Structural–Phase Transformations in Stainless Steel CF8 Under Ion Implantation and Thermal Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Ion Implantation of 57Fe Ions
3.2. Thermal Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yvon, P.; Le Flem, M.; Cabet, C.; Seran, J.-L. Structural materials for next generation nuclear systems: Challenges and the path forward. Nucl. Eng. Des. 2015, 294, 161–169. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Was, G.S. Materials challenges in nuclear energy. Acta Mater. 2013, 61, 735–758. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, A.; María Camacho, A.; Mendoza, C.; Kickhofel, J.; Lomonaco, G. Evolution of Standardized Specifications on Materials, Manufacturing and In-Service Inspection of Nuclear Reactor Vessels. Sustainability 2021, 13, 10510. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19. [Google Scholar] [CrossRef]
- De Bellefon, G.M.; Van Duysen, J.C. Tailoring plasticity of austenitic stainless steels for nuclear applications: Review of mechanisms controlling plasticity of austenitic steels below 400 °C. J. Nucl. Mater. 2016, 475, 168–191. [Google Scholar] [CrossRef]
- Garner, F.A. Radiation-Induced Damage in Austenitic Structural Steels Used in Nuclear Reactors. Compr. Nucl. Mater. 2020, 3, 57–168. [Google Scholar]
- Was, G.S. Fundamentals of Radiation Materials Science: Metals and Alloys, 2nd ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Klueh, R.L.; Gelles, D.S.; Jitsukawa, S.; Kimura, A.; Odette, G.R.; van der Schaaf, B.; Victoria, M. Ferritic/martensitic steels—Overview of recent results. J. Nucl. Mater. 2002, 307–311, 455–465. [Google Scholar] [CrossRef]
- Huang, M.; Wang, L.; Wang, C.; Li, Y.; Wang, J.; Yuan, J.; Hu, J.; Huang, M.; Xu, W. Optimizing crack initiation energy in austenitic steel via controlled martensitic transformation. J. Mater. Sci. Technol. 2024, 198, 231–242. [Google Scholar] [CrossRef]
- Li, Y.; San Martín, D.; Wang, J.; Wang, C.; Xu, W. A review of the thermal stability of metastable austenite in steels: Martensite formation. J. Mater. Sci. Technol. 2021, 91, 200–214. [Google Scholar] [CrossRef]
- Shen, Y.F.; Dong, X.X.; Song, X.T.; Jia, N. Carbon content-tuned martensite transformation in low-alloy TRIP steels. Sci. Rep. 2019, 9, 7559. [Google Scholar] [CrossRef]
- Xue, W.Y.; Zhang, H.F.; Shen, Y.F.; Jia, N. Manganese controlled transformation and twinning of the nanoscale austenite in low-carbon-medium-Mn steel. Mater. Sci. Eng. A 2022, 829, 142162. [Google Scholar] [CrossRef]
- Wang, Y.L.; Shen, Y.F.; Jia, N.; Wang, J.J.; Zhao, S.-X. Acicular martensite induced superior strength-ductility combination in a 20Cr2Ni2MoV steel. Mater. Sci. Eng. A 2022, 848, 143400. [Google Scholar] [CrossRef]
- Luptáková, N.; Svoboda, J.; Bártková, D.; Weiser, A.; Dlouhý, A. The Irradiation Effects in Ferritic, Ferritic-Martensitic and Austenitic Oxide Dispersion Strengthened Alloys: A Review. Materials 2024, 17, 3409. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-X.; Yang, T.-X.; Kimura, A.; Dou, P. Prediction of the tensile properties of ODS ferritic/martensitic steels under various temperature conditions using interpretable machine learning models. Intermetallics 2025, 186, 108962. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Xu, B.; Zhang, J.; Sun, M.; Li, D. Research progress on preparation technology of oxide dispersion strengthened steel for nuclear energy. Int. J. Extreme Manuf. 2021, 3, 032001. [Google Scholar] [CrossRef]
- Tan, F.; Li, L.; Li, J.; Liu, B.; Liaw, P.K.; Fang, Q. Multiscale Modelling of Irradiation Damage Behavior in High-Entropy Alloys. Adv. Powder Mater. 2023, 2, 100114. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Li, J.; Liu, B.; Zhang, R.; Xia, J.; Fang, O. Irradiation Hardening and Creep Modeling of High-Entropy Alloy at High Temperature and Dose. Acta Mech. Solida Sin. 2025, 38, 588–597. [Google Scholar] [CrossRef]
- Vörtler, K.; Juslin, N.; Bonny, G.; Malerba, L.; Nordlund, K. The effect of prolonged irradiation on defect production and ordering in Fe-Cr and Fe-Ni alloys. J. Phys. Condens. Matter 2011, 23, 355007. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Cieslak, J. Short-range order in iron-rich Fe-Cr alloys as revealed by Mössbauer spectroscopy. Phys. Rev. B 2011, 83, 180202. [Google Scholar] [CrossRef]
- Cao, Z.; Liang, X.; Luo, S.; Song, J.; Pu, C.; Pang, Z.; He, W. Improvement of nitrogen ion implantation on the wear and corrosion resistance of bearing steel in NaCl solution. Vacuum 2024, 222, 112995. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, W.; Huang, W.; Tang, J.; Jiang, T.; Shen, X. Mechanical response of carbon ion implanted ferrite via atomic simulations. Int. J. Mech. Sci. 2025, 285, 109837. [Google Scholar] [CrossRef]
- Vojtech, V.; Charilaou, M.; Kovács, A.; Firlus, A.; Gerstl, S.S.A.; Dunin-Borkowski, R.E.; Löffler, J.F.; Schäublin, R.E. Macroscopic magnetic hardening due to nanoscale spinodal decomposition in Fe–Cr. Acta Mater. 2022, 240, 118265. [Google Scholar] [CrossRef]
- Macchi, J.; Nakonechna, O.; Henry, R.; Castro, C.; Edalati, K.; de Geuser, F.; Sauvage, X.; Lefebvre, W. Microstructural design by combining nanograins and spinodal decomposition in a Fe-Cr alloy. Scr. Mater. 2024, 252, 116247. [Google Scholar] [CrossRef]
- Suzuki, Y.; Hashimoto, N. Microstructure change of duplex stainless steels after thermal aging and electron beam irradiation. Nucl. Mater. Energy 2018, 15, 208–213. [Google Scholar] [CrossRef]
- Hyde, J.M.; Miller, M.K.; Hetherington, M.G.; Cerezo, A.; Smith, G.D.W.; Elliott, C.M. Spinodal decomposition in Fe-Cr alloys: Experimental study at the atomic level and comparison with computer models—II. Development of domain size and composition amplitude. Acta Metall. Mater. 1995, 43, 3403–3413. [Google Scholar] [CrossRef]
- Zhou, J.; Odqvist, J.; Thuvander, M.; Hedström, P. Quantitative evaluation of spinodal decomposition in Fe-Cr by atom probe tomography and radial distribution function analysis. Micros. Microanal. 2013, 19, 665–675. [Google Scholar] [CrossRef]
- Miller, M.K.; Stoller, R.E.; Russell, K.F. Effect of neutron-irradiation on the spinodal decomposition of Fe-32% Cr model alloy. J. Nucl. Mater. 1996, 230, 219–225. [Google Scholar] [CrossRef]
- Fujii, K.; Fukuya, K. Effects of radiation on spinodal decomposition of ferrite in duplex stainless steel. J. Nucl. Mater. 2013, 440, 612–616. [Google Scholar] [CrossRef]
- Cao, P. How Does Short-Range Order Impact Defect Kinetics in Irradiated Multiprincipal Element Alloys? Acc. Mater. Res. 2021, 2, 71–74. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Cieslak, J.; Reuther, H. Effect of He+ irradiation on Fe–Cr alloys: Mössbauer-effect study. J. Nucl. Mater. 2013, 434, 235–239. [Google Scholar] [CrossRef]
- Kozlov, K.; Shabashov, V.; Kozlov, A.; Sagaradze, V.; Semyonkin, V.; Panchenko, V.; Zamatovskii, A.; Kataeva, N.; Nikitina, A. Mössbauer spectroscopy investigation of the effect of a high-dose neutron irradiation on the atomic redistribution in the industrial steel EP823. J. Nucl. Mater. 2022, 558, 153384. [Google Scholar] [CrossRef]
- Arkoub, H.; Jin, M. Impact of chemical short-range order on radiation damage in Fe-Ni-Cr alloys. Scr. Mater. 2023, 229, 115373. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Du, Q.; Wang, H.; Wu, Y.; Jiang, S.; Lu, Z. Local chemical ordering and its impact on radiation damage behavior of multi-principal element alloys. J. Mater. Sci. Technol. 2023, 135, 13–15. [Google Scholar] [CrossRef]
- Dubiel, S.M. What can one learn about Fe-Cr alloys using Mössbauer spectroscopy? Crit. Rev. Solid. State Mater. Sci. 2024, 49, 856–907. [Google Scholar] [CrossRef]
- Vereshchak, M.; Manakova, I.; Yeshmanova, G.; Tleubergenov, Z. Structural Transformations in Duplex Stainless Steel CF8 under Intensive Cold Plastic Deformation. Metals 2024, 14, 1449. [Google Scholar] [CrossRef]
- Andrianov, V.A.; Bedelbekova, K.A.; Ozernoy, A.N.; Vereshchak, M.F.; Manakova, I.A.; Dektereva, A.S. Mössbauer study of the implantation of Fe-57 ions into metallic Ta and Mo and stainless steel. J. Surf. Investig. X-Ray Synchrotron Neutron Tech. 2020, 14, 371–375. [Google Scholar] [CrossRef]
- Andrianov, V.A.; Bedelbekova, K.A.; Ozernoy, A.N.; Vereshchak, M.F.; Manakova, I.A. Mössbauer studies of 57Fe implantation in metal Ta and Mo. J. Nucl. Instrum. Methods Phys. Res. Sect. B 2020, 475, 71–76. [Google Scholar] [CrossRef]
- Vereshchak, M.; Manakova, I.; Shokanov, A. Mössbauer and X-ray studies of radiation-induced processes in Nb–Zr alloys implanted with 57Fe Ions. Materials 2023, 16, 3813. [Google Scholar] [CrossRef]
- Aono, Y.; Abe, H.; Kuramoto, E.; Tsukuda, N.; Takenaka, M.; Kinoshita, T.; Yoshida, H. Radiation effects on the mechanical properties of stainless steels. J. Nucl. Mater. 1985, 133–134, 501–505. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, H.; Qiu, S.; Min, S.; Gu, Y.; Kuang, W.; Hou, J. Irradiation damage and corrosion performance of proton irradiated 304 L stainless steel fabricated by laser-powder bed fusion. Mater. Charact. 2023, 202, 113023. [Google Scholar] [CrossRef]
- Shu, S.; Bellon, P.; Averback, R.S. Role of point-defect sinks on irradiation-induced compositional patterning in model binary alloys. Phys. Rev. B 2015, 91, 214107. [Google Scholar] [CrossRef]
- Van Renterghem, W.; Al Mazouzi, A.; Van Dyck, S. Influence of post irradiation annealing on the mechanical properties and defect structure of AISI 304 steel. J. Nucl. Mater. 2011, 413, 95–102. [Google Scholar] [CrossRef]
- Johnson, E. Martensitic transformations in ion implanted stainless steels. MRS Online Proc. Libr. 2011, 157, 759–770. [Google Scholar] [CrossRef]
- Cook, D.C. Strain induced martensite formation in stainless steel. Met. Mater. Trans. A 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Ribis, J. Phase stability in irradiated alloys. J. Comp. Nucl. Mater. 2020, 1, 265–309. [Google Scholar]
- Budzyński, P. Effect of Ion Irradiation on the Properties of Metals and Alloys, 1st ed.; Routledge: London, UK, 2024; p. 174. [Google Scholar]
- Jin, H.-H.; Lim, S.; Kwon, J. Characterization of the martensite phase formed during hydrogen ion irradiation in austenitic stainless steel. Nucl. Instrum. Methods Phys. Res. Sect. B 2017, 409, 318–322. [Google Scholar] [CrossRef]
- Kadyrzhanov, K.K.; Rusakov, V.S.; Turkebaev, T.E. Phase transformation studies in implantation induced iron–metalloid systems studied by Mössbauer spectroscopy. Nucl. Instrum. Methods Phys. Res. Sect. B 2000, 170, 85–97. [Google Scholar] [CrossRef]
- Wang, F.; Wang, F.; Ding, X.; Gao, M.; Zhang, H. Microstructure evolution and electrochemical properties of carburized CSS-42L steel by high dose carbon ion implantation. Thin Solid Films 2023, 771, 139782. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, C.; Huang, J. Growth and agglomeration behaviors of eutectic M7C3 carbide in electroslag remelted martensitic stainless steel. J. Mater. Res. Technol. 2021, 11, 1490–1505. [Google Scholar] [CrossRef]
- Yu, W.-T.; Li, J.; Shi, C.-B.; Zhu, Q.-T. Effect of Electroslag Remelting Parameters on Primary Carbides in Stainless Steel 8Cr13MoV. Mater. Trans. 2016, 9, 1547–1551. [Google Scholar] [CrossRef]
- Zhu, Q.-T.; Li, J.; Zhang, J.; Shi, C.-B.; Huang, J. Precipitation Mechanism and Reduction of Amount of Primary Carbides During Electroslag Remelting of 8Cr13MoV Stainless Steel. Metall. Mater. Trans. B 2019, 50, 1365–1377. [Google Scholar] [CrossRef]
- Was, G.S.; Wharry, J.P.; Frisbie, B.; Wirth, B.D.; Morgan, D.; Tucker, J.D.; Allen, T.R. Assessment of Radiation-Induced Segregation Mechanisms in Austenitic and Ferritic–Martensitic Alloys. J. Nucl. Mater. 2011, 411, 41–50. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.; Kim, T.; Kim, J.H. Microstructure Response and Sodium Corrosion Behavior of Ferritic–Martensitic Steel after Proton Irradiation. Nucl. Eng. Technol. 2024, 56, 5028–5036. [Google Scholar] [CrossRef]
- Barr, C.M.; Thomas, S.; Hart, J.L.; Harlow, W.; Anber, E.; Taheri, M.L. Tracking the Evolution of Intergranular Corrosion through Twin-Related Domains in Grain Boundary Networks. npj Mater. Degrad. 2018, 2, 14. [Google Scholar] [CrossRef]
- Liu, H.; Lei, G.-H.; Huang, H.-F. Review on Synergistic Damage Effect of Irradiation and Corrosion on Reactor Structural Alloys. Nucl. Sci. Tech. 2024, 35, 57. [Google Scholar] [CrossRef]
- Soisson, F.; Jourdan, T. Radiation-Accelerated Precipitation in Fe–Cr Alloys. Acta Mater. 2016, 103, 870–881. [Google Scholar] [CrossRef]
- Senninger, O.; Soisson, F.; Martínez, E.; Nastar, M.; Fu, C.-C.; Bréchet, Y. Modeling Radiation-Induced Segregation in Iron–Chromium Alloys. Acta Mater. 2016, 103, 1–11. [Google Scholar] [CrossRef]
- Pareige, C.; Kuksenko, V.; Pareige, P. Behaviour of P, Si, Ni Impurities and Cr in Self-Ion Irradiated Fe–Cr Alloys—Comparison to Neutron Irradiation. J. Nucl. Mater. 2015, 456, 471–476. [Google Scholar] [CrossRef]
- Stoller, R.E.; Toloczko, M.B.; Was, G.S.; Certain, A.G.; Dwaraknath, S.; Garner, F.A. On the use of SRIM for computing radiation damage exposure. Nucl. Instrum. Methods Phys. Res. Sect. B 2013, 310, 75–80. [Google Scholar] [CrossRef]
- Ivanova, T.; Korenek, M.; Mashlan, M. Using Mössbauer spectroscopy to evaluate the influence of heat treatment on the surface characteristics of additive manufactured 316L stainless steel. Materials 2024, 17, 3494. [Google Scholar] [CrossRef]
- Sedlackova, A.; Ivanova, T.; Mashlan, M.; Dolakova, H. Phase changes in the surface layer of stainless steel annealed at a temperature of 550 °C. Materials 2022, 15, 8871. [Google Scholar] [CrossRef] [PubMed]
- Mijovilovich, A.; Goncalves Viera, A.; Paniago, R.; Pfannes, H.-D.; Mendonca Gonzales, B. Mössbauer study of the retained austenitic phase in multiple steels. Mater. Sci. Eng. A 2000, 283, 65–69. [Google Scholar] [CrossRef]
- Shabashov, V.; Sagaradze, V.; Kozlov, K.; Ustyugov, Y. Atomic order and submicrostructure in iron alloys at megaplastic deformation. Metals 2018, 8, 995. [Google Scholar] [CrossRef]
- Principi, G. The Mössbauer effect: A romantic scientific page. Metals 2020, 10, 992. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Brito, P.P.; Magalhaes, F.C.; Azzi, P.C.; Ardisson, J.D.; Abrao, A.M. Influence of low plasticity burnishing on the formation of strain induced martensite in the surface layer. J. Mater. Res. Technol. 2023, 27, 4573–4594. [Google Scholar] [CrossRef]
- Linderhof, F.; Mashlan, M.; Doláková, H.; Ingr, T.; Ivanova, T. Surface micromorphology and structure of stainless and maraging steel obtained via selective laser melting: A Mössbauer spectroscopy study. Metals 2021, 11, 1028. [Google Scholar] [CrossRef]
- Eymery, J.-P.; Merakeb, N.; Goudeau, P.; Fnidiki, A.; Bouzabata, B. A Mössbauer comparative study of the local environment in metastable 304 stainless steel films depending on the preparation mode. J. Magn. Magn. Mater. 2003, 256, 227–236. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An application for Mössbauer spectra modeling and fitting. AIP Conf. Proc. 2012, 1489, 178–185. [Google Scholar]
- Li, F.-S.; Sun, J.-J.; Chien, C.L. 57Fe Mossbauer study of metastable 304 stainless steel film with BCC structure. J. Phys. Condens. Matter 1995, 7, 1921–1931. [Google Scholar] [CrossRef]
- Kuzmann, E.; Jaen, J.; Vertes, A.; Csöme, L.; Tibiassy, B.; Kaldor, M. Mössbauer investigation of austenite formation together with Cr depletion in aged turbine blade steels. Hyperfine Interact. 1990, 58, 2593–2598. [Google Scholar] [CrossRef]
- Besoky, J.I.; Danon, C.A.; Ramos, C.P. Retained austenite phase detected by Mössbauer spectroscopy in ASTM A335 P91 steel submitted to continuous cooling cycles. J. Mater. Res. Technol. 2019, 8, 1888–1896. [Google Scholar] [CrossRef]
- Vardavoulias, M.; Papadimitriou, G. Mössbauer spectra and hyperfine parameters of iron–chromium carbides in ferritic stainless steels. Phys. Status Solidi 1992, 134, 183. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Theisen, W.; Sirosh, V.V.; Polshin, E.V.; Kortmann, A.; Mogilny, G.S.; Petrov, Y.N.; Tarusin, Y.V. Low-temperature martensitic transformation in tool steels in relation to their deep cryogenic treatment. Acta Mater. 2013, 61, 1705–1715. [Google Scholar] [CrossRef]
- Schaaf, P.; Krämer, A.; Wiesen, S.; Gonser, U. Mössbauer study of iron carbides: Mixed carbides M7C3 and M23C6. Acta Metall. Mater. 1994, 42, 3077–3081. [Google Scholar] [CrossRef]
- Shabashov, V.; Sagaradze, V.; Zamatovskii, A.; Kozlov, K.; Kataeva, N.; Danilov, S. Regulation of the concentration heterogeneity and thermal expansion coefficient in the metastable invar FeNi31.1 alloy. Materials 2022, 15, 8627. [Google Scholar] [CrossRef]
- Nordlund, K.; Zinkle, S.J.; Sand, A.E.; Granberg, F.; Averback, R.S.; Stollerc, R.E.; Suzudo, T.; Malerba, L.; Banhart, F.; Weber, W.J.; et al. Primary radiation damage: A review of current understanding and models. J. Nucl. Mater. 2018, 512, 450–479. [Google Scholar] [CrossRef]
- Kozlov, K.; Shabashov, V.; Zamatovskii, A.; Novikov, E.; Ustyugov, Y. Inversion of the sign of the short-range order as a function of the composition of Fe–Cr alloys at warm severe plastic deformation and electron irradiation. Metals 2020, 10, 659. [Google Scholar] [CrossRef]
- Gavriljuk, V.G.; Sirosh, V.A.; Petrov, Y.N.; Tyshchenko, A.I.; Theisen, W.; Kortmann, A. Carbide precipitation during tempering of a tool steel subjected to deep cryogenic treatment. Metall. Mater. Trans. A 2014, 45, 2453–2465. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Zukrowski, J. Mössbauer effect study of charge and spin transfer in Fe-Cr. J. Magn. Magn. Mater. 1981, 23, 214–228. [Google Scholar] [CrossRef]
- Dubiel, S.M.; Cieslak, J.; Zukrowski, J. Distribution of Cr atoms in the surface zone of Fe-rich Fe–Cr alloys quenched into various media: Mössbauer spectroscopic study. Appl. Surf. Sci. 2015, 359, 526–532. [Google Scholar] [CrossRef]
- Cios, G.; Tokarski, T.; Żywczak, A.; Dziurka, R.; Stępień, M.; Gondek, L.; Marciszko, M.; Pawłowski, B.; Wieczerzak, K.; Bala, P. The Investigation of Strain-Induced Martensite Reverse Transformation in AISI 304 Austenitic Stainless Steel. Metall. Mater. Trans. A 2017, 48, 4999–5008. [Google Scholar] [CrossRef]
- Sunil, S.; Kapoor, R.; Singh, J.B. Reversion of strain induced martensite to achieve high strength and ductility in AISI 304 L. Mater. Charact. 2023, 205, 113353. [Google Scholar] [CrossRef]
- Yeshmanova, G.; Manakova, I.; Vereshchak, M.; Tleubergenov, Z. Kinetics of reverse martensitic-austenitic transformation in duplex steel CF8 under thermal action. Contrib. Phys. 2025, 93, 81–87. [Google Scholar]
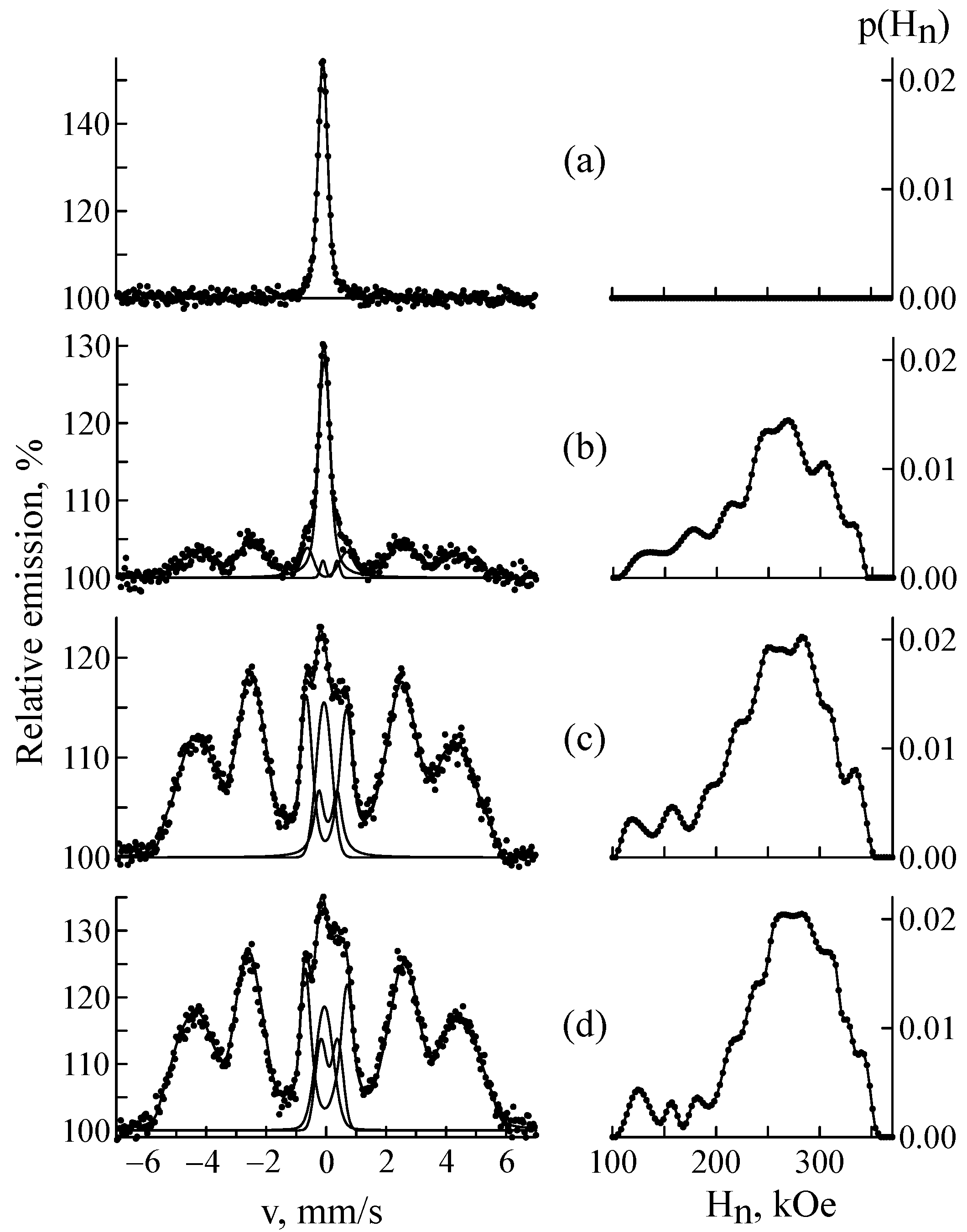
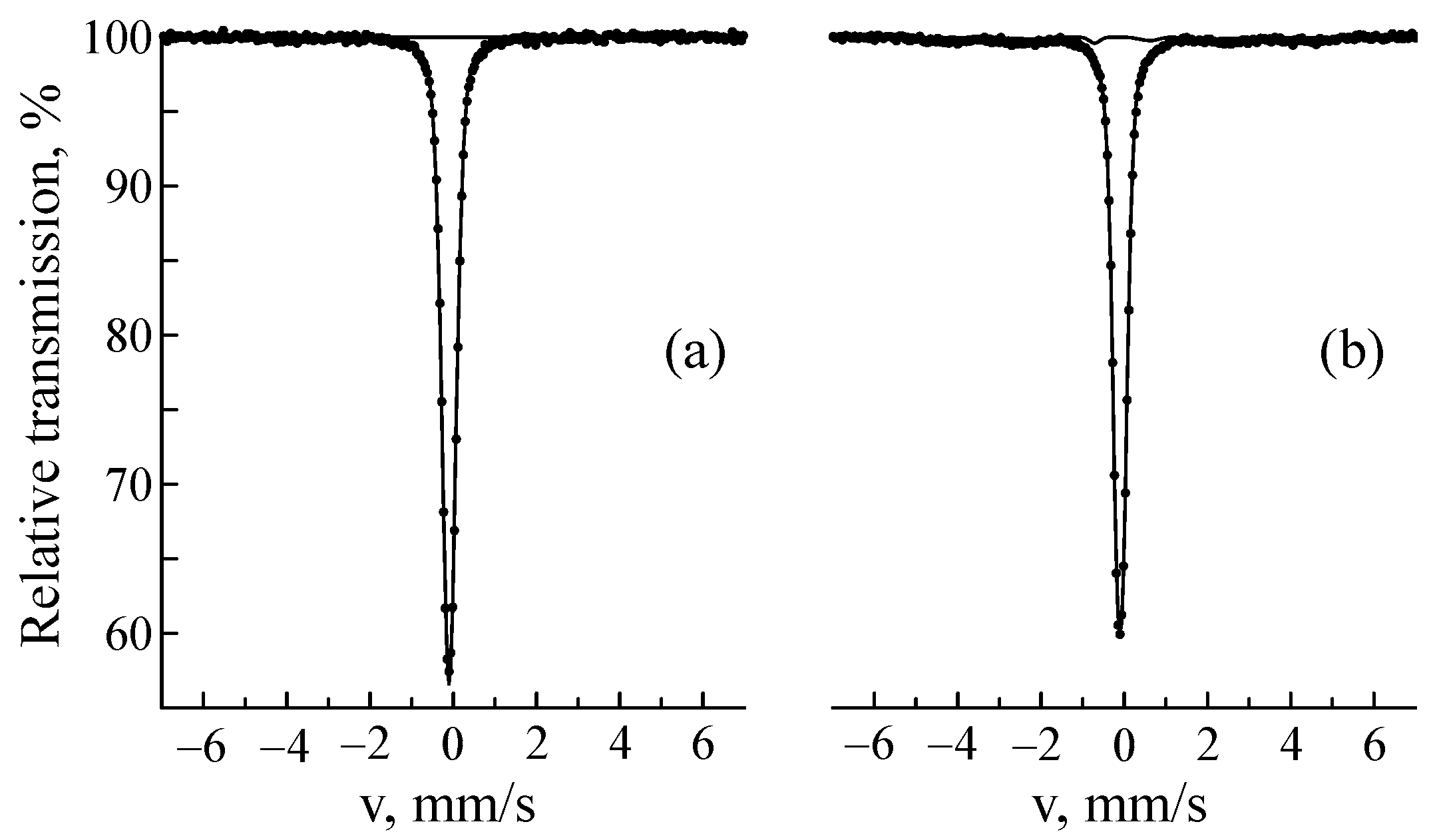
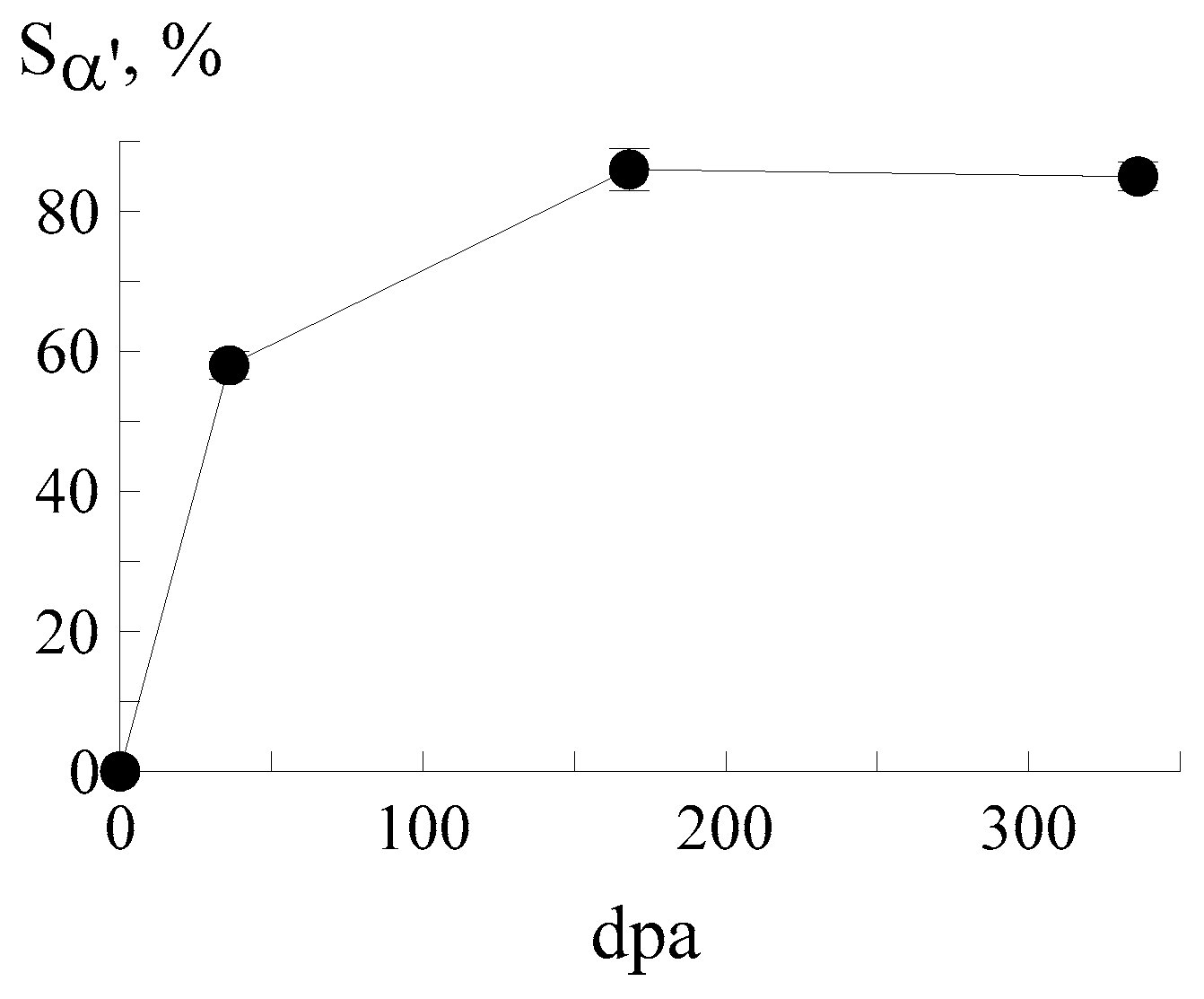
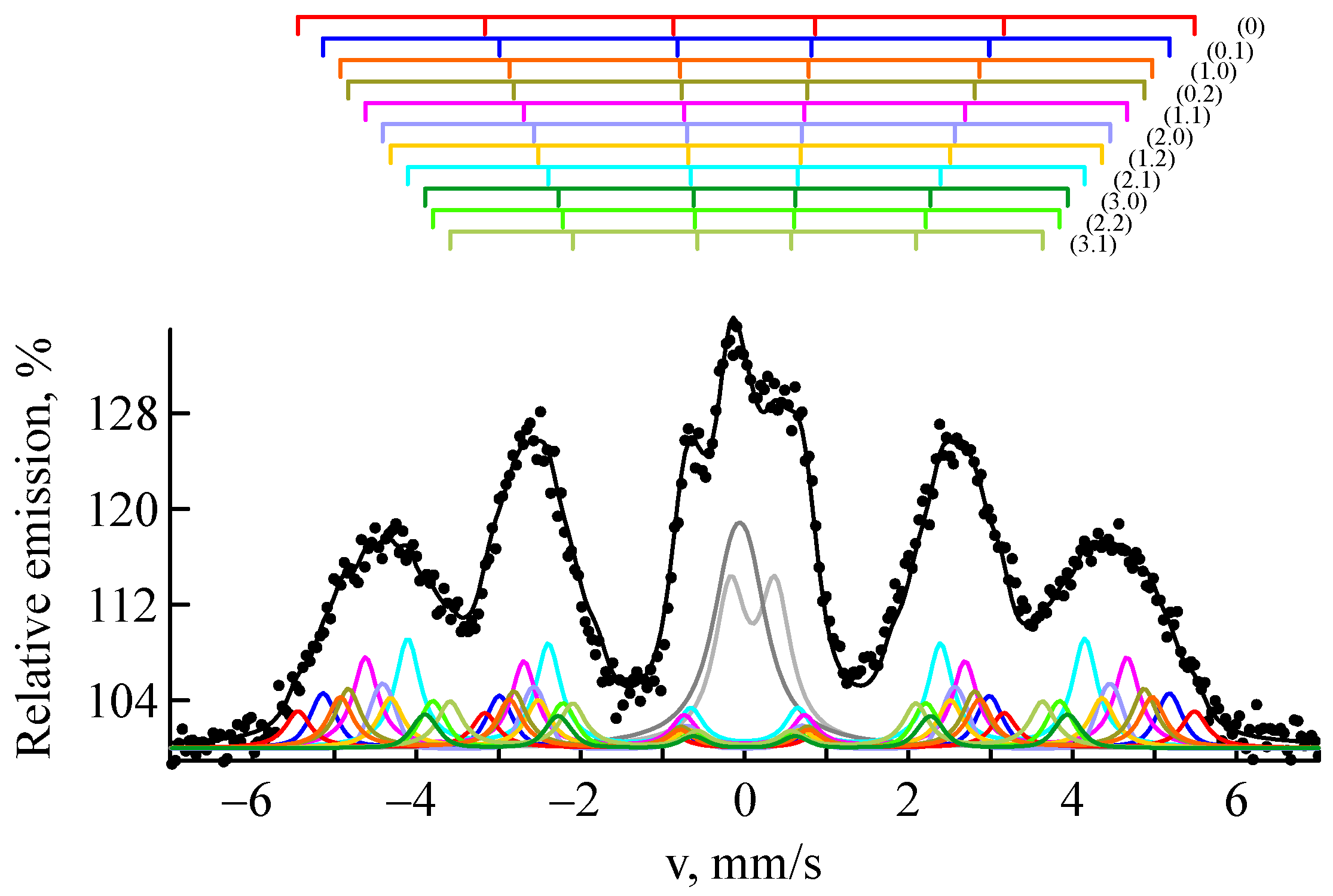
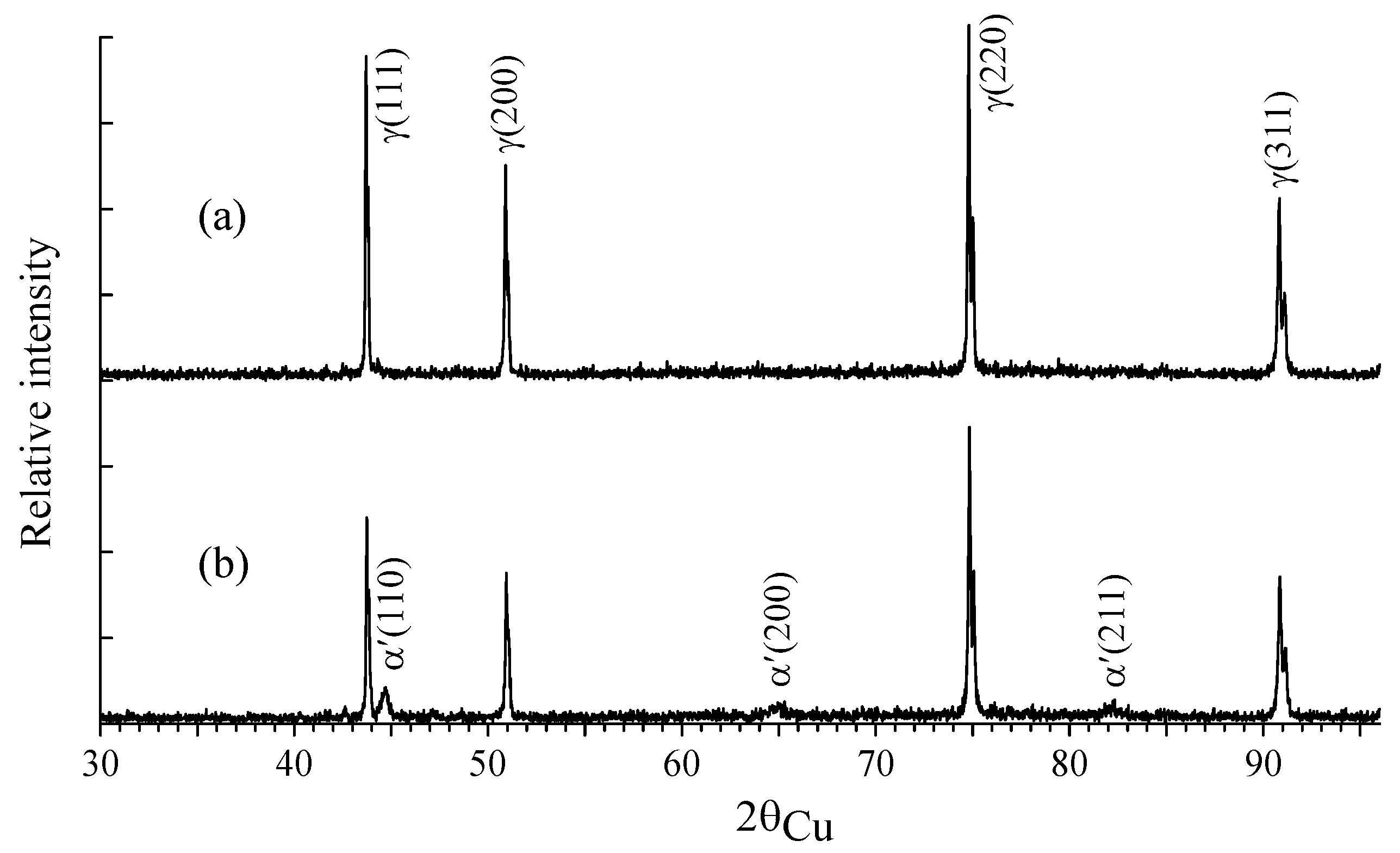
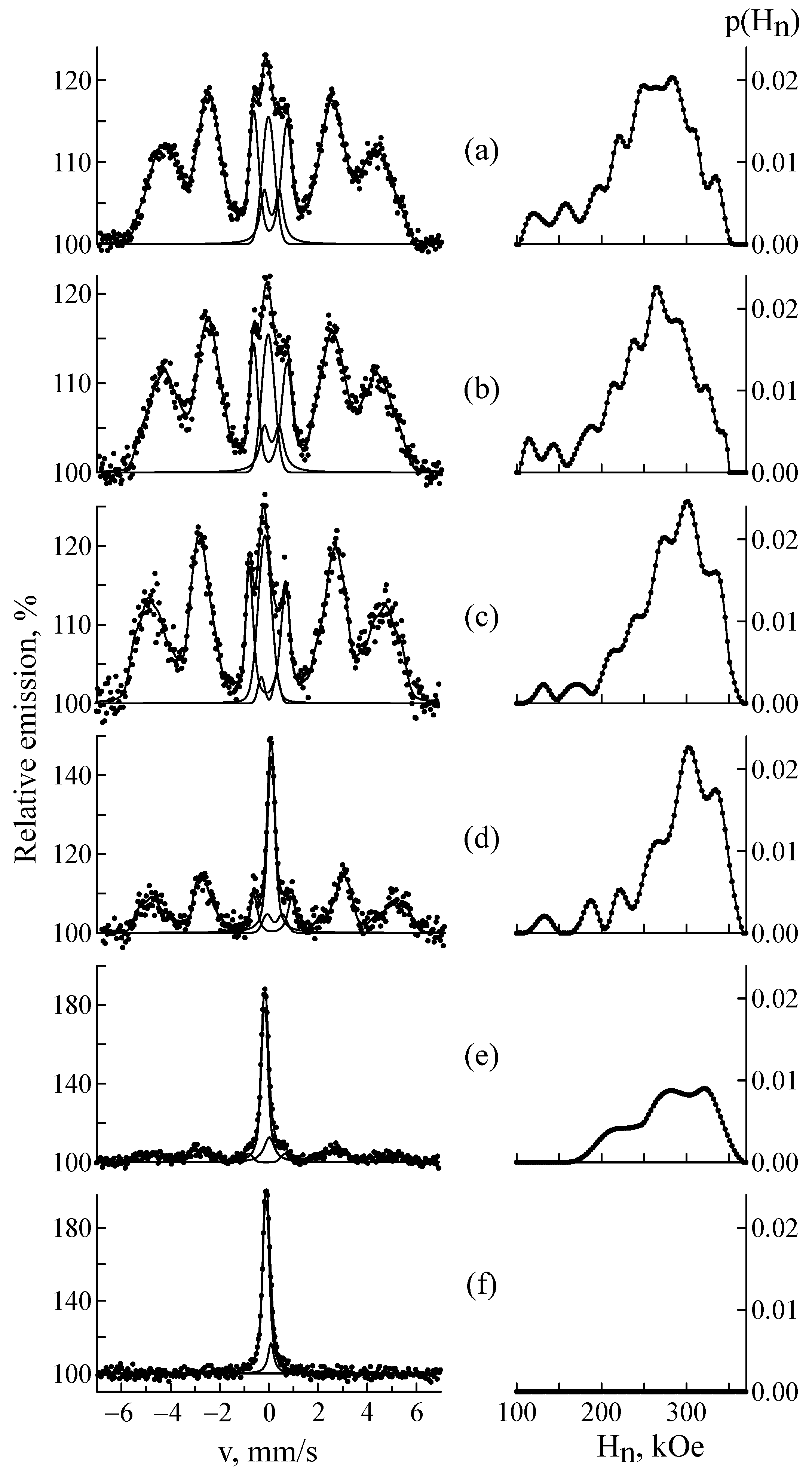

| C | Si | Mn | P | S | Cr | Ni | Fe | |
|---|---|---|---|---|---|---|---|---|
| <0.08 | <2 | <1.5 | <0.04 | <0.04 | 18.0–21.0 | 8.0–11.0 | balance | ASTM A743 |
| 0.07 | 1.6 | 1.0 | - | - | 19.4 | 8.6 | 69.3 | Measured |
| Fluence, ion/cm2 | Sα′, % | Sγ, % | SMeC, % |
|---|---|---|---|
| 1 × 1016 | 58 ± 2 | 40 ± 2 | 2 ± 1 |
| 5 × 1016 | 86 ± 3 | 8 ± 1 | 6 ± 3 |
| 1 × 1017 | 85 ± 2 | 8 ± 2 | 7 ± 1 |
| Atomic Configuration | Is, mm/s (±0.007) | H, kOe (±1) | S, % (±0.9) |
|---|---|---|---|
| 0.0 | 0.062 | 339 | 4.9 |
| 0.1 | 0.053 | 320 | 4.3 |
| 1.0 | 0.030 | 308 | 3.5 |
| 0.2 | 0.020 | 302 | 9.1 |
| 1.1 | 0.015 | 288 | 10.9 |
| 2.0 | 0.018 | 276 | 6.2 |
| 1.2 | 0.000 | 268 | 6.7 |
| 2.1 | −0.001 | 257 | 10.5 |
| 3.0 | 0.000 | 247 | 4.4 |
| 2.2 | −0.011 | 238 | 6.4 |
| 3.1 | −0.026 | 226 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manakova, I.; Vereshchak, M.; Yeshmanova, G.; Tleubergenov, Z. Structural–Phase Transformations in Stainless Steel CF8 Under Ion Implantation and Thermal Treatment. Materials 2025, 18, 5062. https://doi.org/10.3390/ma18215062
Manakova I, Vereshchak M, Yeshmanova G, Tleubergenov Z. Structural–Phase Transformations in Stainless Steel CF8 Under Ion Implantation and Thermal Treatment. Materials. 2025; 18(21):5062. https://doi.org/10.3390/ma18215062
Chicago/Turabian StyleManakova, Irina, Mikhail Vereshchak, Gaukhar Yeshmanova, and Zhandos Tleubergenov. 2025. "Structural–Phase Transformations in Stainless Steel CF8 Under Ion Implantation and Thermal Treatment" Materials 18, no. 21: 5062. https://doi.org/10.3390/ma18215062
APA StyleManakova, I., Vereshchak, M., Yeshmanova, G., & Tleubergenov, Z. (2025). Structural–Phase Transformations in Stainless Steel CF8 Under Ion Implantation and Thermal Treatment. Materials, 18(21), 5062. https://doi.org/10.3390/ma18215062





