Organic Acid-Based Anodization Process to Produce Bioactive Oxides on Titanium Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Titanium Specimen Preparation
2.2. Anodization
2.3. Anodized Oxide Surface Characterization
2.4. Anodized Oxide Thickness Evaluation
2.5. Anodized Oxide Adhesion Quality Assessment
2.6. Osteoblast Culture
2.7. Cell Viability Testing
2.8. Biochemical Analyses
2.9. Statistical Analyses
3. Results and Discussion
3.1. Anodized Oxide Characterization
3.1.1. Oxide Structural Analysis
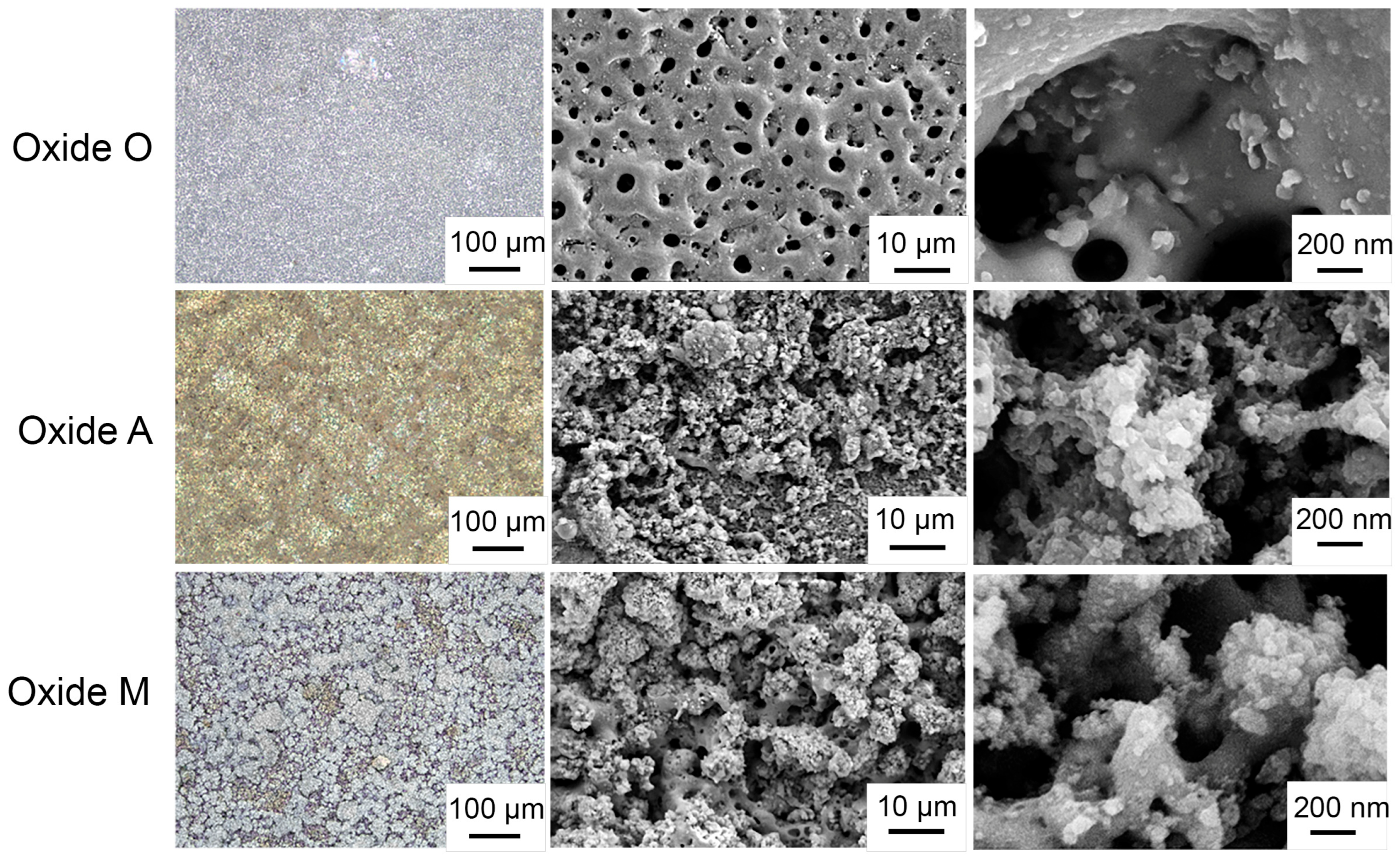
3.1.2. Anodized Oxide Surface Topographies
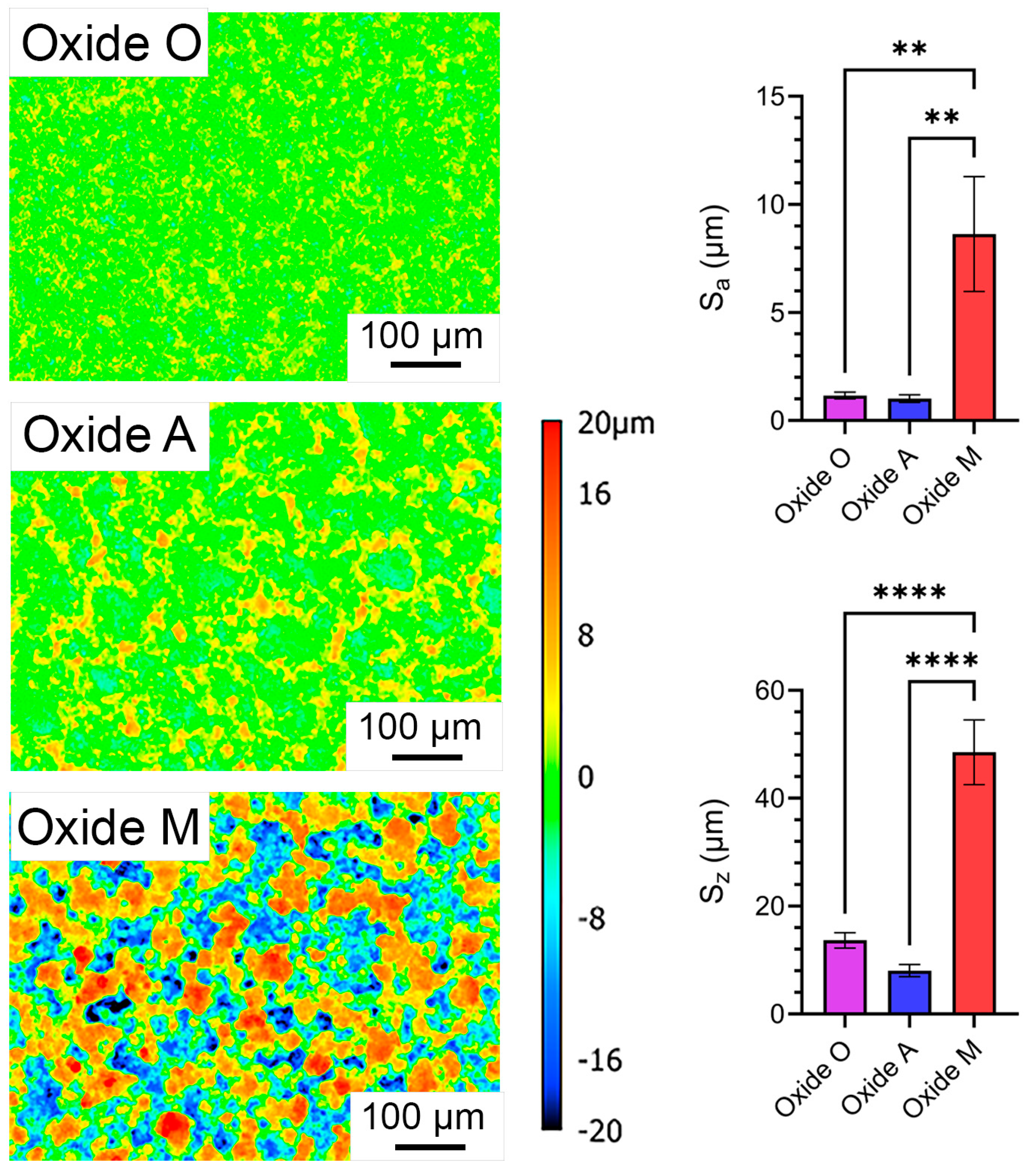
3.1.3. Anodized Oxide Surface Roughness
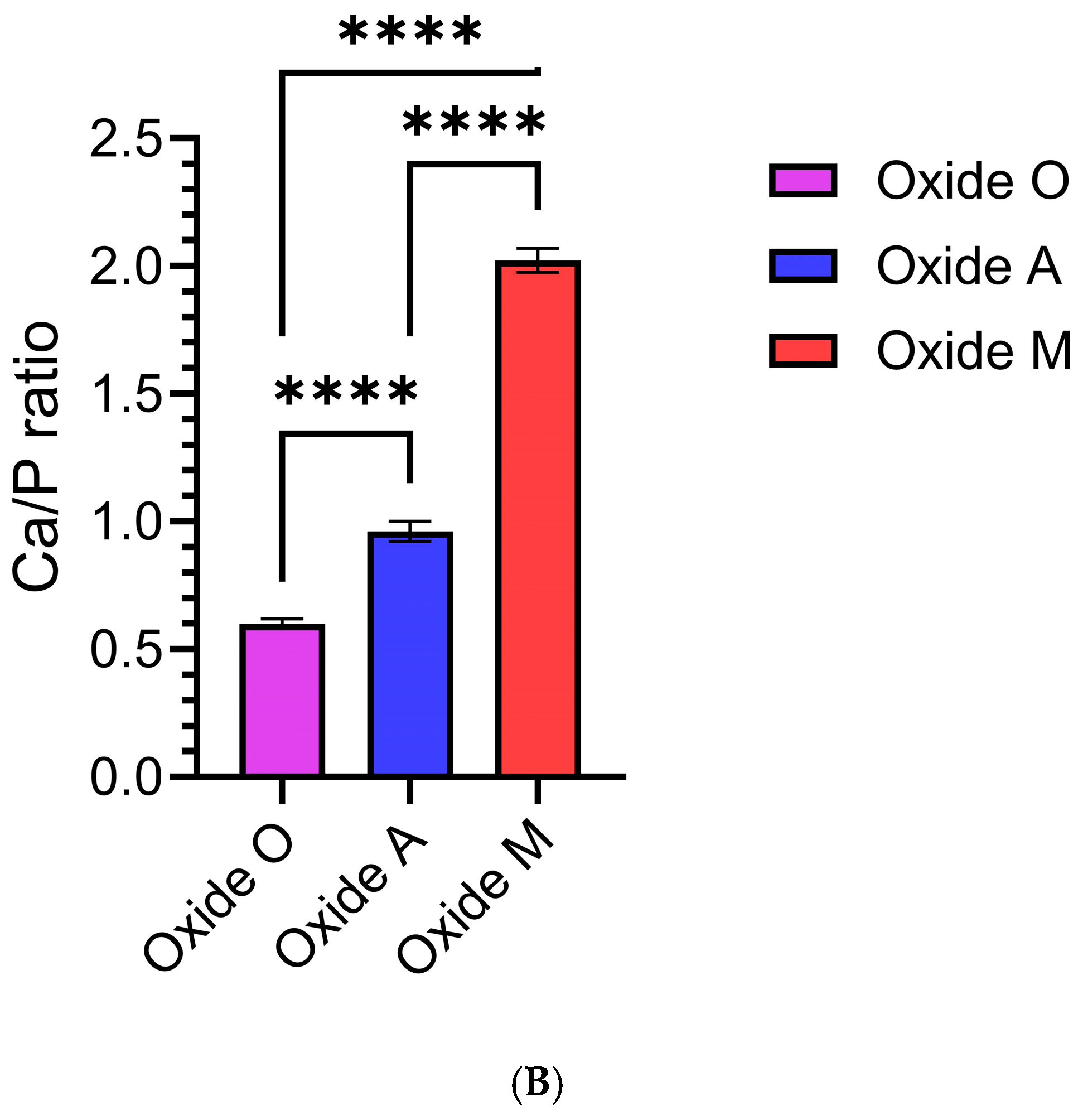
3.1.4. Anodized Oxide Surface Composition
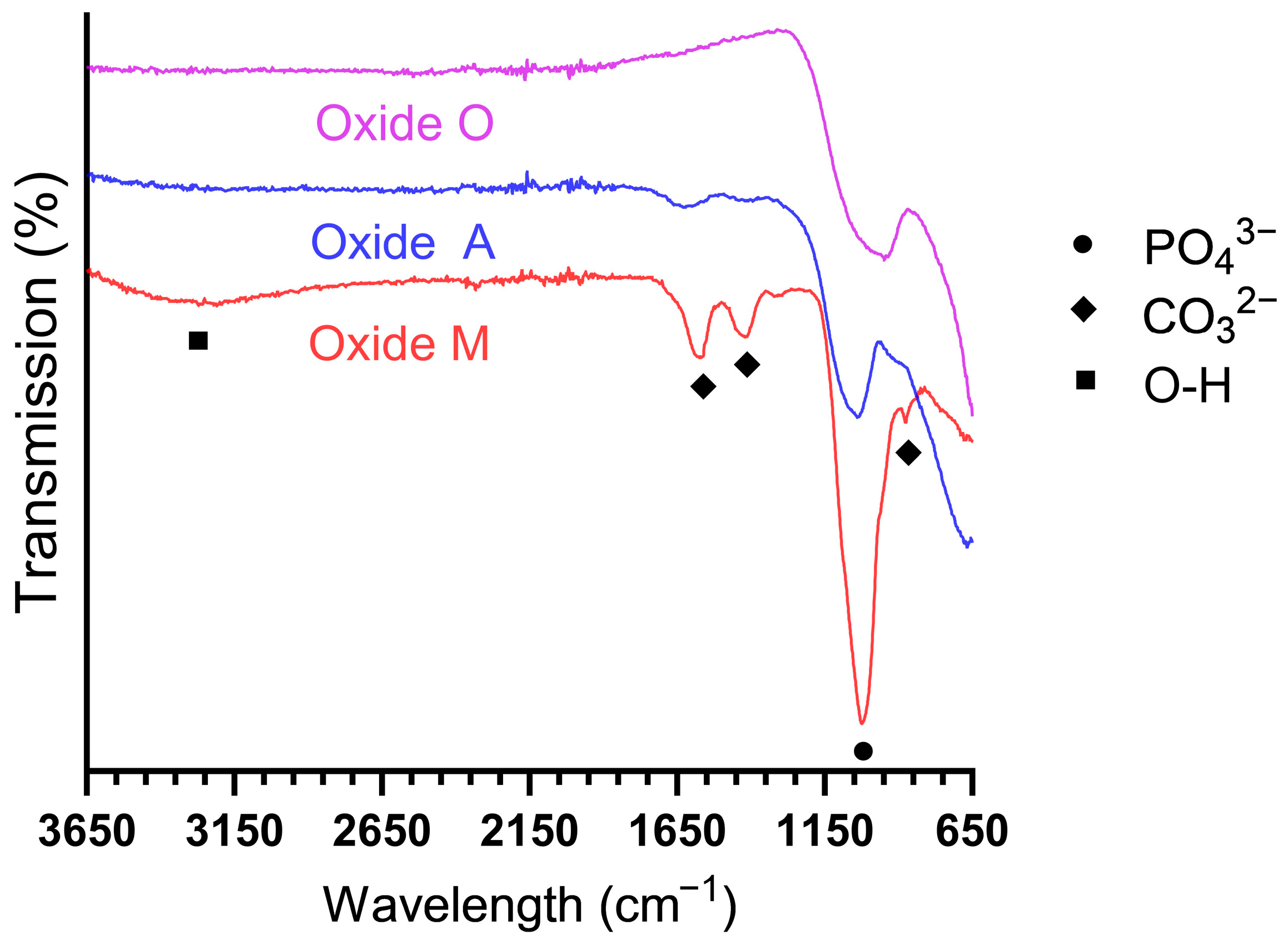
3.1.5. Anodized Oxide Molecular Structure Analyses
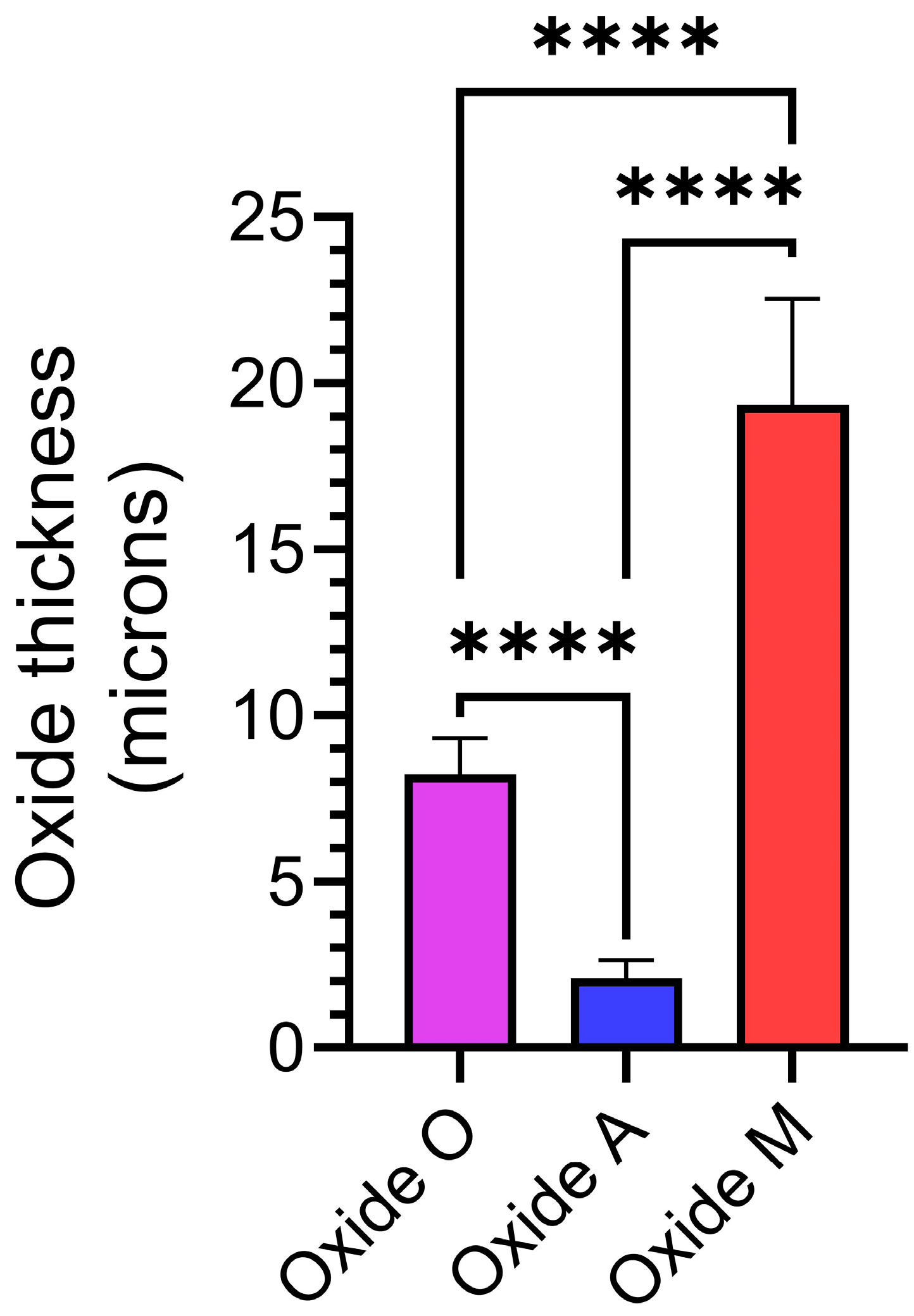
3.2. Anodized Oxide Coating Thickness
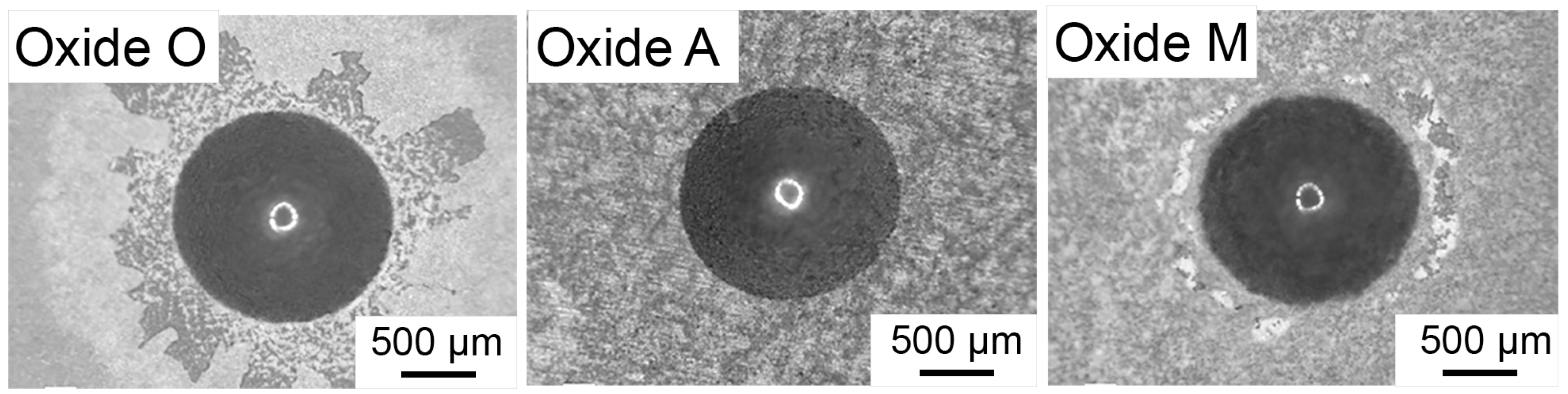
3.3. Oxide Adhesion Quality
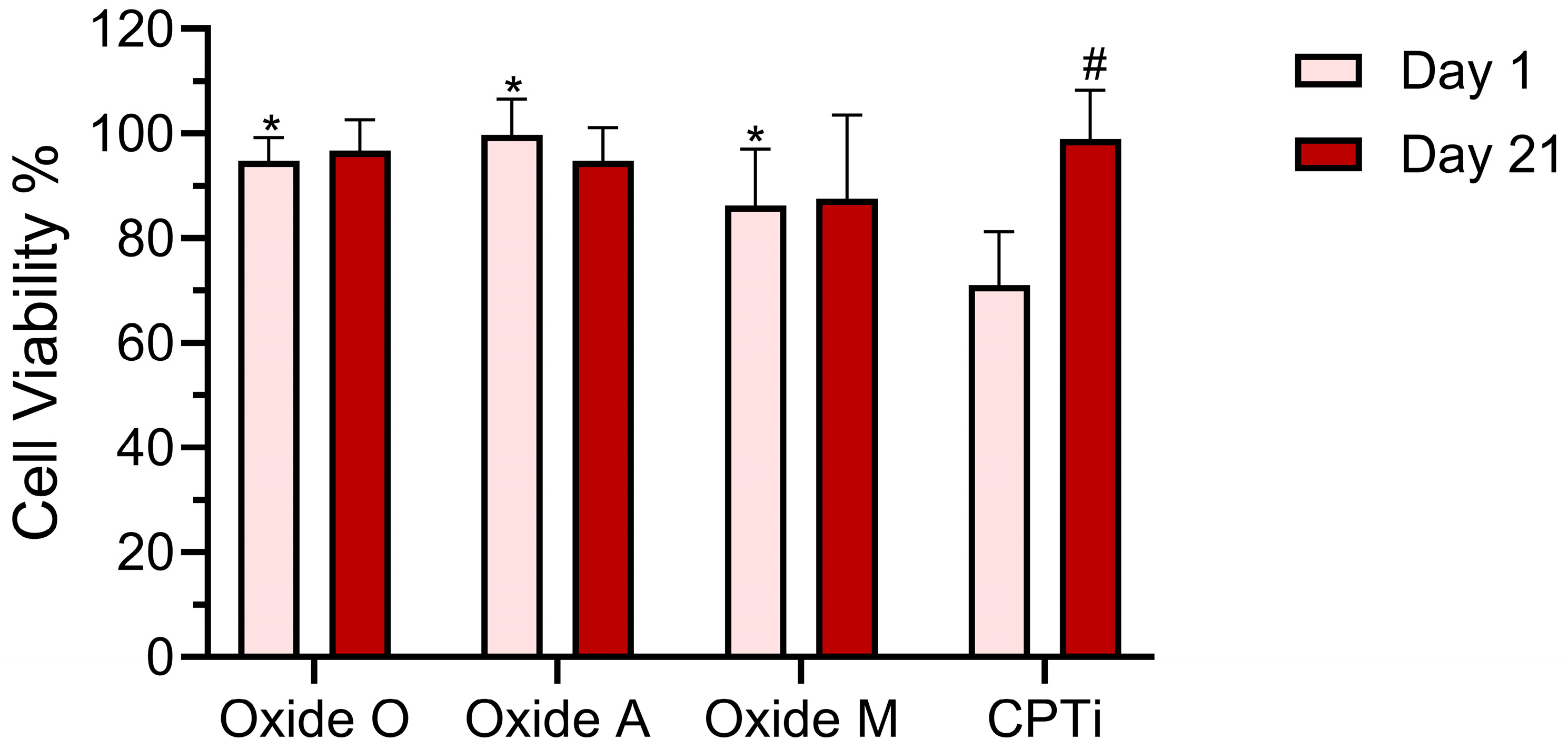
3.4. Cell Viability Test Results
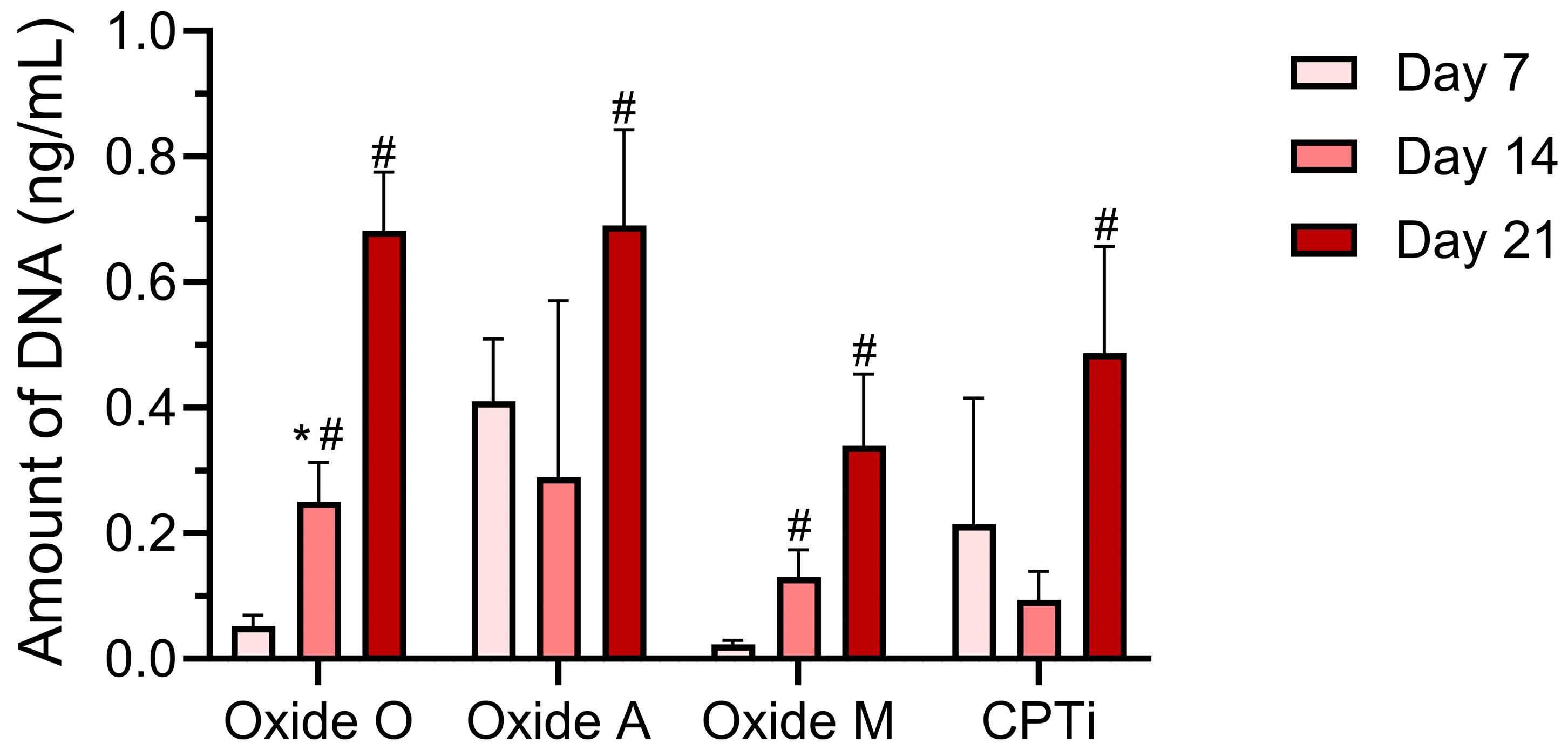
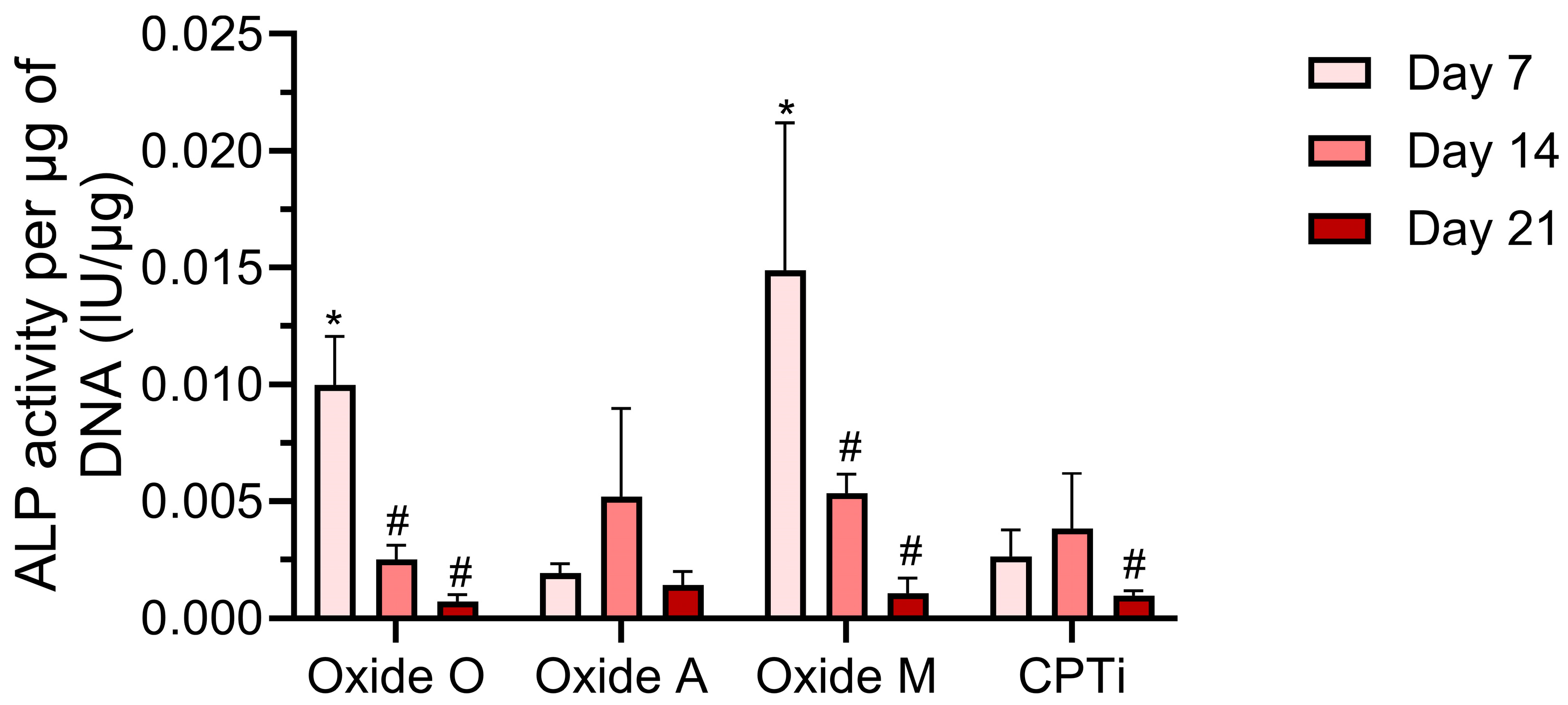
3.5. Biochemical Analyses
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Cooper, L.F.; Shirazi, S. Osseointegration—The biological reality of successful dental implant therapy: A narrative review. Front. Oral Maxillofac. Med. 2021, 4. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact. 2009, 9, 61–71. [Google Scholar]
- Tobias, G.; Chackartchi, T.; Haim, D.; Mann, J.; Findler, M. Dental Implant Survival Rates: Comprehensive Insights from a Large-Scale Electronic Dental Registry. J. Funct. Biomater. 2025, 16, 60. [Google Scholar] [CrossRef]
- Chatzopoulos, G.S.; Wolff, L.F. Retrospective analysis of 50,333 implants on implant failure and associated patient-related factors. J. Stomatol. Oral. Maxillofac. Surg. 2023, 124, 101555. [Google Scholar] [CrossRef]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef] [PubMed]
- El Awadly, T.A.; Wu, G.; Ayad, M.; Radi, I.A.W.; Wismeijer, D.; Abo El Fetouh, H.; Osman, R.B. A histomorphometric study on treated and untreated ceramic filled PEEK implants versus titanium implants: Preclinical in vivo study. Clin. Oral. Implants Res. 2020, 31, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Nayan, N.H.M.; Sahari, N.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. An updated review on surface functionalisation of titanium and its alloys for implants applications. Mater. Today Proc. 2021, 42, 270–282. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Y.; Hu, R.; Shi, B.; Zhang, L.; Huang, Q.; Yang, Y.; Tang, P.; Lin, C. Advanced surface engineering of titanium materials for biomedical applications: From static modification to dynamic responsive regulation. Bioactive Mater. 2023, 27, 15–57. [Google Scholar] [CrossRef]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant. Dent. 2021, 7, 32. [Google Scholar] [CrossRef]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium surface treatment by calcium modification with acid-etching promotes osteogenic activity and stability of dental implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Anitua, E.; Tejero, R. Provisional Matrix Formation at Implant Surfaces—The Bridging Role of Calcium Ions. Cells 2022, 11, 3048. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Hsu, Y.; He, Y.; Wang, F.; Yang, F.; Yan, F.; Xia, D.; Liu, Y. Surface modification of titanium implants with Mg-containing coatings to promote osseointegration. Acta Biomater. 2023, 169, 19–44. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [PubMed]
- Safari, B.; Aghanejad, A.; Roshangar, L.; Davaran, S. Osteogenic effects of the bioactive small molecules and minerals in the scaffold-based bone tissue engineering. Colloids Surf. B Biointerfaces 2021, 198, 111462. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
- Abdullah, Z.S.; Mahmood, M.S.; Abdul-Ameer, F.M.A.; Fatalla, A.A. Effect of commercially pure titanium implant coated with calcium carbonate and nanohydroxyapatite mixture on osseointegration. J. Med. Life 2023, 16, 52–61. [Google Scholar] [CrossRef]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Chudinova, E.A.; Grubova, I.Y.; Korneva, O.S.; Shulepov, I.A.; Teresov, A.D.; Koval, N.N.; Mayer, J.; Oehr, C.; Surmenev, R.A. Effect of pulsed electron beam treatment on the physico-mechanical properties of hydroxyapatite-coated titanium. Ceram. Int. 2016, 42 Part B, 1470–1475. [Google Scholar] [CrossRef]
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2021, 47, 4426–4445. [Google Scholar] [CrossRef]
- Khor, K.A.; Gu, Y.W.; Pan, D.; Cheang, P. Microstructure and mechanical properties of plasma sprayed HA/YSZ/Ti–6Al–4V composite coatings. Biomaterials 2004, 25, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kumar, M.A.; Chowdhary, R. Anodized Dental Implant Surface. Indian. J. Dent. Res. 2017, 28, 76–99. [Google Scholar] [CrossRef]
- Sul, Y.-T.; Johansson, C.B.; Petronis, S.; Krozer, A.; Jeong, Y.; Wennerberg, A.; Albrektsson, T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: The oxide thickness, micropore configurations, surface roughness, crystal structure and chemical composition. Biomaterials 2002, 23, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-J.; Fuh, L.-J.; Chen, W.-C. Nano-morphology, crystallinity and surface potential of anatase on micro-arc oxidized titanium affect its protein adsorption, cell proliferation and cell differentiation. Mater. Sci. Eng. C 2020, 107, 110204. [Google Scholar] [CrossRef]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Jain, S.; Williamson, R.S.; Janorkar, A.V.; Griggs, J.A.; Roach, M.D. Osteoblast response to nanostructured and phosphorus-enhanced titanium anodization surfaces. J. Biomater. Appl. 2019, 34, 419–430. [Google Scholar] [CrossRef]
- Qiao, X.; Yang, J.; Shang, Y.; Deng, S.; Yao, S.; Wang, Z.; Guo, Y.; Peng, C. Magnesium-doped Nanostructured Titanium Surface Modulates Macrophage-mediated Inflammatory Response for Ameliorative Osseointegration. Int. J. Nanomed. 2020, 15, 7185–7198. [Google Scholar] [CrossRef]
- Parekh, A.; Moore, M.; Janorkar, A.V.; Roach, M.D. Mg-Doped Carbonated Hydroxyapatite and Tricalcium Phosphate Anodized Coatings on Titanium Implant Alloys. Appl. Sci. 2024, 14, 11831. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.C.; Koshy, P.; Abdullah, H.Z.; Idris, M.I. Influence of altered Ca-P based electrolytes on the anodised titanium bioactivity. Surf. Coat. Technol. 2021, 412, 127041. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Liang, C.; Wang, H.; Qiao, Z. Formation mechanism and adhesive strength of a hydroxyapatite/TiO2 composite coating on a titanium surface prepared by micro-arc oxidation. Appl. Surf. Sci. 2016, 362, 109–114. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Ning, C.; Nan, K.; Han, Y. Hydroxyapatite coatings produced on commercially pure titanium by micro-arc oxidation. Biomed. Mater. 2007, 2, 196–201. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Zhou, L.; Ma, J.; Zhang, X.; Li, H.; Liang, C.; Liu, S.; Wang, H. Influence of surface structures on biocompatibility of TiO2/HA coatings prepared by MAO. Mater. Chem. Phys. 2018, 215, 339–345. [Google Scholar] [CrossRef]
- Abbasi, S.; Bayati, M.R.; Golestani-Fard, F.; Rezaei, H.R.; Zargar, H.R.; Samanipour, F.; Shoaei-Rad, V. Micro arc oxidized HAp–TiO2 nanostructured hybrid layers—Part I: Effect of voltage and growth time. Appl. Surf. Sci. 2011, 257, 5944–5949. [Google Scholar] [CrossRef]
- Alipal, J.; Saidin, S.; Lo, A.Z.K.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. In vitro surface efficacy of CaP-based anodised titanium for bone implants. Surf. Interfaces 2023, 39, 102872. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. The tribological properties of bioceramic coatings produced on Ti6Al4V alloy by plasma electrolytic oxidation. Ceram. Int. 2014, 40, 3627–3635. [Google Scholar] [CrossRef]
- Sun, J.; Han, Y.; Huang, X. Hydroxyapatite coatings prepared by micro-arc oxidation in Ca- and P-containing electrolyte. Surf. Coat. Technol. 2007, 201, 5655–5658. [Google Scholar] [CrossRef]
- Parekh, A.; Tahincioglu, A.; Walters, C.; Chisolm, C.; Williamson, S.; Janorkar, A.V.; Roach, M.D. Citrus-Fruit-Based Hydroxyapatite Anodization Coatings on Titanium Implants. Materials 2025, 18, 1163. [Google Scholar] [CrossRef]
- Parekh, A.; Janorkar, A.V.; Roach, M.D. Bone-like Carbonated Apatite Titanium Anodization Coatings Produced in Citrus sinensis-Based Electrolytes. Appl. Sci. 2025, 15, 8548. [Google Scholar] [CrossRef]
- Grąz, M. Role of oxalic acid in fungal and bacterial metabolism and its biotechnological potential. World J. Microbiol. Biotechnol. 2024, 40, 178. [Google Scholar] [CrossRef]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res. Ther. 2013, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Thaler, R.; Khani, F.; Sturmlechner, I.; Dehghani, S.S.; Denbeigh, J.M.; Zhou, X.; Pichurin, O.; Dudakovic, A.; Jerez, S.S.; Zhong, J.; et al. Vitamin C epigenetically controls osteogenesis and bone mineralization. Nat. Commun. 2022, 13, 5883. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Gorman, D.; Green, C.; Booth-Downs, F.; Gee, M.; Fry, T. Rockwell Indentation Test for Evaluation of Adhesion of Coatings—Aka Daimler-Benz Adhesion Test; National Physical Laboratory: Teddington, UK, 2022. [Google Scholar]
- Oss Giacomelli, R.; Mattos, J.; Soprano, P.; Salvaro, D.; Binder, C.; Klein, A.; Biasoli de Mello, J.D. Assessment of multifunctional coating adhesion: Comparison between indentation and scratch tests. In Proceedings of the 2nd International Brazilian Conference on Tribology, Guangzhou, China, 10–13 November 2019; pp. 4059–4068. [Google Scholar] [CrossRef]
- Drobný, P.; Mercier, D.; Koula, V.; Škrobáková, S.I.; Čaplovič, Ľ.; Sahul, M. Evaluation of Adhesion Properties of Hard Coatings by Means of Indentation and Acoustic Emission. Coatings 2021, 11, 919. [Google Scholar] [CrossRef]
- Vidakis, N.; Antoniadis, A.; Bilalis, N. The VDI 3198 indentation test evaluation of a reliable qualitative control for layered compounds. J. Mater. Process. Technol. 2003, 143–144, 481–485. [Google Scholar] [CrossRef]
- Parekh, A.; Knotts, P.; Janorkar, A.V.; Roach, M.D. Citrus Fruit-Based Calcium Titanate Anodization Coatings on Titanium Implants. Oxygen 2025, 5, 7. [Google Scholar] [CrossRef]
- Lv, L.; Li, K.; Xie, Y.; Cao, Y.; Zheng, X. Enhanced osteogenic activity of anatase TiO2 film: Surface hydroxyl groups induce conformational changes in fibronectin. Mater. Sci. Eng. C 2017, 78, 96–104. [Google Scholar] [CrossRef]
- Yang, X.F.; Chen, Y.; Yang, F.; He, F.M.; Zhao, S.F. Enhanced initial adhesion of osteoblast-like cells on an anatase-structured titania surface formed by H2O2/HCl solution and heat treatment. Dent. Mater. 2009, 25, 473–480. [Google Scholar] [CrossRef]
- Yu, D.; Tang, Z.; Bao, S.; Guo, S.; Chen, C.; Wu, Q.; Wang, M.; Zheng, Z.; Cao, P.; Xu, B.; et al. Immunoregulatory Neuro-Vascularized Osseointegration Driven by Different Nano-Morphological CaTiO(3) Bioactive Coatings on Porous Titanium Alloy Scaffolds. Adv. Healthc. Mater. 2025, 14, e2404647. [Google Scholar] [CrossRef]
- Cao, H.; Qin, H.; Zhao, Y.; Jin, G.; Lu, T.; Meng, F.; Zhang, X.; Liu, X. Nano-thick calcium oxide armed titanium: Boosts bone cells against methicillin-resistant Staphylococcus aureus. Sci. Rep. 2016, 6, 21761. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Liao, Z.; Wei, Y.; Hang, R.; Huang, D. Engineered functional doped hydroxyapatite coating on titanium implants for osseointegration. J. Mater. Res. Technol. 2023, 27, 122–152. [Google Scholar] [CrossRef]
- Li, K.; Dai, F.; Yan, T.; Xue, Y.; Zhang, L.; Han, Y. Magnetic Silicium Hydroxyapatite Nanorods for Enhancing Osteoblast Response in Vitro and Biointegration in Vivo. ACS Biomater. Sci. Eng. 2019, 5, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, S.; Roy, M.; Bandyopadhyay, A.; Bose, S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015, 17, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.B.; Silva, N.; Marques, J.F.; Mata, A.; Silva, F.S.; Caramês, J. Biomimetic Implant Surfaces and Their Role in Biological Integration—A Concise Review. Biomimetics 2022, 7, 74. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogino, M. Formation and characterization of anodic titanium oxide films containing Ca and P. J. Biomed. Mater. Res. 1995, 29, 65–72. [Google Scholar] [CrossRef]
- Ishizawa, H.; Ogino, M. Characterization of thin hydroxyapatite layers formed on anodic titanium oxide films containing Ca and P by hydrothermal treatment. J. Biomed. Mater. Res. 1995, 29, 1071–1079. [Google Scholar] [CrossRef]
- Cerqueira, A.; García-Arnáez, I.; Romero-Gavilán, F.; Azkargorta, M.; Elortza, F.; Martín de Llanos, J.J.; Carda, C.; Gurruchaga, M.; Goñi, I.; Suay, J. Complex effects of Mg-biomaterials on the osteoblast cell machinery: A proteomic study. Biomater. Adv. 2022, 137, 212826. [Google Scholar] [CrossRef]
- Nelson, J.; Jain, S.; Pal, P.; Johnson, H.A.; Nobles, K.P.; Janorkar, A.V.; Williamson, R.S.; Roach, M.D. Anodized titanium with calcium and phosphorus surface enhancements for dental and orthopedic implant applications. Thin Solid Films 2022, 745, 139117. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy; InTech: London, UK, 2012. [Google Scholar]
- Liu, S.; Yang, X.; Cui, Z.; Zhu, S.; Wei, Q. One-step synthesis of petal-like apatite/titania composite coating on a titanium by micro-arc oxidation. Mater. Lett. 2011, 65, 1041–1044. [Google Scholar] [CrossRef]
- Madupalli, H.; Pavan, B.; Tecklenburg, M.M.J. Carbonate substitution in the mineral component of bone: Discriminating the structural changes, simultaneously imposed by carbonate in A and B sites of apatite. J. Solid State Chem. 2017, 255, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yotsova, R.; Peev, S. Biological Properties and Medical Applications of Carbonate Apatite: A Systematic Review. Pharmaceutics 2024, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Baďurová, B.; Nystøl, K.; Michalič, T.O.; Kucháriková, V.; Statelová, D.; Nováková, S.; Strnádel, J.; Halašová, E.; Škovierová, H. Temporal Profiling of Cellular and Molecular Processes in Osteodifferentiation of Dental Pulp Stem Cells. Biology 2025, 14, 257. [Google Scholar] [CrossRef] [PubMed]


| Group | Distilled Water | Organic Acid Component (M) | Magnesium Phosphate (M) | Calcium Acetate (M) |
|---|---|---|---|---|
| Oxide O | 500 mL | Oxalic acid—0.16 | 0.150 | 0.275 |
| Oxide A | 500 mL | Ascorbic acid—0.18 | 0.150 | 0.275 |
| Oxide M | 500 mL | Malic acid—0.1 | 0.100 | 0.350 |
| Elements | Oxide O (At. %) | Oxide A (At. %) | Oxide M (At. %) |
|---|---|---|---|
| Titanium | 11 ± 0.1 | 10 ± 1 | <1 |
| Oxygen | 64 ± 0.7 | 61 ± 2 | 58 ± 0.5 |
| Calcium | 4 ± 0.1 | 6 ± 0.3 | 18 ± 0.5 |
| Phosphorus | 7 ± 0.2 | 6 ± 0.2 | 9 ± 0.1 |
| Magnesium | 5 ± 0.2 | 2 ± 0.1 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ettuthaiyil Sambasivan, A.; Parekh, A.; Janorkar, A.V.; Roach, M.D. Organic Acid-Based Anodization Process to Produce Bioactive Oxides on Titanium Implants. Materials 2025, 18, 5190. https://doi.org/10.3390/ma18225190
Ettuthaiyil Sambasivan A, Parekh A, Janorkar AV, Roach MD. Organic Acid-Based Anodization Process to Produce Bioactive Oxides on Titanium Implants. Materials. 2025; 18(22):5190. https://doi.org/10.3390/ma18225190
Chicago/Turabian StyleEttuthaiyil Sambasivan, Arunendu, Amisha Parekh, Amol V. Janorkar, and Michael D. Roach. 2025. "Organic Acid-Based Anodization Process to Produce Bioactive Oxides on Titanium Implants" Materials 18, no. 22: 5190. https://doi.org/10.3390/ma18225190
APA StyleEttuthaiyil Sambasivan, A., Parekh, A., Janorkar, A. V., & Roach, M. D. (2025). Organic Acid-Based Anodization Process to Produce Bioactive Oxides on Titanium Implants. Materials, 18(22), 5190. https://doi.org/10.3390/ma18225190










