Macroporous Hydroxyapatite-Based Bone Scaffolds Loaded with CAPE Derivatives: A Strategy to Reduce Oxidative Stress and Biofilm Formation
Abstract
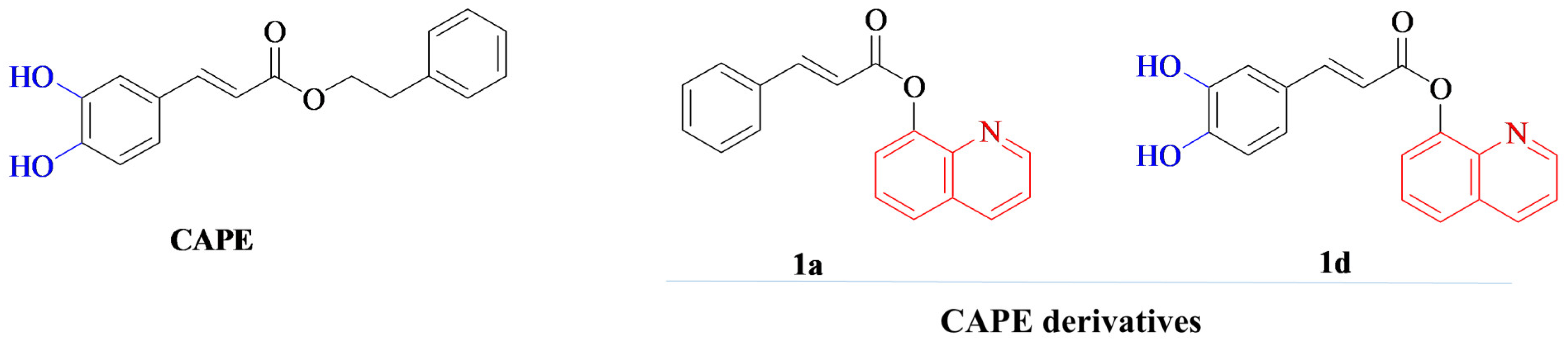
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Bone Scaffolds
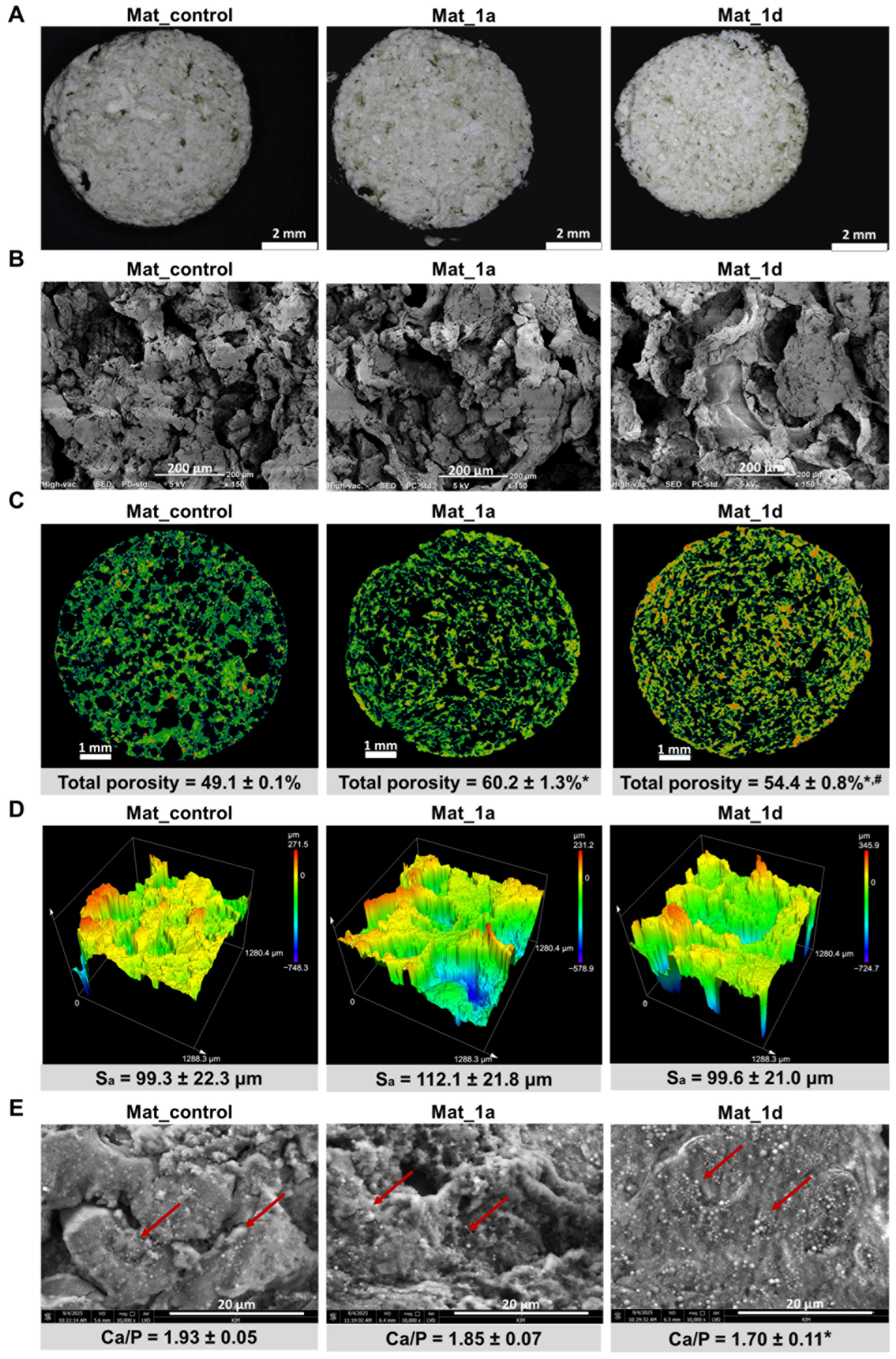
2.3. Microstructure Characterization
2.4. Determination of Mechanical Properties
2.5. Liquid Absorption Ability
2.6. Bioactivity Assessment
2.7. Evaluation of Biological Properties In Vitro
2.7.1. Cells
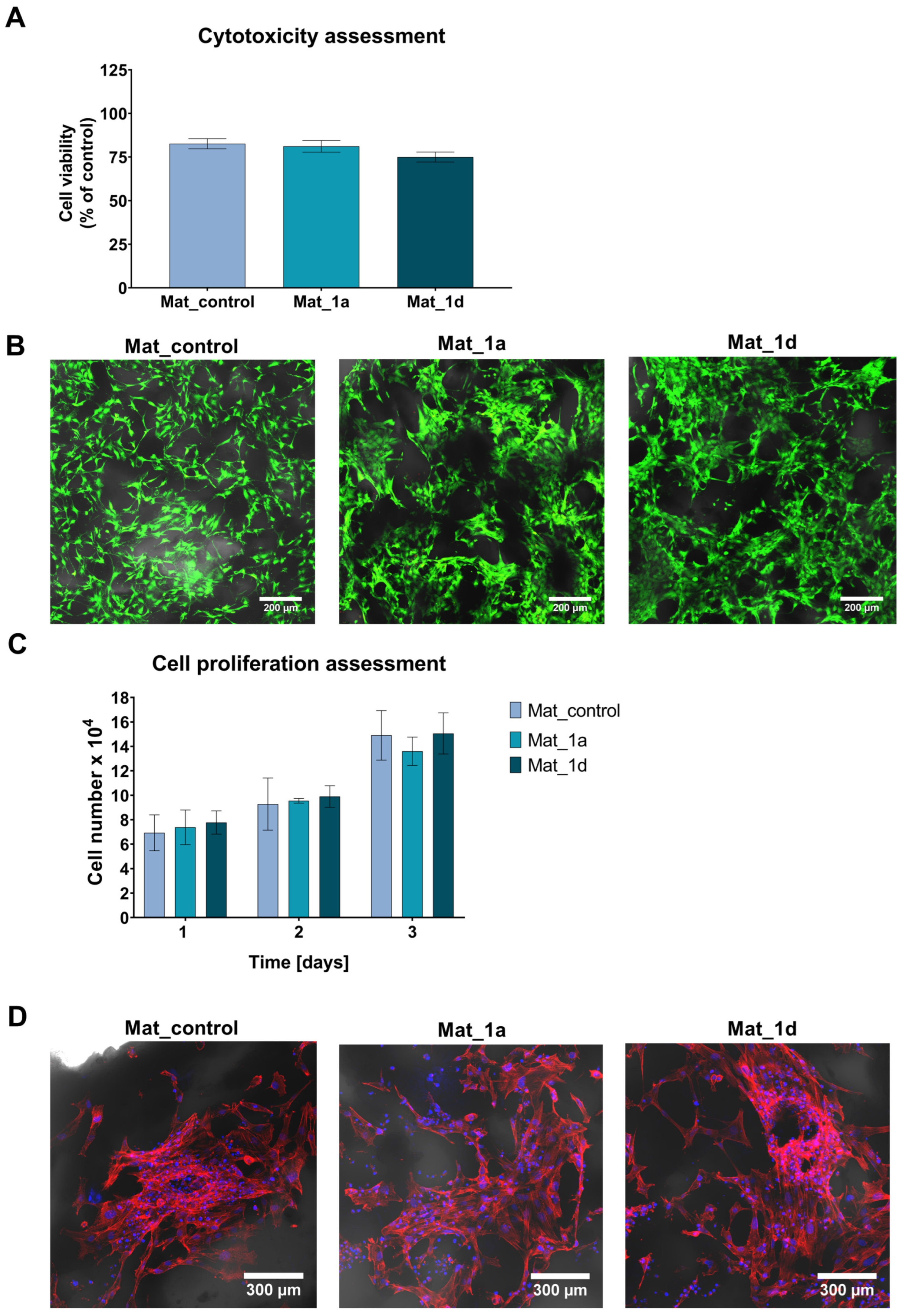
2.7.2. Cytotoxicity Assessment
2.7.3. Cell Proliferation Assessment
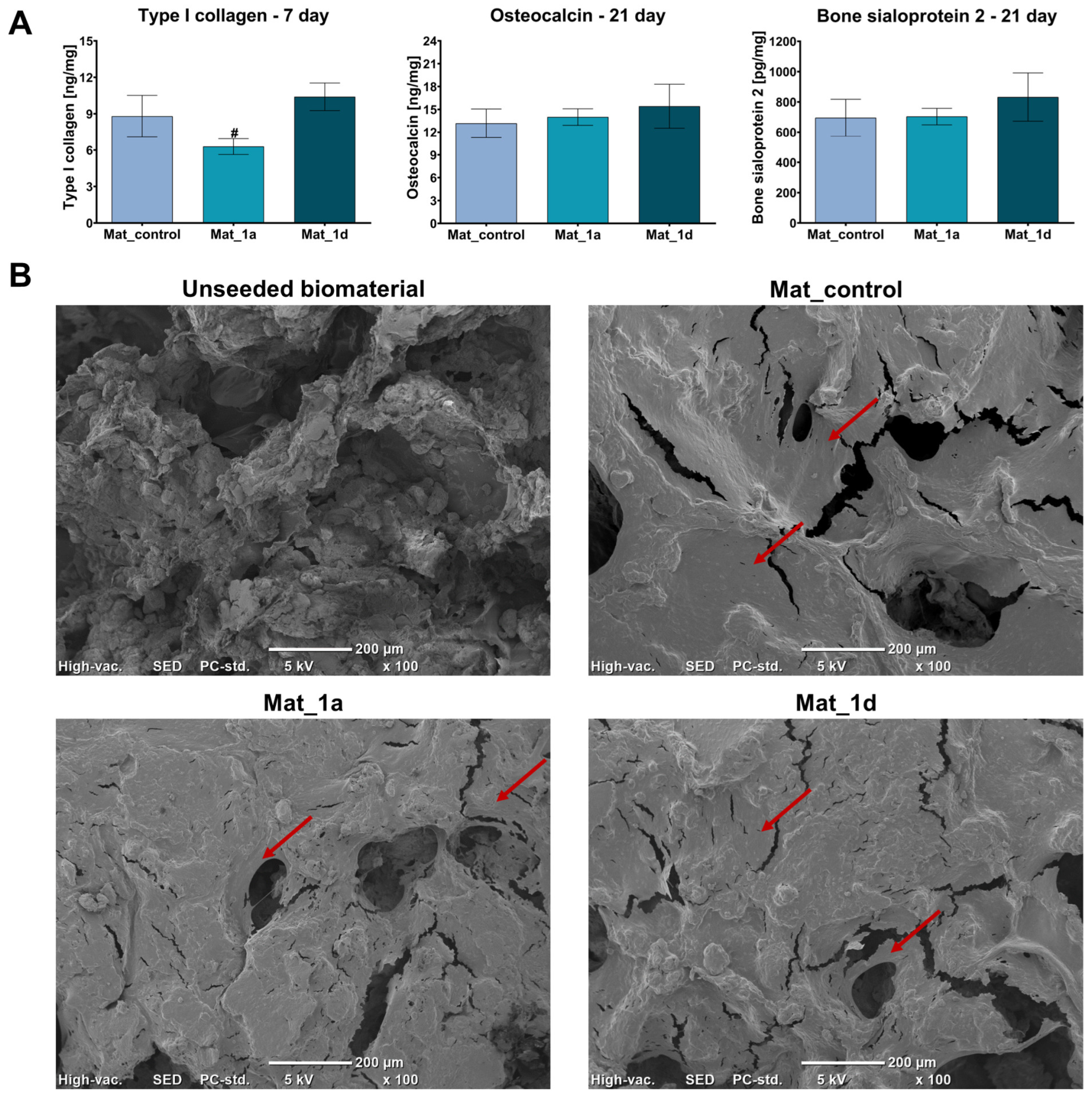
2.7.4. Osteogenic Differentiation
2.7.5. ROS/RNS Generation by Immune Cells
2.7.6. Evaluation of Biofilm Formation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Microstructure, Mechanical Properties, Liquid Absorption Ability, and Bioactivity
3.2. Evaluation of Biological Properties In Vitro
3.2.1. Cytotoxicity and Cell Proliferation Assessment
3.2.2. Osteogenic Differentiation Assessment
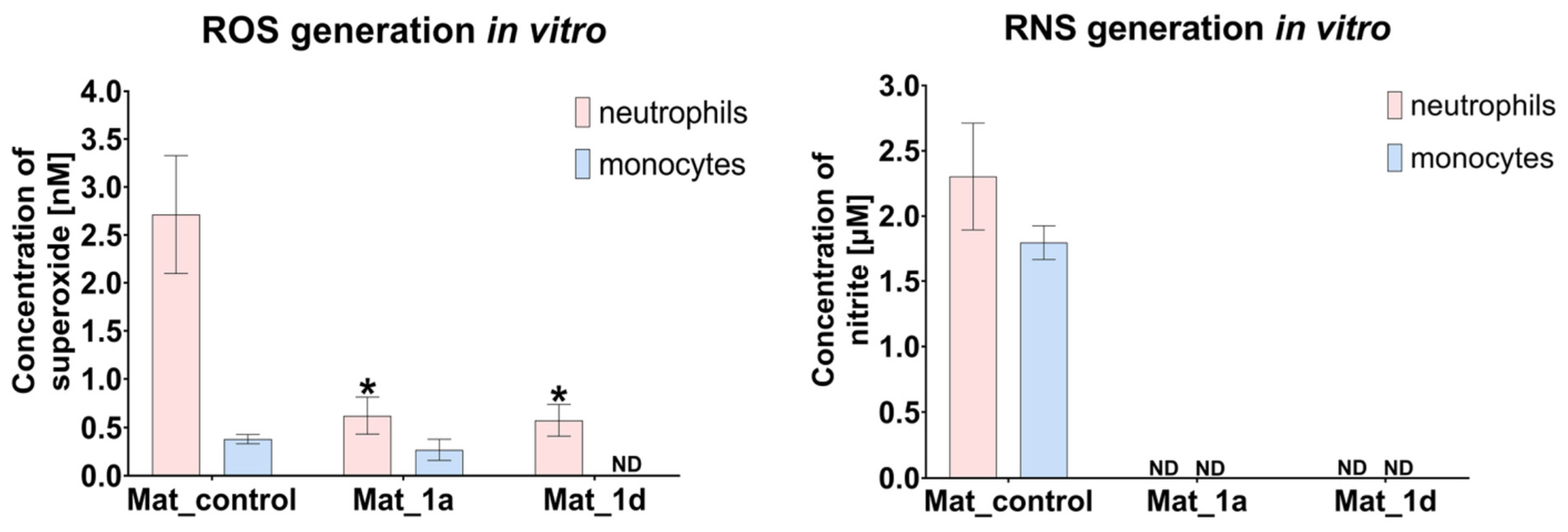
3.2.3. Evaluation of ROS/RNS Generation by Human Immune Cells
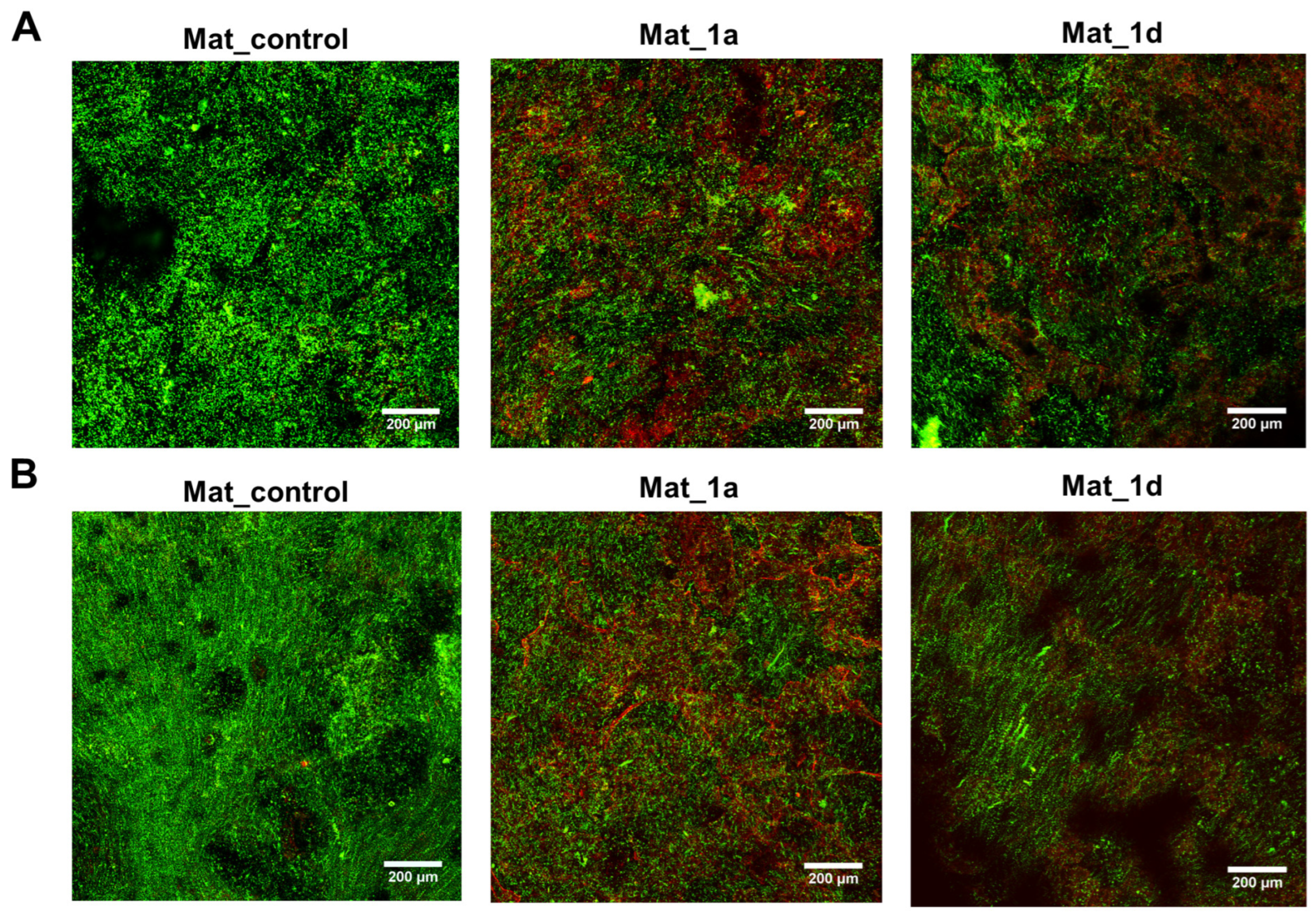
3.2.4. Evaluation of Biofilm Formation
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| bALP | Bone Alkaline Phosphatase |
| CAPE | Caffeic Acid Phenethyl Ester |
| CLSM | Confocal Laser Scanning Microscope |
| ECM | Extracellular Matrix |
| EDS | Energy-Dispersive Spectroscopy |
| EPS | Extracellular Polymeric Substance |
| LDH | Lactate Dehydrogenase |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SBF | Simulated Body Fluid |
References
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Kostadinova, M.; Raykovska, M.; Simeonov, R.; Lolov, S.; Mourdjeva, M. Recent Advances in Bone Tissue Engineering: Enhancing the Potential of Mesenchymal Stem Cells for Regenerative Therapies. Curr. Issues Mol. Biol. 2025, 47, 287. [Google Scholar] [CrossRef]
- Bello, S.A.; Cruz-Lebrón, J.; Rodríguez-Rivera, O.A.; Nicolau, E. Bioactive Scaffolds as a Promising Alternative for Enhancing Critical-Size Bone Defect Regeneration in the Craniomaxillofacial Region. ACS Appl. Bio Mater. 2023, 6, 4465–4503. [Google Scholar] [CrossRef]
- Sachot, N.; Engel, E.; Castano, O. Hybrid Organic-Inorganic Scaffolding Biomaterials for Regenerative Therapies. Curr. Org. Chem. 2014, 18, 2299–2314. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef]
- Balaha, M.; Cataldi, A.; Ammazzalorso, A.; Cacciatore, I.; De Filippis, B.; Di Stefano, A.; Maccallini, C.; Rapino, M.; Korona-Glowniak, I.; Przekora, A.; et al. CAPE Derivatives: Multifaceted Agents for Chronic Wound Healing. Arch. Pharm. 2024, 357, e2400165. [Google Scholar] [CrossRef]
- Al-Hariri, M.; Alsunni, A.; Shaikh, M.H.; Eldin, T.G.; Al Ghamdi, K.; Alharbi, A.F.; Alhawaj, H.; Chathoth, S. Caffeic Acid Phenethyl Ester Reduces pro Inflammatory Cytokines in Moderate Swimming Test in Growing Rats Model. J. Inflamm. Res. 2021, 14, 5653–5657. [Google Scholar] [CrossRef] [PubMed]
- Kȩpa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial Potential of Caffeic Acid against Staphylococcus aureus Clinical Strains. Biomed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, e00251-20. [Google Scholar] [CrossRef]
- Balaha, M.; De Filippis, B.; Cataldi, A.; Di Giacomo, V. Cape and Neuroprotection: A Review. Biomolecules 2021, 11, 176. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef]
- Ekeuku, S.O.; Pang, K.L.; Chin, K.Y. Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review. Drug Des. Dev. Ther. 2021, 15, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bowman, P.D.; Kerwin, S.M.; Stavchansky, S. Stability of Caffeic Acid Phenethyl Ester and Its Fluorinated Derivative in Rat Plasma. Biomed. Chromatogr. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Pagliani, T.; Poggi, A. In Vitro and in Vivo Stability of Caffeic Acid Phenethyl Ester, a Bioactive Compound of Propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Burgos, V.; Marín, V.; Camins, A.; Olloquequi, J.; González-Chavarría, I.; Ulrich, H.; Wyneke, U.; Luarte, A.; Ortiz, L.; et al. Caffeic Acid Phenethyl Ester (CAPE): Biosynthesis, Derivatives and Formulations with Neuroprotective Activities. Antioxidants 2023, 12, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, P.; Benko, A.; Palka, K.; Canal, C.; Kolodynska, D.; Przekora, A. Novel Synthesis Method Combining a Foaming Agent with Freeze-Drying to Obtain Hybrid Highly Macroporous Bone Scaffolds. J. Mater. Sci. Technol. 2020, 43, 52–63. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Benko, A.; Nocun, M.; Przekora, A. Novel Chitosan/Agarose/Hydroxyapatite Nanocomposite Scaffold for Bone Tissue Engineering Applications: Comprehensive Evaluation of Biocompatibility and Osteoinductivity with the Use of Osteoblasts and Mesenchymal Stem Cells. Int. J. Nanomed. 2019, 14, 6615–6630. [Google Scholar] [CrossRef]
- Venkatesan, J.; Pallela, R.; Bhatnagar, I.; Kim, S.K. Chitosan-Amylopectin/Hydroxyapatite and Chitosan-Chondroitin Sulphate/Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Int. J. Biol. Macromol. 2012, 51, 1033–1042. [Google Scholar] [CrossRef]
- ISO 23317:2025; Implants for Surgery—Materials—Simulated Body Fluid (SBF) Preparation Procedure and Test Method to Detect Apatite Formation in SBF for Initial Screening of Bone-Contacting Implant Materials. ISO: Geneva, Switzerland, 2025.
- Wessely-Szponder, J.; Michalska, J.; Szponder, T.; Żylińska, B.; Tarczyńska, M.; Szubstarski, M. The Role of Antimicrobial Neutrophil Extract in Modification of the Inflammatory Response during Osteochondral Autograft and Allograft Transplantation in Rabbits. J. Comp. Pathol. 2020, 175, 49–63. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Faia-Torres, A.B.; Charnley, M.; Goren, T.; Guimond-Lischer, S.; Rottmar, M.; Maniura-Weber, K.; Spencer, N.D.; Reis, R.L.; Textor, M.; Neves, N.M. Osteogenic Differentiation of Human Mesenchymal Stem Cells in the Absence of Osteogenic Supplements: A Surface-Roughness Gradient Study. Acta Biomater. 2015, 28, 64–75. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv. Healthc. Mater. 2022, 11, e2102087. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. Oral Implant Surfaces: Part 1—Review Focusing on Topographic and Chemical Properties of Different Surfaces and in Vivo Responses to Them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A Review of the Mechanical Behavior of CaP and CaP/Polymer Composites for Applications in Bone Replacement and Repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can Bioactivity Be Tested in Vitro with SBF Solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, X.; Darvell, B.W.; Lu, W.W. Apatite-Formation Ability—Predictor of “Bioactivity”? Acta Biomater. 2010, 6, 4181–4188. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The Mechanism of Biomineralization of Bone-like Apatite on Synthetic Hydroxyapatite: An in Vitro Assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The Summary of the Most Important Cell-Biomaterial Interactions That Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Elshahawy, W. Biocompatibility. In Advances in Ceramics—Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment; Sikalidis, C., Ed.; InTech: London, UK, 2011; pp. 359–378. ISBN 978-953-307-350-7. [Google Scholar]
- Williams, D.F. On the Mechanisms of Biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do Novel Cement-Type Biomaterials Reveal Ion Reactivity That Affects Cell Viability in Vitro? Cent. Eur. J. Biol. 2014, 9, 277–289. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Tan, Y.; Fan, H.; Zhang, X. The Material and Biological Characteristics of Osteoinductive Calcium Phosphate Ceramics. Regen. Biomater. 2018, 5, 43–59. [Google Scholar] [CrossRef]
- Valenti, M.T.; Carbonare, L.D.; Mottes, M. Osteogenic Differentiation in Healthy and Pathological Conditions. Int. J. Mol. Sci. 2017, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Effects of Inflammatory and Anti-Inflammatory Cytokines on the Bone. Eur. J. Clin. Investig. 2011, 41, 1361–1366. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Sridharan, R.; Cameron, A.R.; Kelly, D.J.; Kearney, C.J.; O’Brien, F.J. Biomaterial Based Modulation of Macrophage Polarization: A Review and Suggested Design Principles. Mater. Today 2015, 18, 313–325. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Aldawood, E. The Effect of 3D-Printed Bone Tissue Scaffolds Geometrical Designs on Bacterial Biofilm Formation. Int. J. Bioprinting 2024, 10, 1768. [Google Scholar] [CrossRef]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in Surgical Site Infections: Recent Advances and Novel Prevention and Eradication Strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Al Ismail, D.; Di Timoteo, F.; De Angelis, M.; Rosa, L.; Cutone, A.; Venditti, M.; Mascellino, M.T.; Valenti, P.; et al. Challenges in the Microbiological Diagnosis of Implant-Associated Infections: A Summary of the Current Knowledge. Front. Microbiol. 2021, 12, 750460. [Google Scholar] [CrossRef]
- Kokilakanit, P.; Dungkhuntod, N.; Serikul, N.; Koontongkaew, S.; Utispan, K. Caffeic Acid Phenethyl Ester Inhibits Multispecies Biofilm Formation and Cariogenicity. PeerJ 2025, 13, 18942. [Google Scholar] [CrossRef]
- Meyuhas, S.; Assali, M.; Huleihil, M.; Huleihel, M. Antimicrobial Activities of Caffeic Acid Phenethyl Ester. J. Mol. Biochem. 2015, 4, 21–31. [Google Scholar]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierczak, P.; Balaha, M.; Palka, K.; Wessely-Szponder, J.; Wojcik, M.; di Giacomo, V.; De Filippis, B.; Przekora, A. Macroporous Hydroxyapatite-Based Bone Scaffolds Loaded with CAPE Derivatives: A Strategy to Reduce Oxidative Stress and Biofilm Formation. Materials 2025, 18, 5074. https://doi.org/10.3390/ma18225074
Kazimierczak P, Balaha M, Palka K, Wessely-Szponder J, Wojcik M, di Giacomo V, De Filippis B, Przekora A. Macroporous Hydroxyapatite-Based Bone Scaffolds Loaded with CAPE Derivatives: A Strategy to Reduce Oxidative Stress and Biofilm Formation. Materials. 2025; 18(22):5074. https://doi.org/10.3390/ma18225074
Chicago/Turabian StyleKazimierczak, Paulina, Marwa Balaha, Krzysztof Palka, Joanna Wessely-Szponder, Michal Wojcik, Viviana di Giacomo, Barbara De Filippis, and Agata Przekora. 2025. "Macroporous Hydroxyapatite-Based Bone Scaffolds Loaded with CAPE Derivatives: A Strategy to Reduce Oxidative Stress and Biofilm Formation" Materials 18, no. 22: 5074. https://doi.org/10.3390/ma18225074
APA StyleKazimierczak, P., Balaha, M., Palka, K., Wessely-Szponder, J., Wojcik, M., di Giacomo, V., De Filippis, B., & Przekora, A. (2025). Macroporous Hydroxyapatite-Based Bone Scaffolds Loaded with CAPE Derivatives: A Strategy to Reduce Oxidative Stress and Biofilm Formation. Materials, 18(22), 5074. https://doi.org/10.3390/ma18225074











