Nanocellulose-Reinforced Poly(Lactic Acid) and Poly(ε-caprolactone) Bio-Nanocomposites: A Review and Future Outlook for Poly(Lactic Acid)/Poly(ε-caprolactone) Blend Systems
Abstract
1. Introduction
2. Synthesis and Properties of Poly(ε-caprolactone)
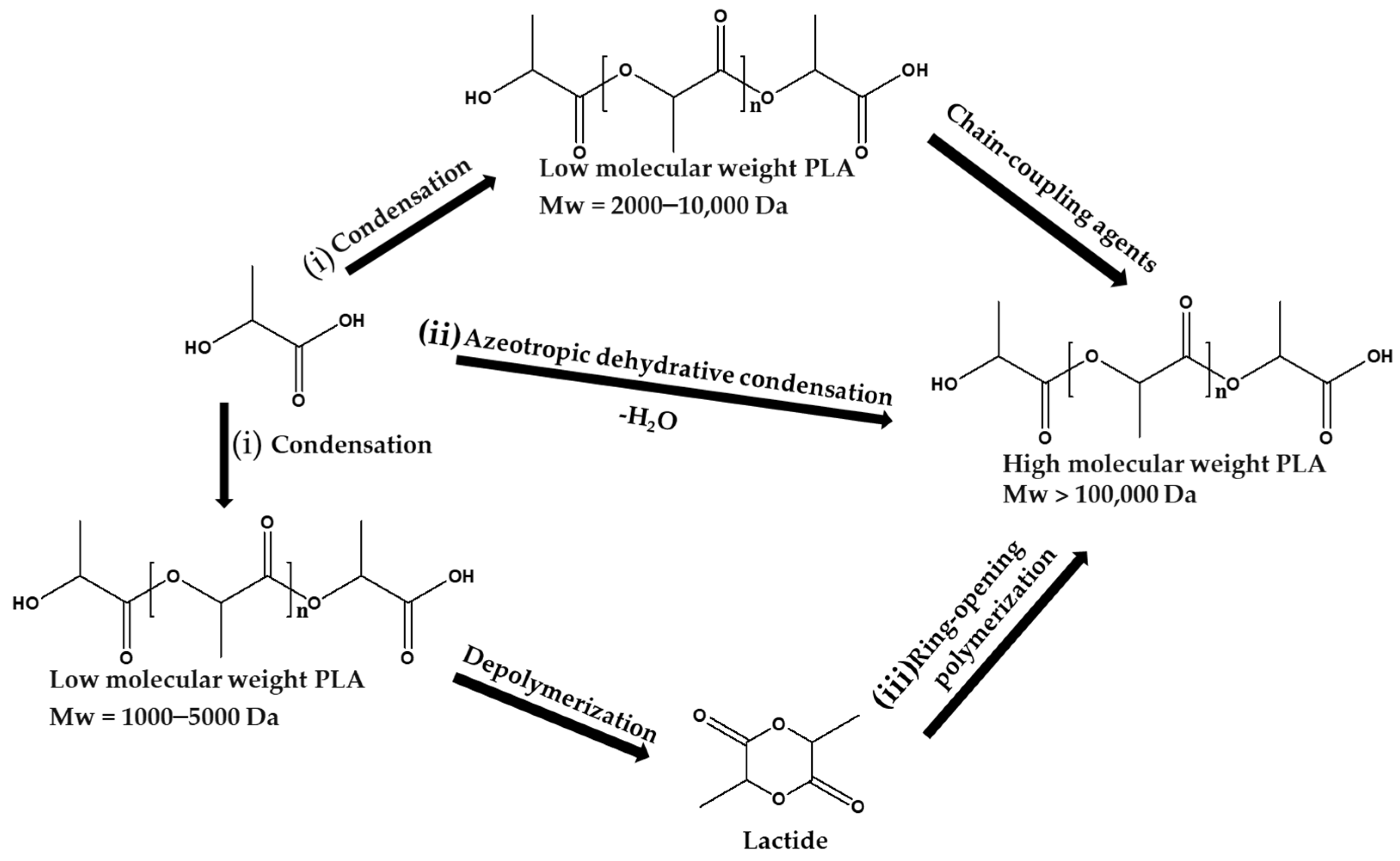
3. Synthesis and Properties of Poly(Lactic Acid)
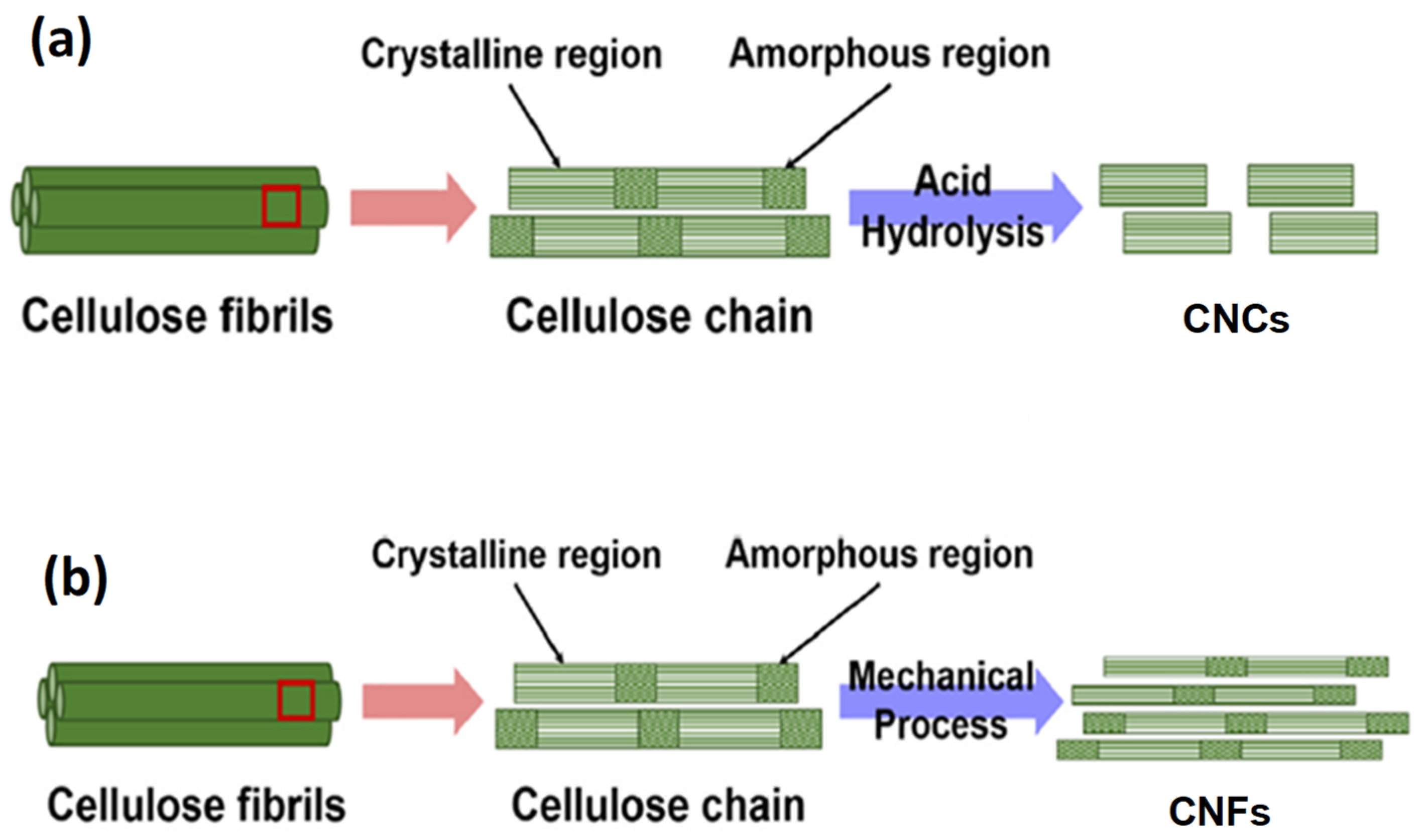
4. Nanocellulose: Extraction Methods and Characterization Techniques
5. Poly(ε-caprolactone)/nanocellulose Bio-Nanocomposites: Preparation and Property Enhancements
| Composite | Source | Filler (wt.%) | Fabrication Method | Enhanced Properties | Application Area | References |
|---|---|---|---|---|---|---|
| PCL/CNCs | Sugarcane bagasse | 2.5, 5.0, 7.5, 10.0, 12.5, and 15.0 | Solution casting | Tensile modulus, storage modulus, biodegradability properties, and moisture barrier. Maximum increase in Young’s modulus was about 77%, and it was achieved at 12.5 wt.% CNCs loading. | Food packaging | [36] |
| PCL/CNCs | Commercial | 10 | Melt blending and pressing | Rheological performance, crosslinking, and thermal stability. | Shape-memory applications | [66] |
| PCL/CNCs | Marine algae biomass | 0.5, 1.0, and 2.0 | Solvent casting | Thermal stability, Young’s modulus, and tensile strength. | Packaging | [67] |
| PCL/CNCs | Purchased | 1.0, 3.0, 5.0, and 7.0 | Solvent casting | Nucleation and crystallinity for all composites. | Energy storage | [68] |
| PCL/CNCs | Sugarcane bagasse | 2.0 | Solution casting | Heterogeneous nucleation, crystallization rate, and improved tensile strength of PCL from 16.5 MPa to 17.8 MPa. | Packaging | [22] |
| PCL/CNCs | Orange peel | 2.0 | Solution casting | Heterogeneous nucleation, crystallization rate, and improved tensile strength of PCL. | Packaging | [22] |
| PCL/micro-cellulose | Wheat bran | 2.0 | Solution casting | Heterogeneous nucleation, crystallization rate, and improved tensile strength of PCL. | Packaging | [22] |
| PCL/CNCs | Starch | 1.0, 3.0, and 5.0 | Solution casting | Tearing strength increased by 68%, and oxygen transmission rate (from 1740 to 1250 cm3/m2 per day) and gas permeability were improved for the composite with 1.0 wt.% CNCs. | Packaging membrane | [23] |
| PCL-CaAlg/CNCs | Cotton | 1.0 and 5.0 | Solution casting | Degradation rate and hydrophilicity. | Wound dressing | [20] |
| PCL/CNFs | Rice straw | 0.5, 1.0, 5.0, 10.0, and 15.0 | Melt blending and pressing | Tensile strength and Young’s modulus increment of 7.5% at 10 wt.% CNFs and 76% at 15 wt.% CNFs, respectively. Hydrophilicity at 15 wt.% CNFs. Nucleated crystallization. | Packaging and biomedical | [21] |
| PCL/CNCs | Cotton waste fiber | 0.5, 1.0, 1.5, 2.5, and 4.0 | Electrospinning | Hydrophilicity and crystallization activation energy at 1.0 wt.% CNCs content. | Tissue engineering and wound dressing | [69] |
| PCL/CNFs-g-PCL | Hardwood kraft pulp | 10.0, 20.0, and 30.0 | 3D printing | Young’s modulus and rigidity improved for all samples. Melting temperature increased. Tensile strength enhanced by 45.5% at CNFs-g-PCL 30.0 wt.% loading. | Sustainable products | [71] |
| PCL/CNCs | Purchased | 1.0, 2.0, and 3.0 wt.% | Wet spinning | Balanced tensile strength and flexibility (Young’s modulus of 0.27 GPa). Biocompatibility. | Tissue engineering and anterior cruciate ligament | [76] |
6. Poly(Lactic Acid)/nanocellulose Bio-Nanocomposites: Preparation and Property Enhancements
| Composite | Source | Filler (wt.%) | Fabrication Method | Enhanced Properties | Application Area | References |
|---|---|---|---|---|---|---|
| PLA/CNCs | Sugarcane bagasse fiber | 10.0 | Solvent casting | Acid hydrolysis of cellulose was performed by sulfuric acid (S–CNCs) and phosphoric acid (P–CNCs). Both P–CNCs and S–CNCs improved thermal stability of PLA. TGA results had shown that PLA/P–CNCs exhibited higher thermal stability than PLA/S–CNCs nanocomposites. | Packaging | [65] |
| PLA/CNFs | Sugarcane bagasse | 1.0, 2.0, 3.0, 4.0, and 5.0 | Injection molding | Improvement in water resistance, thermal stability, and mechanical properties such as tensile and flexural strength, impact resistance, and fracture toughness was observed in nanocomposites with 2 wt.% CNFs loading. | Sustainable products | [16] |
| PLA/CNCs | Sugarcane bagasse | 5.0, 10.0, and 15.0 | Solvent casting | Improved thermal stability and tensile strength at 10.0 wt.% | Packaging | [17] |
| PLA/CNCs | Softwood pulp | 1.0, 2.0, 3.0, and 5.0 | Solution casting and co-extrusion | Improved storage moduli at 3.0 wt.%. | Packaging, Medical | [77] |
| PLA/CNCs | Purchased | 1.0, 3.0, and 5.0 | Melt blending | Improved tensile properties at 3.0 wt.%. | Packaging | [18] |
| PLA/CNCs | Neptune grass | 1.0 and 3.0 | Solvent casting | Accelerated degradation at 3.0 wt.%. | Food packaging | [37] |
| PLA/CNCs | Wood pulp | 0.5 | Melt blending and injection molding | Tensile strength increased from 57.9 to 79.6 MPa. Crystallinity increased from 35.9 to 42.5%. | Packaging | [19] |
| PLA/CNCs | Purchased | 0.75, 1.0, and 2.0 | Single screw extrusion | Thermal stability increased at 2.0 wt.% CNCs, and tensile strength increased by 18.2% at 1.0 wt.% CNCs. | 3D biomedical applications. | [14] |
| PLA/CNCs | Purchased | 2.0 | Solvent-free cast extrusion | Reduced microbial growth, therefore, increased the shelf life of food that is oxygen sensitive. | Food packaging | [78] |
| PLA/CNCs-PEG | Cotton | 0.5, 1.0, 2.0 and 4.0, and 8.0 | Electrospinning | Tensile strength and elongation at break improved by factor of 2.8 and 1.9, respectively, at 4.0 wt.% CNCs-PEG loading, while thermal stability increased with increasing CNCs-PEG content. Hydrophilicity and enzyme degradation rate increased for all CNCs-PEG-containing samples compared to neat PLA. | Sustainable products | [81] |
| PLA/CNFs-g-PLA | Hardwood kraft pulp | 10.0, 20.0, and 30.0 | 3D printing | Young’s modulus significantly increased at 20.0 wt.% CNFs-g-PLA loading. Tensile strength increased by 20.8% at 20.0 wt.% CNFs-g-PLA loading. Thermal stability is also enhanced by 20.0 wt.% CNFs-g-PLA loading. | Sustainable products | [71] |
| PLA/MCNCs | Purchased | 1.25 | Solution casting | Enhanced crystallinity, biodegradation rate under soil burial, and mechanical properties (tensile strength by 34.6% and elongation at break by 84.3%). | Agriculture and packaging | [86] |
| PLA/CNCs and PLA/CNCs-g-ECO | Purchased | 1.0 | Melt blending and (solvent casting followed by melt blending) | CNCs-g-ECO improved dispersion, acted as a nucleating agent, increased crystallinity, and thermal stability. | Sustainable products | [87] |
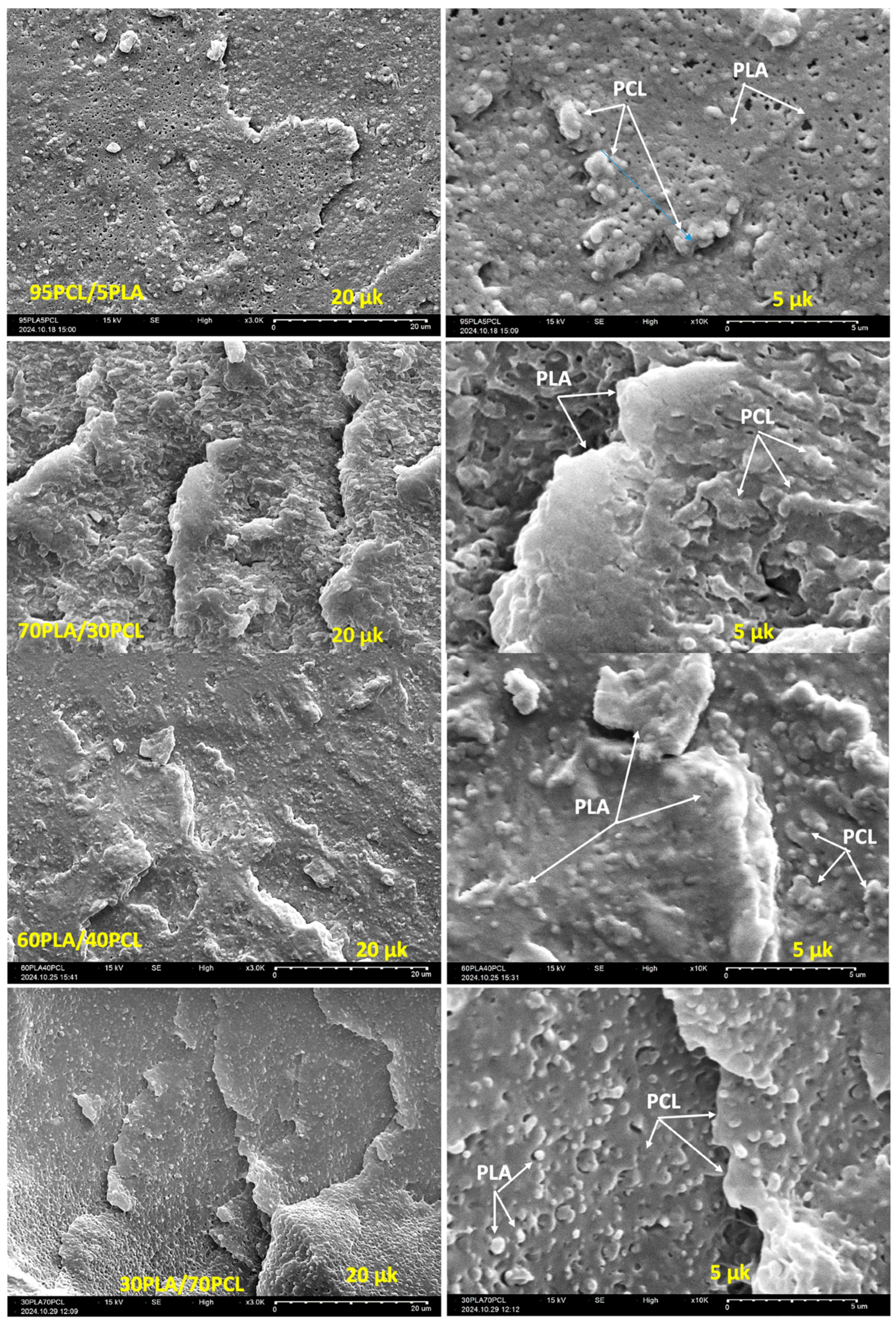
7. Poly(Lactic Acid)/poly(ε-caprolactone) Biopolymer Blends: Morphology, Compatibility and Properties
8. Poly(Lactic Acid)/poly(ε-caprolactone)-Based Composites Hybrid Reinforcements: Processing and Performance
| Composite Name | PLA/PCL Ratios | Filler Type and Source | Filler Content (wt.%) | Fabrication Method | Enhanced Properties | Application Area | References |
|---|---|---|---|---|---|---|---|
| PLA/PCL/TOBC | 100/0, 95/5, 90/10, 85/15, and 80/20 | TEMPO-oxidized bacterial cellulose | 1.5 | 3D printing | 10% PCL content increased tensile strength and elongation at break by 17.4% and 208% compared to that of neat PLA, respectively. Crystallinity increases with increasing PCL content. | Biomedical | [32] |
| PLA/PCL/MCC | 90/10 and 80/20 | Micro-crystalline cellulose (MCC) from cotton | 1.0 | Melt extrusion and blending | Enhanced hydrophilicity and accelerated biodegradation. | Packaging | [33] |
| PLA/PCL/CNCs | 70/30 | CNCs, CNCs-g-PCL and CNCs-g-PLLA | 1.0 | Melt blending | Enhanced shape-memory response, accelerated biodegradation, elastic modulus, and tensile strength. | Biomedicine and food packaging | [97] |
| PLA/PCL/CNCs, PLA/PCL/BCP, and PLA/PCL/BCP-CNCs | 60/40 | Cellulose nanocrystals (CNCs) from cotton and PCL-PEG-PCL (BCP) tri-block copolymer | CNCs (0.5, 1.0 and 2.0) BCP (5.0, 10.0 and 20.0) | Solvent casting | Enhanced water uptake for all samples. BCP10-CNCs1.0 enhanced interfacial interaction. 10.0 wt.% BCP enhanced crystallinity of PCL. Porosity increased with CNCs content in blend. | Biomedical | [28] |
| PLA/PCL/MMT nanoclay | 80/20 | Montmorillonite (MMT) | 2.0, 4.0, and 6.0 | Melt blending | Higher tensile strength and compatibility at 4.0 wt.% MMT. | Printing plates | [25] |
| PLA/PCL/Silk fibroin nanoparticles | 100/0, 90/10, 80/20, and 70/30 | Silk fibroin nanoparticles (SFNPs) from silkworm cocoons | 1.0 | Melt blending | Enhanced thermal stability and compatibility for 70/30 blend by 1.0 wt.% SFNPs. | Food packaging | [27] |
| PLA/PCL/ Pluronic | 100/0, 90/10, 85/15, 80/20, 75/25, and 70/30 | Synthetic Pluronic | 2.5, 5.0, and 7.5 | Melt blending | Improved tensile strength for blends with 10, 15, and 20 wt.% PCL content at 2.5 parts per hundred. | Packaging | [24] |
| PLA/PCL/TAIC | 80/20, 60/40, 40/60, and 20/80 | Triallyl isocyanurate (TAIC) | 3.0 | Melt blending | Improved strength, modulus, and hindered phase separation for 20 PLA/80 PCL. | Packaging and Biomedical | [31] |
| PLA/PCL/MMT | 70/30 | Montmorillonite | 1.0 | Solvent casting, Melt blending | Improved PLA phase dispersion and better interface interaction. | Biomedical | [26] |
| PLA/PCL/ O.MMT | 20/80 | Organophilic Montmorillonite (O.MMT) | 2.0 | Melt blending | Improved indentation modulus by 50% compared to that of PCL. | Packaging | [34] |
| PLA/PCL/CNCs | 70/30 | CNCs (purchased) | 1.0, 2.0, 3.0, and 5.0 | Melt extrusion and blending | Improved compatibility and mechanical properties. | Various applications | [100] |
9. Conclusions, Challenges, and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| CNCs | Cellulose nanocrystals |
| CNFs | Cellulose nanofibers |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared spectroscopy |
| MCC | Microcrystalline cellulose |
| MMT | Montmorillonite |
| NC | Nanocellulose |
| O.MMT | Organically modified montmorillonite |
| PBAT | Poly(butylene adipate–co–terephthalate) |
| PCL | Poly(ε-caprolactone) |
| P–CNCs | Phosphoric acid cellulose nanocrystals |
| PEG | Poly(ethylene) glycol |
| PEO | Poly(ethylene oxide) |
| PVA | Poly(vinyl alcohol) |
| PLA | Poly(lactic acid) |
| ROP | Ring-opening polymerization |
| rROP | Radical ring-opening polymerization |
| S–CNCs | Sulfuric acid cellulose nanocrystals |
| SEM | Scanning electron microscopy |
| SFNPs | Silk fibroin nanoparticles |
| TAIC | Triallyl isocyanurate |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl |
| TGA | Thermogravimetric analysis |
| TOBC | (TEMPO)-oxidized bacterial cellulose |
References
- Wang, W.; Niu, B.; Liu, R.; Chen, H.; Fang, X.; Wu, W.; Wang, G.; Gao, H.; Mu, H. Development of bio-based PLA/cellulose antibacterial packaging and its application for the storage of shiitake mushroom. Food Chem. 2023, 429, 136905. [Google Scholar] [CrossRef]
- Widjaja, T.; Rohmah, A.A.Z.; Nurkhamidah, S.; Ni’mah, H.; Wardhono, E.Y.; Saputra, B.Y.E.; Sari, C.Y. Effectiveness study of Cellulose Nanocrystal (CNC) filler usage on polylactic acid (PLA) properties through plasticizer addition optimization: Application in paper-coated tableware. Case Stud. Chem. Environ. Eng. 2025, 11, 101186. [Google Scholar] [CrossRef]
- Kurakula, M.; Rao, G.K.; Yadav, K.S. Fabrication and characterization of polycaprolactone-based green materials for drug delivery. In Applications of Advanced Green Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 395–423. [Google Scholar]
- Fernández-Tena, A.; Otaegi, I.; Irusta, L.; Sebastián, V.; Guerrica-Echevarria, G.; Müller, A.J.; Aranburu, N. High-Impact PLA in Compatibilized PLA/PCL Blends: Optimization of Blend Composition and Type and Content of Compatibilizer. Macromol. Mater. Eng. 2023, 308, 2300213. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Argyros, A. Optimization of cellulose nanocrystal (CNC) concentration in polycaprolactone bio-composites for bio-plotting: A robust interpretation of the reinforcement mechanisms. Cellulose 2024, 31, 3657–3680. [Google Scholar] [CrossRef]
- Ghanem, A.F.; Yassin, M.A.; Cosquer, R.; Gouanvé, F.; Espuche, E.; Rehim, M.H.A. Polycaprolactone composite films infused with hyperbranched polyester/reduced graphene oxide: Influence on biodegradability, gas/water transport and antimicrobial properties for sustainable packaging. RSC Adv. 2024, 14, 5740–5753. [Google Scholar] [CrossRef]
- Carotenuto, M.R.; Cavallaro, G.; Chinnici, I.; Lazzara, G.; Milioto, S. Thermomechanical properties of bionanocomposites based on polycaprolactone and halloysite clay nanotubes. J. Therm. Anal. Calorim. 2025, 150, 7519–7528. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, W.; Bartolo, P. In vitro investigations on the effects of graphene and graphene oxide on polycaprolactone bone tissue engineering scaffolds. Bio-Des. Manuf. 2024, 7, 651–669. [Google Scholar] [CrossRef]
- Wang, Z.P.; Ruan, W.H.; Rong, M.Z.; Zhang, M.Q. Injection molding of highly filled microcrystalline cellulose/polycaprolactone composites with the aid of reversible Diels-Alder reaction. J. Mater. Sci. Technol. 2024, 170, 246–254. [Google Scholar] [CrossRef]
- Xiao, Y.; Ding, Y.; Qiu, J.; Zhang, X.; Zheng, Y.; Huang, C.; Zhao, L.; Tang, Z.; Chen, Y.; Liu, Y. Treatment effects of 3D-printed PCL/Fe3O4@ ZIF-8 magnetic nanocomposite on infected bone defect. Int. J. Bioprinting 2024, 10, 2271. [Google Scholar] [CrossRef]
- Soriano-Cuadrado, B.; Fontecha-Cámara, M.Á.; Mañas-Villar, M.; Delgado-Blanca, I.; Ramírez-Rodríguez, M.D. Mechanical, Thermal and Morphological Study of Bio-Based PLA Composites Reinforced with Lignin-Rich Agri-Food Wastes for Their Valorization in Industry. Polymers 2024, 16, 2462. [Google Scholar] [CrossRef] [PubMed]
- Şen, İ.; Sever, K. Production and characterization of agricultural waste natural fiber-filled polylactic acid composites. Polym. Bull. 2025, 82, 4051–4074. [Google Scholar] [CrossRef]
- Ntrivala, M.A.; Pitsavas, A.C.; Lazaridou, K.; Baziakou, Z.; Karavasili, D.; Papadimitriou, M.; Ntagkopoulou, C.; Balla, E.; Bikiaris, D.N. Polycaprolactone (PCL): The biodegradable polyester shaping the future of materials–a review on synthesis, properties, biodegradation, applications and future perspectives. Eur. Polym. J. 2025, 234, 114033. [Google Scholar] [CrossRef]
- Ahmad, N.D.; Wildan, M.W. Preparation and properties of cellulose nanocrystals-reinforced Poly (lactic acid) composite filaments for 3D printing applications. Results Eng. 2023, 17, 100842. [Google Scholar] [CrossRef]
- Gond, R.; Gupta, M. Development of PLA Based Nanocellulose Film for Packaging Applications. J. Indian Chem. Soc. 2020, 97, 1621–1625. [Google Scholar]
- Gond, R.; Naik, T.P.; Gupta, M.K.; Singh, I. Development and characterisation of sugarcane bagasse nanocellulose/PLA composites. Mater. Technol. 2022, 37, 2942–2954. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Chow, W.S.; Ismail, H. Tensile, thermal and ultra-violet shielding enhancement of poly (lactic acid) bionanocomposite film using cellulose nanocrystals extracted from sugarcane bagasse. J. Thermoplast. Compos. Mater. 2023, 36, 2543–2561. [Google Scholar] [CrossRef]
- Pracella, M.; Haque, M.M.-U.; Puglia, D. Morphology and properties tuning of PLA/cellulose nanocrystals bio-nanocomposites by means of reactive functionalization and blending with PVAc. Polymer 2014, 55, 3720–3728. [Google Scholar] [CrossRef]
- Wu, J.-j.; Gao, N.; Jiang, L.; Zhong, G.-j.; Deng, C.; Gao, X. The coupling effect of cellulose nanocrystal and strong shear field achieved the strength and toughness balance of Polylactide. Int. J. Biol. Macromol. 2022, 207, 927–940. [Google Scholar] [CrossRef]
- Rashtchian, M.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Simorgh, S. Fabricating alginate/poly (caprolactone) nanofibers with enhanced bio-mechanical properties via cellulose nanocrystal incorporation. Carbohydr. Polym. 2020, 233, 115873. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, Z.; Sun, K. Renewable rice straw cellulose nanofibril reinforced poly (ε-caprolactone) composite films. Mater. Chem. Phys. 2022, 292, 126879. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Deng, L.; Jiang, H.; Yang, Z.; Yang, R.; Wu, D. Preparation and research of PCL/cellulose composites: Cellulose derived from agricultural wastes. Int. J. Biol. Macromol. 2023, 235, 123785. [Google Scholar] [CrossRef]
- Xu, C.; Chen, C.; Wu, D. The starch nanocrystal filled biodegradable poly (ε-caprolactone) composite membrane with highly improved properties. Carbohydr. Polym. 2018, 182, 115–122. [Google Scholar] [CrossRef]
- Rytlewski, P.; Gohs, U.; Stepczyńska, M.; Malinowski, R.; Karasiewicz, T.; Moraczewski, K. Electron-induced structural changes in flax fiber reinforced PLA/PCL composites, analyzed using the rule of mixtures. Ind. Crops Prod. 2022, 188, 115587. [Google Scholar] [CrossRef]
- Rao, R.U.; Venkatanarayana, B.; Suman, K. Enhancement of mechanical properties of PLA/PCL (80/20) blend by reinforcing with MMT nanoclay. Mater. Today Proc. 2019, 18, 85–97. [Google Scholar]
- Zhu, B.; Wang, Y.; Liu, H.; Ying, J.; Liu, C.; Shen, C. Effects of interface interaction and microphase dispersion on the mechanical properties of PCL/PLA/MMT nanocomposites visualized by nanomechanical mapping. Compos. Sci. Technol. 2020, 190, 108048. [Google Scholar] [CrossRef]
- Dadras Chomachayi, M.; Jalali-Arani, A.; Beltrán, F.R.; De la Orden, M.U.; Martínez Urreaga, J. Biodegradable nanocomposites developed from PLA/PCL blends and silk fibroin nanoparticles: Study on the microstructure, thermal behavior, crystallinity and performance. J. Polym. Environ. 2020, 28, 1252–1264. [Google Scholar] [CrossRef]
- Salmani, M.R.; Shirazi, F.; Goodarzi, K.; Noormohammadi, F.; Nourany, M. Improving phase compatibility of PLA-PCL Matrix-Droplet blend using CNC/PCL-PEG-PCL triblock copolymer to prepare porous 3D osteoinductive scaffolds. J. Polym. Environ. 2024, 32, 6405–6424. [Google Scholar] [CrossRef]
- Nourany, M.; Makaremy, A.; Bazrpash, S.; Hosseini, S. Bimodal macroporous 3D scaffolds based on compatibilized PCL and PLA blend using PCL-PEG-PCL block copolymers and cellulose nanocrystals for osteogenic differentiation of hMSCs. Int. J. Biol. Macromol. 2025, 299, 140149. [Google Scholar] [CrossRef]
- Haghgoo, G.; Dadashi, P.; Babaei, A. Effects of Cellulose Nanocrystals Localization on Compatibility Between Polylactic Acid and Polycaprolactone: Correlating the Microstructure and Mechanical Performance. Polym. Adv. Technol. 2025, 36, e70113. [Google Scholar] [CrossRef]
- Malinowski, R. Mechanical properties of PLA/PCL blends crosslinked by electron beam and TAIC additive. Chem. Phys. Lett. 2016, 662, 91–96. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Li, L.; Zhang, H.; Wang, H.; Ke, F. Fully biodegradable PLA composite with improved mechanical properties via 3D printing. Mater. Lett. 2023, 331, 133543. [Google Scholar] [CrossRef]
- Kalita, N.K.; Bhasney, S.M.; Mudenur, C.; Kalamdhad, A.; Katiyar, V. End-of-life evaluation and biodegradation of Poly (lactic acid)(PLA)/Polycaprolactone (PCL)/Microcrystalline cellulose (MCC) polyblends under composting conditions. Chemosphere 2020, 247, 125875. [Google Scholar] [CrossRef] [PubMed]
- Slouf, M.; Ujcic, A.; Nevoralova, M.; Vackova, T.; Fambri, L.; Kelnar, I. Monitoring of morphology and properties during preparation of PCL/PLA microfibrillar composites with organophilic montmorillonite. Front. Mater. 2020, 7, 188. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Hassan, M.L.; Bras, J.; Hassan, E.A.; Fadel, S.M.; Dufresne, A. Polycaprolactone/modified bagasse whisker nanocomposites with improved moisture-barrier and biodegradability properties. J. Appl. Polym. Sci. 2012, 125, E10–E19. [Google Scholar] [CrossRef]
- Luzi, F.; Fortunati, E.; Puglia, D.; Petrucci, R.; Kenny, J.M.; Torre, L. Study of disintegrability in compost and enzymatic degradation of PLA and PLA nanocomposites reinforced with cellulose nanocrystals extracted from Posidonia Oceanica. Polym. Degrad. Stab. 2015, 121, 105–115. [Google Scholar] [CrossRef]
- Ruz-Cruz, M.; Herrera-Franco, P.J.; Flores-Johnson, E.A.; Moreno-Chulim, M.; Galera-Manzano, L.M.; Valadez-González, A. Thermal and mechanical properties of PLA-based multiscale cellulosic biocomposites. J. Mater. Res. Technol. 2022, 18, 485–495. [Google Scholar] [CrossRef]
- Guarino, V.; Gentile, G.; Sorrentino, L.; Ambrosio, L. Polycaprolactone: Synthesis, properties, and applications. Encycl. Polym. Sci. Technol. 2002, 1–36. [Google Scholar] [CrossRef]
- Chaipakdee, N.; Hemvichian, K.; Lertsarawut, P.; Rimdusit, S.; Tiptipakorn, S. Effects of molecular weight of polycaprolactone on the thermal properties of V-fa/PCL blends added with cross-linking agent. IOP Conf. Ser. Mater. Sci. Eng. 2025, 1325, 012003. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Li, Y.; Zhang, C.-J.; Zhang, Y.-Y.; Cao, X.-H.; Zhang, X.-H. Synthesis of high-molecular-weight poly (ε-caprolactone) via heterogeneous zinc-cobalt (III) double metal cyanide complex. Giant 2020, 3, 100030. [Google Scholar] [CrossRef]
- Dias, J.R.; Sousa, A.; Augusto, A.; Bártolo, P.J.; Granja, P.L. Electrospun polycaprolactone (PCL) degradation: An in vitro and in vivo study. Polymers 2022, 14, 3397. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Tena, A.; Pérez-Camargo, R.A.; Coulembier, O.; Sangroniz, L.; Aranburu, N.; Guerrica-Echevarria, G.; Liu, G.; Wang, D.; Cavallo, D.; Müller, A.J. Effect of Molecular Weight on the Crystallization and Melt Memory of Poly (ε-caprolactone)(PCL). Macromolecules 2023, 56, 4602–4620. [Google Scholar] [CrossRef]
- Gong, C.; Li, J.; Yi, C.; Qu, S. Catalytic regulation of oligomers in polycaprolactone. Mol. Catal. 2021, 508, 111594. [Google Scholar] [CrossRef]
- Meneses, J.; van de Kemp, T.; Costa-Almeida, R.; Pereira, R.; Magalhães, F.D.; Castilho, M.; Pinto, A.M. Fabrication of polymer/graphene biocomposites for tissue engineering. Polymers 2022, 14, 1038. [Google Scholar] [CrossRef]
- Kumalo, F.I.; Malimabe, M.A.; Mosoabisane, M.F.T.; Gumede, T.P. Development and Characterization of PBS/EA Cellulose and PCL/EA Cellulose Biocomposites: Structural, Morphological, and Thermal Insights for Sustainable Applications. Polymers 2025, 17, 971. [Google Scholar] [CrossRef]
- Selikane, D.G.A.; Gumede, T.P.; Shingange, K.; Malevu, T.D.; Ngwenya, M.; Kumalo, F. Characterization of Polycaprolactone/Eucomis autumnalis Cellulose Composite: Structural, Thermal, and Mechanical Analysis. J. Biomim. Biomater. Biomed. Eng. 2024, 65, 45–58. [Google Scholar] [CrossRef]
- Ikhtiarini, N.; Kamil, M.Z.; Bukit, B.F.; Juliadmi, D.; Prasetiyo, K.W.; Fransiska, D.; Sedayu, B.B.; Subiyanto, B.; Sulastiningsih, I.M.; Rochima, E. Biocompatible composites based on alginate, polycaprolactone, and nanocellulose-A review. Int. J. Biol. Macromol. 2025, 311, 143423. [Google Scholar] [CrossRef]
- D’Amario, J.; Limsukon, W.; Bher, A.; Auras, R. Impact of hydrolysis pretreatment on the compostability of biodegradable poly (caprolactone) and poly (lactic acid) films. RSC Appl. Polym. 2025, 3, 711–721. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Luyt, A.S.; Malik, S.S. Can biodegradable plastics solve plastic solid waste accumulation? In Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 403–423. [Google Scholar]
- Widjaja, T.; Hendrianie, N.; Nurkhamidah, S.; Altway, A.; Yusuf, B.; Rohma, A.A.Z.; Pahlevi, A. Poly lactic acid production using the ring opening polymerization (ROP) method using Lewis acid surfactant combined iron (Fe) catalyst (Fe(DS)3). Heliyon 2023, 9, e17985. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H.; Sasai, K. Effect of the addition of poly (D-lactic acid) on the thermal property of poly (L-lactic acid). Polymer 2003, 44, 2569–2575. [Google Scholar] [CrossRef]
- Refinetti, D.; Sonzogni, A.; Manenti, F.; Nascimento Lima, N.M. Modeling and simulation of poly (l-lactide) polymerization in batch reactor. In Chemical Engineering Transactions; AIDIC: Milan, Italy, 2014; pp. 691–696. [Google Scholar]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Karpova, S.G.; Olkhov, A.A.; Varyan, I.A.; Khan, O.I.; Botin, A.A.; Naletova, A.V.; Popov, A.A.; Iordanskii, A.L. Electrospun Polylactide—Poly (ε-Caprolactone) Fibers: Structure Characterization and Segmental Dynamic Response. Polymers 2024, 16, 1307. [Google Scholar] [CrossRef]
- Sikhosana, S.T.; Gumede, T.P.; Malebo, N.J.; Ogundeji, A.O.; Motloung, B. Medicinal plants as a cellulose source for the fabrication of poly (lactic acid) composites: A mini-review. Polym. Renew. Resour. 2023, 14, 44–57. [Google Scholar] [CrossRef]
- Chitbanyong, K.; Pisutpiched, S.; Khantayanuwong, S.; Theeragool, G.; Puangsin, B. TEMPO-oxidized cellulose nanofibril film from nano-structured bacterial cellulose derived from the recently developed thermotolerant Komagataeibacter xylinus C30 and Komagataeibacter oboediens R37–9 strains. Int. J. Biol. Macromol. 2020, 163, 1908–1914. [Google Scholar] [CrossRef]
- Mokhena, T.; John, M. Esterified cellulose nanofibres from saw dust using vegetable oil. Int. J. Biol. Macromol. 2020, 148, 1109–1117. [Google Scholar] [CrossRef]
- Motloung, B.; Pfukwa, R.; Klumperman, B. Ion-Mediated Gelation of Thermo-Responsive Cellulose Nanofibril/Poly (N-isopropylacrylamide) Hybrid Hydrogels with Tunable De-Swelling Kinetics. Macromol. Mater. Eng. 2024, 309, 2300457. [Google Scholar] [CrossRef]
- Kurniawan, T.W.; Sulistyarti, H.; Rumhayati, B.; Sabarudin, A. Cellulose nanocrystals (CNCs) and cellulose nanofibers (CNFs) as adsorbents of heavy metal ions. J. Chem. 2023, 2023, 5037027. [Google Scholar] [CrossRef]
- Kusmono, K.; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef]
- Mohomane, S.M.; Motloung, S.V.; Koao, L.F.; Motaung, T.E. Effects of acid hydrolysis on the extraction of cellulose nanocrystals (CNCs): A review. Cellul. Chem. Technol. 2022, 56, 691–703. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Synthesis of phosphoric acid-treated sugarcane bagasse cellulose nanocrystal and its thermal properties enhancement for poly (lactic acid) nanocomposites. J. Thermoplast. Compos. Mater. 2019, 32, 619–634. [Google Scholar] [CrossRef]
- Avella, A.; Idström, A.; Mincheva, R.; Nakayama, K.; Evenäs, L.; Raquez, J.-M.; Re, G.L. Reactive melt crosslinking of cellulose nanocrystals/poly (ε-caprolactone) for heat-shrinkable network. Compos. Part A Appl. Sci. Manuf. 2022, 163, 107166. [Google Scholar] [CrossRef]
- Mondal, K.; Bhagabati, P.; Goud, V.V.; Sakurai, S.; Katiyar, V. Utilization of microalgae residue and isolated cellulose nanocrystals: A study on crystallization kinetics of poly (ɛ-caprolactone) bio-composites. Int. J. Biol. Macromol. 2021, 191, 521–530. [Google Scholar] [CrossRef]
- Li, J.; Wu, D. Nucleation roles of cellulose nanocrystals and chitin nanocrystals in poly (ε-caprolactone) nanocomposites. Int. J. Biol. Macromol. 2022, 205, 587–594. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A.; Siehr, A.; Sahoo, A.; Milan, P.B.; Joghataei, M.T.; Amoupour, M.; Simorgh, S. Cellulose nanocrystal effect on crystallization kinetics and biological properties of electrospun polycaprolactone. Mater. Sci. Eng. C 2021, 121, 111855. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Yu, Y.; Wu, C.; Zhang, H.; Zhao, Y.; Bi, H.; Xu, C. Molecular engineering of nanocellulose copolymer by grafting from homopolymerization of caprolactone: A revisit to temperature condition. Ind. Crops Prod. 2025, 235, 121699. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Q.; Wang, S.; Li, B.; Ma, L.; Zhang, L.; Yu, J.; Fan, Y.; Wang, Z. One-pot cellulose nanofibrillation, surface-modification and grafting by deep eutectic solvent towards high performance 3D printing nanocellulose-enriched bionanocomposites. Ind. Crops Prod. 2024, 210, 118134. [Google Scholar] [CrossRef]
- N’Gatta, K.M.; Assanvo, E.F.; El Hayek, J.; Masquelez, N.; Kamgang Syapnjeu, P.; Deabate, S.; Bonniol, V.; Soussan, L.; Zamora-Ledezma, C.; Elango, J. 3D-Printed Polycaprolactone Scaffolds Reinforced with Cellulose Nanocrystals and Silver Nanoparticles for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2025, 17, 41028–41043. [Google Scholar] [CrossRef] [PubMed]
- Chanthavong, V.; Cabo, M., Jr.; Prabhakar, M.; Woo, L.D.; Song, J.i. Fabrication of polycaprolactone/polyvinyl alcohol green composite film by reinforcing extracted micro cellulose fibers for food packaging applications. Polym. Eng. Sci. 2025, 65, 3673–3686. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.-S.; Park, S.B.; Kim, S.; Lee, M.; Park, S.-A.; Hwang, S.Y.; Koo, J.M.; Oh, D.X.; Park, J. Improved mechanical properties of biodegradable polycaprolactone nanocomposites prepared using cellulose nanocrystals. Cellulose 2023, 30, 11561–11574. [Google Scholar] [CrossRef]
- Hong, J.K.; Cooke, S.L.; Whittington, A.R.; Roman, M. Bioactive cellulose nanocrystal-poly (ε-caprolactone) nanocomposites for bone tissue engineering applications. Front. Bioeng. Biotechnol. 2021, 9, 605924. [Google Scholar] [CrossRef]
- Rocha, J.M.; Sousa, R.P.; Sousa, D.; Tohidi, S.D.; Ribeiro, A.; Fangueiro, R.; Ferreira, D.P. Polycaprolactone-based fibrous scaffolds reinforced with cellulose nanocrystals for anterior cruciate ligament repair. Appl. Sci. 2025, 15, 2301. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. In-situ polymerized cellulose nanocrystals (CNC)—Poly (l-lactide)(PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr. Polym. 2016, 153, 549–558. [Google Scholar] [CrossRef]
- Wongthanaroj, D.; Jessmore, L.A.; Lin, Y.; Bergholz, T.M.; Stark, N.M.; Sabo, R.C.; Matuana, L.M. Sustainable and eco-friendly poly (Lactic acid)/cellulose nanocrystal nanocomposite films for the preservation of oxygen-sensitive food. Appl. Food Res. 2022, 2, 100222. [Google Scholar] [CrossRef]
- Khoshkava, V.; Kamal, M. Effect of surface energy on dispersion and mechanical properties of polymer/nanocrystalline cellulose nanocomposites. Biomacromolecules 2013, 14, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Yong, Q.; Hu, L.; Rosqvist, E.; Peltonen, J.; Hu, Y.; Xu, W.; Xu, C. Molecular engineering of nanocellulose-poly (lactic acid) bio-nanocomposite interface by reactive surface grafting from copolymerization. Int. J. Biol. Macromol. 2025, 306, 141371. [Google Scholar] [CrossRef]
- Gong, W.; Li, M.; Sun, J.; Zhang, F.; Tian, T.; Yin, Y.; Wang, C. Enhancement of Mechanical and Thermal properties of PLA Electrospun Films based on PEG-Grafted Cellulose Nanocrystals. Compos. Commun. 2025, 58, 102501. [Google Scholar] [CrossRef]
- Islam, M.S.; Elahee, G.F.; Fang, Y.; Yu, X.B.; Advincula, R.C.; Cao, C.C. Polylactic acid (PLA)-based multifunctional and biodegradable nanocomposites and their applications. Compos. Part B Eng. 2025, 306, 112842. [Google Scholar] [CrossRef]
- Trivedi, A.K.; Gupta, M. PLA based biodegradable bionanocomposite filaments reinforced with nanocellulose: Development and analysis of properties. Sci. Rep. 2024, 14, 23819. [Google Scholar] [CrossRef]
- Kelnar, I.; Kaprálková, L.; Krejčíková, S.; Dybal, J.; Vyroubalová, M.; Abdel-Mohsen, A. Effect of Polydopamine Coating of Cellulose Nanocrystals on Performance of PCL/PLA Bio-Nanocomposites. Materials 2023, 16, 1087. [Google Scholar] [CrossRef]
- Galera Manzano, L.M.; Ruz Cruz, M.A.; Moo Tun, N.M.; Valadez González, A.; Mina Hernandez, J.H. Effect of cellulose and cellulose nanocrystal contents on the biodegradation, under composting conditions, of hierarchical PLA biocomposites. Polymers 2021, 13, 1855. [Google Scholar] [CrossRef]
- Wu, C.; Wu, B.; Abdalkarim, S.Y.H.; Wang, M.; Zou, Z.; Jin, M.; Yu, H.-Y. Synergistic enhancement and spherulite growth mechanism of PLA composites by multi-dimensional mineralized cellulose nanocrystals. Carbohydr. Polym. 2025, 366, 123926. [Google Scholar] [CrossRef]
- Wahbi, M.; Wen, Y.; Kontopoulou, M.; De France, K.J. Modification of cellulose nanocrystals with epoxidized canola oil for enhancing interfacial compatibility with poly (lactic acid). Carbohydr. Polym. 2025, 370, 124471. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Han, D.H. Viscoelastic properties of PLA/PCL blends compatibilized with different methods. Korea-Aust. Rheol. J. 2017, 29, 295–302. [Google Scholar] [CrossRef]
- Botlhoko, O.J.; Ramontja, J.; Ray, S.S. A new insight into morphological, thermal, and mechanical properties of melt-processed polylactide/poly (ε-caprolactone) blends. Polym. Degrad. Stab. 2018, 154, 84–95. [Google Scholar] [CrossRef]
- Vala, N.S.; Bavaliya, K.J.; Raj, M.; Raj, L. Compatibilization Strategies for PLA/PCL Blends: Tuning Thermal, Mechanical, and Biodegradation Properties via Functionalized PCL Derivatives. Polym.-Plast. Technol. Mater. 2025, 64, 2713–2734. [Google Scholar] [CrossRef]
- Gumede, T.P.; Shingange, K.; Mbule, P.; Motloung, B. Miscibility effect of biodegradable aliphatic poly (butylene succinate)/aromatic polycarbonate blends. Polym. Renew. Resour. 2022, 13, 28–43. [Google Scholar] [CrossRef]
- Eryildiz, M.; Karakus, A.; Demirci, M.; Altintas Kadirhan, O.; Eksi Altan, M. Optimization of Thermal, Mechanical, Biodegradation, and Shape Memory Properties in 4D-Printed PLA/PCL Blends for Spinal Cages. Polym. Adv. Technol. 2025, 36, e70133. [Google Scholar] [CrossRef]
- Ivanov, E.; Kotsilkova, R.; Georgiev, V.; Batakliev, T.; Angelov, V. Advanced rheological, dynamic mechanical and thermal characterization of phase-separation behavior of pla/pcl blends. J. Manuf. Mater. Process. 2025, 9, 35. [Google Scholar] [CrossRef]
- Eryildiz, M.; Karakus, A.; Altan Eksi, M. Development and characterization of PLA/PCL blend filaments and 3D printed scaffolds. J. Mater. Eng. Perform. 2025, 34, 14043–14054. [Google Scholar] [CrossRef]
- Kiani, P.; Sedighi, M.; Kasaeian-Naeini, M.; Jabbari, A. Investigation of mechanical integrity and high-cycle fatigue behavior of 3D-printed PLA/PCL blend after exposure to a physiological environment. J. Mater. Res. Technol. 2025, 36, 3671–3683. [Google Scholar] [CrossRef]
- Ilhan, R.; Gumus, O.Y.; Lekesiz, H. Biodegradable Nanocomposite Filament Based on PLA/PCL/CNCs for FDM 3D Printing: Production, Characterization and Printability. Polym. Compos. 2025, 0, 1–23. [Google Scholar] [CrossRef]
- Sessini, V.; Navarro-Baena, I.; Arrieta, M.P.; Dominici, F.; López, D.; Torre, L.; Kenny, J.M.; Dubois, P.; Raquez, J.-M.; Peponi, L. Effect of the addition of polyester-grafted-cellulose nanocrystals on the shape memory properties of biodegradable PLA/PCL nanocomposites. Polym. Degrad. Stab. 2018, 152, 126–138. [Google Scholar] [CrossRef]
- Sikhosana, S.; Gumede, T.; Malebo, N.; Ogundeji, A.; Motloung, B. The influence of cellulose content on the morphology, thermal, and mechanical properties of poly (lactic acid)/Eucomis autumnalis cellulose biocomposites. Polym. Eng. Sci. 2023, 63, 1411–1422. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Ivanov, E.; Angelov, V.; Kotsilkova, R. Tailored Polylactic Acid/Polycaprolactone Blends with Excellent Strength–Stiffness and Shape Memory Capacities. Processes 2025, 13, 1328. [Google Scholar] [CrossRef]
- Motloung, M.P.; Ojijo, V.; Bandyopadhyay, J.; Ray, S.S. Morphological characteristics and thermal, rheological, and mechanical properties of cellulose nanocrystals-containing biodegradable poly (lactic acid)/poly (ε-caprolactone) blend composites. J. Appl. Polym. Sci. 2020, 137, 48665. [Google Scholar] [CrossRef]

| Polymer | Tensile Strength (MPa) | Elongation at Break (%) | Melting Temperature (°C) | Glass Transition (°C) | Processing Method | Approximate Degradation Time Under Composting Conditions (Months) | Applications |
|---|---|---|---|---|---|---|---|
| PCL | 10–40 | 200–600 | 58–64 | −65 to −60 | Extrusion, injection molding, compression molding, solvent casting, electrospinning | >24 | Drug delivery sutures |
| PLA | 50–70 | 3–6 | 173–178 | 60–65 | Extrusion, injection molding, compression molding, solvent casting | 6 to 12 | Orthopedic surgery, oral and maxillofacial surgery |
| Blend Composition (PLA/PCL wt.%) | Fabrication Technique | Key Processing Characteristics | Findings/Impact on Composite Performance | Applications | References |
|---|---|---|---|---|---|
| 30/70 | Melt blending | High temperature, direct mixing | Incompatible blends; poor interface adhesion; uneven PLA phase dispersion. | Not reported | [26] |
| 100/0, 90/10, 80/20, 70/30, 60/40, 50/50, 40/60, 30/70, 20/80, 10/90, and 0/100 | Melt blending | High temperature, direct mixing | Stiffness, strength, elongation at break, thermal stability, and activation energy balancing were enhanced for (60/40) PLA/PCL blend. However, other blends showed poor compatibility and mechanical properties. | Packaging, Biomedical | [89] |
| 80/20 | Melt blending | High temperature, direct mixing | Large, dispersed particle sizes; poor mechanical properties. | Not reported | [88] |
| 100/0, 90/10, 80/20, 70/30, and 0/100 | Melt blending | High temperature, direct mixing | All blends showed poor compatibility. Based on results, blend containing 30% PCL had superior thermal properties compared to other blend ratios. | Packaging, Biomedical | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwenya, M.; Gumede, T.P.; Pérez Camargo, R.A.; Motloung, B. Nanocellulose-Reinforced Poly(Lactic Acid) and Poly(ε-caprolactone) Bio-Nanocomposites: A Review and Future Outlook for Poly(Lactic Acid)/Poly(ε-caprolactone) Blend Systems. Materials 2025, 18, 5172. https://doi.org/10.3390/ma18225172
Ngwenya M, Gumede TP, Pérez Camargo RA, Motloung B. Nanocellulose-Reinforced Poly(Lactic Acid) and Poly(ε-caprolactone) Bio-Nanocomposites: A Review and Future Outlook for Poly(Lactic Acid)/Poly(ε-caprolactone) Blend Systems. Materials. 2025; 18(22):5172. https://doi.org/10.3390/ma18225172
Chicago/Turabian StyleNgwenya, Mbongeni, Thandi Patricia Gumede, Ricardo Arpad Pérez Camargo, and Bennie Motloung. 2025. "Nanocellulose-Reinforced Poly(Lactic Acid) and Poly(ε-caprolactone) Bio-Nanocomposites: A Review and Future Outlook for Poly(Lactic Acid)/Poly(ε-caprolactone) Blend Systems" Materials 18, no. 22: 5172. https://doi.org/10.3390/ma18225172
APA StyleNgwenya, M., Gumede, T. P., Pérez Camargo, R. A., & Motloung, B. (2025). Nanocellulose-Reinforced Poly(Lactic Acid) and Poly(ε-caprolactone) Bio-Nanocomposites: A Review and Future Outlook for Poly(Lactic Acid)/Poly(ε-caprolactone) Blend Systems. Materials, 18(22), 5172. https://doi.org/10.3390/ma18225172








