Stabilizing Zinc Anodes with Water-Soluble Polymers as an Electrolyte Additive
Highlights
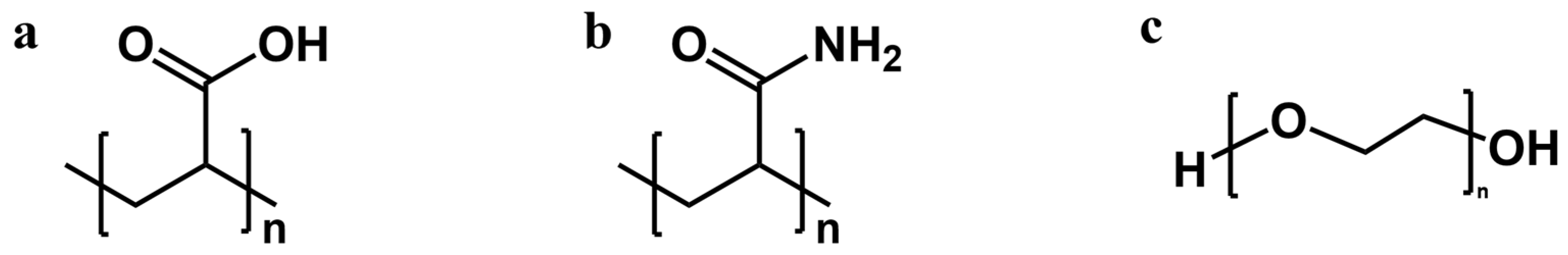
- Different water-soluble polymers can be used as electrolyte additives.
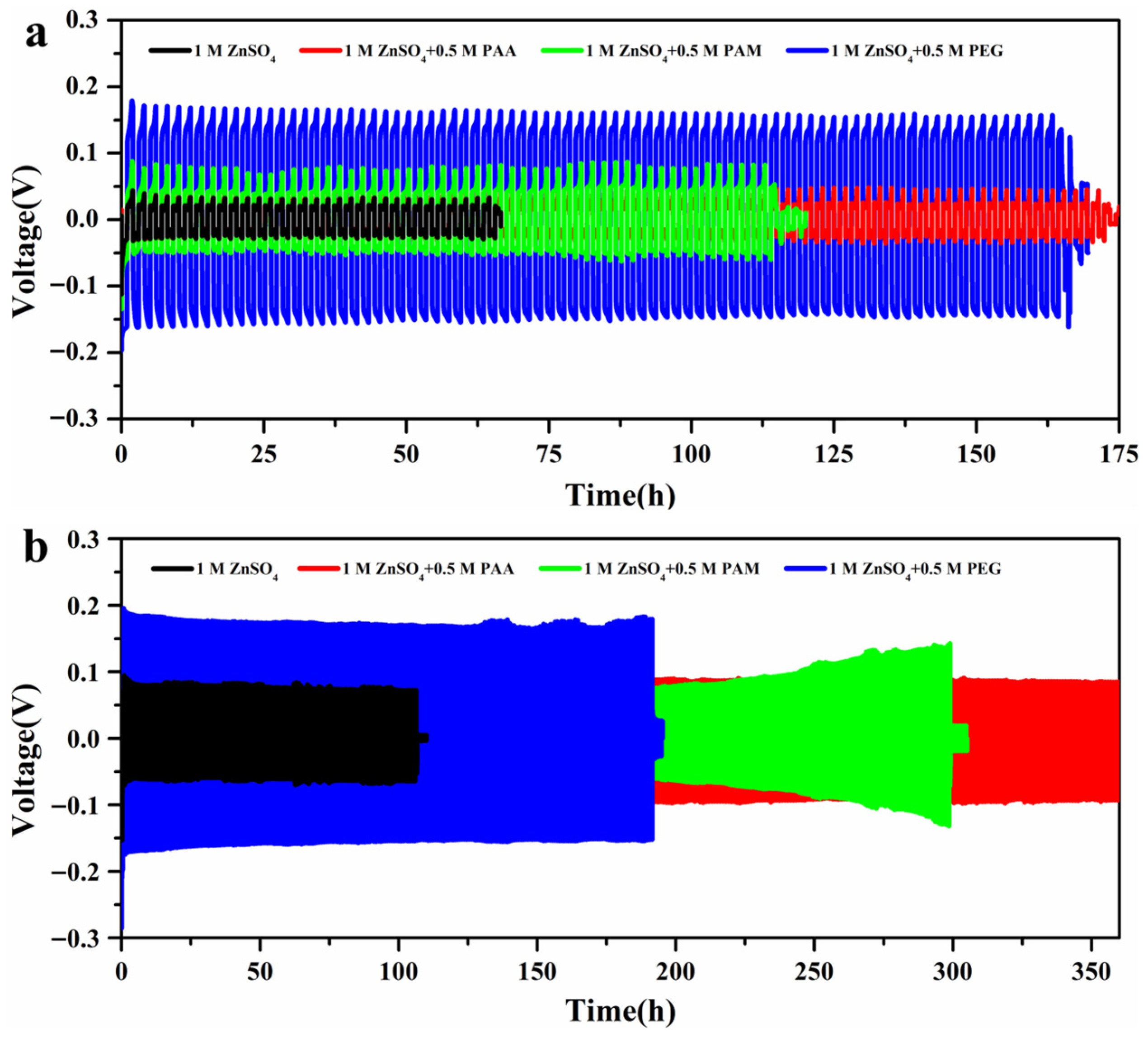
- PAA exhibits stable cycling for more than 360 h at 5 mA cm−2, 2 mA h cm−2.
- PAA exhibits an optimum performance.
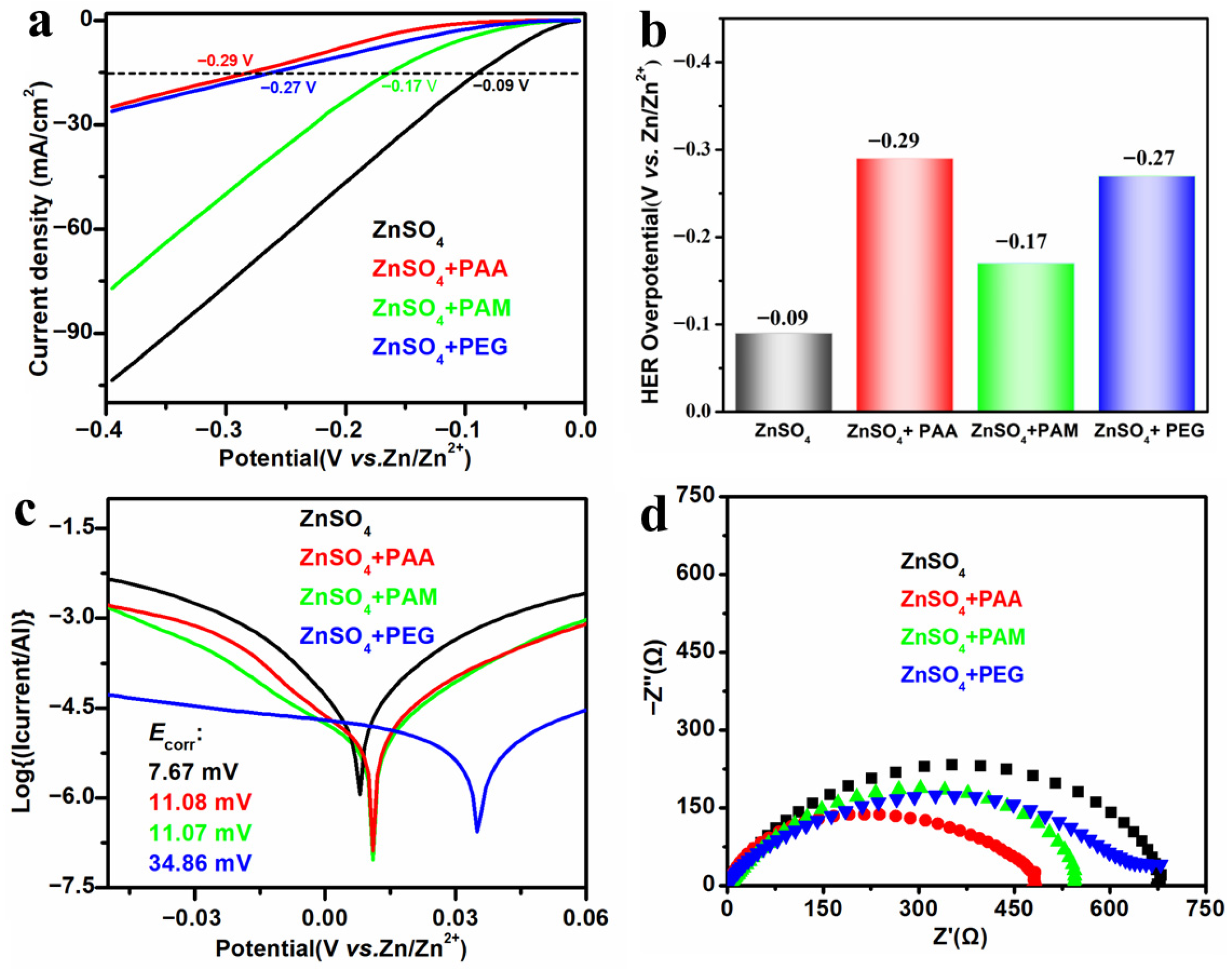
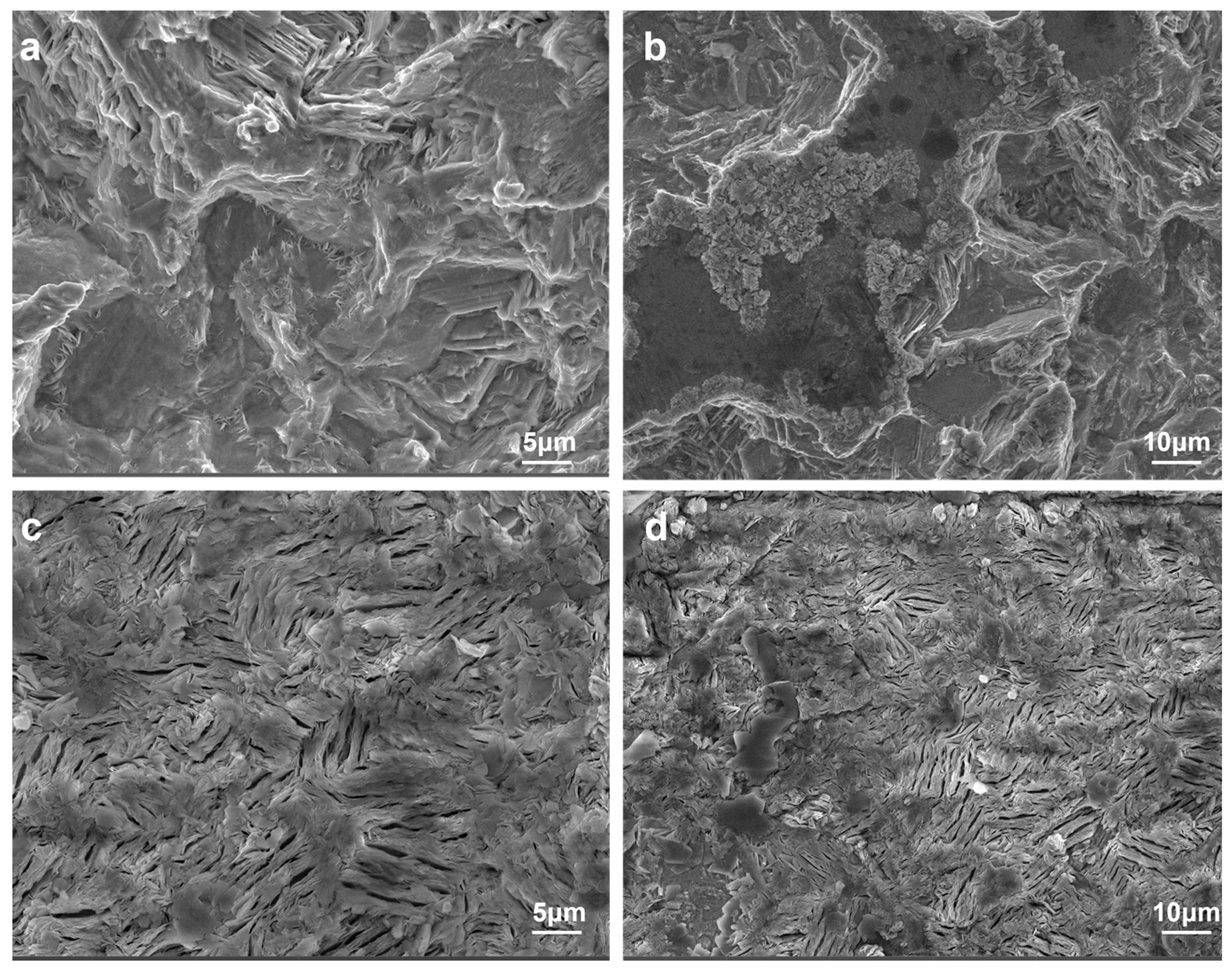
- PAA additive can inhibit hydrogen evolution, dendrite growth, and corrosion on the zinc anode.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Electrochemical Performance Measurements and Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Lyu, C.; Cheng, J.; Guan, C.; Li, X.; Zhang, J. Nitrogen atoms doping strengthens the interaction between Fe3C and carbon support for boosted hydrogen production performance in wide pH range. Int. J. Hydrogen Energy 2024, 93, 1363–1376. [Google Scholar] [CrossRef]
- Cheng, J.; Mai, W.; Lyu, C.; Zhu, H.; Zhou, D.; Liu, Y. Fluorine atoms incorporation strengthens the strong metal-support interaction of PtNi@NFGC for enhanced methanol oxidation reaction performance under alkaline media. Nano Energy 2024, 129, 110013. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Pei, W.; Song, S.; Li, P.; Shi, C.; Wang, J.; Chen, Z. Enhanced trace NO2 gas sensing with functionalized graphene-based nanofibers. Sens. Actuators B Chem. 2024, 417, 136171. [Google Scholar] [CrossRef]
- Wang, H.B.; Wu, Y.T.; Xie, Q.H.; Ma, X.X.; Zou, J.W.; Zheng, A.Y.; Guo, T.L.; Wang, C.; Han, J. An ionic sieve-integrated conductive interfacial design to simultaneously regulate the Zn2+ flux and interfacial resistance for advancing zinc-ion batteries. Adv. Funct. Mater. 2025, 35, 2417145. [Google Scholar] [CrossRef]
- Song, Y.-X.; Chen, X.-J.; Wang, J.; Wang, K.; Zhang, Y.-H.; Zhang, L.-X.; Zhong, X.-B.; Liang, J.-F.; Wen, R. Highly reversible Zn anodes enabled by in-situ construction of zincophilic zinc polyacrylate interphase for aqueous Zn-ion batteries. J. Colloid Interface Sci. 2025, 678, 284. [Google Scholar] [CrossRef]
- Fu, H.; Huang, S.; Wang, C.; Kim, J.S.; Zhao, Y.; Wu, Y.; Xiong, P.; Park, H.S. Exploring hybrid electrolytes for Zn metal batteries. Adv. Energy Mater. 2025, 15, 2501152. [Google Scholar] [CrossRef]
- Long, X.; Ma, Q.; Li, Y.; Bi, H.; Huang, M.; Dai, S.; Liu, R.; Ren, J.; Wang, X.; Luo, D.; et al. Multifunctional polyol electrolyte design toward long lifespan aqueous zinc-ion batteries. Adv. Energy Mater. 2025, 15, 2500957. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, T.; Wang, Y.; Lu, X.; Zhou, H.; Dong, S. Critical design strategy of electrolyte engineering toward aqueous zinc-ion battery. Chem. Eng. J. 2024, 497, 154541. [Google Scholar] [CrossRef]
- Song, Y.-X.; Wang, X.-F.; Liu, C.-B.; Guo, K.-X.; Guo, X.-H.; Zhong, X.-B.; Zhang, Y.-H.; Wang, K.; Guo, H.-J.; Zhang, L.-X.; et al. A widely used nonionic surfactant with desired functional groups as aqueous electrolyte additives for stabilizing Zn anode. Rare Met. 2024, 43, 3692–3701. [Google Scholar] [CrossRef]
- Xiao, X.; Ye, X.; Wu, Z.; Wu, X.; Yu, J.; Gu, L.; Liu, S. Trace small molecular/nano-colloidal multiscale electrolyte additives enable ultra-long lifespan of zinc metal anodes. Adv. Mater. 2024, 36, 2408706. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-X.; Wang, J.; Zhong, X.-B.; Wang, K.; Zhang, Y.-H.; Liu, H.-T.; Zhang, L.-X.; Liang, J.-F.; Wen, R. Interfacial chemistry regulation via dibenzenesulfonamide-functionalized additives enables high-performance Zn metal anodes. Energy Storage Mater. 2023, 58, 85. [Google Scholar] [CrossRef]
- Lv, Y.; Zhao, M.; Du, Y.; Kang, Y.; Xiao, Y.; Chen, S. Engineering a self-adaptive electric double layer on both electrodes for high-performance zinc metal batteries. Energy Environ. Sci. 2022, 15, 4748–4760. [Google Scholar] [CrossRef]
- Lin, Y.; Mai, Z.; Liang, H.; Li, Y.; Yang, G.; Wang, C. Dendrite-free Zn anode enabled by anionic surfactant-induced horizontal growth for highly-stable aqueous Zn-ion pouch cells. Energy Environ. Sci. 2023, 16, 687–697. [Google Scholar] [CrossRef]
- Zhao, K.; Fan, G.; Liu, J.; Liu, F.; Li, J.; Zhou, X.; Ni, Y.; Yu, M.; Zhang, Y.-M.; Su, H. Boosting the Kinetics and Stability of Zn Anodes in Aqueous Electrolytes with Supramolecular Cyclodextrin Additives. J. Am. Chem. Soc. 2022, 144, 11129–11137. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, J.; Lu, T.; Li, X.; Wu, H.; Chen, T.; Zhang, Y.; Wei, J.; Hu, M.; Zheng, X.; et al. Improving Zn2+ migration via designing multiple zincophilic polymer electrolyte for advanced aqueous zinc ion batteries. Chem. Eng. J. 2024, 496, 153815. [Google Scholar] [CrossRef]
- Yan, M.; Dong, N.; Zhao, X.; Sun, Y.; Pan, H. Tailoring the stability and kinetics of Zn anodes through trace organic polymer additives in dilute aqueous electrolyte. ACS Energy Lett. 2021, 6, 3236–3243. [Google Scholar] [CrossRef]
- Xue, P.; Guo, C.; Gong, W.; Chen, Y.; Chen, X.; Li, X.; Yang, J.; Zhang, Q.; Davey, K.; Zhu, K.; et al. Multifunctional polymer interphase with fast kinetics for ultrahigh-rate Zn metal anode. Angew. Chem. Int. Ed. 2025, 64, e202500295. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, J.; Zhang, J.; Yang, S.; Li, Y.; Luo, F.; You, Y.; Li, Y.; Xie, H.; Chen, Y. Multifunctional cellulose nanocrystals electrolyte additive enable ultrahigh-rate and dendrite-free Zn anodes for rechargeable aqueous zinc batteries. Angew. Chem. Int. Ed. 2024, 136, e202319051. [Google Scholar] [CrossRef]
- Zhang, Q.; Luan, J.; Fu, L.; Wu, S.; Tang, Y.; Ji, X.; Wang, H. The three-dimensional dendrite -free zinc anode on a copper mesh with a zinc-oriented polyacrylamide electrolyte additive. Angew. Chem. Int. Ed. 2019, 58, 15841. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Jiao, Y.; Wu, P. Guiding Zn Uniform Deposition with Polymer Additives for Long-lasting and Highly Utilized Zn Metal Anodes. Angew. Chem. Int. Ed. 2023, 62, e202314456. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, J.; Guo, Y.; Li, Z.; Yuan, C.; Ju, A.; Wang, X. Polymers for aqueous zinc-ion batteries: From fundamental to applications across core components. Adv. Energy Mater. 2024, 14, 2303967. [Google Scholar] [CrossRef]
- Nasrun, R.F.B.; Son, D.H.; Kim, J.H. Water-soluble anionic polymer electrolytes based on polyfluorene as the universal interlayer for organic solar cells. Macromol. Res. 2025, 33, 313–320. [Google Scholar] [CrossRef]
- Zhao, K.; Ma, S.; Zhao, J.; Li, H.; Ma, C.; Sheng, J.; Ding, J.; Wang, H.; Luo, H.; Wu, S.; et al. Synergistic interfacial chemistry enabled by a multifunctional zwitterionic polymer additive for highly reversible Zn metal anodes. J. Colloid Interface Sci. 2026, 702, 138847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, T.; Xin, W.; Peng, H.; Yan, Z.; Zhu, Z. Additive engineering for a hydrophilic/zincophilic polymeric layer towards dendrite-free zinc anode. Mater. Today Energy 2022, 29, 101130. [Google Scholar] [CrossRef]
- Zeng, X.; Xie, K.; Liu, S.; Zhang, S.; Hao, J.; Liu, J.; Pang, W.K.; Liu, J.; Rao, P.; Wang, Q.; et al. Bio-inspired design of an in situmultifunctional polymeric solid–electrolyte interphase for Zn metal anode cycling at 30 mA cm−2 and 30 mA h cm−2. Energy Environ. Sci. 2021, 14, 5947–5957. [Google Scholar] [CrossRef]
- Rivas, B.; Pereira, E.; Moreno-Villoslada, I. Water-soluble polymer–metal ion interactions. Prog. Polym. Sci. 2003, 28, 173–208. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; et al. Recent application developments of water-soluble synthetic polymers. J. Ind. Eng. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Wang, P.; Liang, S.; Chen, C.; Xie, X.; Chen, J.; Liu, Z.; Tang, Y.; Lu, B.; Zhou, J. Spontaneous Construction of Nucleophilic Carbonyl-Containing Interphase towards Ultra-Stable Zinc Metal Anodes. Adv. Mater. 2022, 34, 2202733. [Google Scholar] [CrossRef]
- Chen, J.; Chang, H.; Liu, Z.; Chen, Z.; Han, S.; Cao, X.; Liang, S. Anti-corrosive and carbonyl-rich interlayer enables highly reversible zinc anode. J. Power Sources 2024, 613, 234904. [Google Scholar] [CrossRef]
- Qiao, S.; Zhou, J.; Zhao, D.; Sun, G.; Zhang, W.; Zhu, Q. Constructing amphipathic molecular layer to assists de-solvation process for dendrite-free Zn anode. J. Colloid Inter. Sci. 2024, 653, 1085–1093. [Google Scholar] [CrossRef]
- Ouyang, K.; Li, F.; Ma, D.; Wang, Y.; Shen, S.; Yang, M.; Qiu, J.; Wen, W.; Zhao, N.; Mi, H.; et al. Trace-additive-mediated hydrophobic structure editing of aqueous zinc metal batteries for enabling all-climate long-term operation. ACS Energy Lett. 2023, 8, 5229–5239. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Pei, W.; Li, S.; Li, P. Stabilization of zinc metal anodes with polyacrylamide as an electrolyte additive. Mater. Rep. 2024, 38, 24020018. [Google Scholar]
- Zhao, K.; Sheng, J.; Ma, S.; Yin, J.; Luo, N.; Ding, J.; Wang, H.; Wang, S.; Fang, S. Unlocking the effect of alkyl chain length and hydroxyl group of electrolyte additives enabling stabilization of zinc anodes. Chem. Eng. J. 2025, 516, 163910. [Google Scholar] [CrossRef]
- Liu, J.; Song, W.; Wang, Y.; Wang, S.; Zhang, T.; Cao, Y.; Zhang, S.; Xu, C.; Shi, Y.; Niu, J.; et al. A polyamino acid with zincophilic chains enabling high-performance Zn anodes. J. Mater. Chem. A 2022, 10, 20779–20786. [Google Scholar] [CrossRef]
- Dong, H.; Yan, S.; Li, T.; Ming, K.; Zheng, Y.; Liu, Z.; Li, G.; Liu, J.; Li, H.; Wang, Q.; et al. Chelating dicarboxylic acid as a multi-functional electrolyte additive for advanced Zn anode in aqueous Zn-ion batteries. J. Power Sources 2023, 585, 233593. [Google Scholar] [CrossRef]
| Test Conditions | Electrolyte System | |||
|---|---|---|---|---|
| 1 M ZnSO4 | 1 M ZnSO4 + 0.5 g L−1 PAA | 1 M ZnSO4 + 0.5 g L−1 PAM | 1 M ZnSO4 + 0.5 g L−1 PEG | |
| 2 mA cm−2-2 mA h cm−2 | 65 h | 172 h | 114 h | 166 h |
| 5 mA cm−2-2 mA h cm−2 | 107 h | >360 h | 300 h | 192 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, X.; Zhang, S.; Wang, J.; Chen, Z.; Song, Y. Stabilizing Zinc Anodes with Water-Soluble Polymers as an Electrolyte Additive. Materials 2025, 18, 5040. https://doi.org/10.3390/ma18215040
Li X, Chen X, Zhang S, Wang J, Chen Z, Song Y. Stabilizing Zinc Anodes with Water-Soluble Polymers as an Electrolyte Additive. Materials. 2025; 18(21):5040. https://doi.org/10.3390/ma18215040
Chicago/Turabian StyleLi, Xueyan, Xiaojiang Chen, Senlong Zhang, Jinrong Wang, Zhuo Chen, and Yuexian Song. 2025. "Stabilizing Zinc Anodes with Water-Soluble Polymers as an Electrolyte Additive" Materials 18, no. 21: 5040. https://doi.org/10.3390/ma18215040
APA StyleLi, X., Chen, X., Zhang, S., Wang, J., Chen, Z., & Song, Y. (2025). Stabilizing Zinc Anodes with Water-Soluble Polymers as an Electrolyte Additive. Materials, 18(21), 5040. https://doi.org/10.3390/ma18215040







