Simultaneous Recovery of Magnesium and Lithium from Salt Lake Brine by Membrane Electrolysis for Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Mg(OH)2 as a Flame Retardant
2.3. Analysis Methods
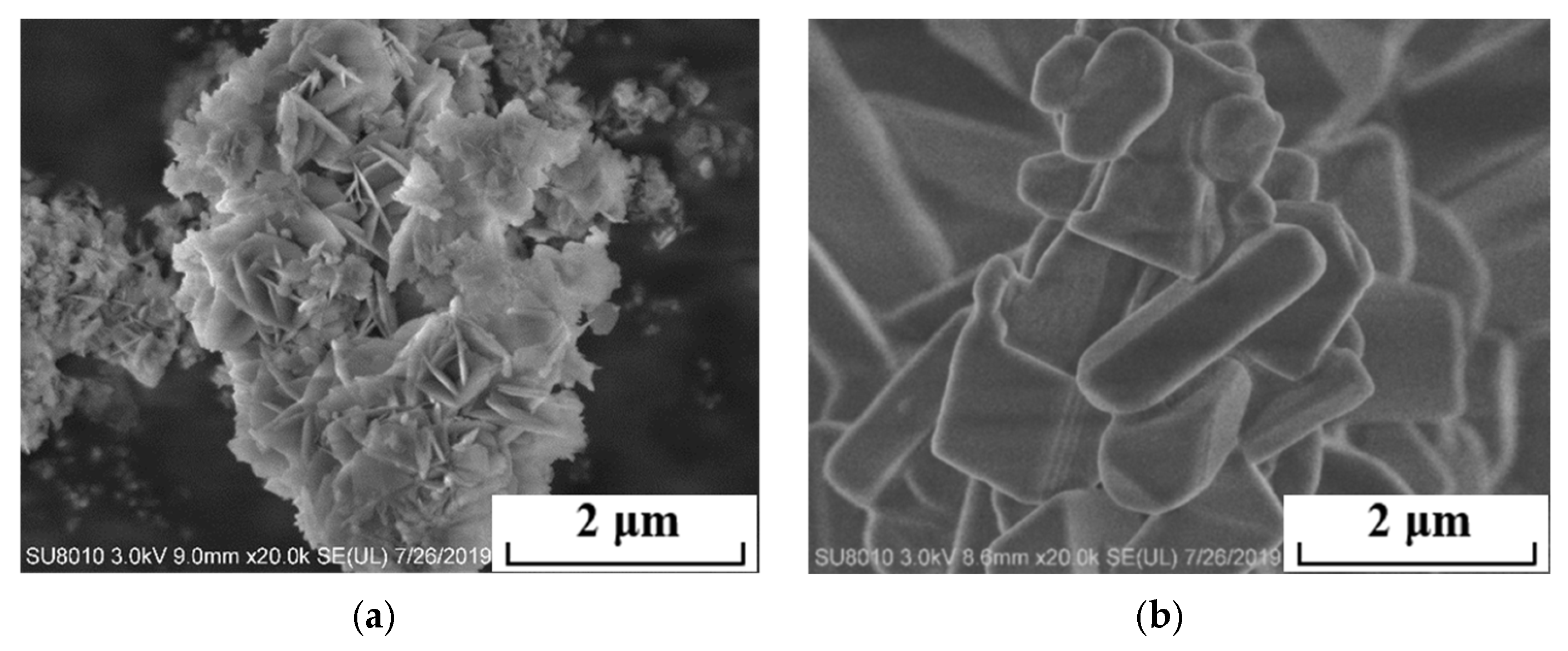
2.3.1. Physicochemical Properties and Morphology Analysis
2.3.2. Data Analysis
3. Results and Discussion
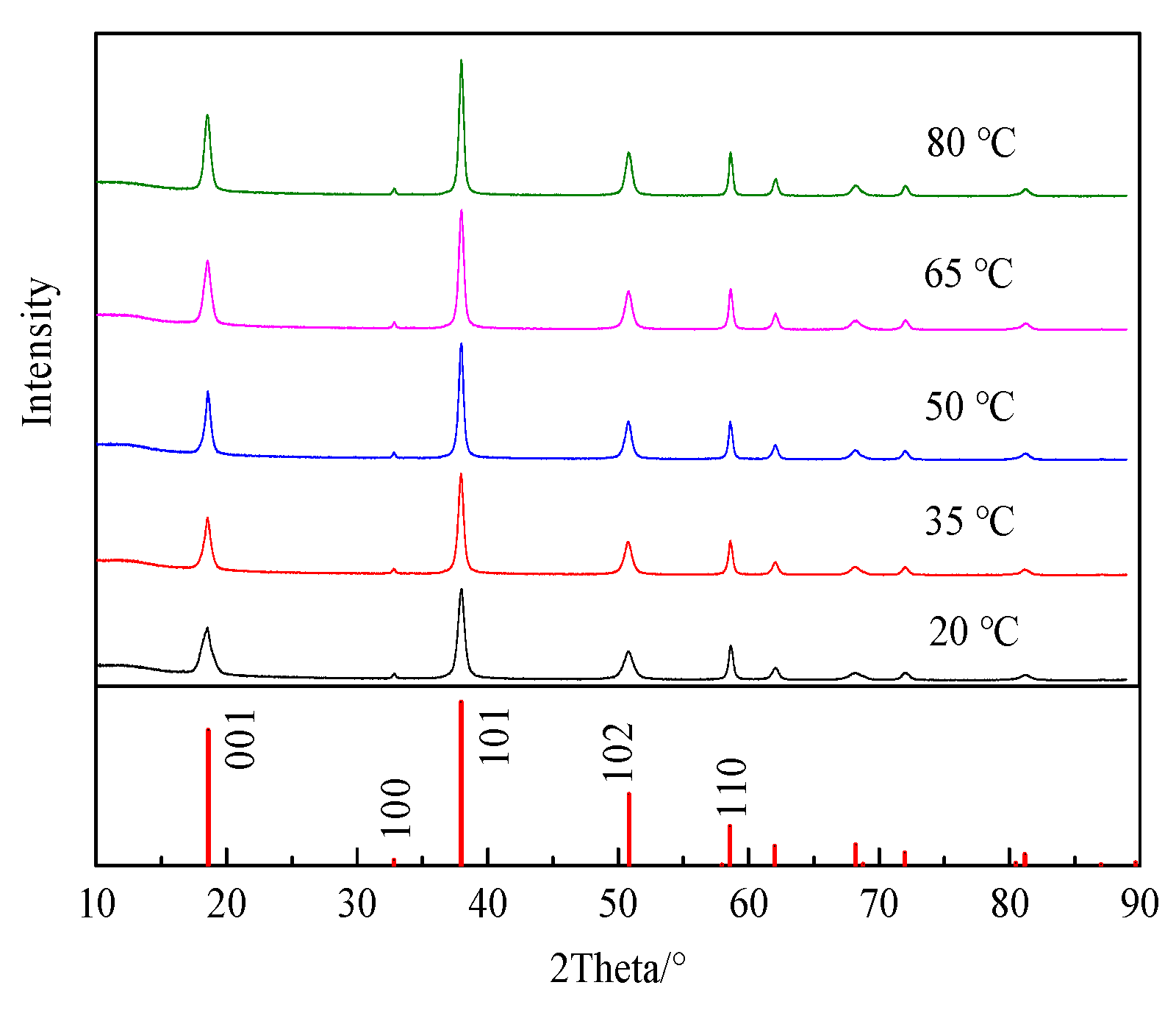
3.1. Effect of Electrolyte Temperature on Physicochemical Properties of Products and the Electrolysis Process
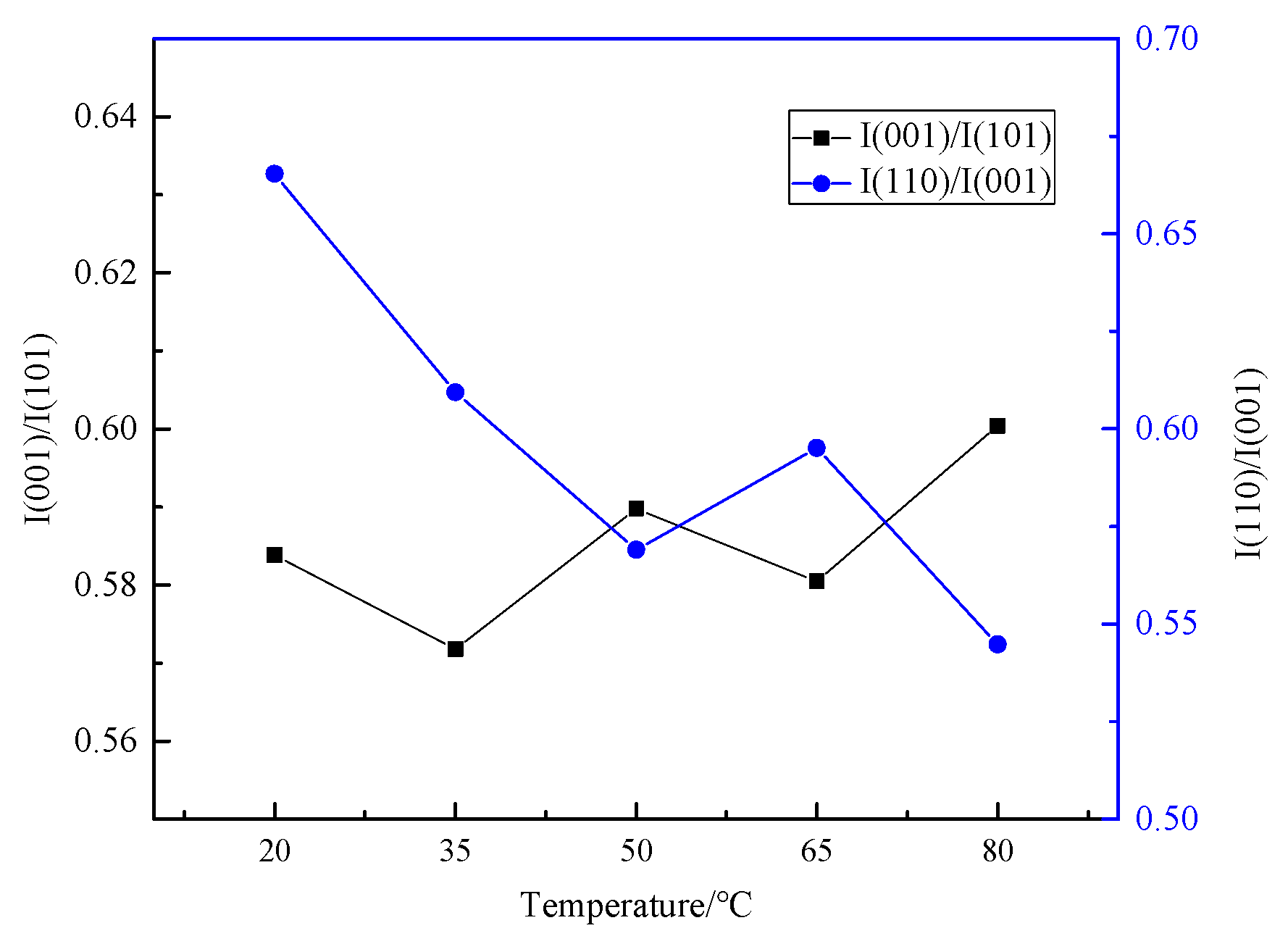
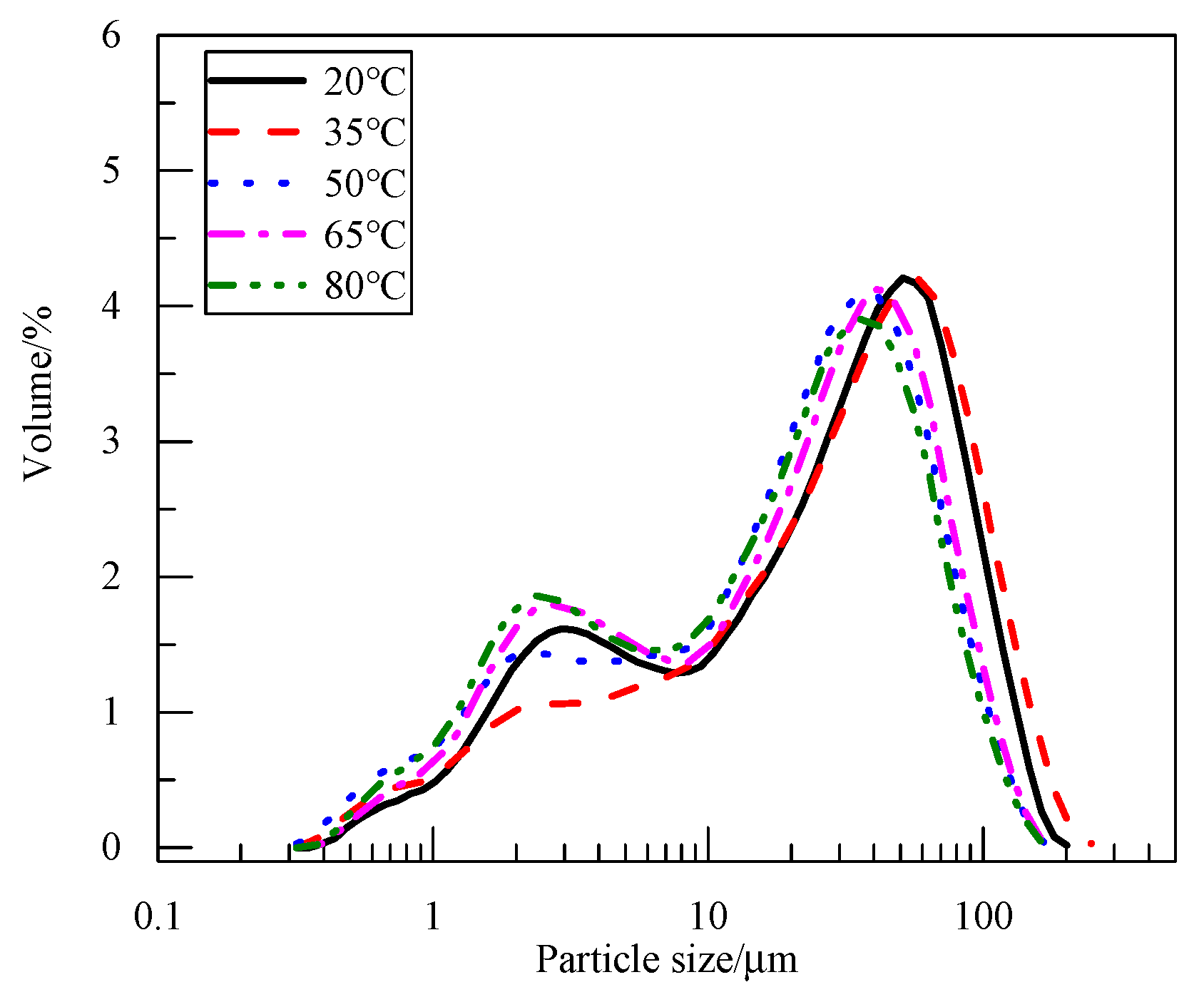
3.1.1. Effect of Electrolyte Temperature on Product Physicochemical Properties
3.1.2. Effect of Electrolyte Temperature on Parameters
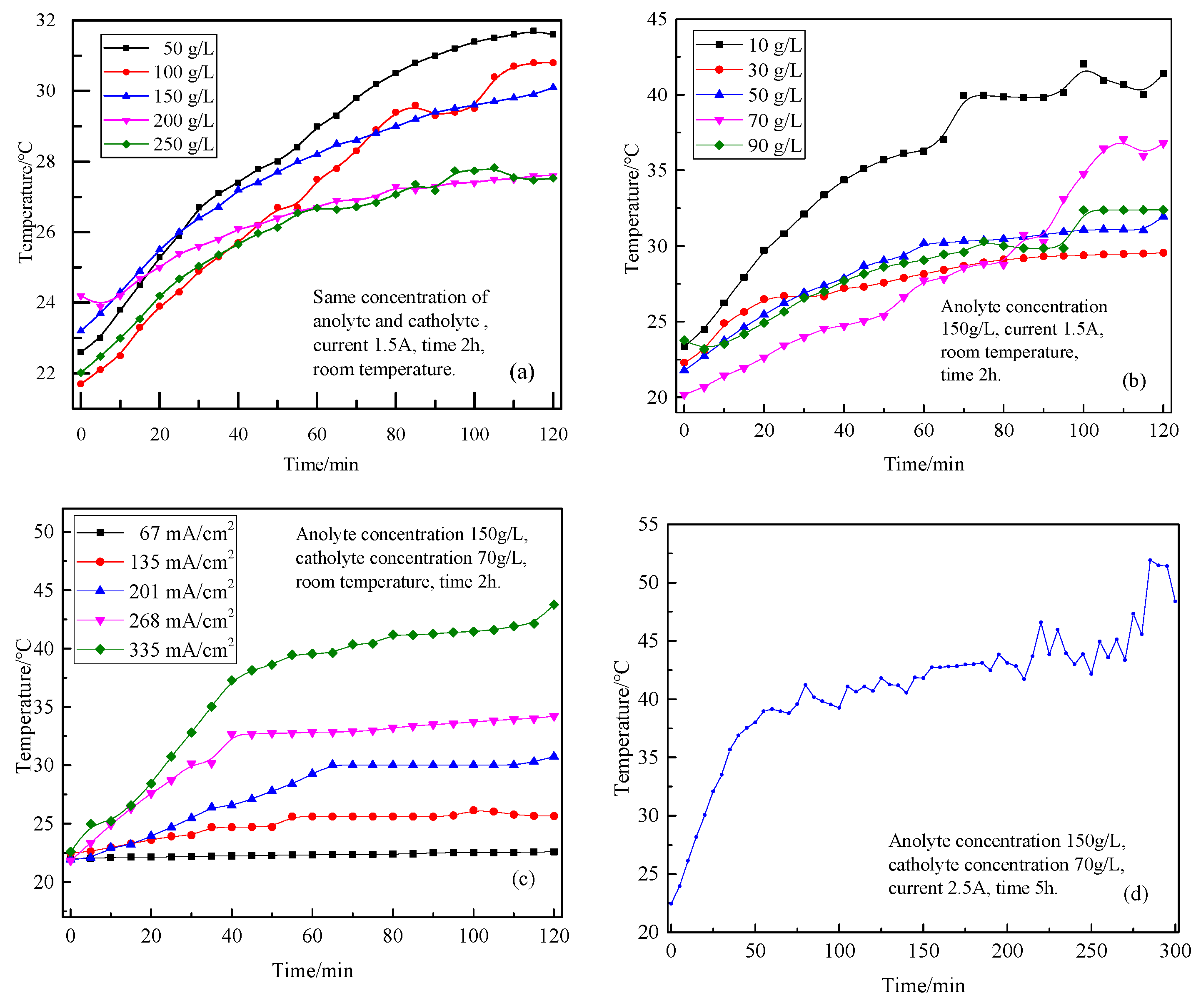
3.2. Effect of Different Experimental Conditions on the Temperature Change During Electrolysis
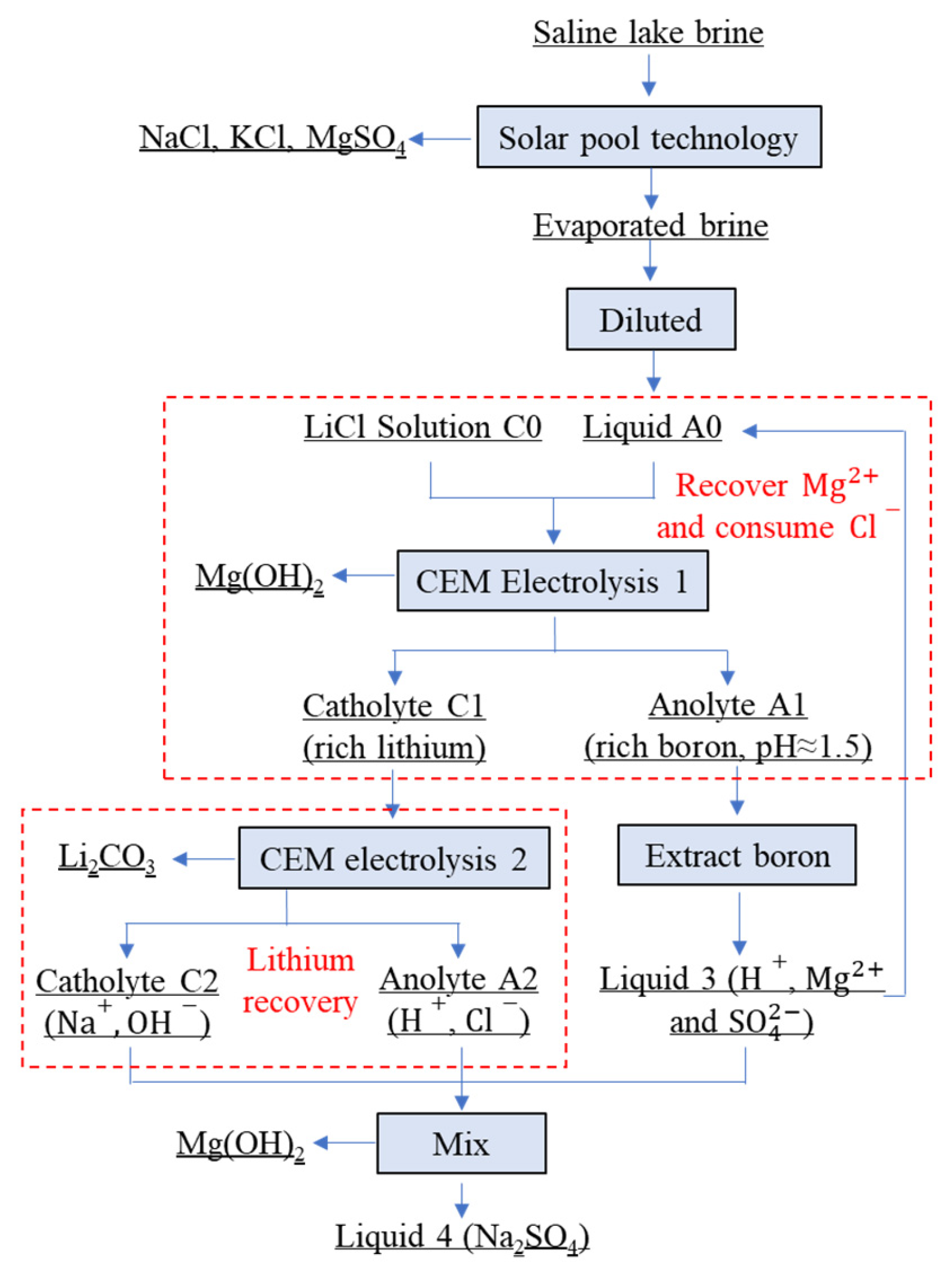
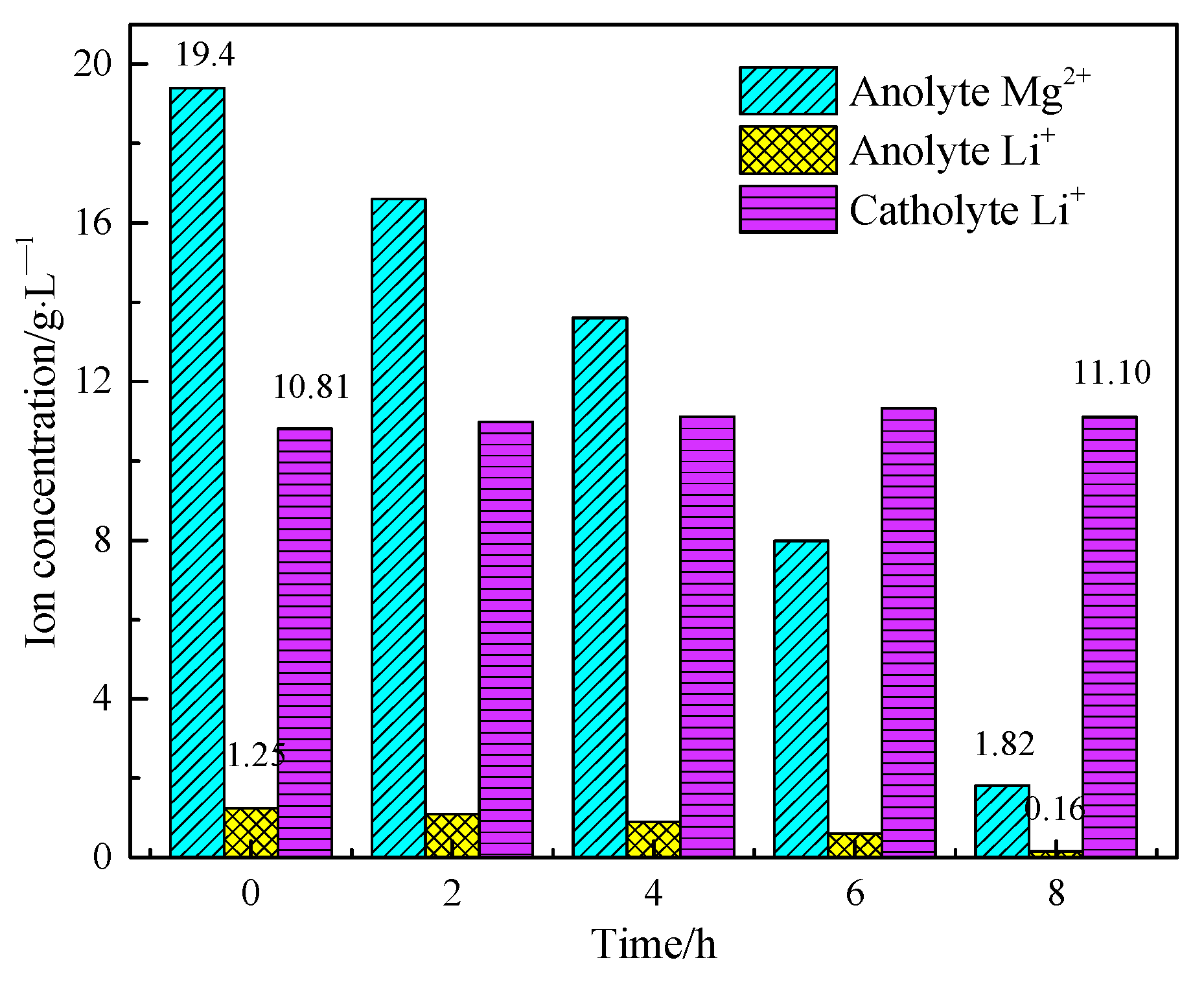
3.3. Separation of Magnesium from Simulating Salt Lake Solution by Cationic Membrane Electrolysis
3.4. Extraction of Lithium from Cathode Liquid of Separating Magnesium
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, N.; Jiang, R.H.; Cheng, Y.F. Chelator auxiliary electrodialysis (CAED) for efficient and sustainable lithium extraction and magnesium co-recovery from salt lake brines. Chem. Eng. J. 2025, 521, 166666. [Google Scholar] [CrossRef]
- Ali, A.; Afrin, S.; Asif, A.H. A comprehensive review on the recovery of lithium from lithium-ion batteries and spodumene. J. Environ. Manag. 2025, 391, 126512. [Google Scholar] [CrossRef]
- Pan, X.J.; Dou, Z.H.; Zhang, T.A.; Meng, D.L.; Fan, Y.Y. Separation of metal ions and resource utilization of magnesium from saline lake brine by membrane electrolysis. Sep. Purif. Technol. 2020, 251, 117316. [Google Scholar] [CrossRef]
- Nieto, C.H.D.; Palacios, N.A.; Verbeeck, K.; Prevoteau, A.; Rabaey, K.; Flexer, V. Membrane electrolysis for the removal of Mg2+ and Ca2+ from lithium rich brines. Water Res. 2019, 154, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.M.; Hu, L.; Wu, P.; Yang, X. Solar evaporation-driven integrated system for saline soil remediation, freshwater recovery. salt resource harvest, and agricultural irrigation. Water Res. 2025, 287 Pt A, 124288. [Google Scholar] [CrossRef] [PubMed]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Pan, X.J.; Dou, Z.H.; Meng, D.L.; Han, X.X.; Zhang, T.A. Electrochemical separation of magnesium from solutions of magnesium and lithium chloride. Hydrometallurgy 2020, 191, 105166. [Google Scholar] [CrossRef]
- Tian, L.; Wei, M.; Mei, H. Adsorption behavior of Li+ onto nano-lithium ion sieve from hybrid magnesium/lithium manganese oxide. Chem. Eng. J. 2010, 156, 134–140. [Google Scholar] [CrossRef]
- Xie, Y. China Salt Lake Industry Development. Qinghai Sci. Technol. 2015, 4, 22–25. [Google Scholar]
- Lim, Y.J.; Goh, K.; Goto, A.; Zhao, Y.; Wang, R. Uranium and lithium extraction from seawater: Challenges and opportunities for a sustainable energy future. J. Mater. Chem. A 2023, 11, 22551–22589. [Google Scholar] [CrossRef]
- Heidari, N.; Momeni, P. Selective adsorption of lithium ions from Urmia Lake onto aluminum hydroxide. Environ. Earth Sci. 2017, 76, 551. [Google Scholar] [CrossRef]
- Xiao, J.L.; Sun, S.Y.; Song, X.; Li, P.; Yu, J.G. Lithium ion recovery from brine using granulated polyacrylamide–MnO2 ion-sieve. Chem. Eng. J. 2015, 279, 659–666. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Peng, X.; Zhang, L.; Zhang, Y. Lithium extraction from low-grade salt lake brine with ultrahigh Mg/Li ratio using TBP–kerosene–FeCl3 system. Sep. Purif. Technol. 2019, 211, 303–309. [Google Scholar] [CrossRef]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Liu, S.C.; Li, Y.; Guo, H.F.; Liu, X.W.; Cao, J.L. Preparation of nano-hexagonal flake magnesium hydroxide from seawater brine and the crystallization-based separation of inorganic salt products from the mother liquor. Desalination 2025, 612, 118954. [Google Scholar] [CrossRef]
- Pan, X.J.; Dou, Z.H.; Zhang, T.A.; Meng, D.L.; Han, X.X. Basic study on direct preparation of lithium carbonate powders by membrane electrolysis. Hydrometallurgy 2020, 191, 105193. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.W.; Ghahreman, A. Novel approaches for lithium extraction from salt lake brines: A review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Wang, X.F.; Ji, L.M.; Shi, D.; Li, J.F.; Li, L.J.; Zhang, Y.Z.; Song, F.G. High efficiency extraction of lithium from the high magnesium brine by P248 with FeCl3 co-extractant. J. Environ. Chem. Eng. 2025, 13, 115276. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Sun, Z.; Song, X.; Yu, J.G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2016, 403, 128–135. [Google Scholar] [CrossRef]
- Pan, X.J.; Yang, Z.Q.; Xu, Y.; Wang, M.; Huang, X.W.; Feng, Z.Y. Separation and purification of Yb2O3 by ion exchange chromatography and preparation of ultra-high purity Yb2O3. Rare Met. 2023, 42, 2725–2735. [Google Scholar] [CrossRef]
- Pan, X.J.; Zhang, T.A.; Dou, Z.H.; Lyu, G.Z.; Han, X.X.; Zhang, J.J. A new method for direct synthesis of Li2CO3 powders by membrane electrolysis. Rare Met. 2018, 37, 716–722. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, P.; Li, W.X. Thermodynamic modeling and phase diagram prediction of salt lake brine systems II. Aqueous Li+-Na+-K+-SO42− and its subsystems. Chin. J. Chem. Eng. 2021, 34, 134–149. [Google Scholar] [CrossRef]
- Gu, J.; Zhou, G.L.; Chen, L.L. Particle size control and electrochemical lithium extraction performance of LiMn2O4. J. Electroanal. Chem. 2023, 940, 117487. [Google Scholar] [CrossRef]





| Ions/g·L−1 | Li+ | Mg2+ | Na+ | K+ | Cl− | SO42− | B2O3 |
|---|---|---|---|---|---|---|---|
| Initial | 0.3–5 | 8–22 | 68–114 | 6.7–14 | 144–231 | 3–47 | 0.8–17 |
| Evaporated | 2–8 | 100–120 | 0.3–3 | 0.2–1.8 | 300–340 | 23–54 | 10–43 |
| g/L | Li+ | Na+ | K+ | Mg2+ | Cl− | B | SO42− |
|---|---|---|---|---|---|---|---|
| A0 | 1.25 | 1.39 | 1.53 | 19.4 | 57.1 | 1.00 | 10.6 |
| C0 | 10.81 | - | - | - | 54.19 | - | - |
| Time/h | Mg | Li | Na | K | Cl | B | SO42− | Mg(OH)2 |
|---|---|---|---|---|---|---|---|---|
| 2 | 41.4 | 0.011 | 0.015 | <0.001 | 0.12 | 0.13 | 0.009 | 99.715 |
| 4 | 41.7 | 0.02 | 0.016 | 0.001 | 0.16 | 0.12 | 0.007 | 99.676 |
| 6 | 40.3 | 0.035 | 0.088 | 0.003 | 0.13 | 0.24 | 0.018 | 99.496 |
| 8 | 39.7 | 0.11 | 0.025 | 0.012 | 0.21 | 0.12 | 0.007 | 99.516 |
| Time/h | Li | C | Na | K | Cl | Li2CO3 |
|---|---|---|---|---|---|---|
| 1 | 18.4 | 16.3 | 0.004 | 0.004 | 0.008 | 99.984 |
| 2 | 18.2 | 16.0 | 0.004 | 0.004 | 0.004 | 99.988 |
| 3 | 18.3 | 16.1 | 0.004 | 0.005 | 0.003 | 99.988 |
| 4 | 18.6 | 15.8 | 0.008 | 0.004 | 0.007 | 99.981 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.; Jia, J.; Han, Y.; Li, W.; Li, X. Simultaneous Recovery of Magnesium and Lithium from Salt Lake Brine by Membrane Electrolysis for Resource Utilization. Materials 2025, 18, 5077. https://doi.org/10.3390/ma18225077
Pan X, Jia J, Han Y, Li W, Li X. Simultaneous Recovery of Magnesium and Lithium from Salt Lake Brine by Membrane Electrolysis for Resource Utilization. Materials. 2025; 18(22):5077. https://doi.org/10.3390/ma18225077
Chicago/Turabian StylePan, Xijuan, Jingyu Jia, Yu Han, Wencheng Li, and Xiang Li. 2025. "Simultaneous Recovery of Magnesium and Lithium from Salt Lake Brine by Membrane Electrolysis for Resource Utilization" Materials 18, no. 22: 5077. https://doi.org/10.3390/ma18225077
APA StylePan, X., Jia, J., Han, Y., Li, W., & Li, X. (2025). Simultaneous Recovery of Magnesium and Lithium from Salt Lake Brine by Membrane Electrolysis for Resource Utilization. Materials, 18(22), 5077. https://doi.org/10.3390/ma18225077




