Structure and Energetics of Chemically Functionalized Silicene: Combined Density Functional Theory and Machine Learning Approach
Abstract
1. Introduction
2. Methods
3. Results and Discussion
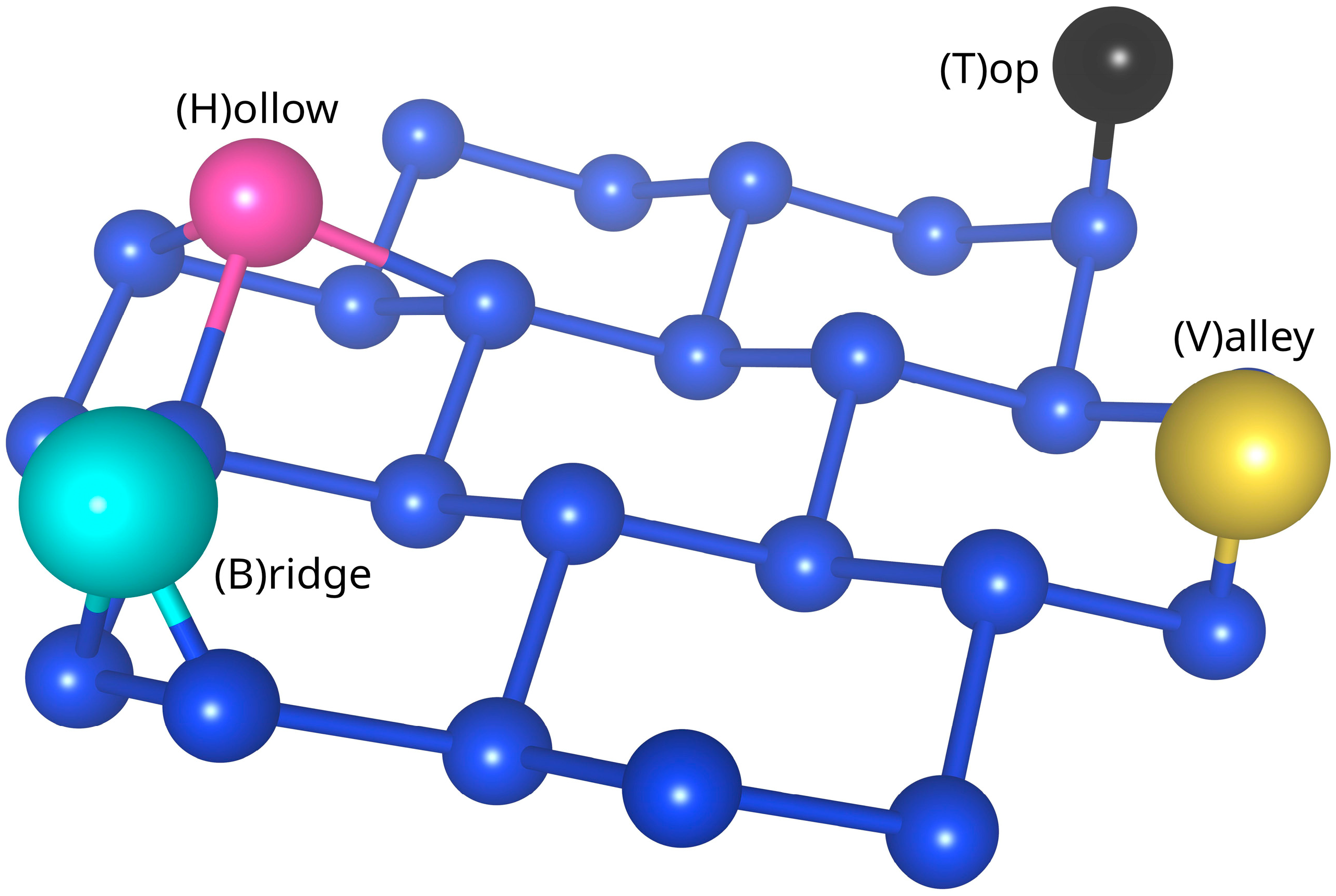
3.1. Input Data Preparation
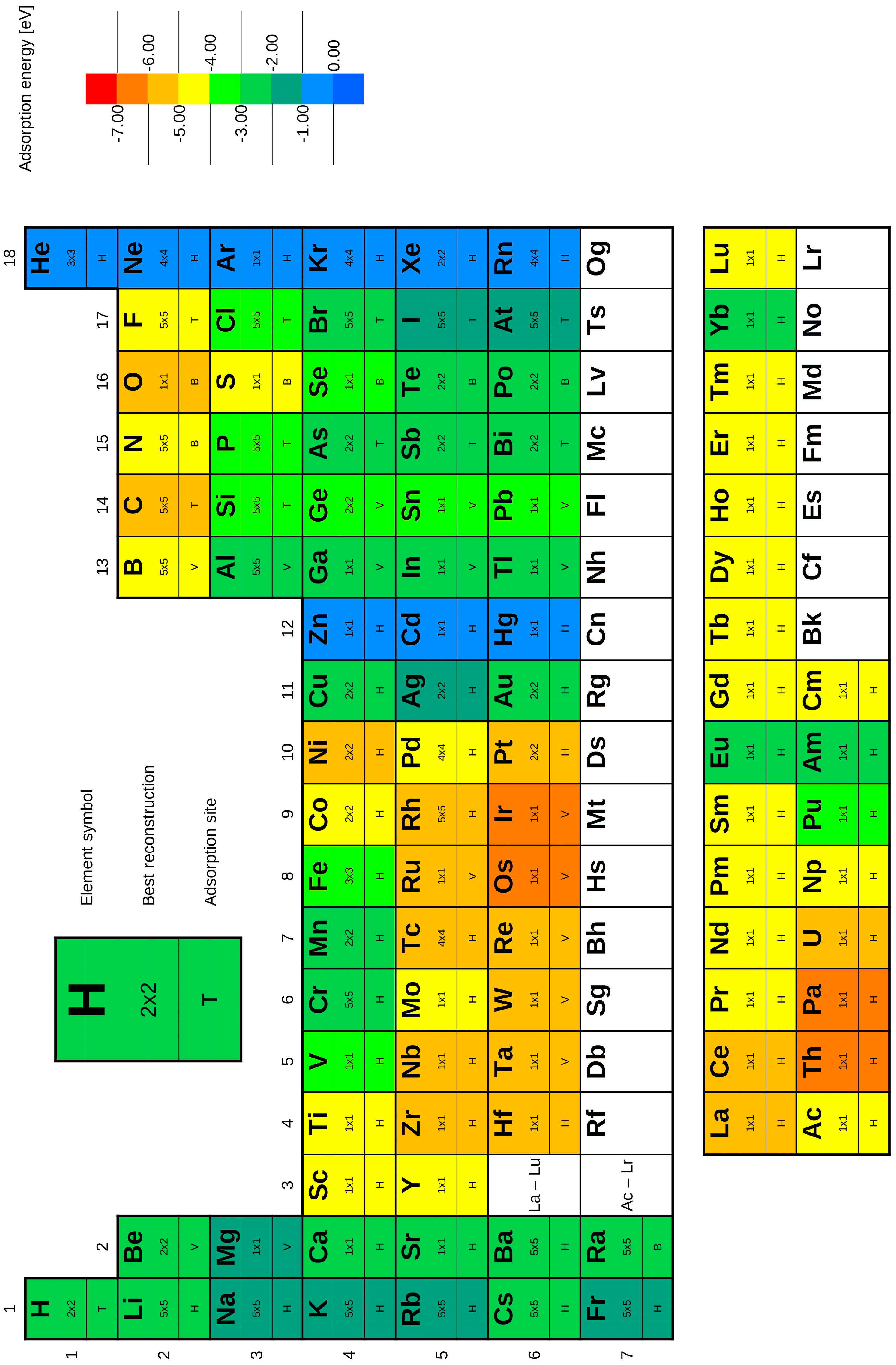
3.2. DFT Calculations
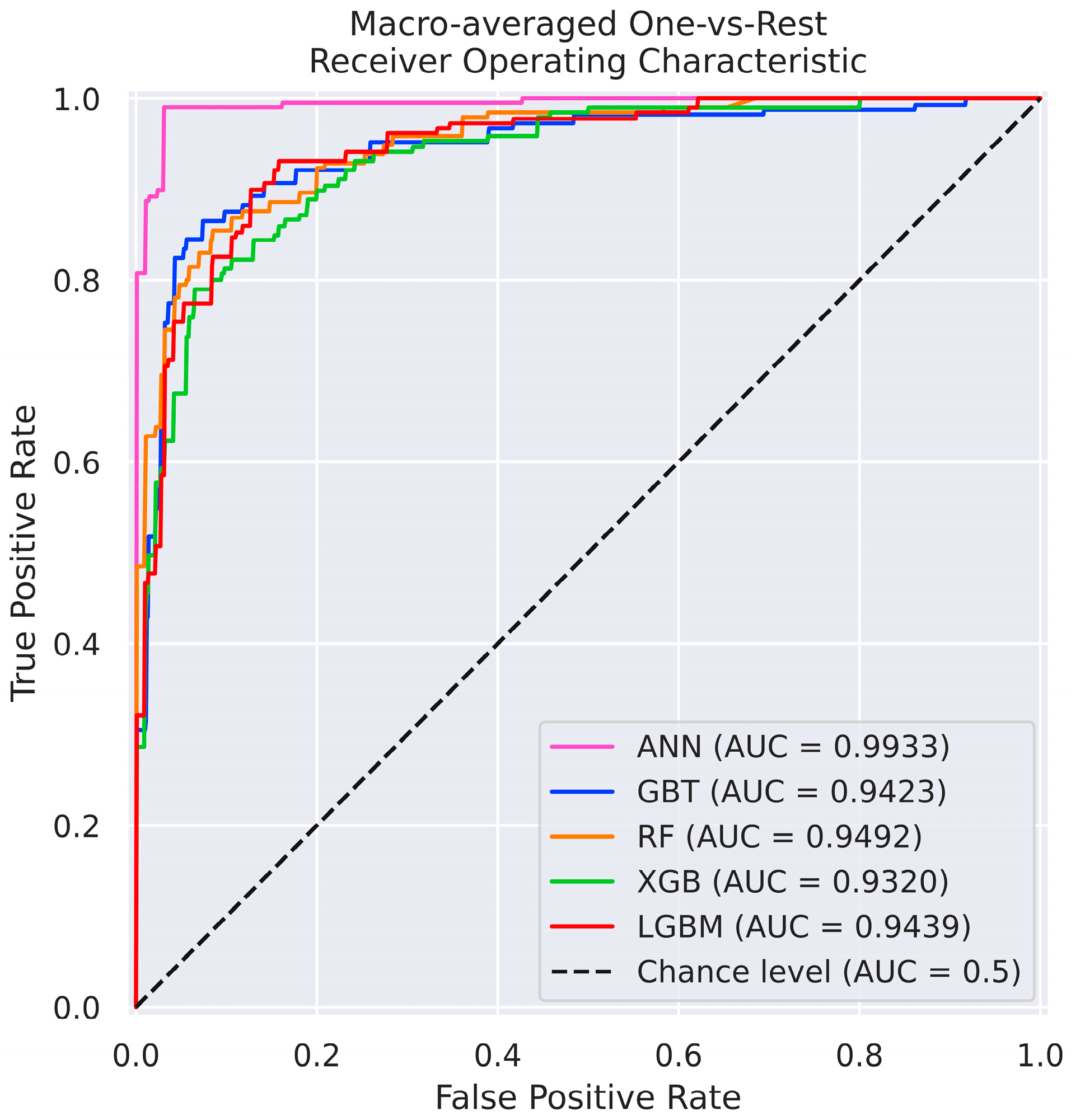
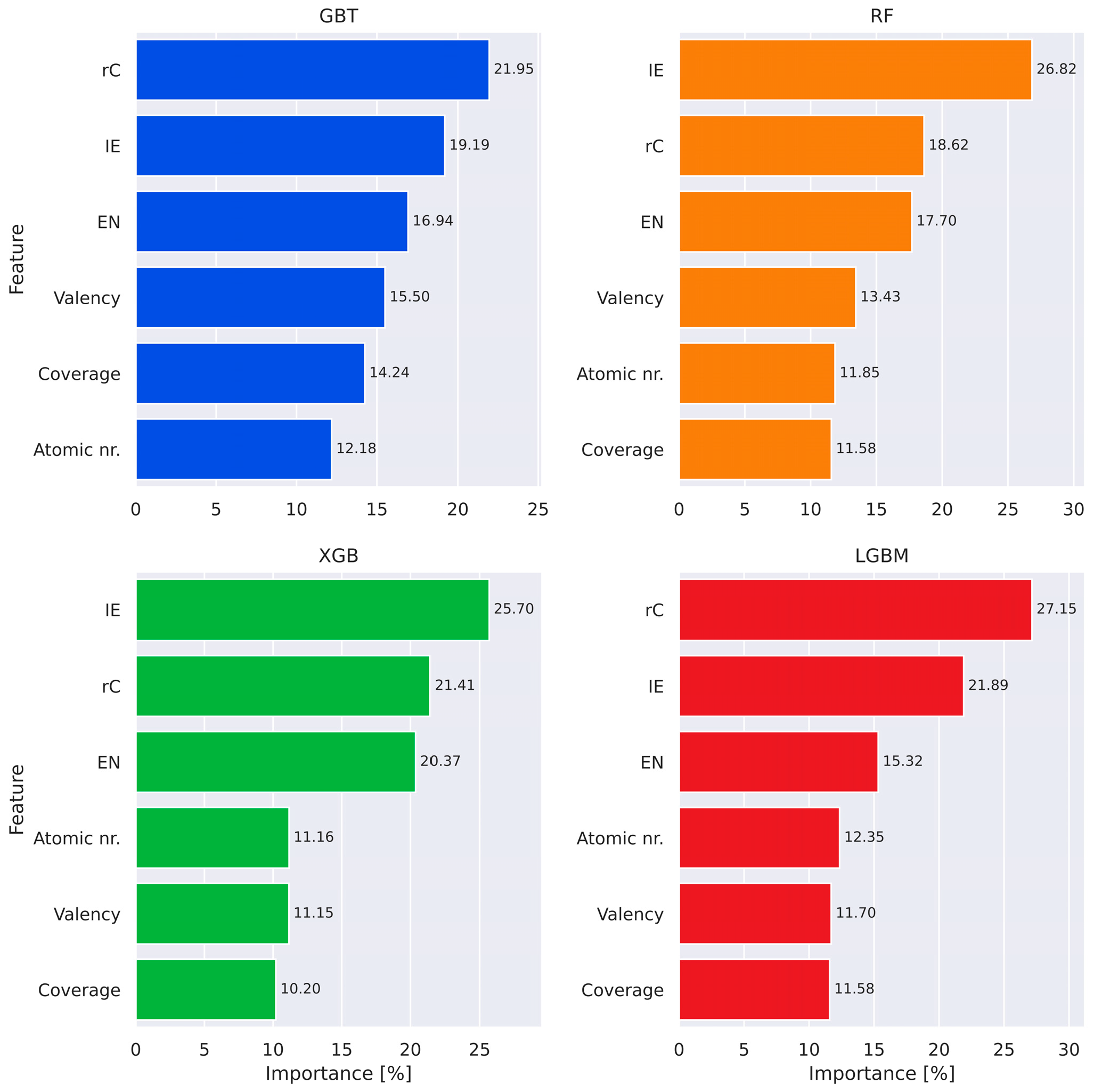
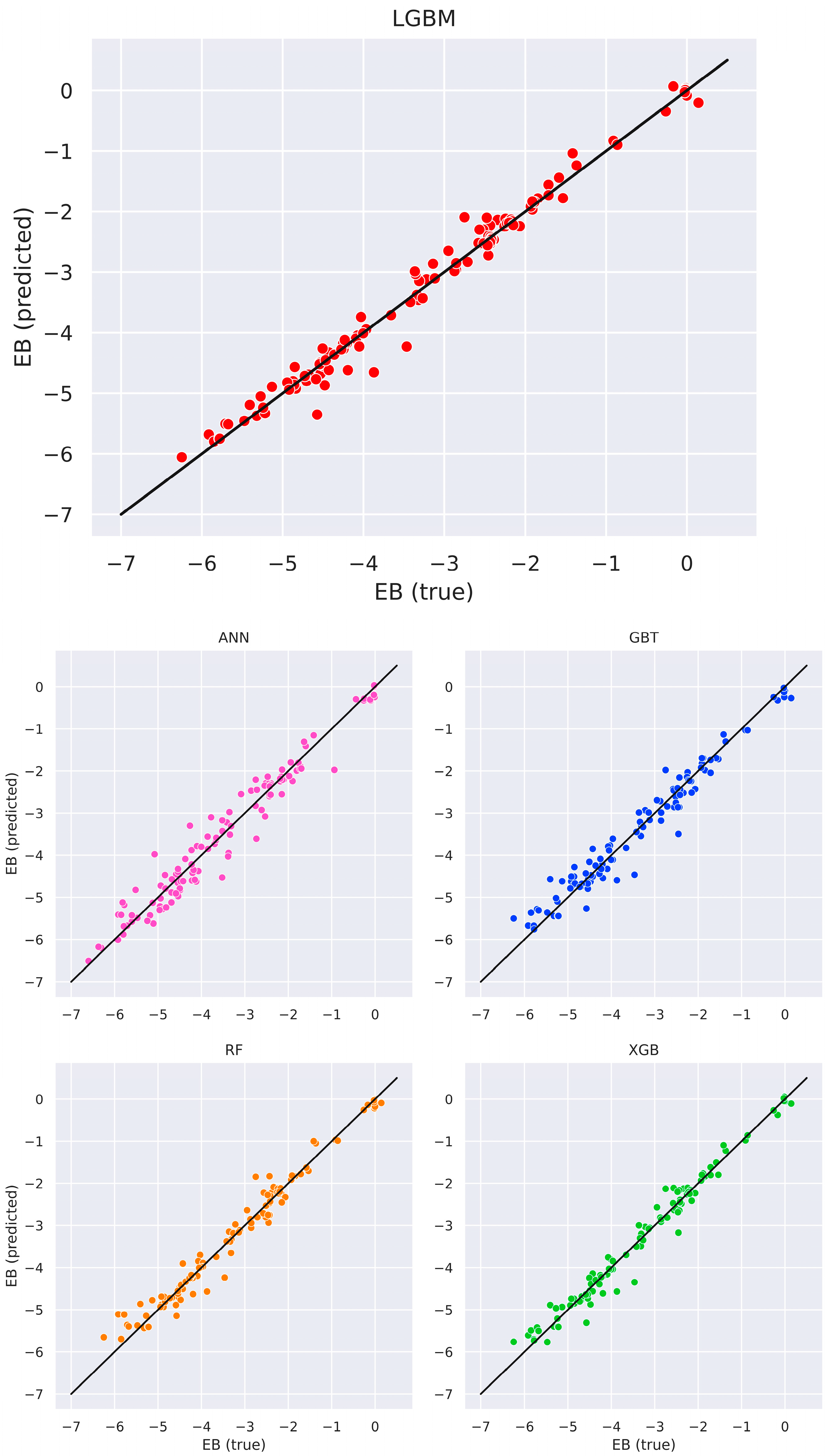
3.3. ML Models Benchmarking
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kharadi, M.A.; Malik, G.F.A.; Khanday, F.A.; Shah, K.A.; Mittal, S.; Kaushik, B.K. Review—Silicene: From Material to Device Applications. ECS J. Solid State Sci. Technol. 2020, 9, 115031. [Google Scholar] [CrossRef]
- Giri, S.; Nanda, S.K.; Parida, A. Recent Experimental and Theoretical Progress in Silicene Based Electrode Materials for Rechargeable Batteries and Supercapacitors. J. Energy Storage 2025, 113, 115521. [Google Scholar] [CrossRef]
- Ghosal, S.; Bandyopadhyay, A.; Chowdhury, S.; Jana, D. A Review on Transport Characteristics and Bio-Sensing Applications of Silicene. Rep. Prog. Phys. 2023, 86, 096502. [Google Scholar] [CrossRef]
- Galashev, A.Y. Prospects for Using Silicene as an Anode for Lithium-Ion Batteries. A Review. J. Energy Storage 2024, 93, 112281. [Google Scholar] [CrossRef]
- Kopciuszyński, M.; Stȩpniak-Dybala, A.; Zdyb, R.; Krawiec, M. Emergent Dirac Fermions in Epitaxial Planar Silicene Heterostructure. Nano Lett. 2024, 24, 2175–2180. [Google Scholar] [CrossRef]
- Stȩpniak-Dybala, A.; Krawiec, M. Formation of Silicene on Ultrathin Pb(111) Films. J. Phys. Chem. C 2019, 123, 17019–17025. [Google Scholar] [CrossRef]
- Stępniak-Dybala, A.; Dyniec, P.; Kopciuszyski, M.; Zdyb, R.; Jałochowski, M.; Krawiec, M. Planar Silicene: A New Silicon Allotrope Epitaxially Grown by Segregation. Adv. Funct. Mater. 2019, 29, 1906053. [Google Scholar] [CrossRef]
- Krawiec, M. Functionalization of Group-14 Two-Dimensional Materials. J. Phys. Condens. Matter 2018, 30, 233003. [Google Scholar] [CrossRef]
- Ni, Z.; Liu, Q.; Tang, K.; Zheng, J.; Zhou, J.; Qin, R.; Gao, Z.; Yu, D.; Lu, J. Tunable Bandgap in Silicene and Germanene. Nano Lett. 2012, 12, 113–118. [Google Scholar] [CrossRef]
- Ezawa, M. Valley-Polarized Metals and Quantum Anomalous Hall Effect in Silicene. Phys. Rev. Lett. 2012, 109, 055502. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lui, C.H.; Shan, J.; Heinz, T.F. Observation of an Electric-Field-Induced Band Gap in Bilayer Graphene by Infrared Spectroscopy. Phys. Rev. Lett. 2009, 102, 256405. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Zhu, Z.Y.; Schwingenschlögl, U. Doped Silicene: Evidence of a Wide Stability Range. Europhys. Lett. 2011, 95, 17005. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y. Strain-Induced Self-Doping in Silicene and Germanene from First-Principles. Solid State Commun. 2013, 155, 6–11. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Makkar, P.; Ghosh, N.N. A Review on the Use of DFT for the Prediction of the Properties of Nanomaterials. RSC Adv. 2021, 11, 27897–27924. [Google Scholar] [CrossRef]
- Liang, J.; Zhen, P.; Gan, P.; Li, Y.; Tong, M.; Liu, W. DFT Calculation of Nonperiodic Small Molecular Systems to Predict the Reaction Mechanism of Advanced Oxidation Processes: Challenges and Perspectives. ACS EST Eng. 2024, 4, 4–18. [Google Scholar] [CrossRef]
- Chafiq, M.; Thari, F.Z.; Lee, H.; Chaouiki, A.; Salghi, R.; Ko, Y.G.; Karrouchi, K.; Bougrin, K.; Ali, I.H.; Lgaz, H. Experimental and First-Principles DFT Insights into the Corrosion Protection Mechanism of Carbon Steel in an HCl Medium by Two Thiazolidinedione Compounds. Mater. Today Commun. 2022, 32, 103841. [Google Scholar] [CrossRef]
- Tu, H.; Tian, B.; Zhao, Z.; Guo, R.; Wang, Y.; Chen, S.; Wu, J. Research on the Influence of G-C3N4 Microstructure Changes on the Efficiency of Visible Light Photocatalytic Degradation. Water Res. X 2025, 28, 100315. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Din, H.U.; Nawaz, S.; Nguyen, S.-T.; Nguyen, C.Q.; Nguyen, C.V. First Principles Study of the Adsorption of Alkali Metal Ions (Li, Na, and K) on Janus WSSe Monolayer for Rechargeable Metal-Ion Batteries. Appl. Surf. Sci. 2023, 632, 157545. [Google Scholar] [CrossRef]
- Kodratoff, Y.; Moustakis, V.; Graner, N. Can Machine Learning Solve My Problem? Appl. Artif. Intell. 1994, 8, 1–31. [Google Scholar] [CrossRef]
- Ward, L.; Agrawal, A.; Choudhary, A.; Wolverton, C. A General-Purpose Machine Learning Framework for Predicting Properties of Inorganic Materials. Npj Comput. Mater. 2016, 2, 16028. [Google Scholar] [CrossRef]
- Tao, Q.; Xu, P.; Li, M.; Lu, W. Machine Learning for Perovskite Materials Design and Discovery. Npj Comput. Mater. 2021, 7, 23. [Google Scholar] [CrossRef]
- Allen, A.E.A.; Lubbers, N.; Matin, S.; Smith, J.; Messerly, R.; Tretiak, S.; Barros, K. Learning Together: Towards Foundation Models for Machine Learning Interatomic Potentials with Meta-Learning. Npj Comput. Mater. 2024, 10, 154. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F.; Yi, L.; Yi, H.; Yu, Q.; Zhao, S.; Zhou, Y.; Wang, Y.; Tang, X. Machine Learning Perspective: Revealing Deep Mechanisms and New Advances in Adsorption and Catalysis of Gaseous Molecules. Appl. Energy 2025, 396, 126241. [Google Scholar] [CrossRef]
- Lian, L.; Li, W.; Zhang, Y.; Gong, X.; Hu, W.; Liu, Y. Design of Ni-Based Single Crystal Superalloys by Machine Learning Based on Data-Driven Multi-Task Optimization. Comput. Mater. Sci. 2025, 256, 113969. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; Zhang, Y.; Wang, G.; Sun, K.; Xu, M.; Chen, D.; Jia, P. Comprehensive Screening-Driven Mo2-C6N8 Monolayer Design for Ultra-Sensitive GIS Insulation Failure Gas Sensing: Mechanistic Insights into Gas-Solid Interactions and Multifunctional Sensor Validation. Surf. Interfaces 2025, 69, 106801. [Google Scholar] [CrossRef]
- Rupp, M.; Tkatchenko, A.; Müller, K.-R.; von Lilienfeld, O.A. Fast and Accurate Modeling of Molecular Atomization Energies with Machine Learning. Phys. Rev. Lett. 2012, 108, 058301. [Google Scholar] [CrossRef]
- Xu, P.; Ji, X.; Li, M.; Lu, W. Small Data Machine Learning in Materials Science. npj Comput. Mater. 2023, 9, 42. [Google Scholar] [CrossRef]
- Li, Q.; Fu, N.; Omee, S.S.; Hu, J. MD-HIT: Machine Learning for Material Property Prediction with Dataset Redundancy Control. npj Comput. Mater. 2024, 10, 245. [Google Scholar] [CrossRef]
- Wang, Y.; Ghaffari, B.; Taylor, C.; Lekakh, S.; Li, M.; Fan, Y. Predicting the Energetics and Kinetics of Cr Atoms in Fe-Ni-Cr Alloys via Physics-Based Machine Learning. Scr. Mater. 2021, 205, 114177. [Google Scholar] [CrossRef]
- Lan, J.; Palizhati, A.; Shuaibi, M.; Wood, B.M.; Wander, B.; Das, A.; Uyttendaele, M.; Zitnick, C.L.; Ulissi, Z.W. AdsorbML: A Leap in Efficiency for Adsorption Energy Calculations Using Generalizable Machine Learning Potentials. npj Comput. Mater. 2023, 9, 172. [Google Scholar] [CrossRef]
- Liu, J.; Lv, H.; Wang, P.; Hou, G.; Zhao, Y.; Zhang, X.; Huang, Q. Synergistic Machine Learning and DFT Screening Strategy: Accelerating Discovery of Efficient Perovskite Passivators. J. Energy Chem. 2026, 112, 56–63. [Google Scholar] [CrossRef]
- Hakala, M.; Kronberg, R.; Laasonen, K. Hydrogen Adsorption on Doped MoS2 Nanostructures. Sci. Rep. 2017, 7, 15243. [Google Scholar] [CrossRef]
- Fung, V.; Hu, G.; Wu, Z.; Jiang, D. Descriptors for Hydrogen Evolution on Single Atom Catalysts in Nitrogen-Doped Graphene. J. Phys. Chem. C 2020, 124, 19571–19578. [Google Scholar] [CrossRef]
- Park, H.; Geum, D.-M.; Kim, H.J. Accelerating Hydrogen Evolution Catalyst Discovery via Data-Driven Strategy for High-Performance Single-Atom Catalysts Embedded in h-BN. J. Energy Chem. 2025, 107, 750–758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Friedman, J.H. Greedy Function Approximation: A Gradient Boosting Machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, New York, NY, USA, 13–17 August 2016; Association for Computing Machinery: New York, NY, USA, 2016; pp. 785–794. [Google Scholar] [CrossRef]
- Ke, G.; Meng, Q.; Finley, T.; Wang, T.; Chen, W.; Ma, W.; Ye, Q.; Liu, T.-Y. LightGBM: A Highly Efficient Gradient Boosting Decision Tree. In Proceedings of the 31st International Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Curran Associates Inc.: Red Hook, NY, USA, 2017; pp. 3149–3157. [Google Scholar]
- Abadi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. TensorFlow: A System for Large-Scale Machine Learning. In Proceedings of the 12th USENIX Conference on Operating Systems Design and Implementation, Savannah, GA, USA, 2–4 November 2016; USENIX Association: Berkeley, CA, USA, 2016; pp. 265–283. [Google Scholar]
- Chollet, F. Keras. GitHub Repos. 2015. Available online: https://github.com/fchollet/keras (accessed on 17 April 2025).
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent Radii Revisited. Dalton Trans. 2008, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- PubChem Ionization Energy|Periodic Table of Elements. Available online: https://pubchem.ncbi.nlm.nih.gov/periodic-table/ionization-energy (accessed on 18 March 2025).
- PubChem Electronegativity|Periodic Table of Elements. Available online: https://pubchem.ncbi.nlm.nih.gov/periodic-table/electronegativity (accessed on 18 March 2025).
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1997; ISBN 978-0-7506-3365-9. [Google Scholar]
- Allred, A.L.; Rochow, E.G. A Scale of Electronegativity Based on Electrostatic Force. J. Inorg. Nucl. Chem. 1958, 5, 264–268. [Google Scholar] [CrossRef]
- Mulliken, R.S. A New Electroaffinity Scale; Together with Data on Valence States and on Valence Ionization Potentials and Electron Affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Structures of Molecules XI. Electroaffinity, Molecular Orbitals and Dipole Moments. J. Chem. Phys. 1935, 3, 573–585. [Google Scholar] [CrossRef]
- Maździarz, M. Uncertainty of DFT Calculated Mechanical and Structural Properties of Solids Due to Incompatibility of Pseudopotentials and Exchange–Correlation Functionals. J. Chem. Theory Comput. 2024, 20, 9734–9740. [Google Scholar] [CrossRef]
- Lejaeghere, K.; Van Speybroeck, V.; Van Oost, G.; Cottenier, S. Error Estimates for Solid-State Density-Functional Theory Predictions: An Overview by Means of the Ground-State Elemental Crystals. Crit. Rev. Solid State Mater. Sci. 2014, 39, 1–24. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Tran, N.T.T.; Chiu, Y.-H.; Lin, M.-F. Concentration-Diversified Magnetic and Electronic Properties of Halogen-Adsorbed Silicene. Sci. Rep. 2019, 9, 13746. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sheng, S.; Chen, J.; Cheng, P.; Chen, L.; Wu, K. Ordered Chlorinated Monolayer Silicene Structures. Phys. Rev. B 2016, 93, 155410. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, A.; Wang, Z. Transition Metal Atom (Ti, V, Mn, Fe, and Co) Anchored Silicene for Hydrogen Evolution Reaction. RSC Adv. 2019, 9, 26321–26326. [Google Scholar] [CrossRef]
- Lin, X.; Ni, J. Much Stronger Binding of Metal Adatoms to Silicene than to Graphene: A First-Principles Study. Phys. Rev. B 2012, 86, 075440. [Google Scholar] [CrossRef]
- Li, W.-Z.; Liu, M.-Y.; Gong, L.; Zhang, M.-L.; Cao, C.; He, Y. The Electronic Properties and Catalytic Activity of Precious-Metals Adsorbed Silicene for Hydrogen Evolution Reaction and Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 560, 150041. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Schwingenschlögl, U. Effects of Heavy Metal Adsorption on Silicene. Phys. Status Solidi RRL—Rapid Res. Lett. 2014, 8, 685–687. [Google Scholar] [CrossRef]
- Huang, J.; Chen, M.; Xue, J.; Li, M.; Cheng, Y.; Lai, Z.; Hu, J.; Zhou, F.; Qu, N.; Liu, Y.; et al. A Study of the Adsorption Properties of Individual Atoms on the Graphene Surface: Density Functional Theory Calculations Assisted by Machine Learning Techniques. Materials 2024, 17, 1428. [Google Scholar] [CrossRef]


| ACC | PPV | TPR | F1 | ROC AUC | |
|---|---|---|---|---|---|
| ANN | 0.9667 | 0.9626 | 0.9782 | 0.9693 | 0.9933 |
| RF | 0.8333 | 0.8393 | 0.8322 | 0.8348 | 0.9542 |
| GBT | 0.8167 | 0.8152 | 0.8122 | 0.8092 | 0.9454 |
| LGBM | 0.7833 | 0.7549 | 0.7843 | 0.7670 | 0.9472 |
| XGB | 0.7583 | 0.7289 | 0.7843 | 0.7418 | 0.9374 |
| MAE | RMSE | R2 | |
|---|---|---|---|
| LGBM | 0.1294 | 0.2012 | 0.9843 |
| XGB | 0.1567 | 0.2298 | 0.9796 |
| RF | 0.1745 | 0.2620 | 0.9734 |
| GBT | 0.2185 | 0.2984 | 0.9655 |
| ANN | 0.2438 | 0.3291 | 0.9586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowski, P.; Bobyk, A.; Krawiec, M. Structure and Energetics of Chemically Functionalized Silicene: Combined Density Functional Theory and Machine Learning Approach. Materials 2025, 18, 5228. https://doi.org/10.3390/ma18225228
Wojciechowski P, Bobyk A, Krawiec M. Structure and Energetics of Chemically Functionalized Silicene: Combined Density Functional Theory and Machine Learning Approach. Materials. 2025; 18(22):5228. https://doi.org/10.3390/ma18225228
Chicago/Turabian StyleWojciechowski, Paweł, Andrzej Bobyk, and Mariusz Krawiec. 2025. "Structure and Energetics of Chemically Functionalized Silicene: Combined Density Functional Theory and Machine Learning Approach" Materials 18, no. 22: 5228. https://doi.org/10.3390/ma18225228
APA StyleWojciechowski, P., Bobyk, A., & Krawiec, M. (2025). Structure and Energetics of Chemically Functionalized Silicene: Combined Density Functional Theory and Machine Learning Approach. Materials, 18(22), 5228. https://doi.org/10.3390/ma18225228





