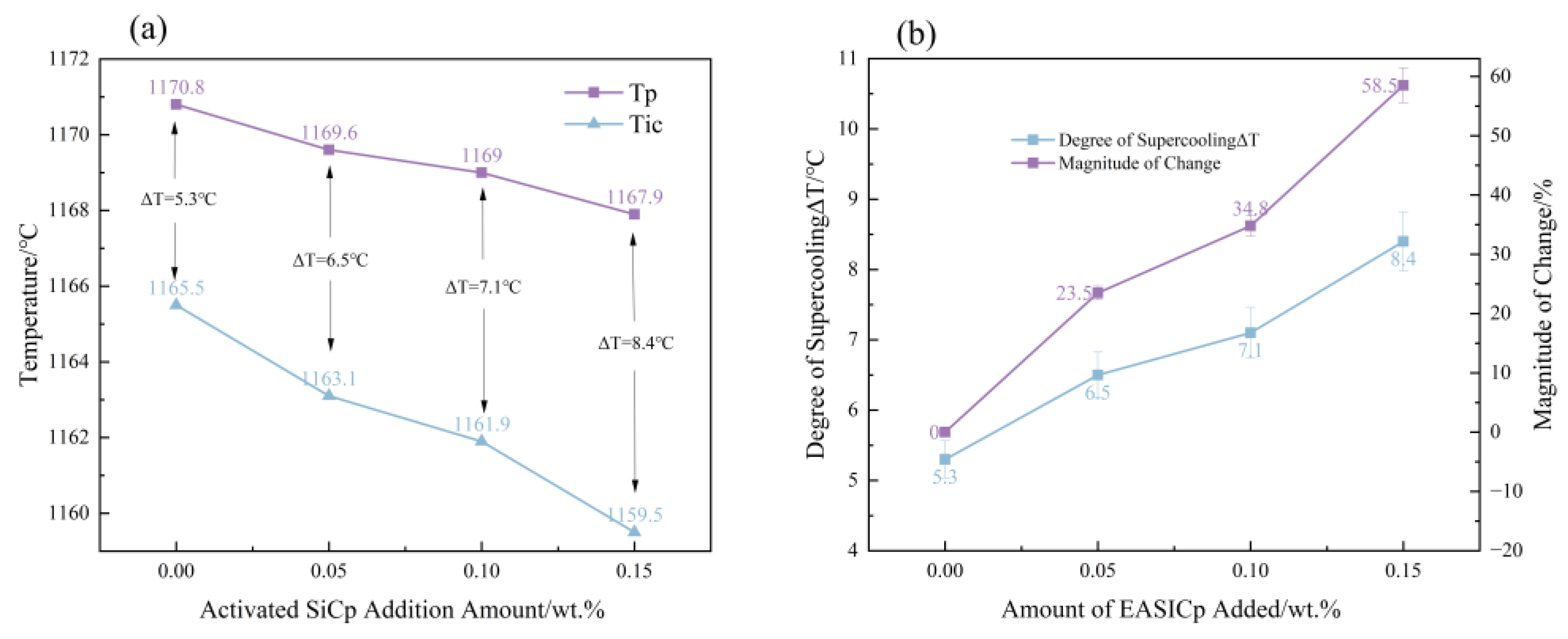
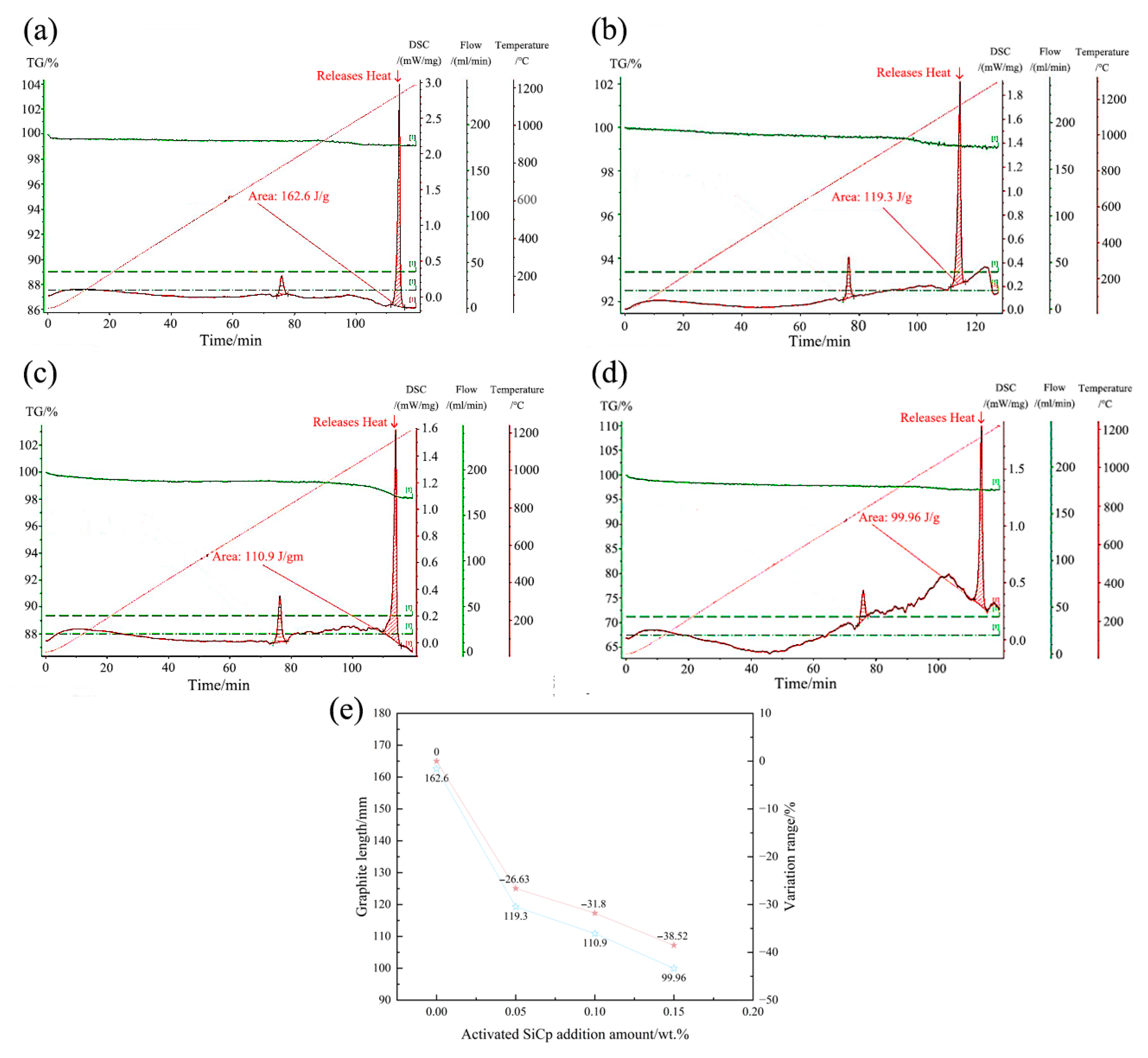
Significant changes in solidification kinetics: The addition of EASiCp effectively regulated the solidification kinetic parameters, increasing the undercooling from 5.3 °C to 8.4 °C and significantly lowering the latent heat of crystallization from 162.6 J/g to 99.96 J/g. This was primarily due to its ongoing endothermic reaction (SiC + Fe → FeSi + C) and strong heterogeneous nucleation effects.
- (4)
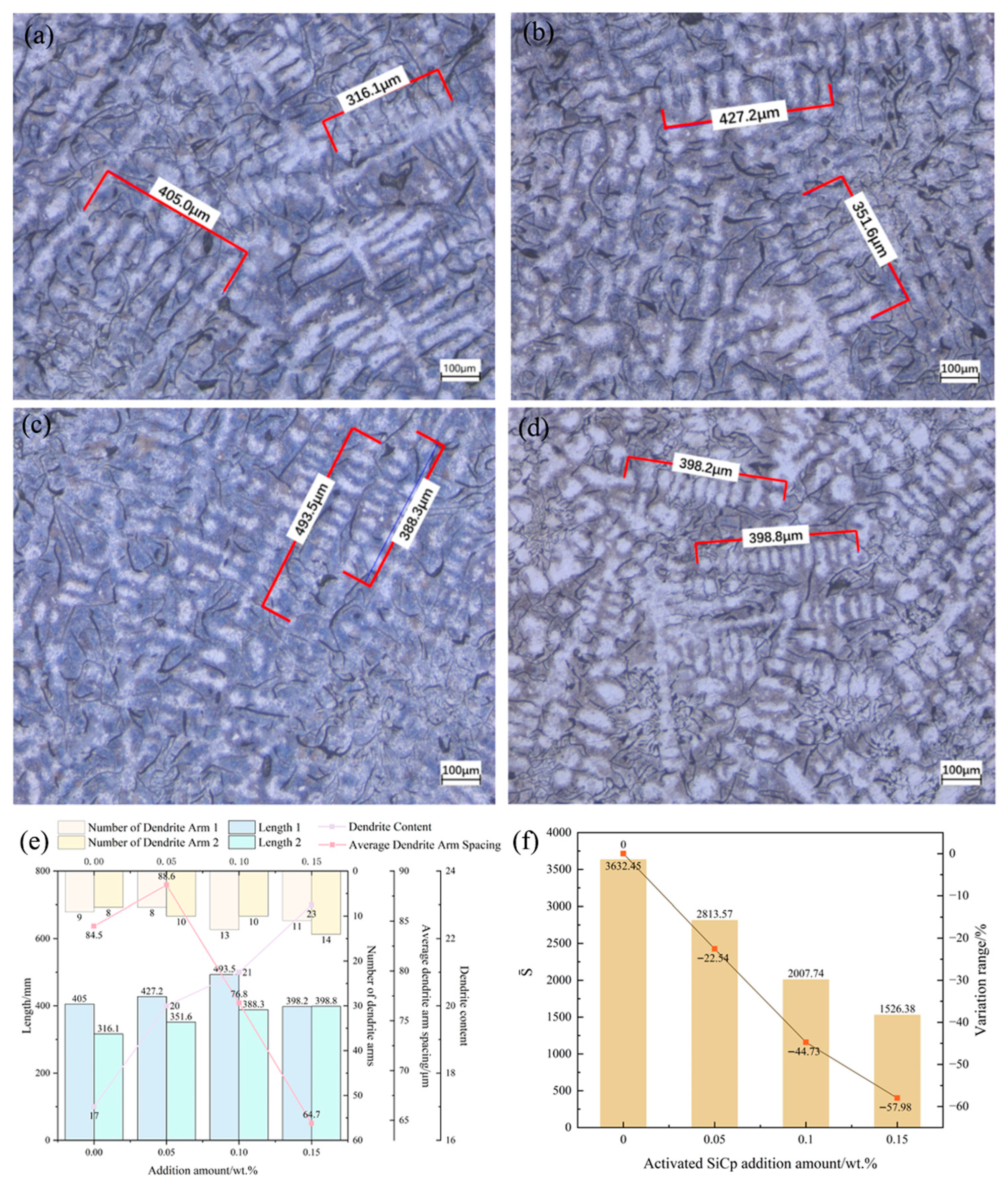
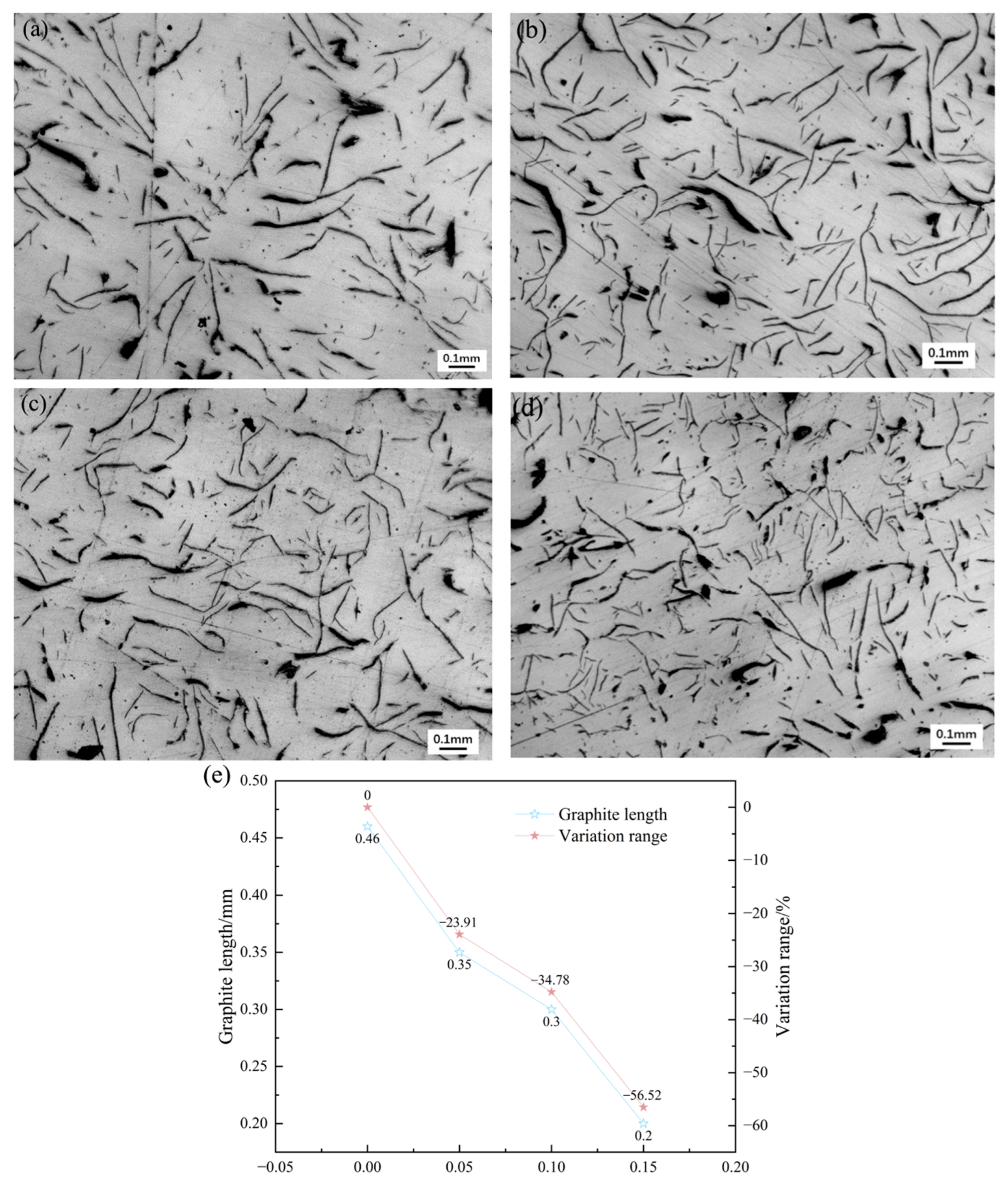
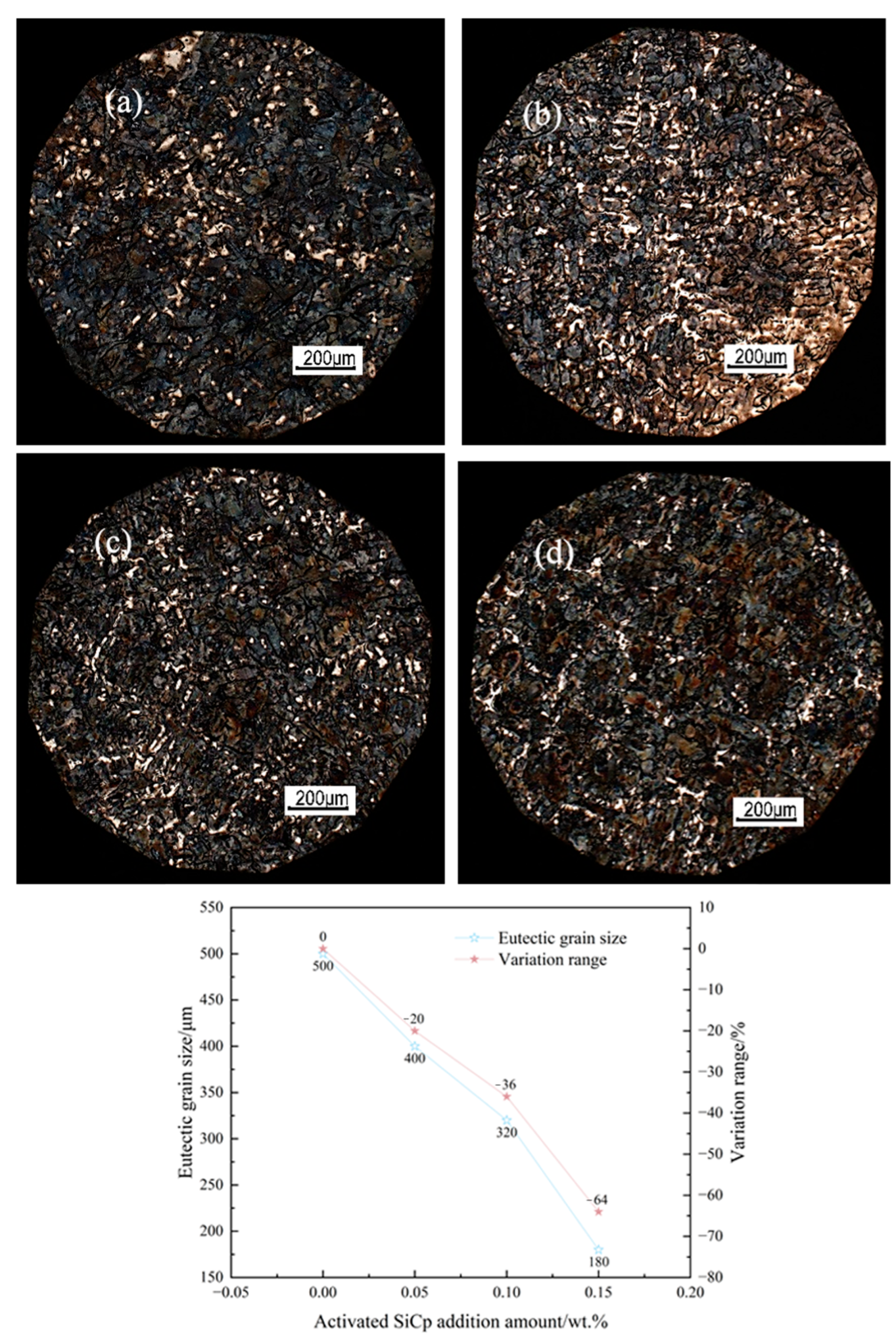
Synergistic mechanism dominates the microstructural refinement: EASiCp optimizes the microstructure through a synergistic mechanism of “promoting nucleation and inhibiting growth.” At the optimal addition amount of 0.15 wt.%, the primary austenite dendrite content increased by 35.29%, and the secondary dendrite arm spacing decreased by 57.98%. The A-type graphite flake length reduced from 0.46 mm to 0.20 mm, and the eutectic structure size was refined from over 500 μm to approximately 180 μm. This mechanism is attributed to the particles’ direct nucleation effect, the additional nucleation sites provided by the “micro-region carbon enrichment” from the reaction products, and the pinning effect on grain boundary migration.
- (5)
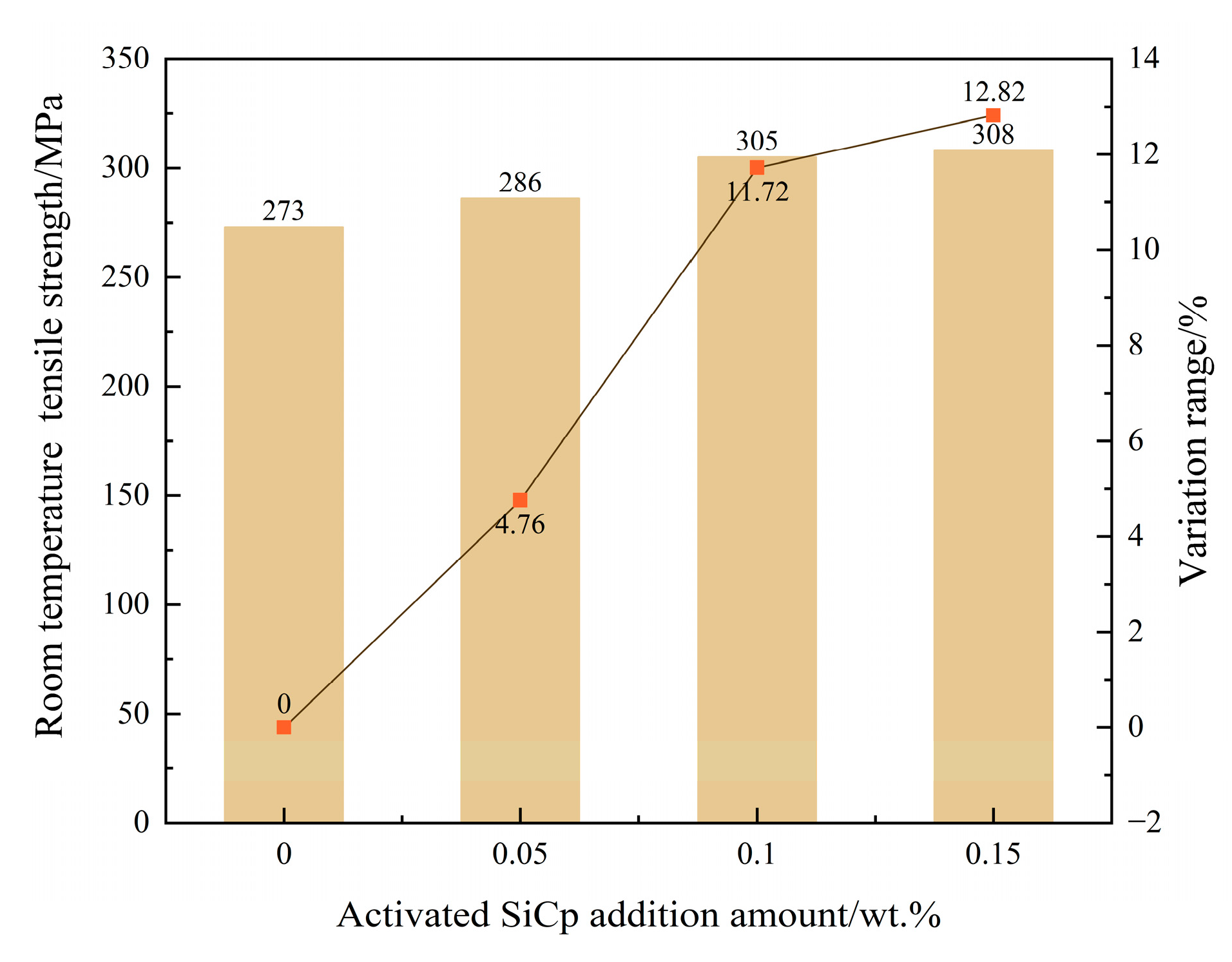
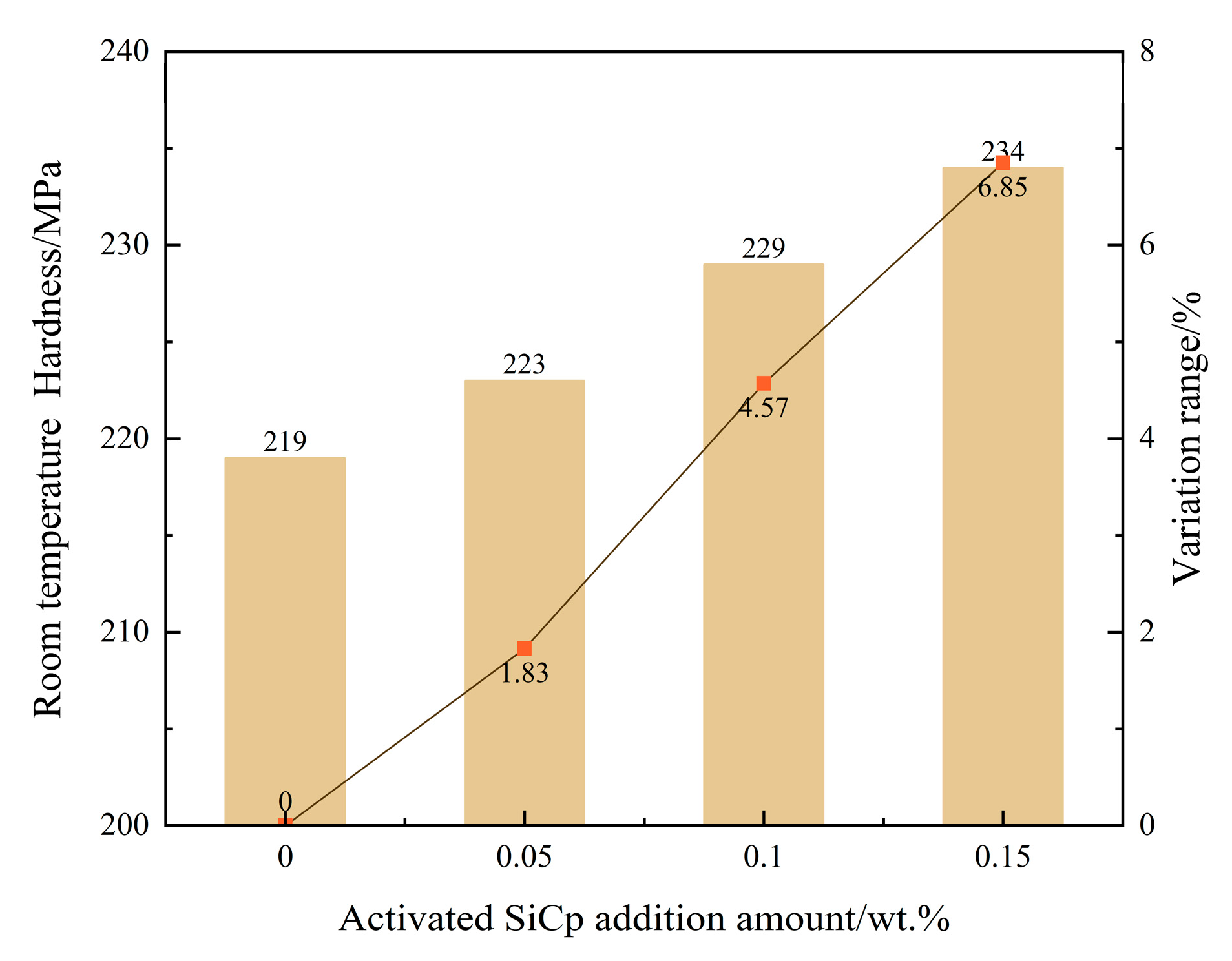
Simultaneous enhancement of mechanical properties: The synergistic optimization of the microstructure directly translated into an improvement in mechanical properties, with the material’s room-temperature tensile strength reaching 308 MPa, a 12.82% increase compared to the unmodified sample. This performance enhancement results from the combined effects of grain refinement, dislocation strengthening, and graphite morphology optimization.
In conclusion, the introduction of EASiCp provides an efficient and controllable technical pathway for regulating the solidification process of hypoeutectic gray cast iron. The “nucleation-growth” synergistic regulation strategy not only significantly refines the microstructure and enhances mechanical properties, but also lays a solid theoretical and experimental foundation for the development of high-performance cast iron components. This study identified an optimal effect within a SiC addition concentration of 0.15 wt.%. However, there may be an upper limit to the SiC addition concentration, beyond which particle agglomeration and performance degradation could occur. Determining the best processing window will be key for future industrial applications.