Ultra-Strong Transparent ZnAl2O4 Glass-Ceramics via Controlled Crystallization and Ion Exchange
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Composition and Batching
2.2. Melting, Casting, and Annealing
2.3. Controlled Crystallization
2.4. Ion Exchange Treatment
2.5. Characterization of Structure and Properties
3. Results and Discussion
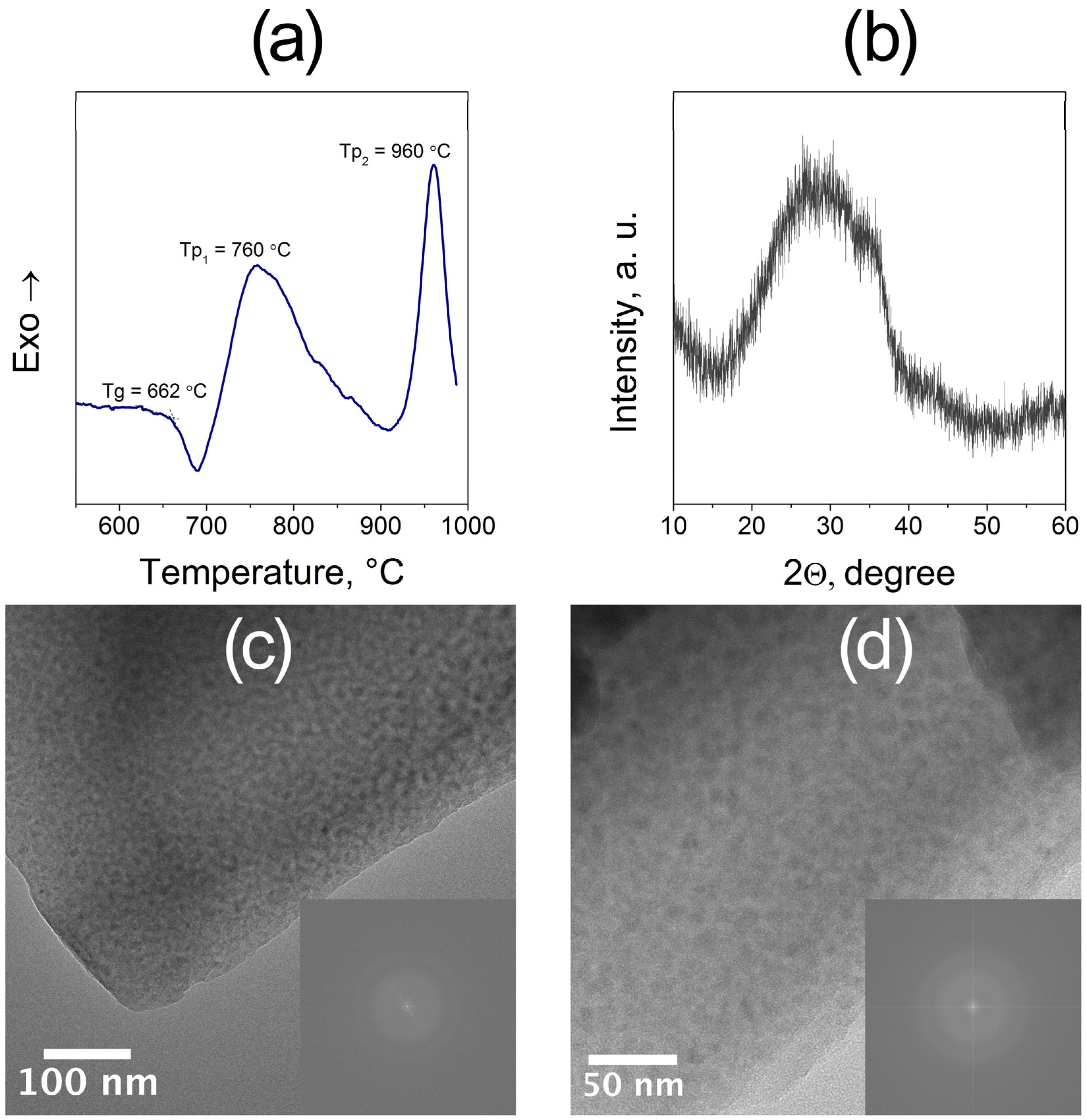
3.1. Structure and Thermal Behavior of the Parent Glass
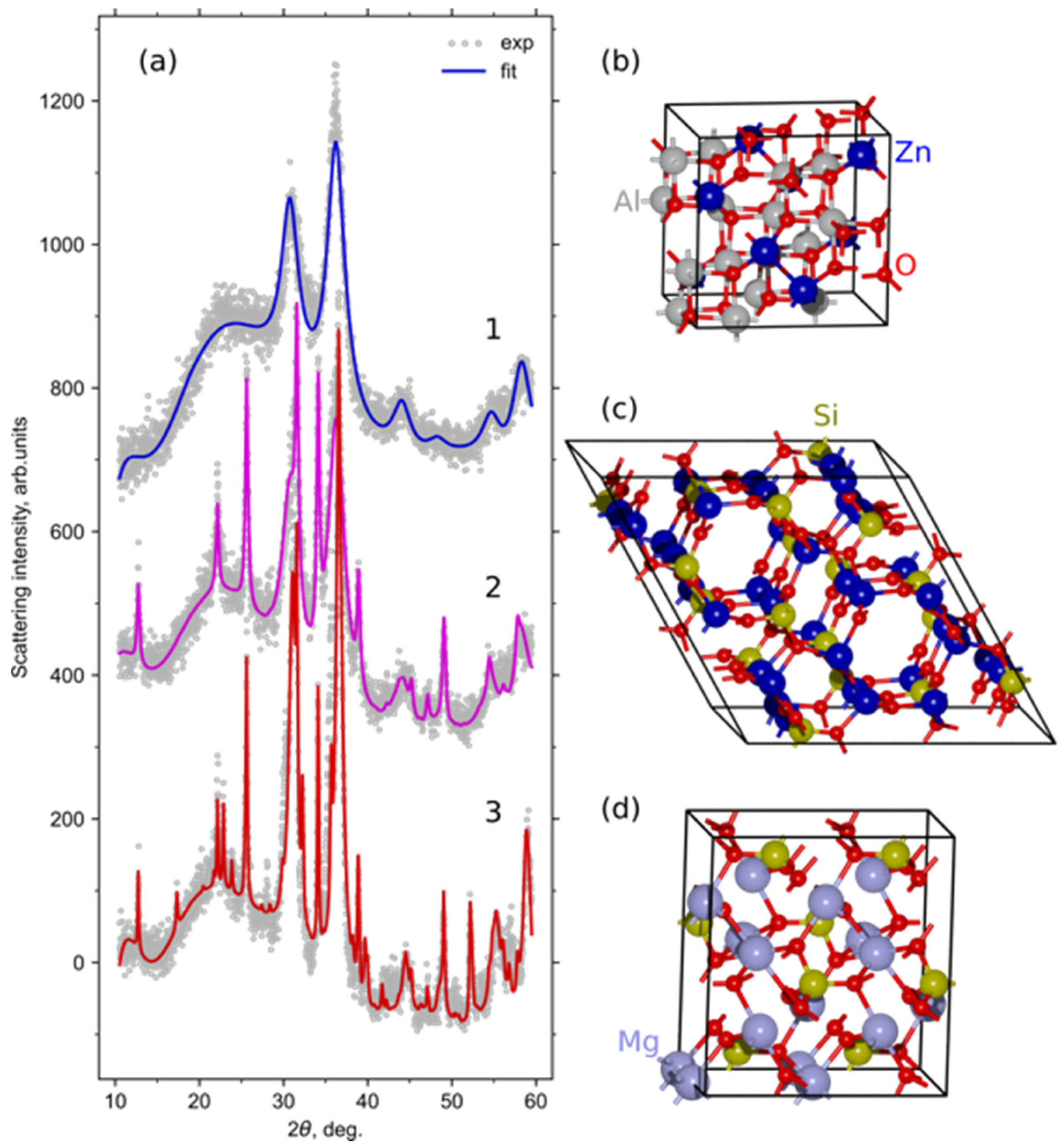
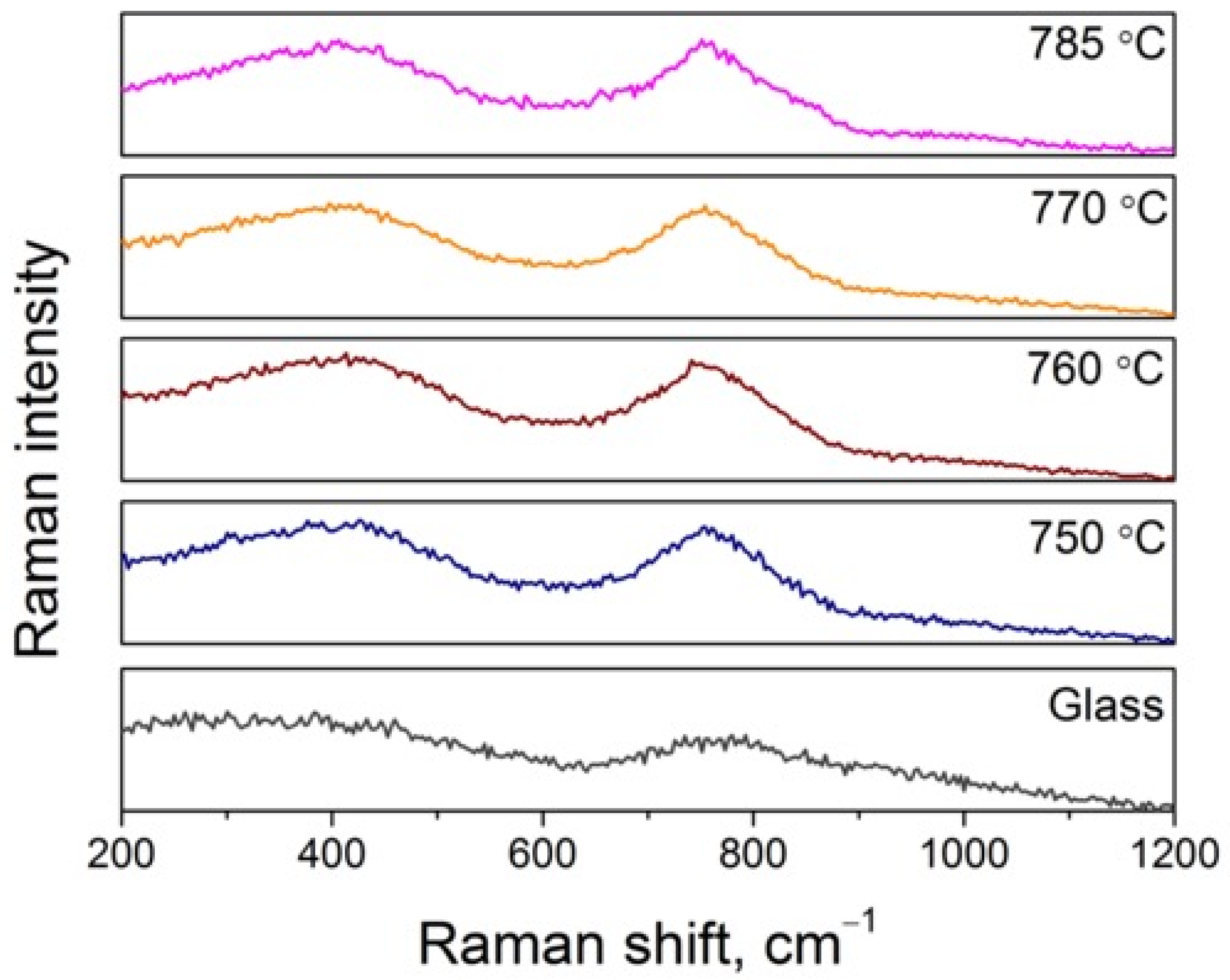
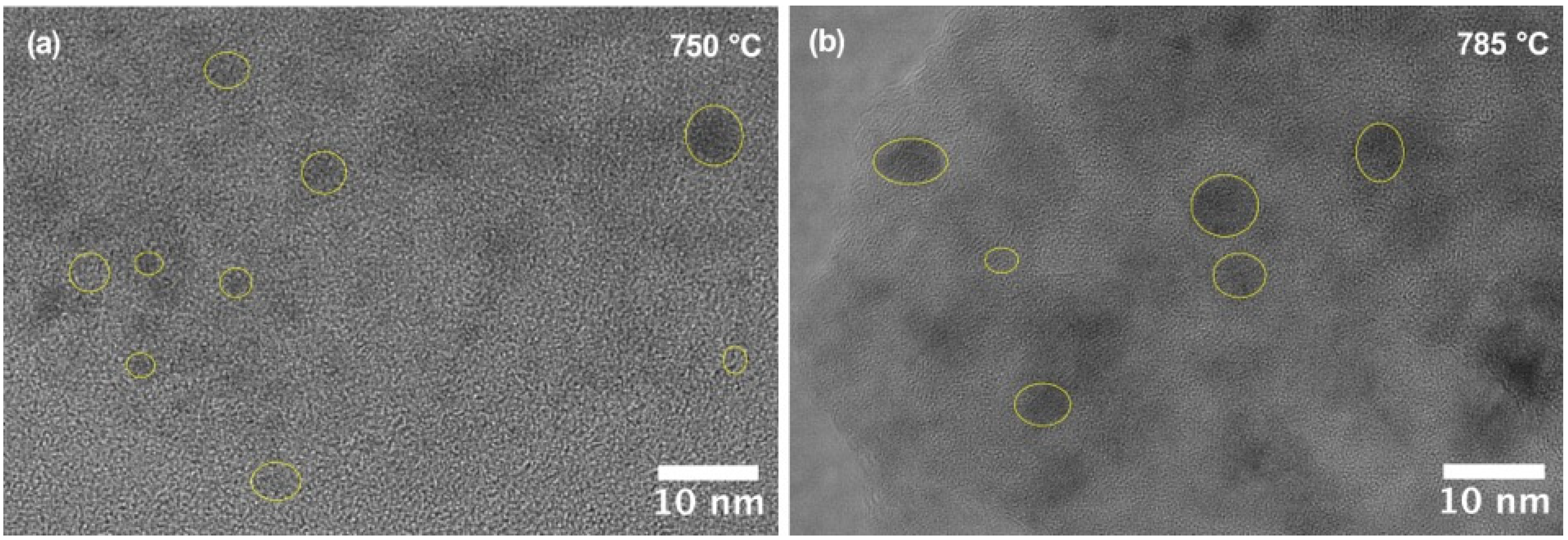
3.2. Crystallization Behavior and Phase Evolution
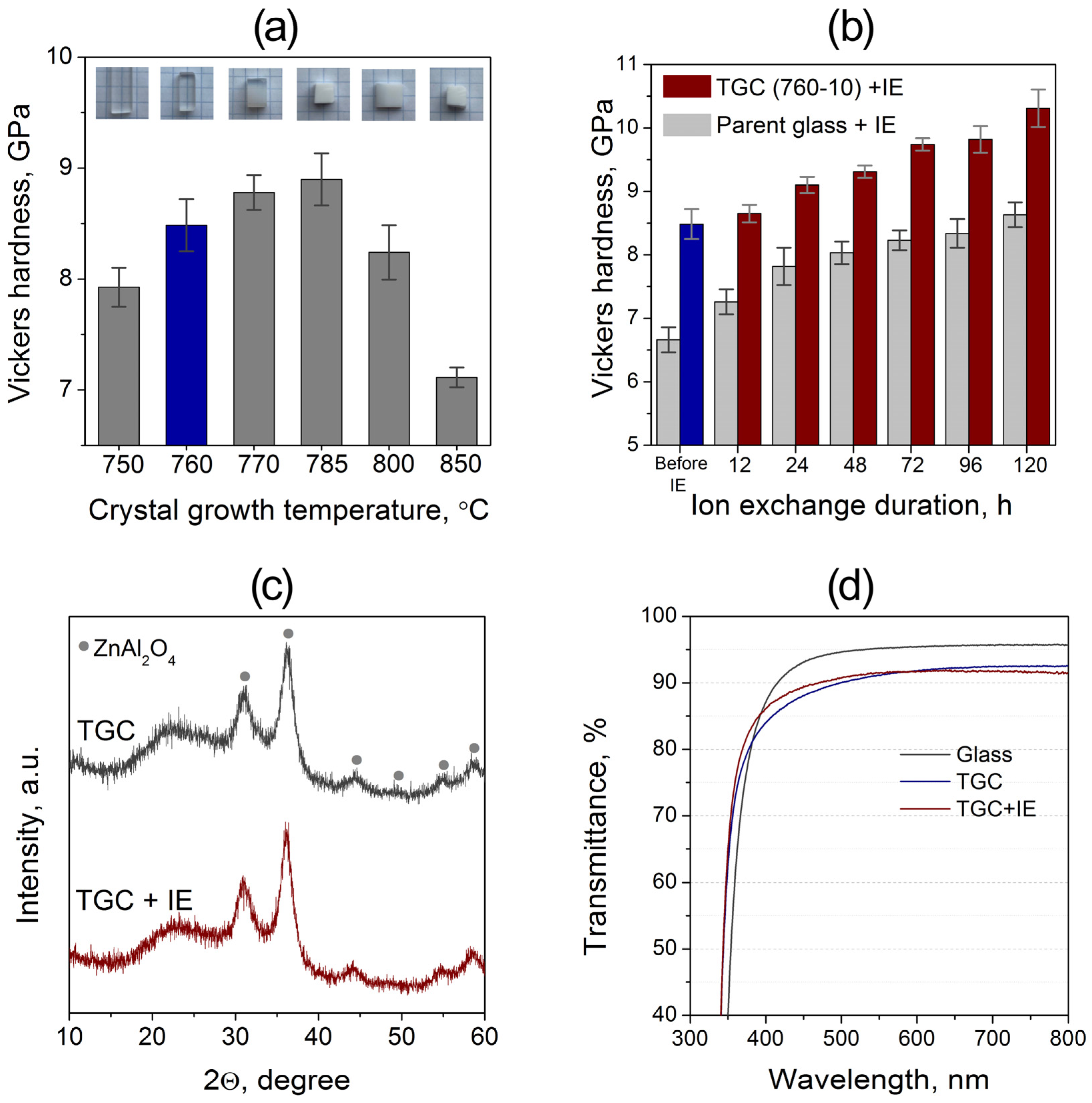
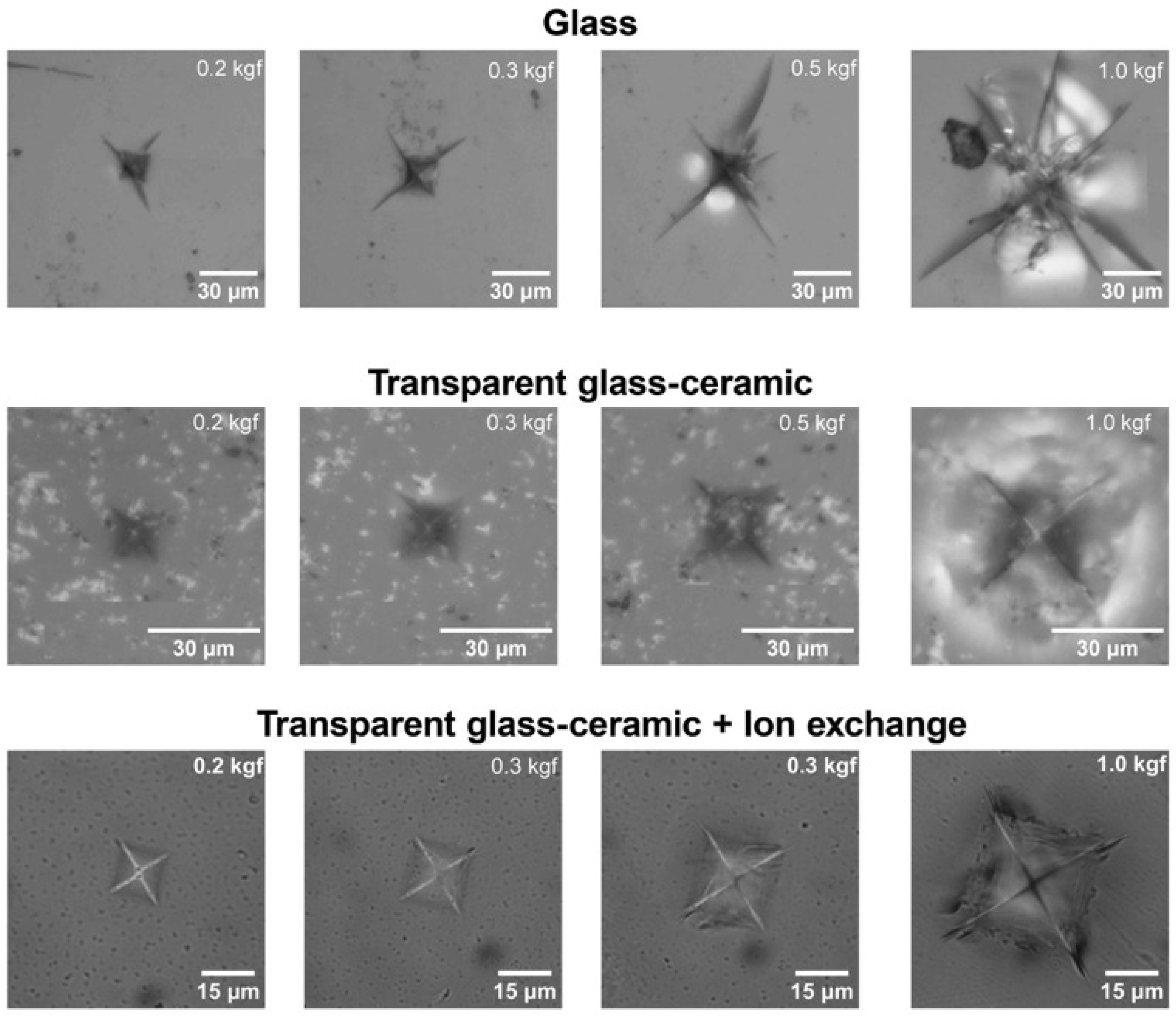
3.3. Mechanical Properties and Ion-Exchange Strengthening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, P.; Wang, Y.; Li, X.; Han, T.; Shi, X. Effect of Physical Tempering on the Mechanical Properties of Aluminosilicate Glass. J. Non Cryst. Solids 2023, 619, 122574. [Google Scholar] [CrossRef]
- Berneschi, S.; Righini, G.C.; Pelli, S. Towards a Glass New World: The Role of Ion-Exchange in Modern Technology. Appl. Sci. 2021, 11, 4610. [Google Scholar] [CrossRef]
- Zhen, W.; Zhenbiao, H.; Tao, S.; Fenghua, Z.; Yulong, L.; Sheikh, M.Z.; Xiang, W.; Yinmao, W. A Comparative Study on the Effect of Loading Speed and Surface Scratches on the Flexural Strength of Aluminosilicate Glass: Annealed vs. Chemically Strengthened. Ceram. Int. 2018, 44, 11239–11256. [Google Scholar] [CrossRef]
- Banapour Ghaffari, O.; Eftekhari Yekta, B.; Zakeri-Nasrabadi, M. Estimating “Depth of Layer” (DOL) in Ion-Exchanged Glasses Using Explainable Machine Learning. Materialia 2024, 33, 102027. [Google Scholar] [CrossRef]
- Seidel, S.; Dittmer, M.; Wisniewski, W.; Höland, W.; Rüssel, C. Effect of the ZrO2 Concentration on the Crystallization Behavior and the Mechanical Properties of High-Strength MgO–Al2O3–SiO2 Glass–Ceramics. J. Mater. Sci. 2017, 52, 1955–1968. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Li, Y.; Zhou, Y.; Jiang, W. Effect of Metastable Phase on the Crystallization and Mechanical Properties of MgO-Al2O3-SiO2 Glass-Ceramics without Nucleating Agents. Ceram. Int. 2023, 49, 7737–7745. [Google Scholar] [CrossRef]
- Beall, G.H.; Comte, M.; Dejneka, M.J.; Marques, P.; Pradeau, P.; Smith, C. Ion-Exchange in Glass-Ceramics. Front. Mater. 2016, 3, 41. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Ojovan, M.I. Inhomogeneities in Glass: From Defects to Functional Nanostructures. Encyclopedia 2025, 5, 136. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Han, J.; Wang, J.; Liu, C.; Ruan, J. The Structure and Properties of Chemical Strengthened Transparent Lithium Disilicate Glass Ceramics with Various P2O5 Contents. J. Non Cryst. Solids 2022, 588, 121626. [Google Scholar] [CrossRef]
- Mu, Y.; Qiu, J.; Wu, L.; Tian, X.; Wang, J.; Li, L.; Han, J. Ion-Exchange Induced Mechanical Properties Enhancement and Microstructural Modifications of Transparent Lithium Aluminosilicate Glass-Ceramics. Ceram. Int. 2024, 50, 14878–14883. [Google Scholar] [CrossRef]
- Zheng, W.; Gao, Z.; Huang, M.; Zhang, H.; Yuan, J.; Tian, P.; Peng, Z.; Du, X. Chemical Strengthening of Lithium Aluminosilicate Glass-Ceramic with Different Crystallinity. J. Non Cryst. Solids 2022, 598, 121940. [Google Scholar] [CrossRef]
- Li, X.C.; Meng, M.; Li, D.; Wei, R.; He, L.; Zhang, S.F. Strengthening and Toughening of a Multi-Component Lithium Disilicate Glass-Ceramic by Ion-Exchange. J. Eur. Ceram. Soc. 2020, 40, 4635–4646. [Google Scholar] [CrossRef]
- Li, W.; Xiang, W.; Li, C.; Wang, J.; Li, L.; Han, J. Influence of Pre-Heating Treatment on the Crystallization and Ion-Exchange Behavior of MgO-Al2O3-SiO2 Glass-Ceramics. Ceram. Int. 2024, 50, 42933–42938. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.; Lu, Y.; Liu, C.; Deng, Z.; Han, N.; Cui, J. Microstructure and Ion-Exchange Properties of Transparent Glass-Ceramics Containing Zn2TiO4/α-Zn2SiO4 Nanocrystals. J. Eur. Ceram. Soc. 2022, 42, 3595–3602. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Ruan, J.; Han, J.; Xie, J.; Liu, C. Microstructure and Ion-Exchange Properties of Glass-Ceramics Containing ZnAl2O4 and β-Quartz Solid Solution Nanocrystals. J. Eur. Ceram. Soc. 2021, 41, 5331–5340. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Wang, J.; Ruan, J.; Xie, J.; Han, J.; Deng, Z.; Zhao, X. Effects of Alkali Oxides and Ion-Exchange on the Structure of Zinc-Alumino-Silicate Glasses and Glass-Ceramics. J. Eur. Ceram. Soc. 2022, 42, 576–588. [Google Scholar] [CrossRef]
- Shakhgildyan, G.; Avakyan, L.; Atroshchenko, G.; Vetchinnikov, M.; Zolikova, A.; Ignat’eva, E.; Ziyatdinova, M.; Subcheva, E.; Bugaev, L.; Sigaev, V. Ultra-Broadband Plasmon Resonance in Gold Nanoparticles Precipitated in ZnO-Al2O3-SiO2 Glass. Ceramics 2024, 7, 562–578. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Zhu, J.; Zheng, Q.; Ding, L.; Jiang, W. Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method. Materials 2021, 14, 5477. [Google Scholar] [CrossRef]
- Shakhgildyan, G.Y.; Alekseev, R.O.; Golubev, N.V.; Savinkov, V.I.; Naumov, A.S.; Presnyakova, N.N.; Sigaev, V.N. One-Step Crystallization of Gahnite Glass-Ceramics in a Wide Thermal Gradient. ChemEngineering 2023, 7, 37. [Google Scholar] [CrossRef]
- Alzahrani, A.S. Novel Ion-Exchangeable Nepheline Glass-Ceramics for Dental Application. J. Eur. Ceram. Soc. 2023, 43, 5682–5690. [Google Scholar] [CrossRef]
- Tian, X.; Chen, Z.; Mu, Y.; Ji, C.; Li, L.; Wu, L.; Qiu, J.; Wang, J.; Han, J. Promotion of Phase Transformation on Ion-Exchange: Transparent Glass-Ceramics with Ultra-High Flexural Strength and Impact Resistance. J. Eur. Ceram. Soc. 2026, 46, 117885. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, J.; He, H.; Dong, J.; Liu, J.; Wang, F.; Liao, Q.; Li, M.; Gu, Y.; Liu, L.; et al. The Effect of Ion Exchange on the Properties of MgO-Al2O3-SiO2 Transparent Glass-Ceramics. Ceram. Int. 2025, 51, 33245–33253. [Google Scholar] [CrossRef]
- Shakhgil’dyan, G.Y.; Savinkov, V.I.; Shakhgil’dyan, A.Y.; Alekseev, R.O.; Naumov, A.S.; Lopatkina, E.V.; Sigaev, V.N. Effect of Sitallization Conditions on the Hardness of Transparent Sitalls in the System ZnO–MgO–Al2O3–SiO2. Glass Ceram. 2021, 77, 426–428. [Google Scholar] [CrossRef]
- Veselov, I.A.; Naumov, A.S.; Savinkov, V.I.; Fedotov, S.S.; Alekseev, R.O.; Ignatieva, E.S.; Shakhgildyan, G.Y.; Sigaev, V.N. Ion Exchange Strengthening of Glasses of the ZnO–MgO–Al2O3–SiO2 Glass-Ceramic-Forming System with an Increased Content of Na2O. Glass Ceram. 2024, 81, 11–16. [Google Scholar] [CrossRef]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Crystallogr. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An Open-Access Collection of Crystal Structures and Platform for World-Wide Collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Golubkov, V.V.; Dymshits, O.S.; Petrov, V.I.; Shashkin, A.V.; Tsenter, M.Y.; Zhilin, A.A.; Kang, U. Small-Angle X-Ray Scattering and Low-Frequency Raman Scattering Study of Liquid Phase Separation and Crystallization in Titania-Containing Glasses of the ZnO–Al2O3–SiO2 System. J. Non Cryst. Solids 2005, 351, 711–721. [Google Scholar] [CrossRef]
- Alekseeva, I.; Baranov, A.; Dymshits, O.; Ermakov, V.; Golubkov, V.; Tsenter, M.; Zhilin, A. Influence of CoO Addition on Phase Separation and Crystallization of Glasses of the ZnO–Al2O3–SiO2–TiO2 System. J. Non Cryst. Solids 2011, 357, 3928–3939. [Google Scholar] [CrossRef]
- Ghose, S.; Choudhury, N.; Chaplot, S.L.; Pal Chowdhury, C.; Sharma, S.K. Lattice Dynamics and Raman Spectroscopy of Protoenstatite Mg2Si2O6. Phys. Chem. Miner. 1994, 20, 469–477. [Google Scholar] [CrossRef]
- Okuno, M.; Zotov, N.; Schmücker, M.; Schneider, H. Structure of SiO2–Al2O3 Glasses: Combined X-Ray Diffraction, IR and Raman Studies. J. Non Cryst. Solids 2005, 351, 1032–1038. [Google Scholar] [CrossRef]

| Glass System and Alkali Oxide Content (mol.%) | Main Crystalline Phase | Salt Composition and IE Treatment Conditions | Vickers Hardness, GPa (Load, kgf) | Ref. |
|---|---|---|---|---|
| LAS Li2O—21 Na2O—1 | Li2Si2O5 LiAlSi4O10 | Before IE Na0.95K0.05NO3 410 °С—4 h 410 °С—6 h 410 °С—8 h 410 °С—10 h | 7.78 (n/a) 8.53 (n/a) 8.87 (n/a) 8.64 (n/a) 8.55 (n/a) | [9] |
| LAS Li2O—15 Na2O—9 K2O—1 | LiAlSiO4 | Before IE (Na0.8K0.2)NO3 420 °C—4 h | 7.03 (0.2) 7.62 (0.2) | [10] |
| LAS Li2O—21.6 | LiAlSi4O10 | Before IE NaNO3 450 °C—6 h | 6.95 (0.2) 7.45 (0.2) | [11] |
| LS Li2O—27.5 | Li2Si2O5 | Before IE NaNO3 315 °C—12 h 385 °С—1 h 450 °С—1 h | 5.9 (0.05) 6.5 (0.05) 6.3 (0.05) 6.2 (0.05) | [12] |
| ZMAS Na2O—8 | ZnAl2O4 MgAl2O4 | Before IE KNO3 440 °C—4 h | 7.2 (0.2) 8.3 (0.2) | [13] |
| ZAS Na2O—8.5 | Zn2TiO4 Zn2SiO4 | Before IE KNO3 410 °C—4 h 430 °С—4 h 450 °С—4 h | 6.6 (0.2) 7.8 (0.2) 7.7 (0.2) 7.6 (0.2) | [14] |
| LZAS Li2O—5.95 | ZnAl2O4 LiAlSi4O10 | Before IE NaNO3 460 °C—4 h -> KNO3 460 °C—4 h | 7.45 (0.2) 8.1 (0.2) | [15] |
| ZAS Na2O–10.5 | ZnO Zn2SiO4 | Before IE KNO3 460 °C—8 h | 6.3 (0.2) 7.6 (0.2) | [16] |
| LAS Li2O—12.4 Na2O—2.8 | β-SiO2 LiAlSi2O6 Li2SiO3 | Before IE KNO3 400 °C—10 h 420 °C—10 h 440 °C—10 h | 5.2 (0.01) 7.3 (0.01) 7.4 (0.01) 7.7 (0.01) | [18] |
| NAS Na2O—15 | NaAlSiO4 | Before IE KNO3 600 °C—8 h | 4.4 (n/d) 6.3(n/d) | [20] |
| LAS Li2O—15 Na2O—9 | LiAlSiO4 NaAlSiO4 | Before IE Na0.85K0.15NO3 430 °C—4 h 450 °C—4 h 470 °C—4 h 490 °C—4 h -> KNO3 380 °C—4 h 400 °C—4 h 420 °C—4 h 440 °C—4 h | 7.4 (0.2) 7.5 (0.2) 7.5 (0.2) 7.5 (0.2) 7.5 (0.2) 8.0 (0.2) 8.1 (0.2) 8.2 (0.2) 8.1 (0.2) | [21] |
| ZMAS Na2O—5.9 | MgAl2O4 ZnAl2O4 SnO2 ZrO2 | Before IE Na0.02K0.98NO3 450 °C—5 h 470 °C—5 h 490 °C—5 h | 7.0 (0.2) 8.0 (0.2) 8.1 (0.2) 8.2 (0.2) | [22] |
| Sample | Crystalline Phase | Phase Fraction, wt.% | Cell Parameters, Å | Crystallite Size, nm | Site Occupancy Zn:Mg | GOF |
|---|---|---|---|---|---|---|
| 5Na-750-10 | ZnAl2O4 | 100 | 8.223 (3) | 3.7 (1) | 55:45 (2) | 1.52 |
| 5Na-760-10 | ZnAl2O4 | 100 | 8.207 (3) | 4.1 (1) | 52:48 (2) | 1.27 |
| 5Na-750-25 | ZnAl2O4 | 100 | 8.234 (3) | 3.8 (1) | 48:52 (2) | 1.27 |
| 5Na-750-110 | ZnAl2O4 | 83 | 8.220 (3) | 3.9 (1) | 57:43 (2) | 1.35 |
| Zn2SiO4 | 17 | 13.905 (4), 9.302 (2) | 25.8 (7) | 45:55 (5) | ||
| 5Na-800-10 | ZnAl2O4 | 86 | 8.205 (2) | 5.0 (1) | 62:38 (1) | 1.39 |
| Zn2SiO4 | 5 | 13.94 (2), 9.28 (1) | 12 (1) | 100:0 * | ||
| Mg2SiO4 | 9 | 4.762 (4), 10.222 (7), 6.000 (5) | 28.8 (4) | 0:100 * | ||
| 5Na-850-10 | ZnAl2O4 | 72 | 8.141 (2) | 9.9 (2) | 81:19 (3) | 2.07 |
| Zn2SiO4 | 10 | 13.909 (4), 9.307 (2) | 66 (5) | 80:20 (10) | ||
| Mg2SiO4 | 18 | 4.769 (2), 10.239 (4), 5.982 (3) | 56 (6) | 15:85 (3) | ||
| 5Na-700-24 | ZnAl2O4 | 80 | 8.204 (6) | 3.3 (1) | 49:51 (3) | 1.12 |
| Zn2SiO4 | 7 | 14.51 (3), 8.38(1) | 3 (1) | 100:0 * | ||
| Mg2SiO4 | 13 | 4.69 (3), 10.09 (6), 6.08 (4) | 7 (2) | 0:100 * | ||
| Reference | ZnAl2O4 | - | 8.085 | - | - | - |
| Zn2SiO4 | - | 13.971, 9.334 | - | - | ||
| Mg2SiO4 | - | 4.752, 10.193, 5.977 | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselov, I.; Shakhgildyan, G.; Savinkov, V.; Golubev, N.; Tregubov, K.; Vinogradov, D.; Avakyan, L.; Ojovan, M.; Ghosh, M.; Sigaev, V. Ultra-Strong Transparent ZnAl2O4 Glass-Ceramics via Controlled Crystallization and Ion Exchange. Materials 2025, 18, 5230. https://doi.org/10.3390/ma18225230
Veselov I, Shakhgildyan G, Savinkov V, Golubev N, Tregubov K, Vinogradov D, Avakyan L, Ojovan M, Ghosh M, Sigaev V. Ultra-Strong Transparent ZnAl2O4 Glass-Ceramics via Controlled Crystallization and Ion Exchange. Materials. 2025; 18(22):5230. https://doi.org/10.3390/ma18225230
Chicago/Turabian StyleVeselov, Ivan, Georgiy Shakhgildyan, Vitaliy Savinkov, Nikita Golubev, Kirill Tregubov, Daniil Vinogradov, Leon Avakyan, Michael Ojovan, Manasi Ghosh, and Vladimir Sigaev. 2025. "Ultra-Strong Transparent ZnAl2O4 Glass-Ceramics via Controlled Crystallization and Ion Exchange" Materials 18, no. 22: 5230. https://doi.org/10.3390/ma18225230
APA StyleVeselov, I., Shakhgildyan, G., Savinkov, V., Golubev, N., Tregubov, K., Vinogradov, D., Avakyan, L., Ojovan, M., Ghosh, M., & Sigaev, V. (2025). Ultra-Strong Transparent ZnAl2O4 Glass-Ceramics via Controlled Crystallization and Ion Exchange. Materials, 18(22), 5230. https://doi.org/10.3390/ma18225230








