Nanostructured Oxides Obtained by Anodizing Aluminum Intermetallic Alloys
Abstract
1. Introduction
2. Anodization of Purple Gold
3. Anodization of Ti-Al Alloys
4. Anodization of Ni3Al
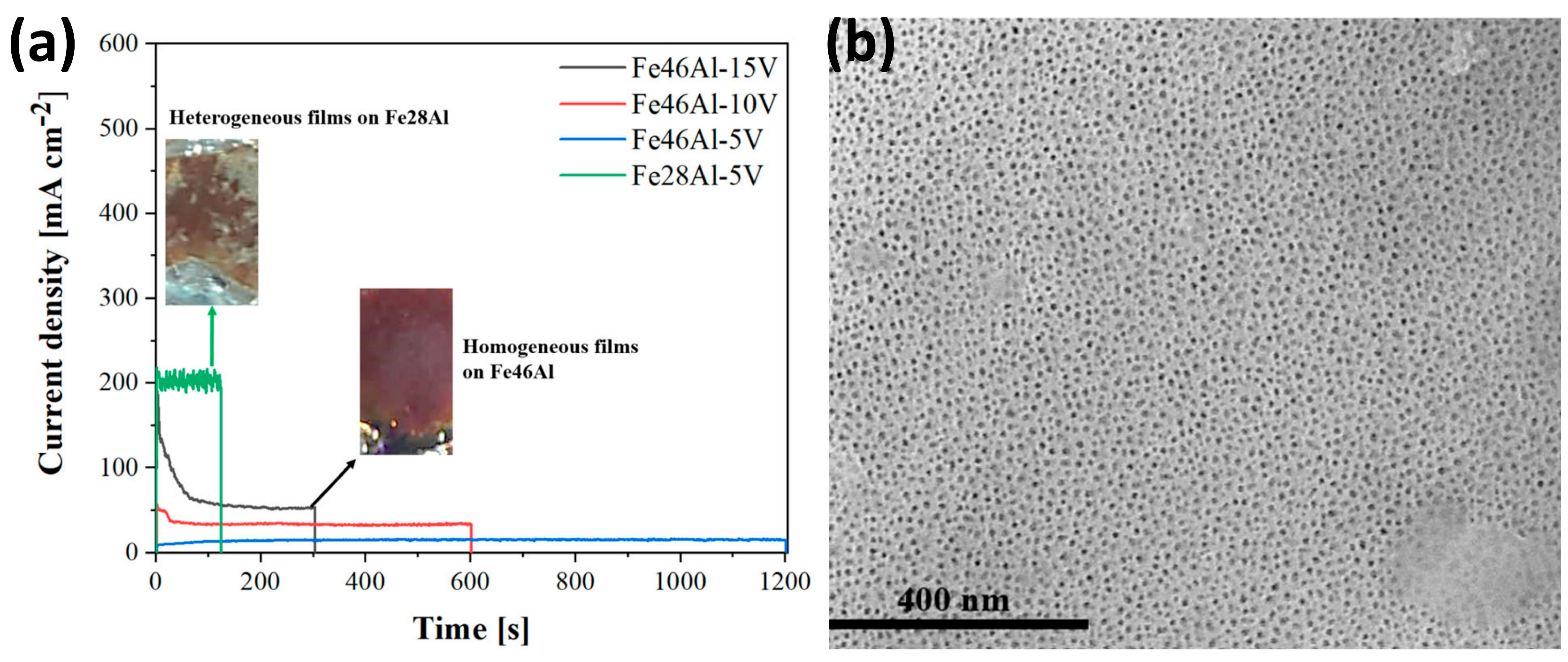
5. Anodization of Fe-Al Intermetallic Alloys
| Type of Material | Bath Composition | Anodizing Conditions | The Most Prominent Findings | Reference |
|---|---|---|---|---|
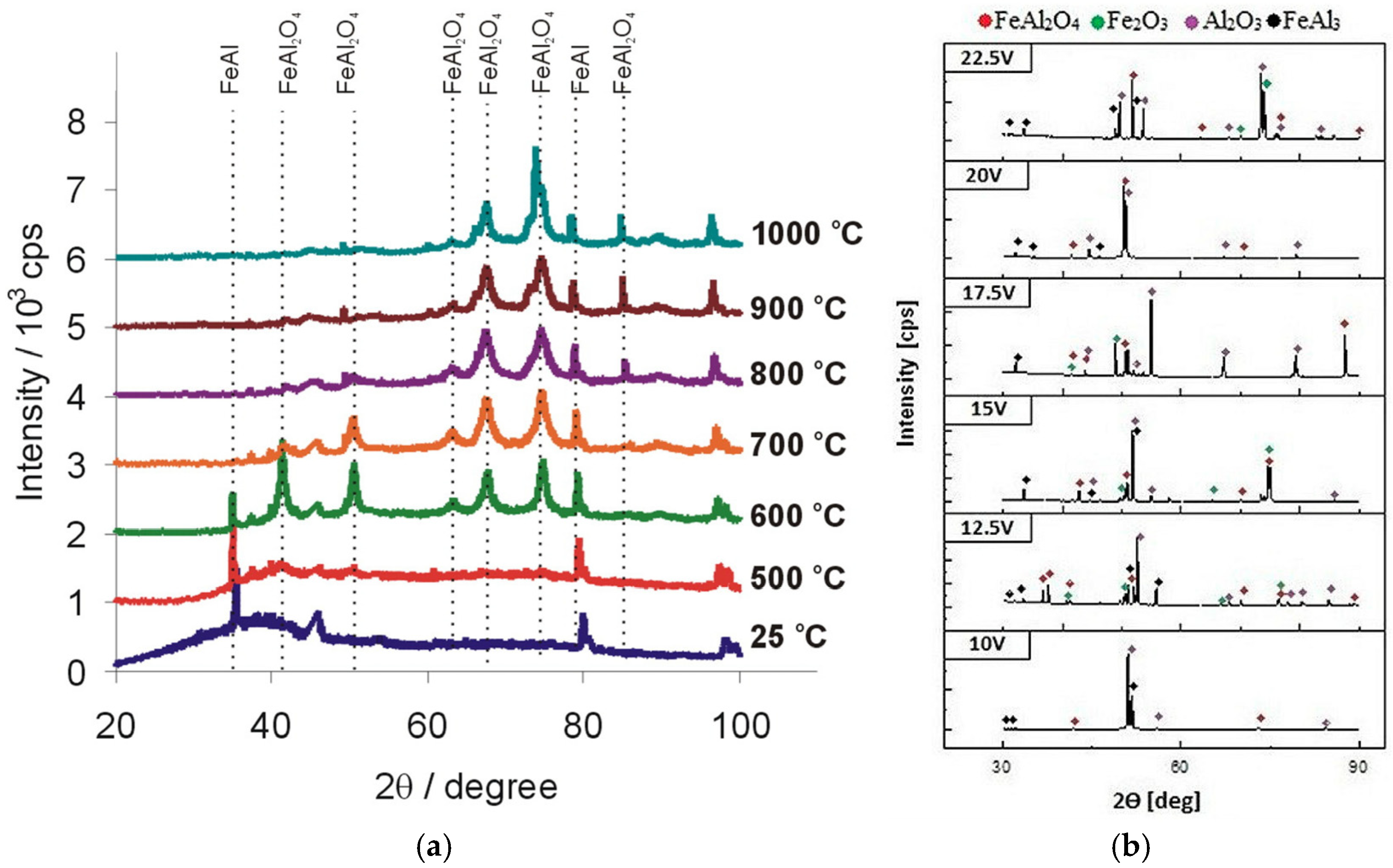
| FeAl: 58.11 at.% of Fe, 41.62 at.% of Al and 0.06 at.% of Zr | 20% wt. H2SO4 | 5–20 V, 0 °C, 60 s. | One and two-step anodizing, as for aluminum, was tested. High current densities, reaching 10 A/cm2, were recorded. Both Al and Fe were oxidized and formed nanoporous oxides. | [75] |
| The greater the anodizing voltage, the better the ordering of the oxide nanopores. | [76] | |||
| FeAl3: 22.47 at% of Fe and 77.53 at% | 10.0–22.5 V, 0 °C, 60 s. | Two-step anodizing was applied; relatively good ordering of the pores was attained; pore diameter and interpore distance grow linearly with applied voltage. Fe2O3, and FeAl2O4 were detected with XRD. | [77] | |
| XPS and XRD confirm the presence of the iron oxides in the products of anodizing; the greater the anodizing voltage, the lower the band gap of the formed oxides. | [78] | |||
| FeAl: 58.11 at.% of Fe, 41.62 at.% of Al and 0.06 at.% of Zr | 0.3 M (COOH)2 with 20% vol. Ethylene glycol | 10–25, and 40 V, 0 °C, 300 s. | Adding ethylene glycol to oxalic acid allowed to decrease the oxide growth rate to decrease; however, the morphology of the formed nanoporous oxides was poorly organized. | [79] |
| Fe3Al; wt.%: 28.0 Al, 5.0 Cr, 0.08 Zr, 0.04 B, and Fe balance | 0.5 M H2SO4 + 1 M tartaric acid, 0, 25, or 50% of ethylene glycol | 10, 15, or 20 V, 10 °C, time varied in order to attain the same charge in the circuit | Addition of ethylene glycol (EG) slowed down the oxide growth rate; even sub-10 nm nanoporous oxides were formed; the formed oxides, after annealing (900 °C), were composed of: α-Fe2O3, Fe3O4, FeAl2O4, FeAlO3, and γ-Al2O3. Depending on the anodizing conditions, the band gap of the formed oxides ranged from 1.91 eV to 2.30 eV. | [83] |
| 0.25 M boric acid + 1 M H2SO4 | 5, 10, 15 V, 10 °C, | No stable, homogeneous layer on Fe28Al was obtained. | [80] | |
| Fe46Al; wt.%: 46 Al, 0.04 B, 0.05 Zr, and Fe balance | The nanoporous oxides were sealed with CuSO4. Before sealing, the band gap ranged from 2.00 to 2.10 eV (after annealing), and after the sealing, it was 1.90 eV (after annealing). | |||
| Fe40Al; wt.%: 40 Al, 0.08 Zr, 0.04 B, and Fe balance | 0.3 M editronic acid; various amounts of ethylene glycol: 0, 50, 75, and 100 vol. % | 10–400 V, 25 °C, 1 or 4 h | Traditionally, die-cast and sintered materials were subjected to anodizing. The as-cast alloys were difficult to anodize—inhomogeneous oxides were formed; materials formed via sintering were found to be much better for anodizing. Phosphorous species were incorporated into the grown oxides; the band gap of the formed oxides ranged from 2.45 to 3.00 eV. | [82] |
| 0.3 M editronic acid with EG, 3:1 vol. ratio | 75 V, 20 °C, 2.5 h | Material obtained by the powder metallurgy process was anodized. Nanotubes were formed. Anodizing was followed by annealing and chemical etching. It allowed the obtaining of material with a developed surface area and relatively high photocurrents recorded in 1.0 M NaOH under illumination. | [84] |
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abrahami, S.T.; de Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- Bononi, M.; Conte, M.; Giovanardi, R.; Bozza, A. Hard Anodizing of AA2099-T8 Aluminum-lithium-copper Alloy: Influence of Electric Cycle, Electrolytic Bath Composition and Temperature. Surf. Coat. Technol. 2017, 325, 627–635. [Google Scholar] [CrossRef]
- Mohammadi, I.; Ahmadi, S.; Afshar, A. Effect of pulse current parameters on the mechanical and corrosion properties of anodized nanoporous aluminum coatings. Mater. Chem. Phys. 2016, 183, 490–498. [Google Scholar] [CrossRef]
- Bozza, A.; Giovanardi, R.; Manfredini, T.; Mattioli, P. Pulsed current effect on hard anodizing process of 7075-T6 aluminium alloy. Surf. Coat. Technol. 2015, 270, 139–144. [Google Scholar] [CrossRef]
- Tomczyk, K.; Stępniowski, W.J. Incorporation of Anions into Anodic Alumina—A New Track in Cr(VI) Anodizing Substitution? Materials 2024, 17, 2938. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- de Almeida, T.F.; Prada Ramirez, O.M.; Lanzutti, A.; Rodrigues, C.L.; Brabetz, M.; Kremmer, T.M.; Hammer, P.; de Melo, H.G. Addition of molybdate ions to the anodizing bath to improve the corrosion resistance of clad 2024-T3 alloy anodized in tartaric-sulfuric acid. Surf. Coat. Technol. 2024, 482, 130682. [Google Scholar] [CrossRef]
- Machado, T.V.; Dick, P.A.; Knörnschild, G.H.; Dick, L.F.P. The effect of different carboxylic acids on the sulfuric acid anodizing of AA2024. Surf. Coat. Technol. 2020, 383, 125283. [Google Scholar] [CrossRef]
- Del Olmo, R.; Tiringer, U.; Milosev, I.; Visser, P.; Arrabal, R.; Matykina, E.; Mol, J.M.C. Hybrid sol-gel coatings applied on anodized AA2024-T3 for active corrosion protection. Surf. Coat. Technol. 2021, 419, 127251. [Google Scholar] [CrossRef]
- Ji, M.; Li, W.; Liu, H.; Zhu, L.; Chen, H.; Li, W. Effect of titanium sol on sulfuric-citric acids anodizing of 7150 aluminum alloy. Surf. Interfaces 2020, 19, 100479. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef]
- Sulka, G.D.; Parkoła, K. Anodising potential influence on well-ordered nanostructures formed by anodisation of aluminium in sulphuric acid. Thin Solid Film. 2006, 515, 338–345. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Bojar, Z. Synthesis of anodic aluminum oxide (AAO) at relatively high temperatures. Study of the influence of anodization conditions on the alumina structural features. Surf. Coat. Technol. 2011, 206, 265–272. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nakajima, D.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of anodic porous alumina via anodizing in cyclicoxocarbon acids. Appl. Surf. Sci. 2014, 313, 276–285. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl. Surf. Sci. 2014, 321, 364–370. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Forbot, D.; Norek, M.; Michalska-Domańska, M.; Król, A. The impact of viscosity of the electrolyte on the formation of nanoporous anodic aluminum oxide. Electrochim. Acta 2014, 133, 57–64. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Moneta, M.; Norek, M.; Michalska-Domańska, M.; Scarpellini, A.; Salerno, M. The influence of electrolyte composition on the growth of nanoporous anodic alumina. Electrochim. Acta 2016, 211, 453–460. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Bojar, Z. Quantitative arrangement analysis of anodic alumina formed by short anodizations in oxalic acid. Mater. Charact. 2013, 78, 79–86. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Presz, A.; Czujko, T.; Varin, A. The effects of time and temperature on the arrangement of anodic aluminum oxide nanopores. Mater. Charact. 2014, 91, 1–9. [Google Scholar] [CrossRef]
- Siemiaszko, D.; Norek, M. The mean angle of displacement (MAD) as a simple parameter for determining the degree of ordering of hexagonal arrays. Acta Mater. 2025, 296, 121300. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Moneta, M.; Karczewski, K.; Michalska-Domańska, M.; Czujko, T.; Mol, J.M.C.; Buijnsters, J.G. Fabrication of copper nanowires via electrodeposition in anodic aluminum oxide templates formed by combined hard anodizing and electrochemical barrier layer thinning. J. Electroanal. Chem. 2018, 809, 59–66. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Florkiewicz, W.; Michalska-Domańska, M.; Norek, M.; Czujko, T. A comparative study of electrochemical barrier layer thinning for anodic aluminum oxide grown on technical purity aluminum. J. Electroanal. Chem. 2015, 741, 80–86. [Google Scholar] [CrossRef]
- Ganapathi, A.; Swaminathan, P.; Neelakantan, L. Anodic Aluminum Oxide Template Assisted Synthesis of Copper Nanowires using a Galvanic Displacement Process for Electrochemical Denitrification. ACS Appl. Nano Mater. 2019, 2, 5981–5988. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chen, H.-Y.; Liao, C.-N. Growth of nanotwinned Cu nanowires in modified anodic aluminum oxide templates. Mater. Lett. 2021, 288, 129381. [Google Scholar] [CrossRef]
- Hopkins, P.D.; Farrer, R.A. A green method to produce nanoporous aluminum oxide templates and the direct application in the synthesis of nanowires. Appl. Mater. Today 2023, 32, 101768. [Google Scholar] [CrossRef]
- Zheng, B.-C.; Shi, J.-B.; Lin, H.-S.; Hsu, P.-Y.; Lee, H.-W.; Lin, C.-H.; Lee, M.-W.; Kao, M.-C. Growth of Less than 20 nm SnO Nanowires Using an Anodic Aluminum Oxide Template for Gas Sensing. Micromachines 2020, 11, 153. [Google Scholar] [CrossRef]
- Kuo, C.-G.; Chang, H.; Wang, J.-H. Fabrication of ZnO Nanowires Arrays by Anodization and High-Vacuum Die Casting Technique, and Their Piezoelectric Properties. Sensors 2016, 16, 431. [Google Scholar] [CrossRef]
- Brock, L.; Sheng, J. Robust Fabrication of Polymeric Nanowire with Anodic Aluminum Oxide Templates. Micromachines 2020, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, A.; Wan, Q.; Li, Z. Controllable fabrication of polymeric nanowires by NIL technique and self-assembled AAO template for SERS application. Sci. Rep. 2021, 11, 14929. [Google Scholar] [CrossRef] [PubMed]
- Dickey, M.D.; Weiss, E.A.; Smythe, E.J.; Chiechi, R.C.; Capasso, F.; Whitsides, G.M. Fabrication of Arrays of Metal and Metal Oxide Nanotubes by Shadow Evaporation. ACS Nano 2008, 2, 800–808. [Google Scholar] [CrossRef]
- Norek, M.; Zaleszczyk, W.; Łuka, G.; Budner, B.; Zasada, D. Tailoring UV emission from a regular array of ZnO nanotubes by the geometrical parameters of the array and Al2O3 coating. Ceram. Int. 2017, 43, 5693–5701. [Google Scholar] [CrossRef]
- Norek, M.; Putkonen, M.; Zaleszczyk, W.; Budner, B.; Bojar, Z. Morphological, structural and optical characterization of SnO2 nanotube arrays fabricated using anodic alumina (AAO) template-assisted atomic layer deposition. Mater. Charact. 2018, 136, 52–59. [Google Scholar] [CrossRef]
- Yang, S.M.; Gu, J.J.; Wang, S.X.; Qi, Y.K. Study on physical properties of anodic aluminum oxide films modified by Co nanodots. Thin Solid Film. 2018, 660, 82–87. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Szultka-Młyńska, M.; Buszewski, B.; Sulka, G.D. Epinephrine sensing at nanostructured Au electrode and determination its oxidative metabolism. Sens. Actuators B 2016, 237, 206–215. [Google Scholar] [CrossRef]
- Kurowska, E.; Brzózka, A.; Jarosz, M.; Sulka, G.D.; Jaskuła, M. Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochim. Acta 2013, 104, 439–447. [Google Scholar] [CrossRef]
- Sulka, G.D.; Hnida, K.; Brzózka, A. pH sensors based on polypyrrole nanowire arrays. Electrochim. Acta 2013, 104, 536–541. [Google Scholar] [CrossRef]
- Brzózka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G.D. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
- Brzózka, A.; Jeleń, A.; Brzudzisz, A.M.; Marzec, M.M.; Sulka, G.D. Electrocatalytic reduction of chloroform at nanostructured silver electrodes. Electrochim. Acta 2017, 225, 574–583. [Google Scholar] [CrossRef]
- Brudzisz, A.; Sulka, G.D.; Brzózka, A. A facile approach to silver nanowire array electrode preparation and its application for chloroform reduction. Electrochim. Acta 2020, 362, 137110. [Google Scholar] [CrossRef]
- Brudzisz, A.; Rajska, D.; Gajewska, M.; Sulka, G.D.; Brzózka, A. Controlled synthesis and characterization of AgPd nanowire arrays for electrocatalytic applications. J. Electroanal. Chem. 2020, 873, 114373. [Google Scholar] [CrossRef]
- Brzózka, A.; Fic, K.; Bogusz, J.; Brudzisz, A.M.; Marzec, M.M.; Gajewska, M.; Sulka, G.D. Polypyrrole–Nickel Hydroxide Hybrid Nanowires as Future Materials for Energy Storage. Nanomaterials 2019, 9, 307. [Google Scholar] [CrossRef]
- Rajska, D.; Hnida-Gut, K.E.; Gajewska, M.; Chlebda, D.; Brzózka, A.; Sulka, G.D. Adjusting the crystal size of InSb nanowires for optical band gap energy modification. Mater. Chem. Phys. 2020, 254, 123498. [Google Scholar] [CrossRef]
- Marinko, Ž.; Suhadolnik, L.; Samardžija, Z.; Kovač, J.; Čeh, M. The Influence of a Surface Treatment of Metallic Titanium on the Photocatalytic Properties of TiO2 Nanotubes Grown by Anodic Oxidation. Catalysts 2020, 10, 803. [Google Scholar] [CrossRef]
- Suhadolnik, L.; Marino, Ž.; Ponikvar-Svet, M.; Tavčar, G.; Kovač, J.; Čeh, M. Influence of Anodization-Electrolyte Aging on the Photocatalytic Activity of TiO2 Nanotube Arrays. J. Phys. Chem. C 2020, 124, 4073–4080. [Google Scholar] [CrossRef] [PubMed]
- Krivec, M.; Žagar, K.; Suhadolnik, L.; Čeh, M.; Dražić, G. Highly efficient TiO2-based microreactor for photocatalytic applications. ACS Appl. Mater. Interfaces 2013, 5, 9088–9094. [Google Scholar] [CrossRef]
- Paulose, M.; Shankar, K.; Varghese, O.K.; Mor, G.K.; Hardin, B.; Grimes, C.A. Backside illuminated dye-sensitized solar cells based on titania nanotube array electrodes. Nanotechnology 2006, 17, 1446. [Google Scholar] [CrossRef]
- Xie, Z.B.; Adams, S.; Blackwood, D.J.; Wang, J. The effects of anodization parameters on titania nanotube arrays and dye sensitized solar cells. Nanotechnology 2008, 19, 405701. [Google Scholar] [CrossRef]
- Santos, J.S.; Sikora, M.S.; Trivinho-Strixino, F.; Praserthdam, S.; Praserthdam, P. A comprehensive review of anodic TiO2 films as heterogeneous catalysts for photocatalytic and photoelectrocatalytic water disinfection. J. Water Process Eng. 2025, 69, 106589. [Google Scholar] [CrossRef]
- Ohse, S.T.; Morais, A.; Felsner, M.L.; Galli, A.; de Souza Sikora, M. Nanostructured TiO2-X/CuXO-based electrochemical sensor for ultra-sensitive glyphosate detection in real water samples. Microchem. J. 2024, 205, 111316. [Google Scholar] [CrossRef]
- Mbisike, S.C.; Tsiamis, A.; Lomax, P.; Cheung, R. Anodic tantalum: Fabrication, breakdown characteristics of capacitor and integration with a WSe2 field effect transistor. Solid State Electron. 2022, 196, 108423. [Google Scholar] [CrossRef]
- Mohapatra, B.D.; Pawlik, K.; Darowska, I.; Gondek, Ł.; Pisarek, M.; Sulka, G.D. Understanding the morphological evolution of anodic tantalum oxide nanostructures in acidic medium. Mater. Adv. 2024, 5, 6560–6571. [Google Scholar] [CrossRef]
- Yoo, J.E.; Choi, J. Electrochemical surface enlargement of a niobium foil for electrolytic capacitor applications. Electrochem. Commun. 2011, 13, 298–301. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Syrek, K.; Sulka, G.D.; Pisarek, M.; Janik-Czachor, M. The effect of foil purity on morphology of anodized nanoporous ZrO2. Appl. Surf. Sci. 2016, 388, 799–804. [Google Scholar] [CrossRef]
- Cao, J.; Gao, Z.; Wang, C.; Muzammal, H.M.; Wang, W.; Gu, Q.; Dong, C.; Ma, H.; Wang, Y. Morphology evolution of the anodized tin oxide film during early formation stages at relatively high constant potential. Surf. Coat. Technol. 2020, 388, 125592. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kawashima, J.; Natsui, S.; Suzuki, R.O. Fabrication of porous tungsten oxide via anodizing in an ammonium nitrate/ethylene glycol/water mixture for visible light-driven photocatalyst. Appl. Surf. Sci. 2017, 422, 130–137. [Google Scholar] [CrossRef]
- Tantray, A.M.; Shah, M.A. Photo electrochemical stability response of ZnO nanoflowers fabricated through single step electrochemical anodization. Chem. Pap. 2021, 75, 1739–1747. [Google Scholar] [CrossRef]
- Tantray, A.M.; Shah, M.A. Photo electrochemical ability of dense and aligned ZnO nanowire arrays fabricated through electrochemical anodization. Chem. Phys. Lett. 2020, 747, 13734. [Google Scholar] [CrossRef]
- Giziński, D.; Brudzisz, A.; Lee, J.; Harishchandre, R.; Choi, J.; Stȩpniowski, W.J.; Ziegler, K.J. Exploring the Impact of Chelating Agents on Copper Oxide Layer Formation and Morphology. Inorg. Chem. 2025, 64, 7437–7449. [Google Scholar] [CrossRef]
- Tomc, B.; Bele, M.; Plut, M.; Kostelec, M.; Popović, S.; Nazrulla, M.A.; Ruiz-Zepeda, F.; Kamšek, A.R.; Šala, M.; Elbataioui, A.; et al. Recognizing the Universality of Copper Reconstruction Via Dissolution–Redeposition at the Onset of CO2 Reduction. J. Phys. Chem. Lett. 2025, 16, 9553–9560. [Google Scholar] [CrossRef]
- Acuña-Bedoya, J.D.; Luévano-Hipólito, E.; Cedillo-González, E.I.; Domínguez-Jaimes, L.P.; Hurtado, A.M.; Hernández-López, J.M. Boosting visible-light photocatalytic degradation of polystyrene nanoplastics with immobilized CuxO obtained by anodization. J. Environ. Chem. Eng. 2021, 9, 106208. [Google Scholar] [CrossRef]
- Anantharaj, S.; Sugime, H.; Yamaoka, S.; Noda, S. Pushing the Limits of Rapid Anodic Growth of CuO/Cu(OH)2 Nanoneedles on Cu for the Methanol Oxidation Reaction: Anodization pH Is the Game Changer. ACS Appl. Energy Mater. 2021, 4, 899–912. [Google Scholar] [CrossRef]
- Rajendran, S.L.; Viswanathan, A. One-dimensional titanium dioxide nanotube arrays for hydrogen generation. J. Chem. Sci. 2025, 137, 79. [Google Scholar] [CrossRef]
- Nishio, K.; Kondo, T.; Masuda, H. Fabrication of Au Fine Structures of Nanoparticles by Anodization of Purple Gold (AuAl2). Chem. Lett. 2009, 38, 1148–1149. [Google Scholar] [CrossRef]
- Nishio, K.; Masuda, H. Anodization of Gold in Oxalate Solution to Form a Nanoporous Black Film. Angew. Chem. Int. Ed. 2011, 50, 1603–1607. [Google Scholar] [CrossRef]
- Berger, S.; Tsuchiya, H.; Schmuki, P. Transition from Nanopores to Nanotubes: Self-Ordered Anodic Oxide Structures on Titanium-Aluminides. Chem. Mater. 2008, 20, 3245–3247. [Google Scholar] [CrossRef]
- Jozwik, P.; Polkowski, W.; Bojar, Z. Applications of Ni3Al Based Intermetallic Alloys—Current Stage and Potential Perceptivities. Materials 2015, 8, 2537–2568. [Google Scholar] [CrossRef]
- Sulka, G.; Jóźwik, P. Electrochemical behavior of Ni3Al-based intermetallic alloys in NaOH. Intermetallics 2011, 19, 974–981. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Cieślak, G.; Norek, M.; Karczewski, K.; Michalska-Domańska, M.; Zasada, D.; Polkowski, W.; Jóźwik, P.; Bojar, Z. Nanoporous alumina formed by self-organized two-step anodization of Ni3Al intermetallic alloy in citric acid. Appl. Surf. Sci. 2013, 264, 605–610. [Google Scholar] [CrossRef]
- Salerno, M.; Stępniowski, W.J.; Cieślak, G.; Norek, M.; Michalska-Domańska, M.; Karczewski, K.; Chilimoniuk, P.; Polkowski, W.; Jóźwik, P.; Bojar, Z. Advanced Image Analysis of the Surface Pattern Emerging in Ni3Al Intermetallic Alloys on Anodization. Front. Mater. 2016, 3, 34. [Google Scholar] [CrossRef]
- Sulka, G.D.; Stępniowski, W.J. Structural features of self-organized nanopore arrays formed by anodization of aluminum in oxalic acid at relatively high temperatures. Electrochim. Acta 2009, 54, 3683–3691. [Google Scholar] [CrossRef]
- Quitério, P.; Apolinário, A.; Mendes, A.; Araújo, J.P.; Sousa, C.T. The role of mild and hard anodization regimes of iron oxide nanotubes in the photoelectrochemical performance. J. Electroanal. Chem. 2022, 926, 116903. [Google Scholar] [CrossRef]
- Sarma, B.; Jurovitzki, A.L.; Ray, R.S.; Smith, Y.R.; Mohanty, S.K.; Misra, M. Electrochemical capacitance of iron oxide nanotube (Fe-NT): Effect of annealing atmospheres. Nanotechnology 2015, 26, 265401. [Google Scholar] [CrossRef] [PubMed]
- Matysik, P.; Jóźwiak, S.; Czujko, T. Characterization of Low-Symmetry Structures from Phase Equilibrium of Fe-Al System—Microstructures and Mechanical Properties. Materials 2015, 8, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, S.; Karczewski, K.; Bojar, Z. The effect of loading mode changes during the sintering process on the mechanical properties of FeAl intermetallic sinters. Intermetallics 2013, 33, 99–104. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Choi, J.; Yoo, H.; Oh, K.; Michalska-Domańska, M.; Chilimoniuk, P.; Czujko, T.; Łyszkowski, R.; Jóźwiak, S.; Bojar, Z.; et al. Anodization of FeAl intermetallic alloys for bandgap tunable nanoporous mixed aluminum-iron oxide. J. Electroanal. Chem. 2016, 771, 37–44. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Choi, J.; Yoo, H.; Oh, K.; Michalska-Domańska, M.; Chilimoniuk, P.; Czujko, T. Quantitative fast Fourier transform based arrangement analysis of porous anodic oxide formed by self-organized anodization of FeAl intermetallic alloy. Mater. Lett. 2016, 164, 176–179. [Google Scholar] [CrossRef]
- Chilimoniuk, P.; Michalska-Domańska, M.; Czujko, T. Formation of Nanoporous Mixed Aluminum-Iron Oxides by Self-Organized Anodizing of FeAl3 Intermetallic Alloy. Materials 2019, 12, 2299. [Google Scholar] [CrossRef]
- Chilimoniuk, P.; Socha, R.P.; Czujko, T. Nanoporous Anodic Aluminum-Iron Oxide with a Tunable Band Gap Formed on the FeAl3 Intermetallic Phase. Materials 2020, 13, 3471. [Google Scholar] [CrossRef]
- Chilimoniuk, P.; Michalska-Domańska, M.; Stępniowski, W.J.; Czujko, T. Formation of nanoporous oxide by self-organized anodizing of FeAl intermetallic alloy in oxalic acid solution containing glycol. Mater. Lett. 2018, 224, 9–12. [Google Scholar] [CrossRef]
- Del Olmo, R.; Pisarek, M.; Durejko, T.; Michalska-Domańska, M. Anodization of FeAl Alloy in Boric-Sulfuric Acid: Band Gap Tuning Via Copper Doping and Annealing. Metall. Mater. Trans. A 2025, 56, 4424–4435. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Siemiaszko, D.; Norek, M. Influence of Ethanol on Porous Anodic Alumina Growth in Etidronic Acid Solutions at Various Temperatures. Materials 2022, 15, 8595. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, R.; Łazińska, M.; Durejko, T.; Antolak-Dudka, A.; Tynkevych, O.; Zaraska, L.; Michalska-Domańska, M. Anodization of cast and sintered Fe40Al alloy in etidronic acid: Morphological and semiconductive properties of the oxide films. J. Alloys Compd. 2024, 1005, 176033. [Google Scholar] [CrossRef]
- Del Olmo, R.; Łazińska, M.; Czerwiński, M.; Durejko, T.; Michalska-Domańska, M. Morphological and semiconductive properties of the anodic oxide layers made on Fe3Al alloy by anodizing in tartaric-sulfuric acid mixture. Sci. Rep. 2023, 13, 15135. [Google Scholar] [CrossRef] [PubMed]
- Zaraska, L.; Gurgul, M.; Tynkevych, O.; Pisarek, M.; Kozieł, M.; Czerwiński, M.; Lazińska, M.; Michalska-Domańska, M. Physicochemical and photoelectrochemical characterization of nanostructured oxide layers grown by anodic oxidation of Fe40Al in etidronic acid-based electrolyte. Electrochim. Acta 2025, 528, 146261. [Google Scholar] [CrossRef]

| Type of Material | Bath Composition | Anodizing Conditions | The Most Prominent Findings | Reference |
|---|---|---|---|---|
| AuAl2 | 0.3 M oxalic acid (COOH)2 | 5 or 20 V, 30 or 90 min. | Anodization of purple gold allowed the formation of gold nanoparticles (ca. 30 nm in diameter); application: detection of pyridine (Surface-Enhanced Raman Spectroscopy) | [63] |
| Type of Material | Bath Composition | Anodizing Conditions | The Most Prominent Findings | Reference |
|---|---|---|---|---|
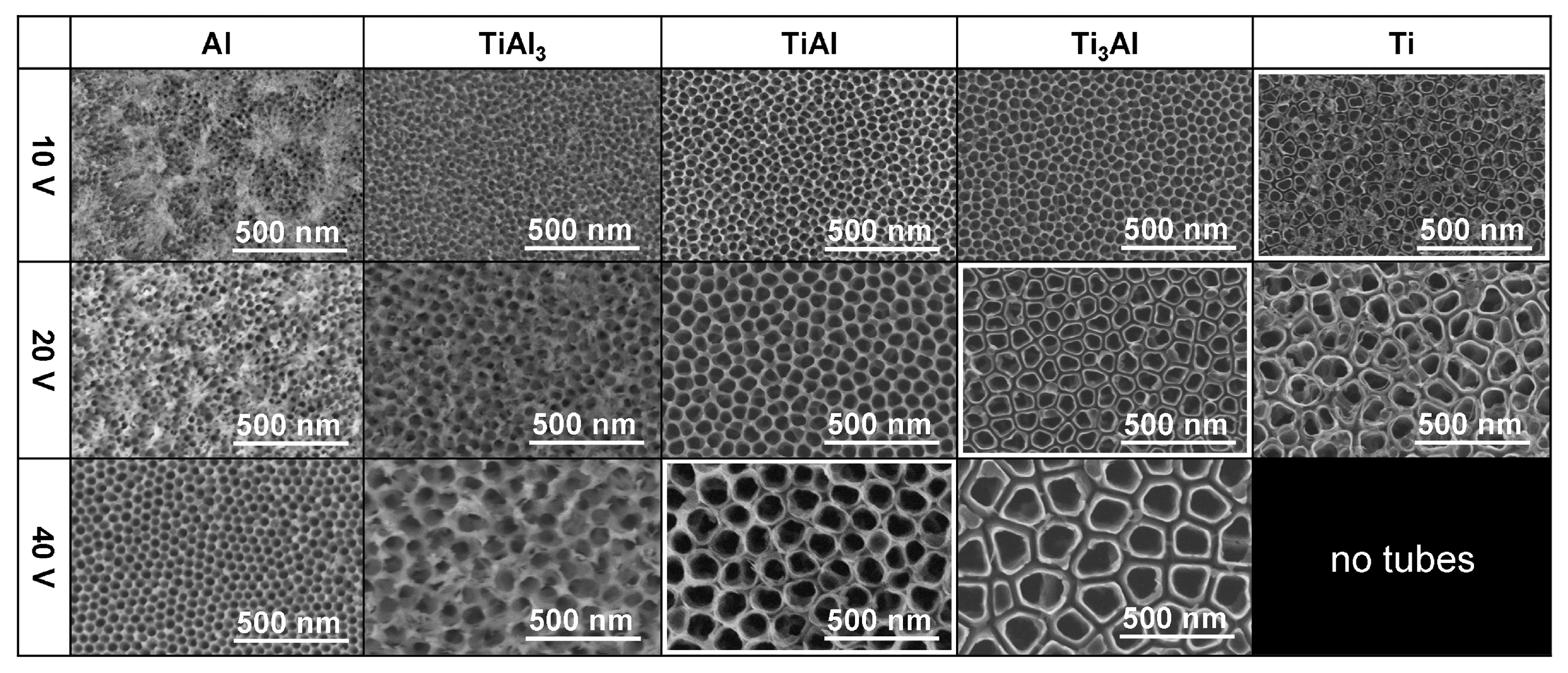
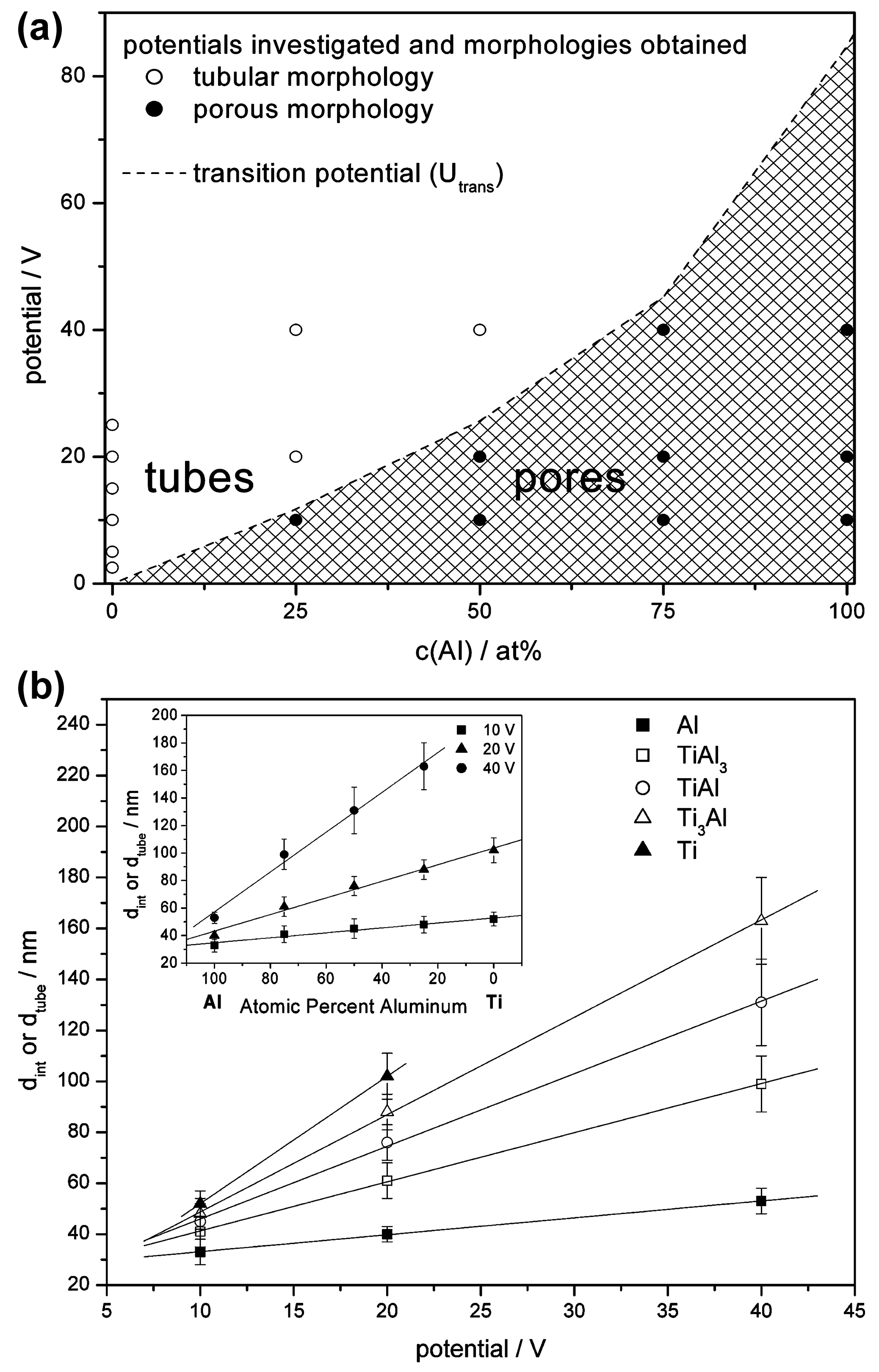
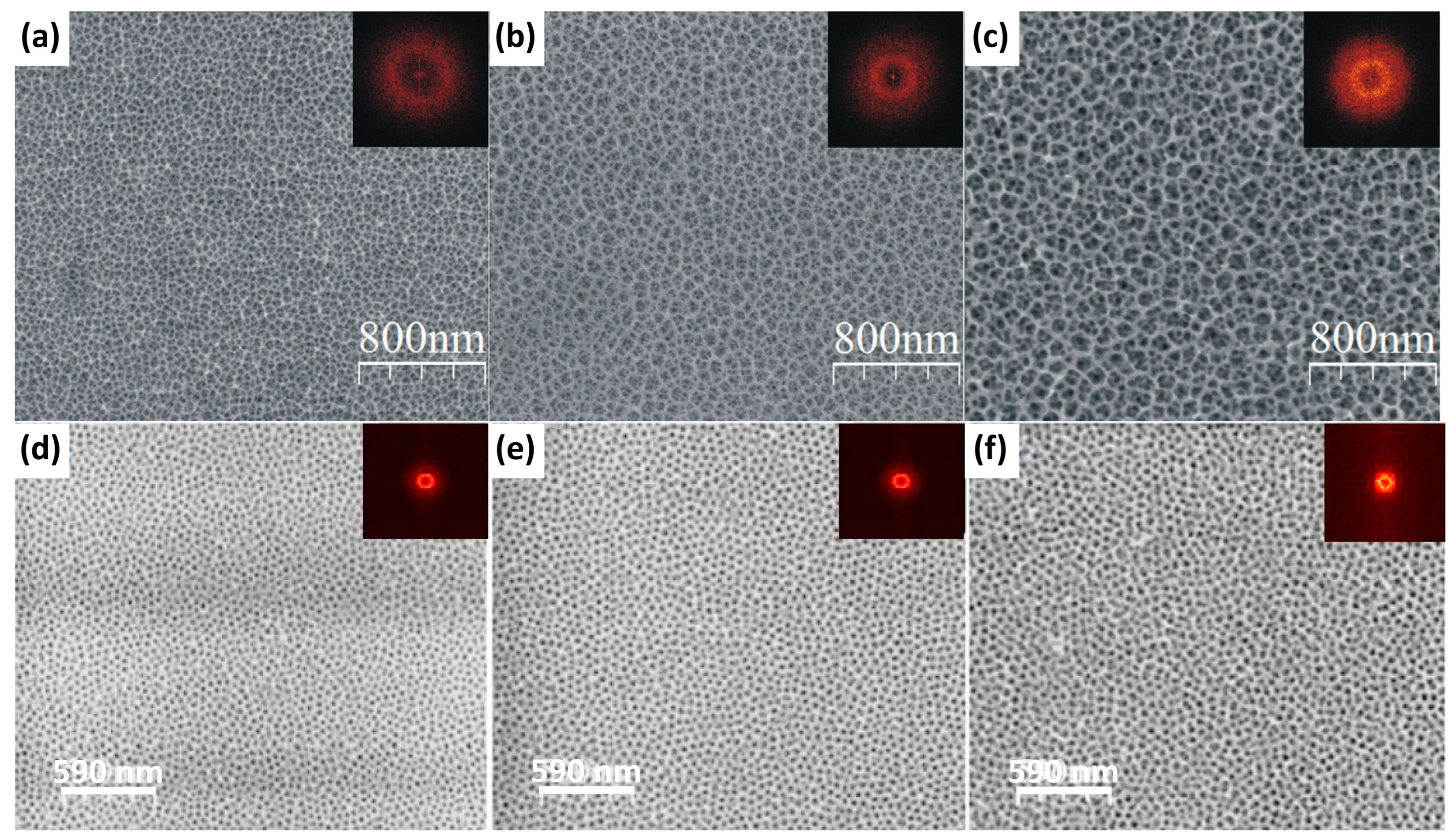
| Al, TiAl3, TiAl, Ti3Al, Ti | 1 M H2SO4 with 0.15 wt.% HF | 10, 20, or 40 V, 30 or 90 min. | Depending on the Ti:Al ratio and anodizing voltage, a nanoporous or nanotubular oxide is formed. | [65] |
| Type of Material | Bath Composition | Anodizing Conditions | The Most Prominent Findings | Reference |
|---|---|---|---|---|
| Ni3Al | 0.3 M citric acid | 2–12 V, 0 or 30 °C, 12 h | The formed oxide was poorly ordered; pore diameter and interpore distance increased linearly with anodizing voltage. | [68] |
| An attempt to quantitatively study the ordering of the formed pores | [69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilimoniuk-Szwarc, P.; Dobroń, P.; Stępniowski, W.J. Nanostructured Oxides Obtained by Anodizing Aluminum Intermetallic Alloys. Materials 2025, 18, 5192. https://doi.org/10.3390/ma18225192
Chilimoniuk-Szwarc P, Dobroń P, Stępniowski WJ. Nanostructured Oxides Obtained by Anodizing Aluminum Intermetallic Alloys. Materials. 2025; 18(22):5192. https://doi.org/10.3390/ma18225192
Chicago/Turabian StyleChilimoniuk-Szwarc, Paulina, Piotr Dobroń, and Wojciech Jerzy Stępniowski. 2025. "Nanostructured Oxides Obtained by Anodizing Aluminum Intermetallic Alloys" Materials 18, no. 22: 5192. https://doi.org/10.3390/ma18225192
APA StyleChilimoniuk-Szwarc, P., Dobroń, P., & Stępniowski, W. J. (2025). Nanostructured Oxides Obtained by Anodizing Aluminum Intermetallic Alloys. Materials, 18(22), 5192. https://doi.org/10.3390/ma18225192






