In Search of Ultra-Black Ceramic Pigments Using Microwaves: Delafossite Cuprates CuMO2 (M = Mn, Fe, Cr)
Abstract
1. Introduction
2. Materials and Methods
- -
- Category A deals with pigments suspended in glass matrixes which require the highest degree of heat stability and chemical resistance to withstand the attack of molten glass.
- -
- Category B deals with pigments suspended in plastics and other polymers, which require only moderate heat stability.
- -
- Category C deals with pigments suspended in liquid vehicles, which require little, or no heat stability.
3. Results and Discussion
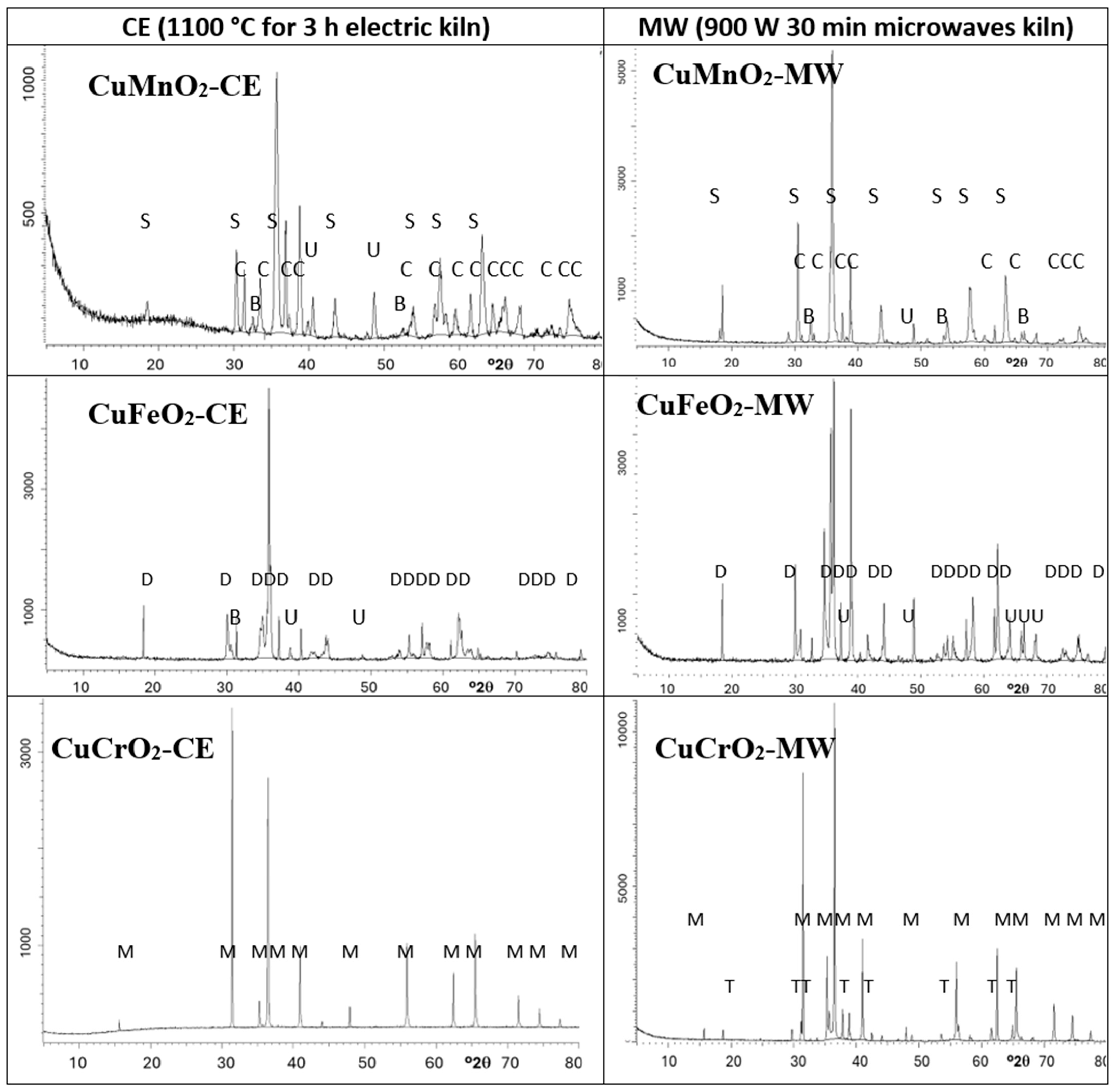
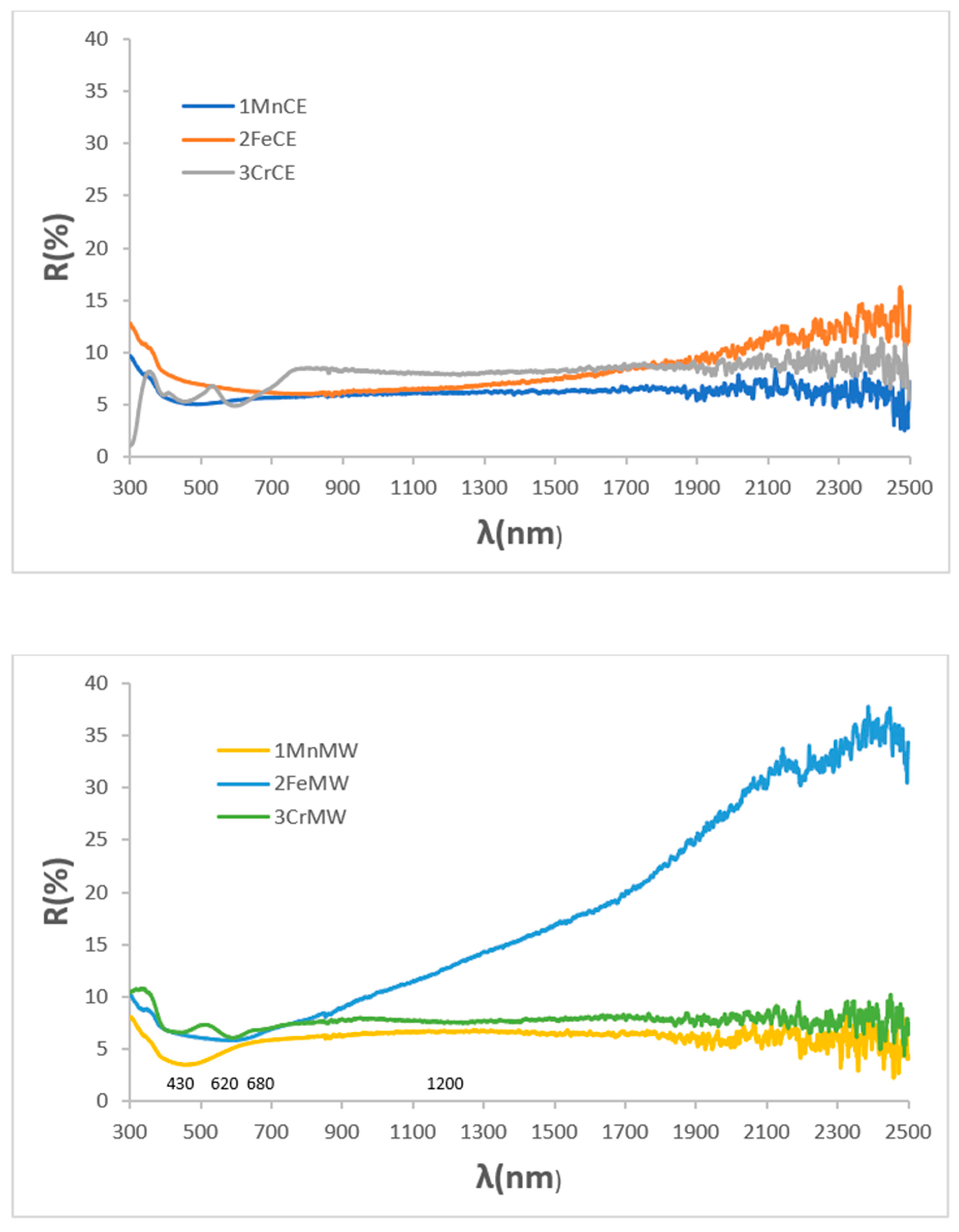
3.1. Characterization of Powders: Effect of Microwaves Firing
3.2. Characterization of 5 wt% Glazed Samples in Double Firing Frit with Maturation Point at 1000 °C, Zinc-Free, Using Electric Firing for Glazing
3.3. Characterization of 5 wt% Glazed Samples in Double Firing Frit with Maturation Point at 1000 °C, Zinc-Free, Using Microwaves Firing for Glazing
3.4. Effect of Temperature and Microwaves Power in Blackness of Mcconnellite
3.5. Characterization of Mcconellite Doped with Iron and Manganese
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSA | Selective solar absorber |
| IR | Infrared |
| XRD | X-ray Diffraction |
| SEM | Scanning electron microscopy with energy-dispersive spectroscopy |
| ICDD | International Centre for Diffraction Data |
| UV-Vis-NIR | Ultraviolet-visible-near-infrared |
References
- Friedel, C. Sur une combinaison naturelle des oxydes de fer et de cuivre, et sur la reproduction de l’atacamite. Comptes Rendus De L’académie Des Ciences i 1873, 77, 211–214. [Google Scholar]
- Shannon, R.D.; Rogers, D.B.; Prewitt, C.T. Chemistry of noble metal oxides. I. Syntheses and properties of ABO2 delafossite compounds. Inorg. Chem. 1971, 10, 713–718. [Google Scholar] [CrossRef]
- Tanaka, M.; Hasegawa, M.; Higuchi, T. Origin of the metallic conductivity in PdCoO2 with delafossite structure. Physica B 1998, 245, 157–163. [Google Scholar] [CrossRef]
- Marquardt, M.A.; Ashmore, N.A.; Cann, D.P. Crystal chemistry and electrical properties of the delafossite structure. Thin Solid Film. 2006, 496, 146–156. [Google Scholar] [CrossRef]
- Kawazoe, H.; Yasukawa, M.; Hyodo, H.; Kurita, M.; Yanagi, H.; Hosono, H. P-type electrical conduction in transparent thin films of CuAlO2. Nature 1997, 389, 939–942. [Google Scholar] [CrossRef]
- Daoua, R.; Frésarda, R.; Eyerta, V.; Héberta, S.; Maignana, A. Unconventional aspects of electronic transport in delafossite oxides. Sci. Technol. Adv. Mater. 2017, 18, 919–938. [Google Scholar] [CrossRef]
- Pabst, A. Crystal structure and density of delafossite. Am. Mineral. 1938, 23, 175–176. [Google Scholar]
- Kohler, B.U.; Jansen, M. Synthesis and crystal structure of 2H-CuAlO2. Z. Krist. 1983, 165, 313–314. [Google Scholar] [CrossRef]
- Poienar, M.; Banica, R.; Sfirloaga, P.; Ianasi, C.; Mihali, C.V.; Vlazan, P. Microwave-assisted hydrothermal synthesis and catalytic activity study of crednerite-type CuMnO2 materials. Ceram. Int. 2018, 44, 6157–6161. [Google Scholar] [CrossRef]
- Jackson, J.J.; Puretzky, A.A.; More, K.L.; Rouleau, C.M.; Eres, G.; Geohegan, D.B. Pulsed growth of vertically aligned nanotube arrays with variable density. ACS Nano 2010, 4, 7573–7581. [Google Scholar] [CrossRef] [PubMed]
- Monrós, G. Black Inorganic Pigments: From Black to Ultra-Black Behaviour, A Microstructural Approach. J. Miner. Mater. Sci. 2025, 6, 1119. [Google Scholar] [CrossRef]
- Marques, V.; Krings, W.; Reis, J.; Gorb, S.; Guillermo-Ferreira, R. Ultrablack color in velvet ant cuticle. Beilstein J. Nanotechnol. 2024, 15, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Calbo, J.; Sorlí, S.; Llusar, M.; Tena, M.A.; Monrós, G. Minimisation of toxicity in nickel ferrite black pigment. Br. Ceram. Trans. 2004, 103, 3–9. [Google Scholar] [CrossRef]
- Calbo, J.; Mestre, A.; García, A.; Tena, M.A.; Llusar, M.; Monrós, G. Multicomponent black coloured spinels from alkoxides. J. Sol-Gel Sci. Technol. 2003, 26, 191–194. [Google Scholar] [CrossRef]
- Monrós, G.; Delgado, C.; Monrós-Andreu, G.; Badenes, J.; Llusar, M. Black Coloured Glazes with Tetragonal CuCr2O4 Ceramic Pigment as Selective Solar Absorbers for Integral Ceramic Solar Collectors. J. Sol. Energy Res. Updates 2024, 11, 78–92. [Google Scholar] [CrossRef]
- Monrós, G.; Badenes, J.; Delgado, C.; Monrós-Andreu, G.; Llusar, M. New Mcconnellite Ceramic Pigment as a Selective Solar Absorber: Effects of Microwave Firing and Rare Earth Doping. Materials 2025, 18, 1520. [Google Scholar] [CrossRef]
- Feng, G.; Liu, T.; Mu, J.; Jiang, F.; Guo, J.; Chiao, L.; Wu, Q.; Zhang, X.; Liu, J.; Liang, J. Effect of mineralizer on the synthesis of (Ni, Mn) co-doping CuFe5O8 pigment and its glazing performance. Open Ceram. 2025, 23, 100822. [Google Scholar] [CrossRef]
- Monrós, G.; Cerro, S.; Badenes, J.A.; Llusar, M. Black Cool Pigments for Urban Heat Island (UHI) Control: From Cr-Hematite to Mn-Melilite. J. Sol. Energy Res. Updates 2021, 8, 27–44. [Google Scholar] [CrossRef]
- Calbo, J.; Gargori, C.; Sorlí, S.; Badenes, J.; Tena, M.A.; Monrós, G. Síntesis de pigmentos cerámicos mediante radiación microondas. Bol. Soc. Esp. Ceram. Vidr. 2007, 46, 14–20. [Google Scholar] [CrossRef]
- Blosi, M.; Dondi, M.; Albonetti, S.; Baldi, G.; Barzanti, A.; Zanelli, C. Microwave-assisted synthesis of Pr–ZrSiO4, V–ZrSiO4 and Cr–YAlO3 ceramic pigments. J. Eur. Cer. Soc. 2009, 29, 2951–2957. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. A review on the susceptor assisted microwave processing of materials. Energy 2016, 97, 306–338. [Google Scholar] [CrossRef]
- CIE Comission International de l’Eclairage. Recommendations on Uniform Color Spaces, Colour Difference Equations, Psychometrics Colour Terms; Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- ASTM G173-03; Standard Tables for Reference Solar Spectral Irradiance at Air Mass 1.5: Direct Normal and Hemispherical for a 37 Degree Tilted Surface. American Society for Testing and Materials (ASTM) International: Conshohocken, PA, USA, 2020.
- CPMA. Classification and Chemical Description of the Complex Inorganic Color Pigments, 4th ed.; Alexandria Dry Color Manufaturers Association: Alexandria, VA, USA, 2010. [Google Scholar]
- Monrós, G. Pigment, Ceramic, in Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-1-4419-8070-0/978-3-642-27851-8. Available online: https://link.springer.com/rwe/10.1007/978-1-4419-8071-7_181 (accessed on 1 October 2025).
- ASTM D3265-21; Standard Test Method for Carbon Black—Tint Strength. American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 2021.
- ISO 5435:2017(E); Rubber Compounding Ingredients, Carbon Black, Determination of Tinting Strength. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Ferch, H.; Seibold, K. Zur Bedeutung und Existenz von Primärteilchen bei hochdispersen Stoffen. Farbe Lack 1984, 90, 88–98. [Google Scholar]
- Ross, C.; Nwaubani, S. Increasing Pigment Black Jetness with Color Pigment Blending, DCL Corporation. Available online: https://www.pigments.com/wp-content/uploads/RETEC-2020-Increasing-Pigment-Black-Jetness-with-Color-Pigment-Blending.pdf (accessed on 1 July 2025).
- Driessens, F.C.M.; Rieck, G.D. Phase Equilibria in the System Cu-Mn-O. Z. Anorg. Und Allg. Chem. 1967, 351, 48–62. [Google Scholar] [CrossRef]
- Trari, M.; Topferb, J.; Dordorc, P.; Grenierc, J.C.; Pouchardc, M.; Doumercc, J.P. Preparation and physical properties of the solid solutions Cu1+xMn1-xO2 (0<x<0.2). J. Solid State Chem. 2005, 178, 2751–2758. [Google Scholar] [CrossRef]
- Huang, X.; Ni, C.; Zhao, G.; Irvine, J.T.S. Oxygen storage capacity and thermal stability of the CuMnO2–CeO2 composite system. J. Mater. Chem. A 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Gabes, Y.; Bouguelia, A.; Trari, M. The physical and photo electrochemical characterization of the crednerite CuMnO2. J. Mater. Sci. 2007, 42, 6469–6476. [Google Scholar] [CrossRef]
- Solsona, B.; Hutchings, G.J.; Garcia, T.; Taylor, S.H. Improvement of the catalytic performance of CuMnOx catalysts for CO oxidation by the addition of Au. New J. Chem. 2004, 28, 708–711. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Huanga, Y.; Wangan, S.; Tsaib, A.; Kameoka, S. Reduction behaviors and catalytic properties for methanol steam reforming of Cu based spinel compounds CuX2O4 (X=Fe, Mn, Al, La). Ceram. Int. 2014, 40, 4541–4551. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, M.; Sunada, K.; Miyauchi, M.; Hashimoto, K. A facile one-step hydrothermal synthesis of rhombohedral CuFeO2 crystals with antivirus property. Chem. Commun. 2012, 48, 7365–7367. [Google Scholar] [CrossRef]
- Matasov, A.; Bush, A.; Kozlov, V.; Stash, A. 3R-CuCrO2 delafosite: Crystal growth, crystal structure, dielectric and DC conductivity properties. Discov. Mater. 2024, 4, 73. [Google Scholar] [CrossRef]
- Sanam, P.K.J.; Shah, M.; Pradyumnan, P.P. Structure induced modification on thermoelectric and optical properties by Mg doping in CuCrO2 nanocrystals. Solid State Commun. 2022, 353, 114855. [Google Scholar] [CrossRef]
- Dondi, M.; Zanelli, C.; Ardit, M.; Cruciani, G.; Mantovani, L.; Tribaudino, M.; Andreozzi, G.B. Ni-free, black ceramic pigments based on Co—Cr—Fe—Mn spinels: A reappraisal of crystal structure, colour and technological behaviour. Ceram. Int. 2013, 39, 9533–9547. [Google Scholar] [CrossRef]
- Prasanna, T.R.S. Angle dependence of the integrated intensity in thin film X-ray diffraction. arXiv 2017, arXiv:1612.01805v2. [Google Scholar] [CrossRef]
- Verger, L.; Olivier, D.; Rousse, G.; Cotte, M.; Cormier, M.L. The Stability of Gahnite Doped with Chromium Pigments in Glazes from the French Manufacture of Sèvres. J. Am. Ceram. Soc. 2016, 100, 86–95. [Google Scholar] [CrossRef]
- Monrós, G.; Badenes, J.A.; Llusar, M.I. Ecofriendly High NIR Reflectance Ceramic Pigments Based on Rare Earths Compared with Classical Chromophores Prepared by DPC Method. Ceramics 2022, 5, 614–641. [Google Scholar] [CrossRef]
- Schorne-Pinto, J.; Cassayre, L.; Presmanes, L.; Barnabé, A. Insights on the Stability and Cationic Nonstoichiometry of CuFeO2 Delafossite. Inorg. Chem. 2019, 58, 6431–6444. [Google Scholar] [CrossRef]
- Li, Y.; Lin, C.; Huang, J.; Chi, C.; Huang, B. Spectrally Selective Absorbers/Emitters for Solar Steam Generation and Radiative Cooling-Enabled Atmospheric Water Harvesting. Glob. Chall. 2021, 5, 2000058. [Google Scholar] [CrossRef]
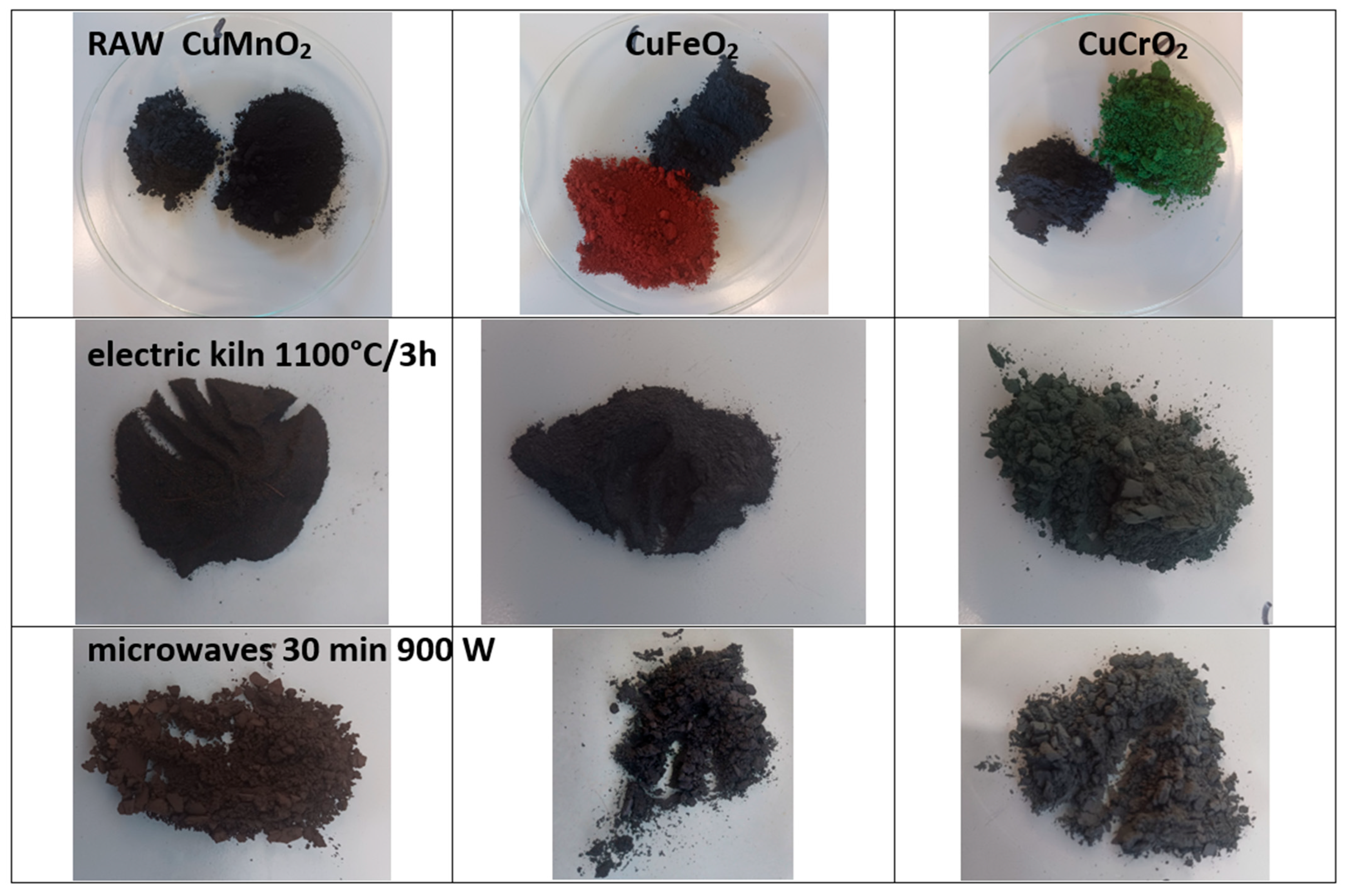
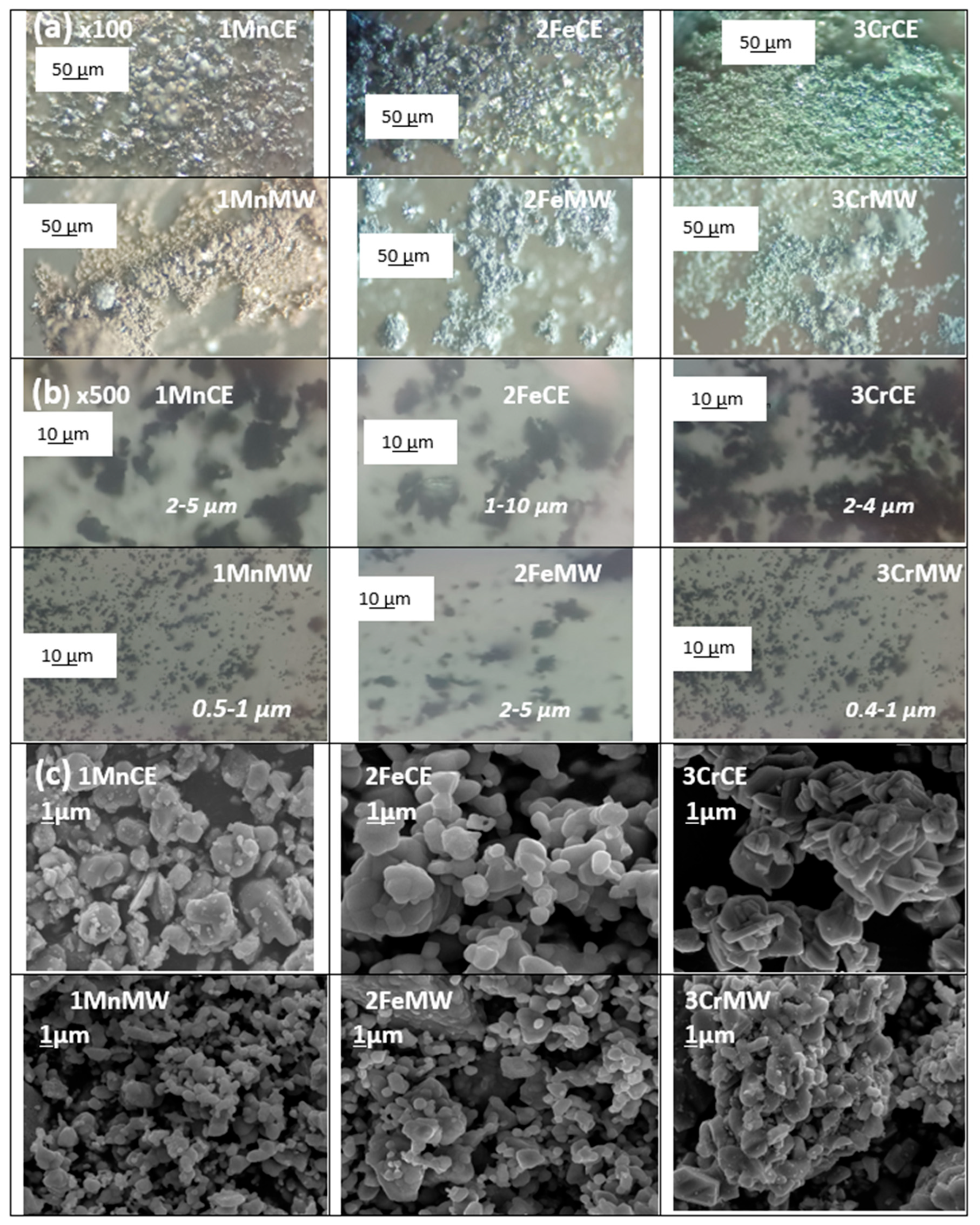
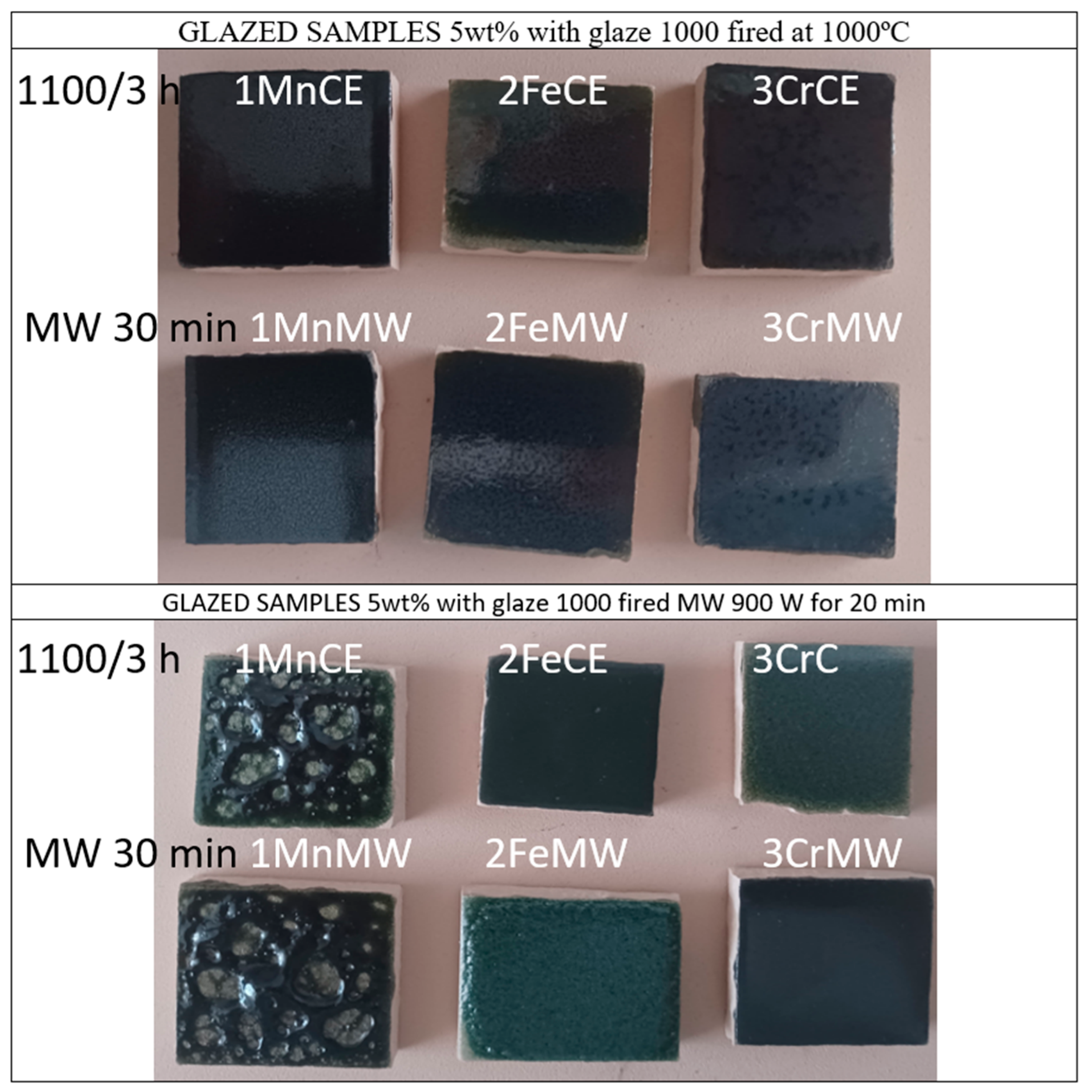

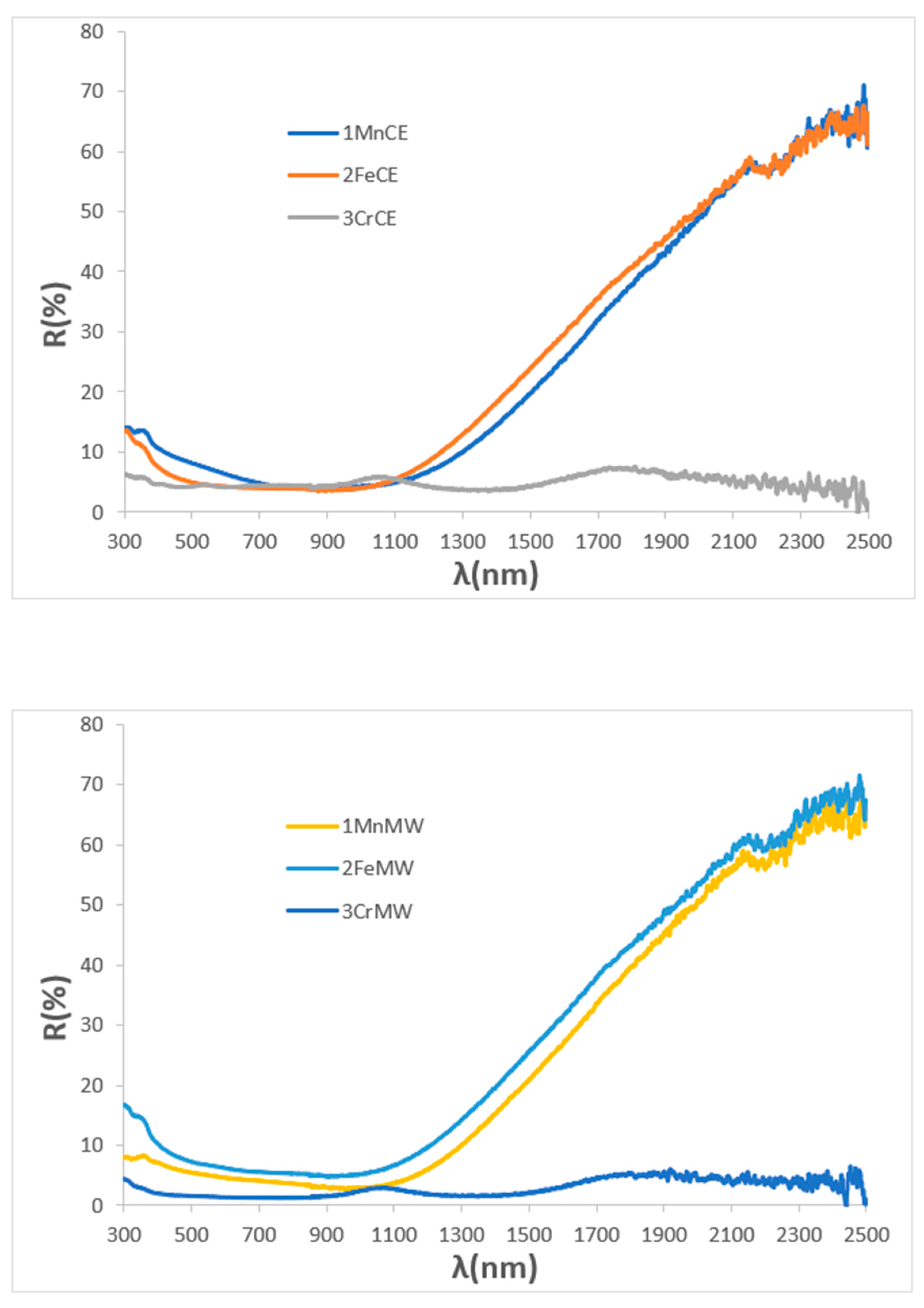
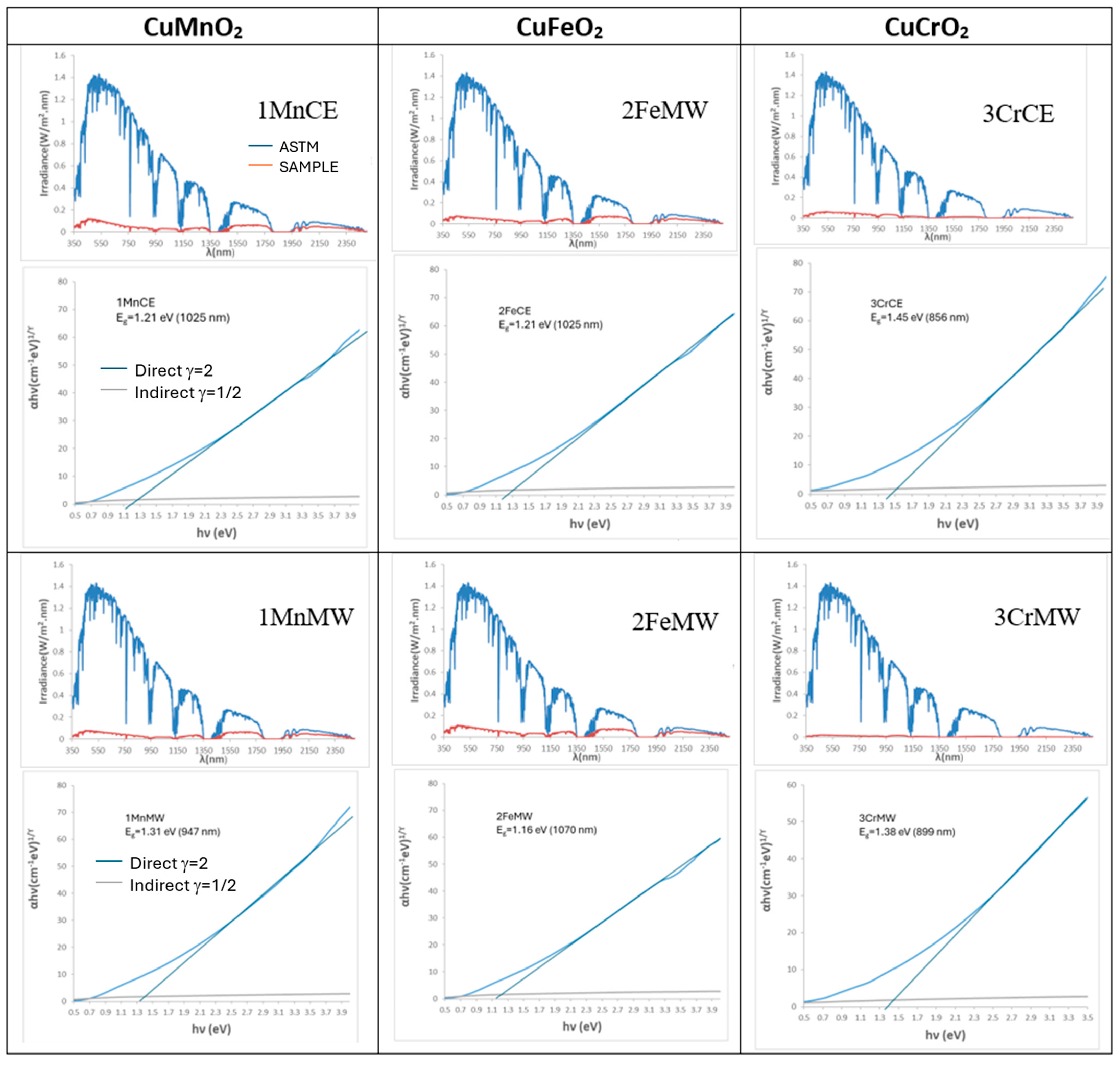
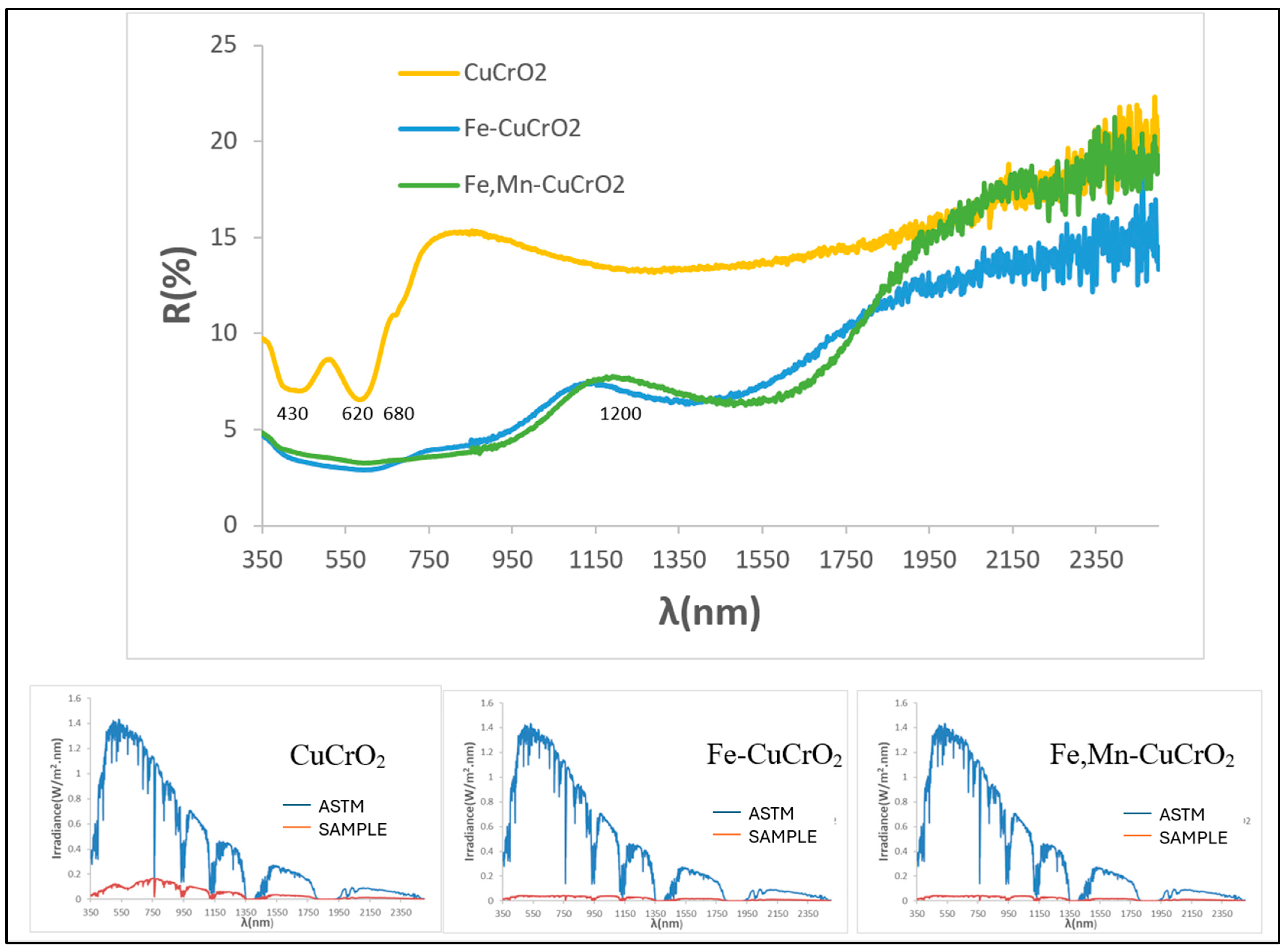

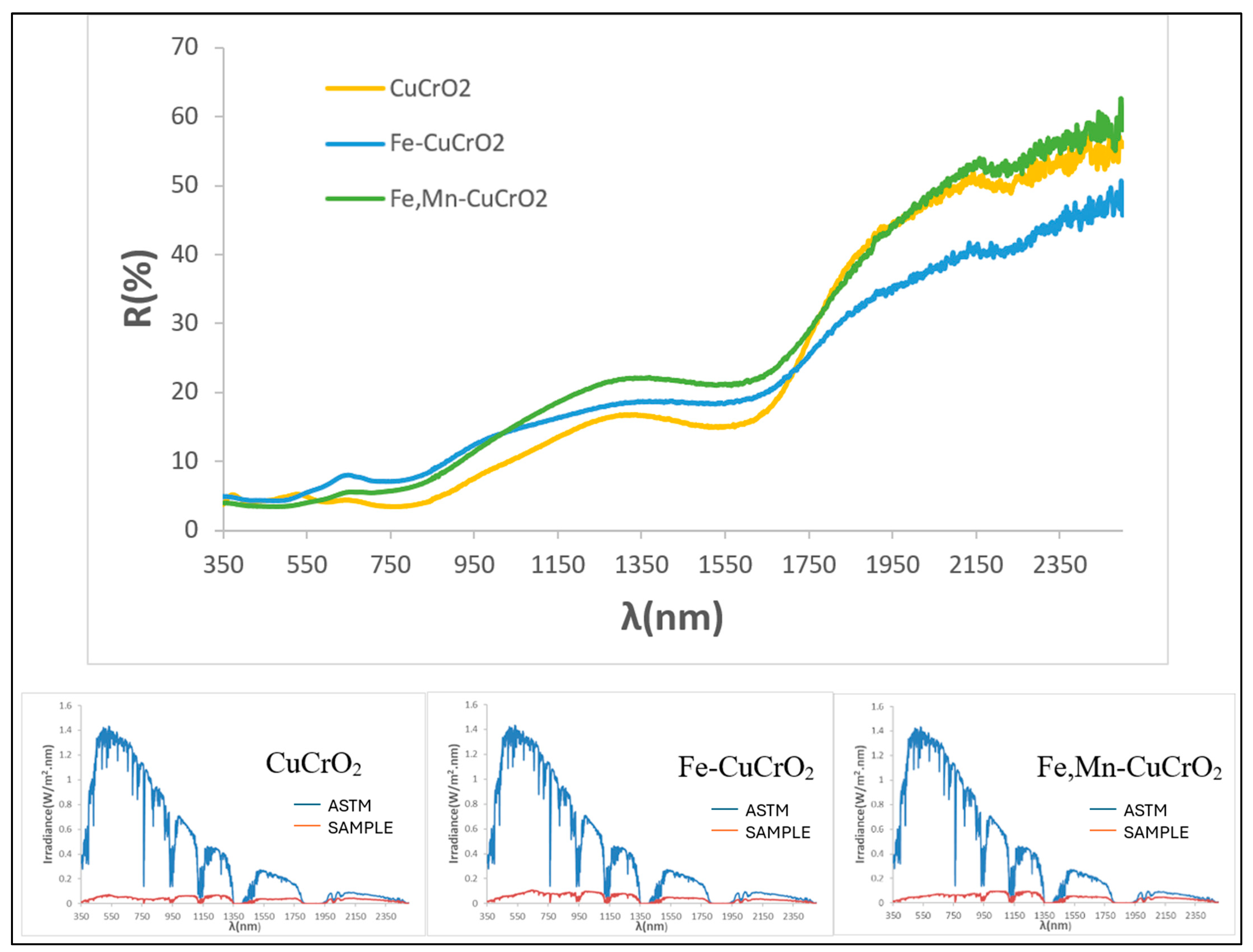
| Powder | Glaze | ||||
|---|---|---|---|---|---|
| Reference | L*a*b* | RVis/RNIR/R (%) | L*a*b* wt%(T°C) | RVis/RNIR/R (%) | |
| Vantablack | [10] | 0.2/0.9/−1 | 0.035/-/- | -- | -- |
| Carbon Black | [11] | 20.2/0.1/0.1 | 3/3/3 | -- | -- |
| Ferrochromite (Co,Ni)(Fe,Cr)2O4 | [13,14] | 41.0/1.0/1.0 | 3.5/-/- | 33.0/1.0/0.05 5(1080) | 4.5/-/- |
| CuCr2O4 | [16] | 40.5/0.1/−0.1 | 4.2/3.4/3.8 | 26.3/−0.3/−0.5 0.5(800) | 3.8/4.5/4.1 |
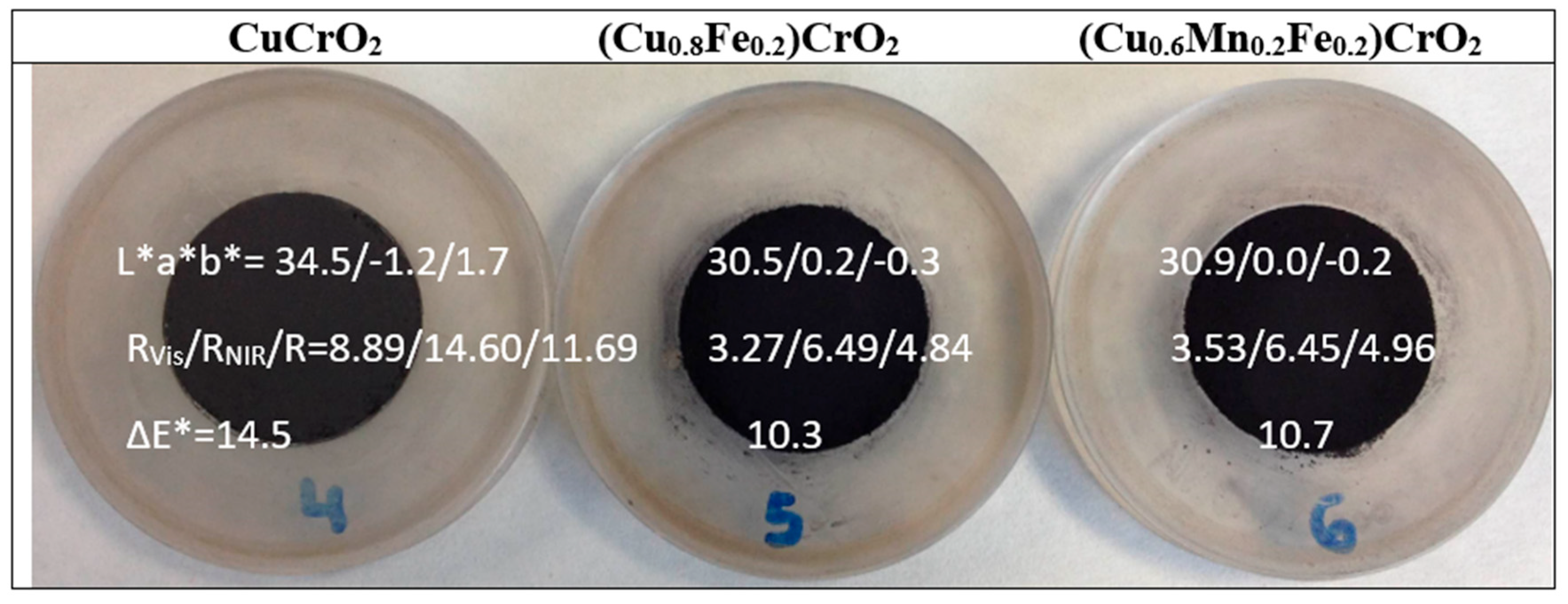
| CuCrO2 | [17] | 36.5/−4.8/−0.8 | 9.9/11.2/10.5 | 11.3/−0.6/−3.05 5(1000) | 1.4/1.9/1.6 |
| Mn,Ni-CuFe5O8 | [18] | 21.36/1.1/−0.24 | 16.95/−0.18/−0.12 | ||
| Fe1.2Cr0.8O3 | [19] | 42.3/−0.3/0.6 | 4/23/14 | 34.1/2.6/−3.75 5(1000) | 4/17/10 |
| YMnO3 | [19] | 27.7/−1.2/−3.5 | 3/39/19 | 31.9/1.6/1.15 5(1000) | 7/31/18 |
| Sr4CuMn2O9 | [19] | 43.9/7.2/6.3 | 7/51/29 | 57.9/2.8/0.25 5(1000) | 25/41/33 |
| Sr2(Mg0.5Mn0.5)Ge2O7 | [19] | 31.8/−0.1/−8.3 | 5/32/17 | 49.6/12.8/13.75 5(1000) | 25/68/44 |
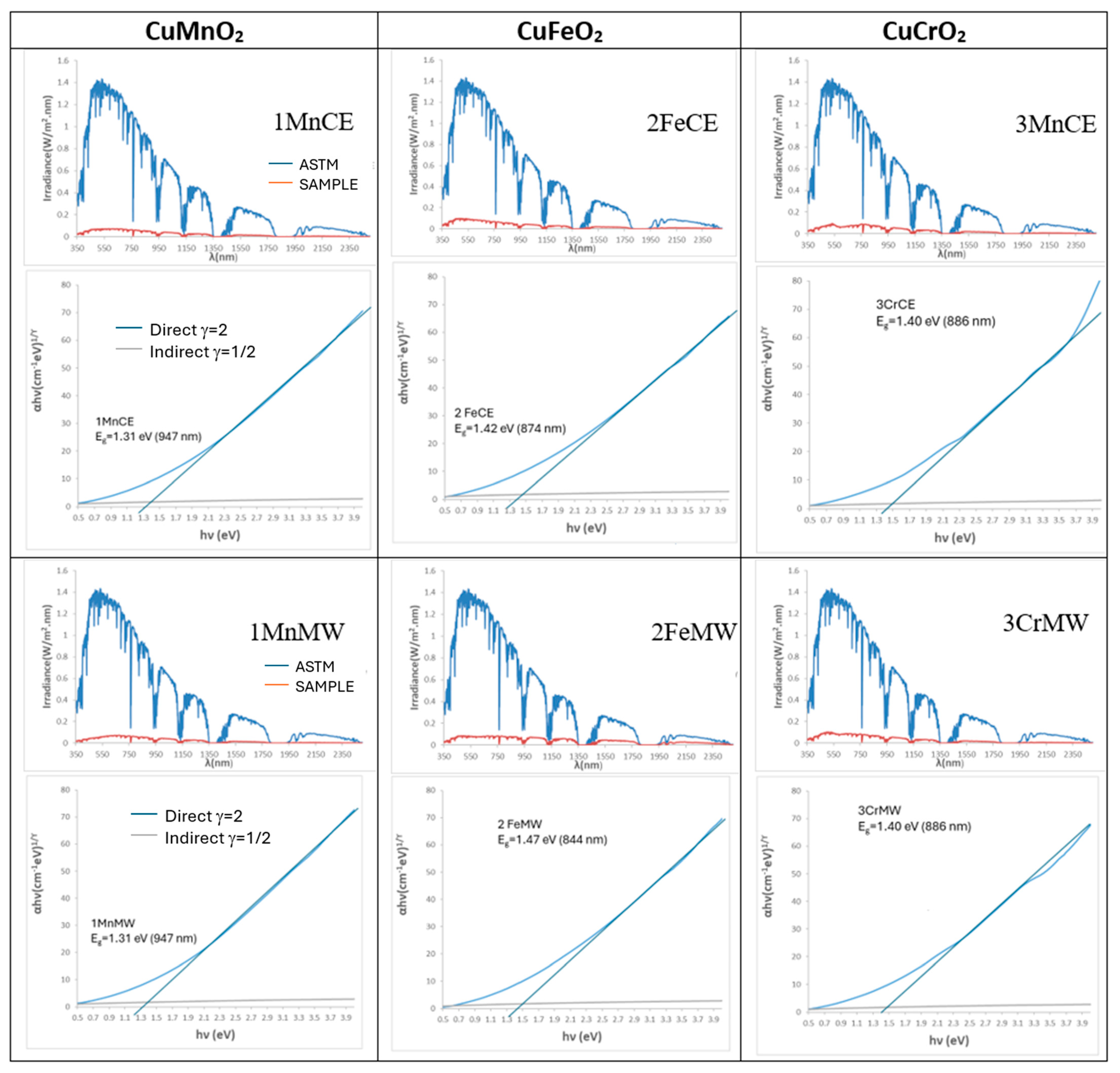
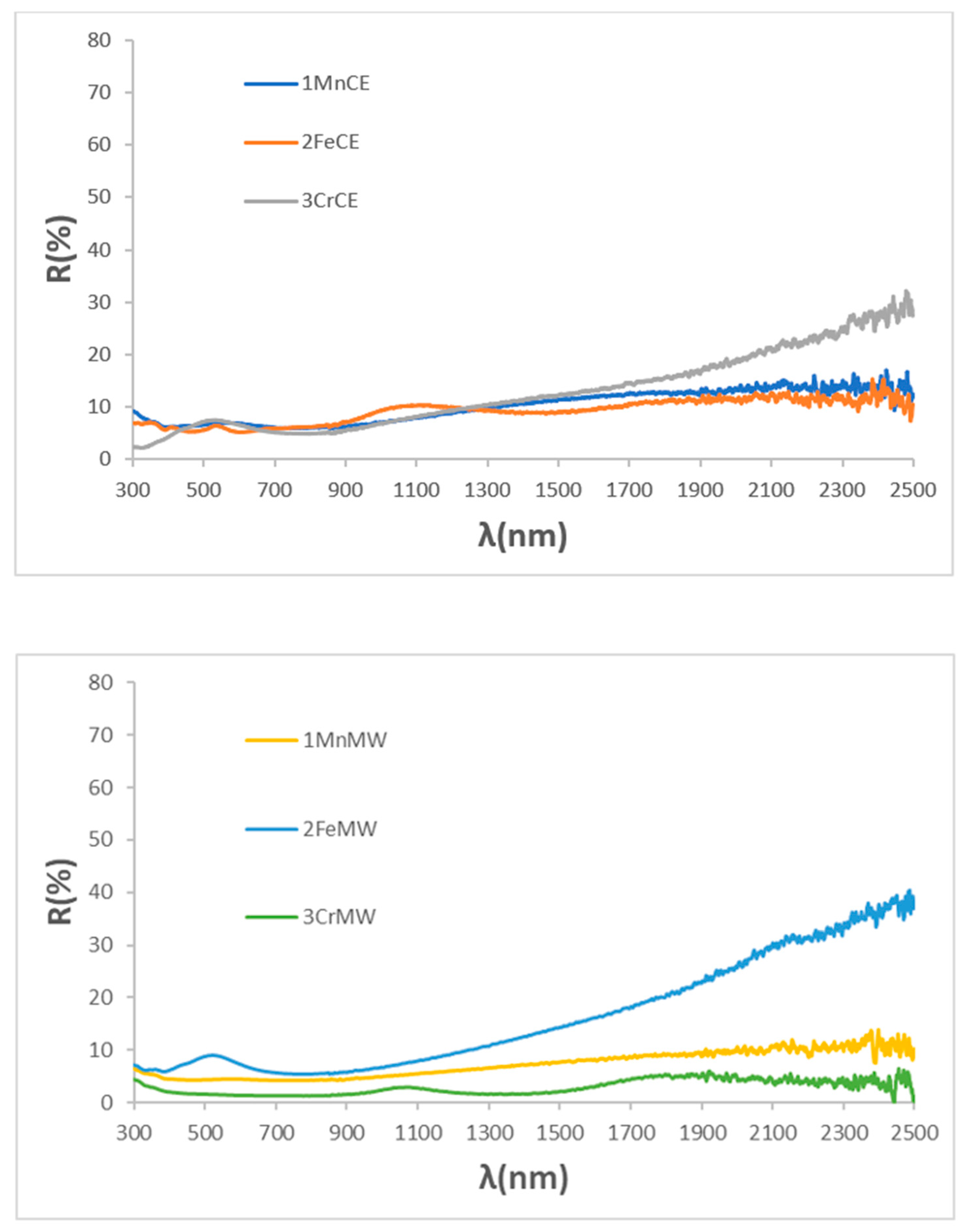
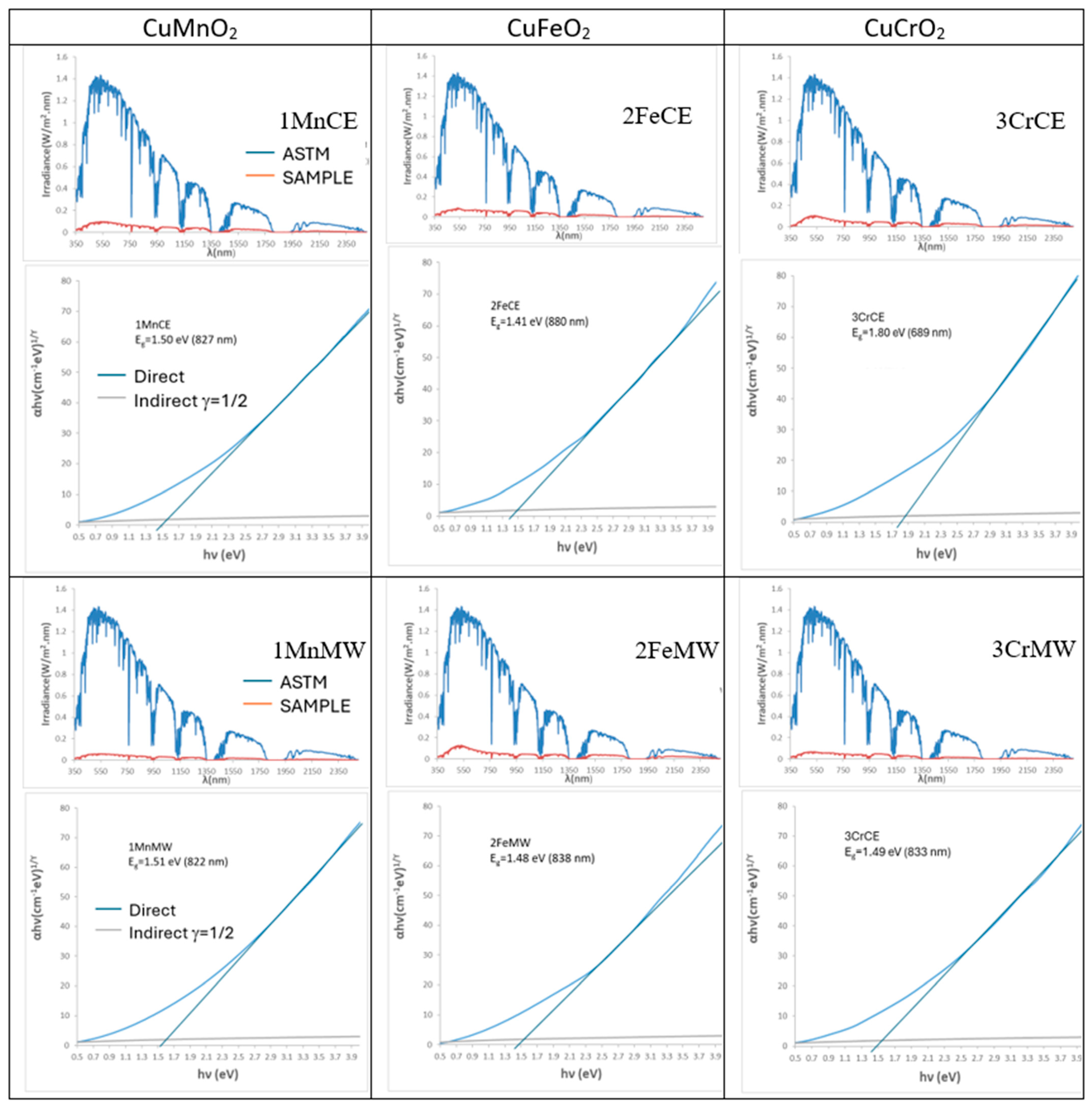
| SAMPLE | L*a*b* | RVis/RNIR/R (%) | Eg (eV) | ΔE* |
|---|---|---|---|---|
| POWDER | ||||
| 1MnCE | 27.32/1.18/0.40 | 5.47/6.06/5.73 | 1.31 | 7.2 |
| 2FeCE | 31.14/−0.29/−2.47 | 6.87/7.10/6.98 | 1.42 | 11.2 |
| 3CrCE | 28.66/−4.17/1.17 | 6.10/8.26/7.03 | 1.40 | 9.5 |
| 1MnMW | 25.23/3.25/5.18 | 4.85/6.36/5.51 | 1.31 | 7.8 |
| 2FeMW | 29.18/0.56/−1.70 | 6.45/13.40/9.52 | 1.47 | 9.2 |
| 3CrMW | 31.05/−2.39/−0.15 | 6.98/7.73/7.31 | 1.40 | 11.1 |
| GLAZED ELECTRIC KILN 5 wt% with glaze 1000 | ||||
| 1MnCE | 32.49/−1.93/−6.73 | 7.23/13.11/9.88 | 1.21 | 14.2 |
| 2FeCE | 25.53/0.60/−5.93 | 5.02/13.99/9.01 | 1.21 | 8.1 |
| 3CrCE | 24.70/−0.77/0.32 | 4.40/4.81/4.58 | 1.45 | 4.6 |
| 1MnMW | 26.80/−0.63/−4.90 | 5.10/12.51/8.41 | 1.31 | 8.3 |
| 2FeMW | 31.25/−0.12/−6.04 | 7.14/15.40/10.82 | 1.16 | 12.6 |
| 3CrMW | 23.52/−0.02/−0.98 | 1.41/2.14/1.77 | 1.38 | 3.5 |
| GLAZED MICROWAVES 5 wt% with glaze 1000 | ||||
| 1MnCE | 31.10/−0.77/1.77 | 6.37/8.50/7.32 | 1.50 | 11.1 |
| 2FeCE | 28.60/−2.63/1.25 | 5.70/8.79/7.06 | 1.41 | 8.9 |
| 3CrCE | 31.94/−4.67/3.88 | 5.95/9.42/7.50 | 1.80 | 13.2 |
| 1MnMW | 24.64/0.02/0.40 | 4.27/5.93/5.01 | 1.51 | 4.5 |
| 2FeMW | 34.30/−5.94/2.31 | 6.99/10.66/8.64 | 1.48 | 15.4 |
| 3CrMW | 24.25/−1.41/−0.23 | 2.63/6.63/5.42 | 1.49 | 4.3 |
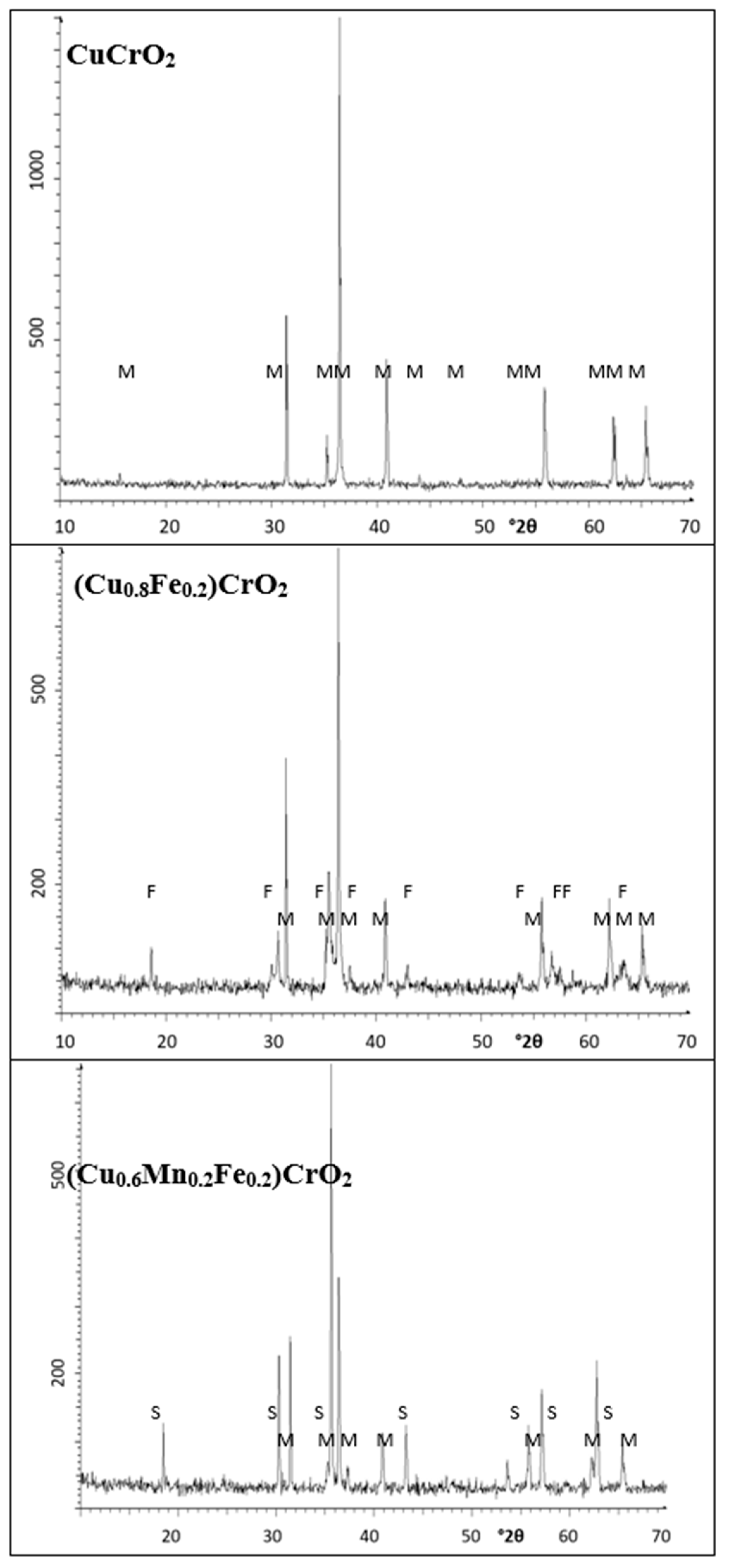
| SAMPLE | Crystalline Phases |
|---|---|
| 11MnCE | S (vs) C (m) U, B (vw) |
| 2FeCE | D (vs) U, B (vw) |
| 3CrCE | M (s) E, U (vw) |
| 1MnMW | S (s) C (w) U, B (vw) |
| 2FeMW | D (vs) U (s) |
| 3CrMW | M (s) T (vw) |
| Sample | Identified Crystalline Phases | References | |
|---|---|---|---|
| CuMnO2-CE | CuMn2O4 cubic (ICDD 74–2422) a = 8.308(1) Å | CuMnO2 monoclinic (ICDD 50-0860) β = 104.08° a = 5.677(1) Å b = 2.801(2) Å c = 6.063(2) Å V = 93.5(3) Å3 | [34] CuMnO2 monoclinic (C2/m ICDD 50-0860) β = 104.03(3)° a = 5.592(3) Å b = 2.883(1) Å c= 5.892(3) Å V = 92.1(1) Å3 [35] β = 104.02(3)° a = 5.596(2) Å b = 2.880(1) Å c = 5.899(2) Å [38] CuMn2O4 cubic Fd3m ICDD 74-2422 a = 8.308 Å |
| CuMnO2-MW | CuMn2O4 cubic ICDD 74–2422) a= 8.308(1) Å | -- | |
| CuFeO2-CE | CuFeO2 (ICDD-75-2146) a = b = 3.093(1) Å c = 17.109(1) Å V = 136.0(3) Å3 | -- | [39] (ICDD-75-2146) a = b = 3.04 Å c = 17.15 Å V = 136.8668 Å3 |
| CuFeO2-MW | CuFeO2 (ICDD-75-2146) a = b = 3.002(1) Å c = 17.392(1) Å V = 135.8(3) Å3 | -- | |
| CuCrO2-CE | CuCrO2 (ICDD 39-0247) a = b = 2.968(5) Å c = 17.002(3) Å V= 129.7(4) Å3 | -- | [40,41] ICDD 39-0247 a = 2.9480(2) Å c = 17.033(2) Å V = 127.9731 Å3 |
| CuCrO2-MW | CuCrO2 (ICDD 39-0247) a = b = 2.967(4) Å c = 17.021(2) Å V = 129.9(5) Å3 | -- | |
| SAMPLE | L*a*b* | RVis/RNIR/R (%) | Eg (eV) | ΔE* |
|---|---|---|---|---|
| POWDER | ||||
| Electric kiln | ||||
| 3CrCE 1000 °C/3 h | 42.6/−5.4/0.1 | 13.8/14.1/13.9 | 1.26 | 23.1 |
| 3CrCE 1100 °C/3 h | 28.66/−4.17/1.17 | 6.10/8.26/7.03 | 1.40 | 9.5 |
| Microwave Kiln | ||||
| 3CrMW 800 W 30 min | 36.5/−4.8/−0.8 | 9.9/11.2/10.5 | 1.30 | 17 |
| 3CrMW 900 W 30 min | 31.05/−2.39/−0.15 | 6.98/7.73/7.31 | 1.40 | 11.1 |
| GLAZED ELECTRIC KILN 5 wt% with glaze 1000 | ||||
| Electric kiln | ||||
| 3CrCE 1000 °C/3 h | 26.9/−0.1/−2.3 | 5.3/5.0/5.2 | 1.0 | 7.2 |
| 3CrCE 1100 °C/3 h | 24.70/−0.77/0.32 | 4.40/4.81/4.58 | 1.45 | 4.6 |
| Microwave Kiln | ||||
| 3CrMW 800 W 30 min | 11.3/−0.6/−3.0 | 1.4/1.9/1.6 | 0.93 | 9.5 |
| 3CrMW 900 W 30 min | 23.52/−0.02/−0.98 | 1.41/2.14/1.77 | 1.38 | 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monrós, G.; Esteve, V.; Delgado, C.; Monrós-Andreu, G.; Llusar, M. In Search of Ultra-Black Ceramic Pigments Using Microwaves: Delafossite Cuprates CuMO2 (M = Mn, Fe, Cr). Materials 2025, 18, 4910. https://doi.org/10.3390/ma18214910
Monrós G, Esteve V, Delgado C, Monrós-Andreu G, Llusar M. In Search of Ultra-Black Ceramic Pigments Using Microwaves: Delafossite Cuprates CuMO2 (M = Mn, Fe, Cr). Materials. 2025; 18(21):4910. https://doi.org/10.3390/ma18214910
Chicago/Turabian StyleMonrós, Guillermo, Vicente Esteve, Carolina Delgado, Guillem Monrós-Andreu, and Mario Llusar. 2025. "In Search of Ultra-Black Ceramic Pigments Using Microwaves: Delafossite Cuprates CuMO2 (M = Mn, Fe, Cr)" Materials 18, no. 21: 4910. https://doi.org/10.3390/ma18214910
APA StyleMonrós, G., Esteve, V., Delgado, C., Monrós-Andreu, G., & Llusar, M. (2025). In Search of Ultra-Black Ceramic Pigments Using Microwaves: Delafossite Cuprates CuMO2 (M = Mn, Fe, Cr). Materials, 18(21), 4910. https://doi.org/10.3390/ma18214910










