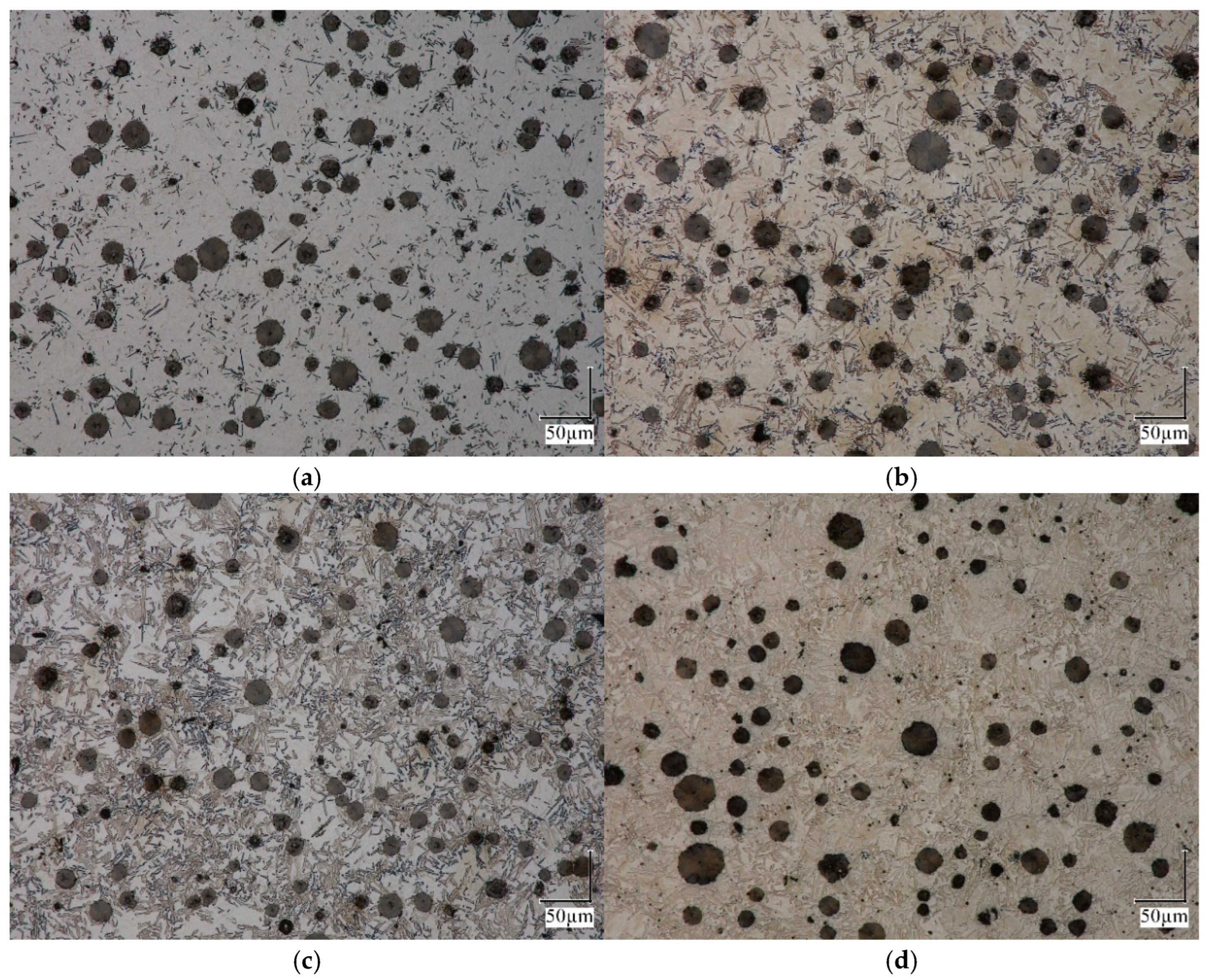
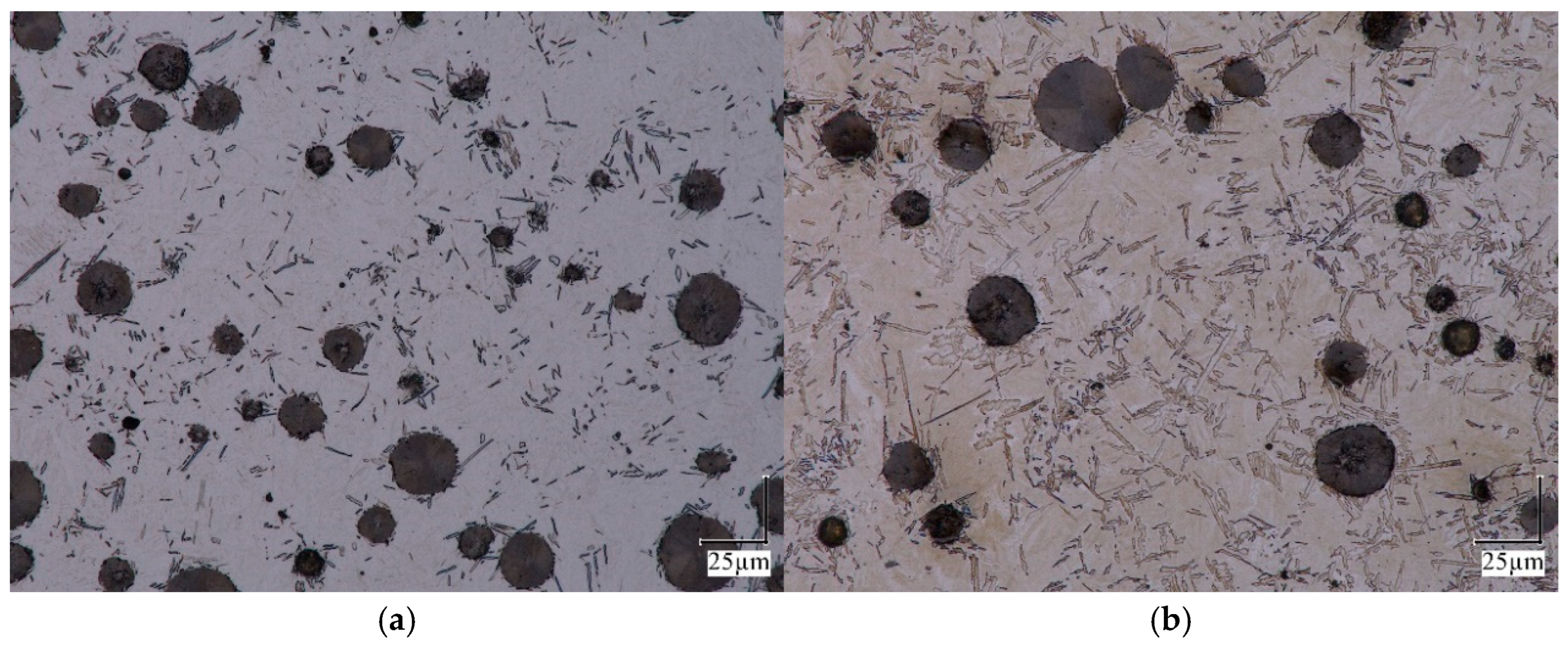
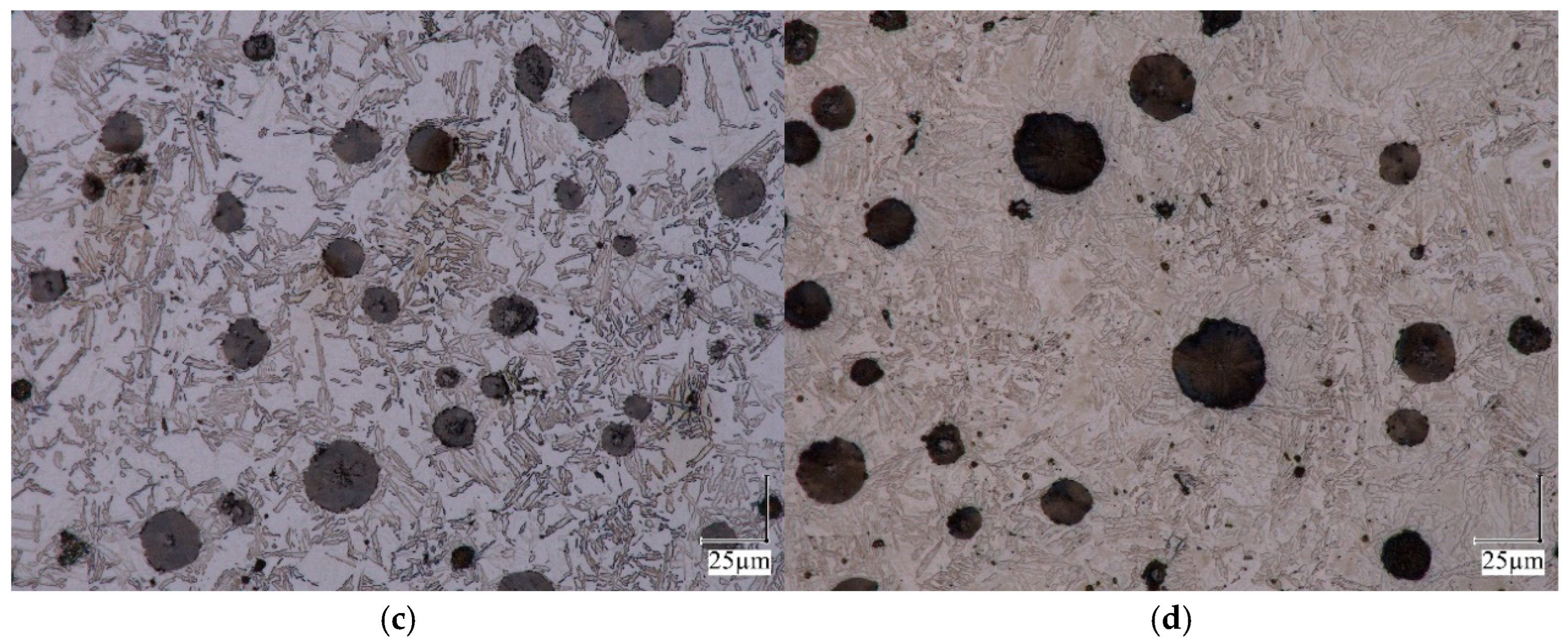
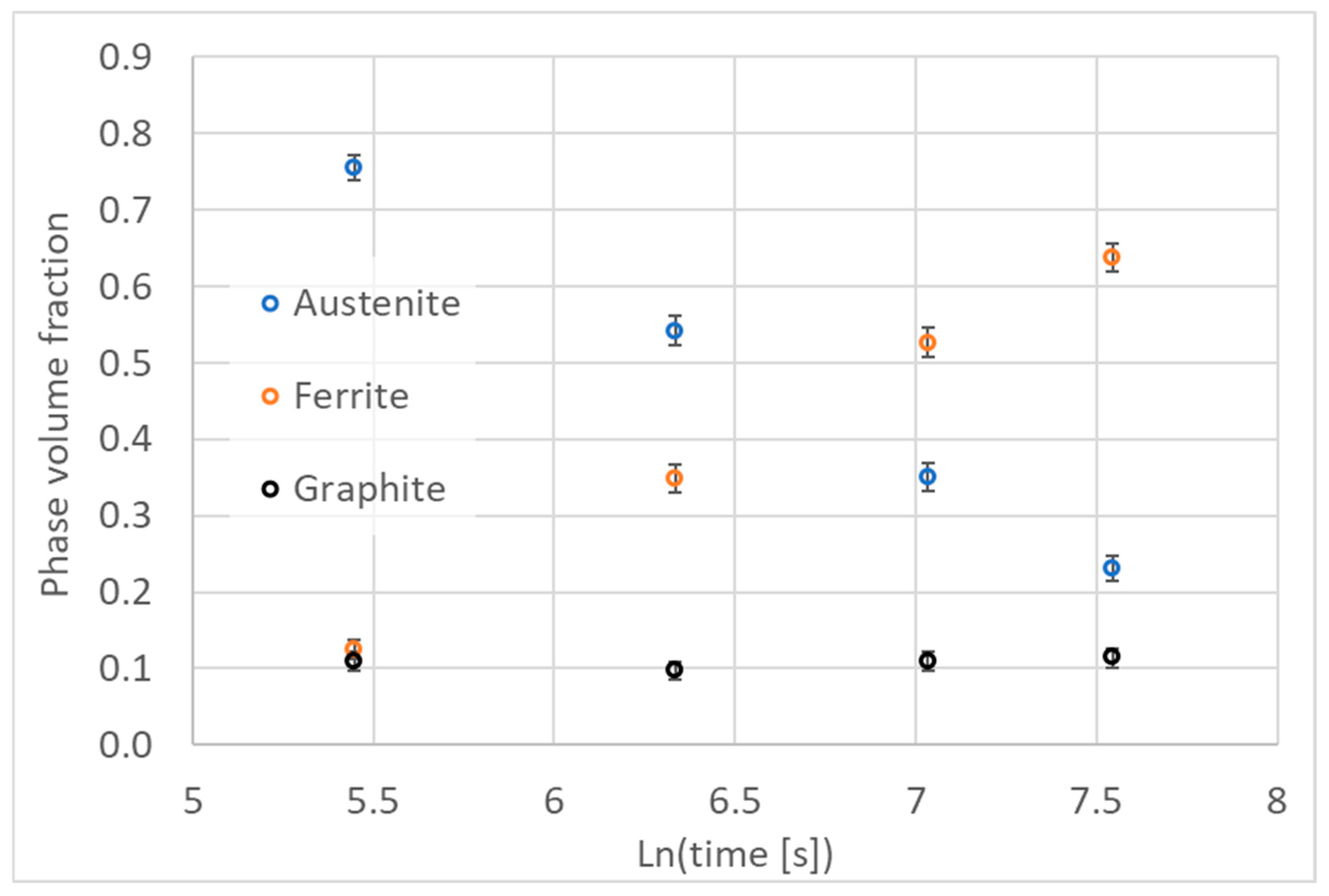
2.1. Dilatometric Studies of Austenite Decomposition in ADI
To directly assess the effect of process conditions (temperature and quenching time) on the development of phase transformations during the formation of the metal matrix microstructure, ADI, Santos et al. [
16] used a quenching dilatometer. The kinetics of austenite decomposition during cooling and isothermal transformation have been described using a graphical relationship between the change in relative elongation and the logarithm of time. Based on these relationships, the transformation time was determined by four temperature levels of isothermal processing. The logarithm of the transformation time was found to be linearly related to the temperature of the isothermal treatment.
The authors of the study introduced a correction for the transition process, i.e., the cooling time in the range from the austenitizing temperature to the isothermal transformation temperature. This correction considers the apparent linear coefficient of thermal expansion. The diagrams obtained from these relations have the shape of a smooth sigmoidal function. However, the article does not provide information on how to select the moments of time corresponding to the beginning and end of austenite decomposition.
Optimization of the two-step ADI heat treatment was presented by Gazda et al. [
17]. A quantitative dilatometric analysis combined with the calorimetric (DSC) method was used for the analysis of the ausferrite decomposition kinetics. These tools allow excellent mechanical properties of ADI after the temperature step-down heat treatment. The relations between relative elongation and temperature obtained in the experiments were reconvoluted by PeakFit software. The results of the dilatometric tests are presented in terms of temperature dependence.
The dilatometry studies for the developed ADI alloys presented by Ahmed et al. [
18] showed that the time required for the completion of the ausferrite formation in the alloys solidify under the conditions of the ultrasonic melt treatment was four times shorter than that required for statically solidified SG irons.
In a publication by Lachart et al. [
19], it was proven that the dilatometric method can predict microstructure variation in heavy section parts with small samples only. Dilatometric tests were performed on small samples for cooling rates corresponding to the conditions found at different locations in the test casting with varying wall thicknesses. Based on the results obtained, it is possible to predict the risk of perlitic structure in areas with low rates of temperature change during quenching.
Górny et al. [
20] used the dilatometric method in combination with other methods of structure studies to evaluate the effect of the temperature of the austenitizing procedure on the course and rate of phase transformations occurring in the isothermal hardening process. Two chemical compositions of ADI and four levels of austenitization temperature were analyzed. The influence of the above parameters was presented in terms of the transformation rate versus time relationship, the maximum solidification rate and the time at which the maximum rate was reached.
2.2. Mathematical Models of Austenite Decomposition Kinetics in ADI
The fundamentals of the general statistical theory of phase transitions involving nucleation and growth of new phase grains were published by Kolmogorov [
21]. Based on an analysis of the probability that a random point remains in the parent phase, this article proposes a general solution for determining the volume fraction of transformation products,
f. The solution relates to the uniform in space random nucleation of grains with varying speed. The grains were assumed to have a convex shape, and it was further assumed that grains can have anisotropic geometry, provided they have the same spatial orientation. The migration speed of the interfacial “solid–liquid” boundary can be variable. In addition to the general solution, two partial solutions for three-dimensional growth with constant velocity are presented in [
21]. Both solutions were obtained for a constant interfacial migration velocity. One concerns a constant nucleation rate. The other is for so-called instantaneous nucleation (constant grain number). These solutions take the form of
where
t is time and the
k and
n parameters depend on the shape of the grains, the migration rate of the interfacial boundary and the nucleation rate.
Equations of this type, proposed in the 1930s and 1940s and also in publications [
22,
23,
24,
25], are now assumed to be called Kolmogorov–Johanson–Mehl–Avrami (KJMA) equations. The Kolmogorov model can be used to analyze both crystallization processes and solid-state phase transformations. Moreover, it is applicable not only in material science but also in the biology of ecological landscapes [
26]. This model describes the kinetics of the processes involving the emergence of growth centers and the subsequent increase in the size of the areas surrounding these centers.
The first mathematical model of the kinetics of the isothermal transformation of austenite in the temperature range of the bainitic transformation was obtained by Austin and Rickett (AR) based on the approximation of experimental data [
27]:
where
f is the volume share of transformation products,
fmax is the maximum share that can be achieved at the analyzed temperature, and
k and
n are the temperature-dependent constants.
Avrami [
24] represents this equation in the following form:
Starink [
28] compared the accuracy of mapping the kinetics of a diffusion-controlled precipitation reaction using the KJMA and AR equations under isothermal conditions for six different alloys. It is shown that, if only one type of precipitation process occurs simultaneously, the AR equation immediately provided a better fit to the experimental data sets. The paper [
28] noted that the reason for the deviation of the prediction using the KJMA equation from the experimental data is the failure to meet the assumptions made in [
21].
The article by Starink [
29] presents a unique kinetic model:
which, in addition to the parameters
k and
n described above, also includes the impingement parameter η
i. The purpose of this parameter is to account for the influence of the shielding effect on the growth of ferrite grains shaped like wheat ears.
An exponential relation between the transformed volume fraction
f and process time
t was proposed in [
20]:
where
k is a curve shape constantly valid for a given transformation condition.
2.3. Description of Model Used: KJMA Equation and Avrami Diagram
The KJMA equation in the form of Equation (3) was used for the numerical simulation of in situ heat treatment of austempering ductile iron in [
30] to calculate the amounts of pearlite and of ausferrite. The parameters of this equation have been determined at each temperature from the “start” and “end” of the isothermal transformation curves on the TTT diagram.
The numerical simulation of isothermal austempering heat treatment of a ductile cast iron presented by Boccardo et al. [
31] used the KJMA equation with a constant exponent. The model with an exponent of
n = 2.1 correctly predicts the course of austenite decomposition compared with the experimental results course obtained in the described experiment.
Pereira et al. [
32] used the KJMA equation for the kinetics of ferrite growth during austempering for three different austenitization routes: two-step, conventional and rapid austenitization. For each of the analyzed methods, eight samples were used to measure the volume fraction of ferrite. These samples were rapidly cooled from the austempering temperature after holding for 15 min to 6 h. The exponent
n in the range 1.5…1.7 was estimated using nonlinear regression—Origin
® 2019 software on the base of collected data.
Suchocki et al. [
33] underscore that a significant improvement in the performance of the JMAK model was achieved when the kinetic constants were determined using the least squares method. In this work, the Scilab software was used to process the data from the dilatometric measurements. The obtained exponent
n values are placed in the range from 0.948 (for austenite decomposition in the temperature 500 °C) to 2.1082 (for 270 °C).
To better understand the physical meaning of the exponent
n in Equation (4), let us look at the general solution proposed by Kolmogorov [
21], which can be expressed as follows:
where
c is the grain shape constant, α(
t′) is the time-varying rate of nucleation,
u(τ) is the time-dependent migration speed of the boundary of the growing grain, and
n is the dimensionality of the growth.
Avrami clarified the physical meaning of the argument of the exponential function in the Kolmogorov Equation (8) by introducing the concept of extended volume
Vex, “which is the total transformed volume if we neglect overlapping of growing grains” [
23]. For a constant number of spherical grains (
Ndim = 3) and their growth at a constant velocity
u, the extended volume is calculated as follows:
where
Ng is the grain number per unit volume, and
Avrami [
27] noted that, for constants
n and
k, applying a double logarithmic transformation of Equation (3) leads to the following linear equation relationship:
The application of Equation (11) for the analysis of the kinetics of austempering reactions in ductile irons for four types of cast iron is described in [
33]. The experimental results obtained using the dilatometric method were analyzed. In the publication [
34], the parameters of the Avrami equation for the analysis of the kinetics of the isothermal decomposition of austenite were determined based on nine measurements of changes in the hardness of the samples. These samples were subjected to isothermal treatment interrupted after a period of 1 min to 180 min.
Comprehensive studies of phase transition kinetics in ADI using neutron diffraction, dilatation measurements, atom probe tomography and metallographic methods are presented in [
35]. It has been shown that this equation does not comprehensively describe stage I and stage II of isothermal transformation, but it was shown that dilatometry is a well-established method that can determine the time for maximum C enrichment in ADI during austempering.
An evaluation of different models of the transformation kinetics of isothermal austempering transformation was presented in [
36]. To this purpose, a series of dilatometric tests were carried out on ADI.
The physical meaning of parameter
k in this equation can be derived from a compilation of Equations (10) and (11). For spherical growth with a constant velocity, the equation is as follows:
Starink [
29] pointed out the dependence of the exponent
n on the nucleation rate and changes in growth velocity of transformation products as follows:
For continuous nucleation with a constant rate, B = 1, whereas for instantaneous nucleation, B = 0. If the boundary migrates at a constant rate, the value of g is 1. If the linear growth rate increases (e.g., due to increased undercooling), g > 1.
If the growth rate changes over time according to a power function
, the extended volume will be equal to
For 3D growth limited by diffusion, theoretically, n′ = −1/2, which means that the value of the exponent n = 1½.
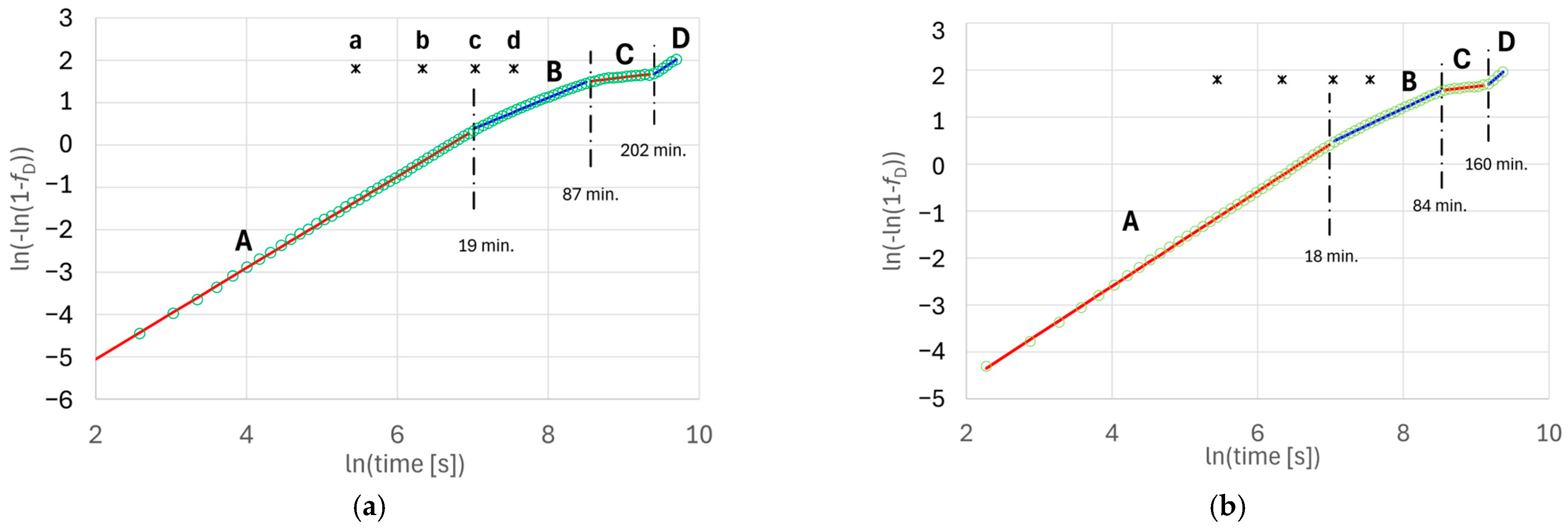
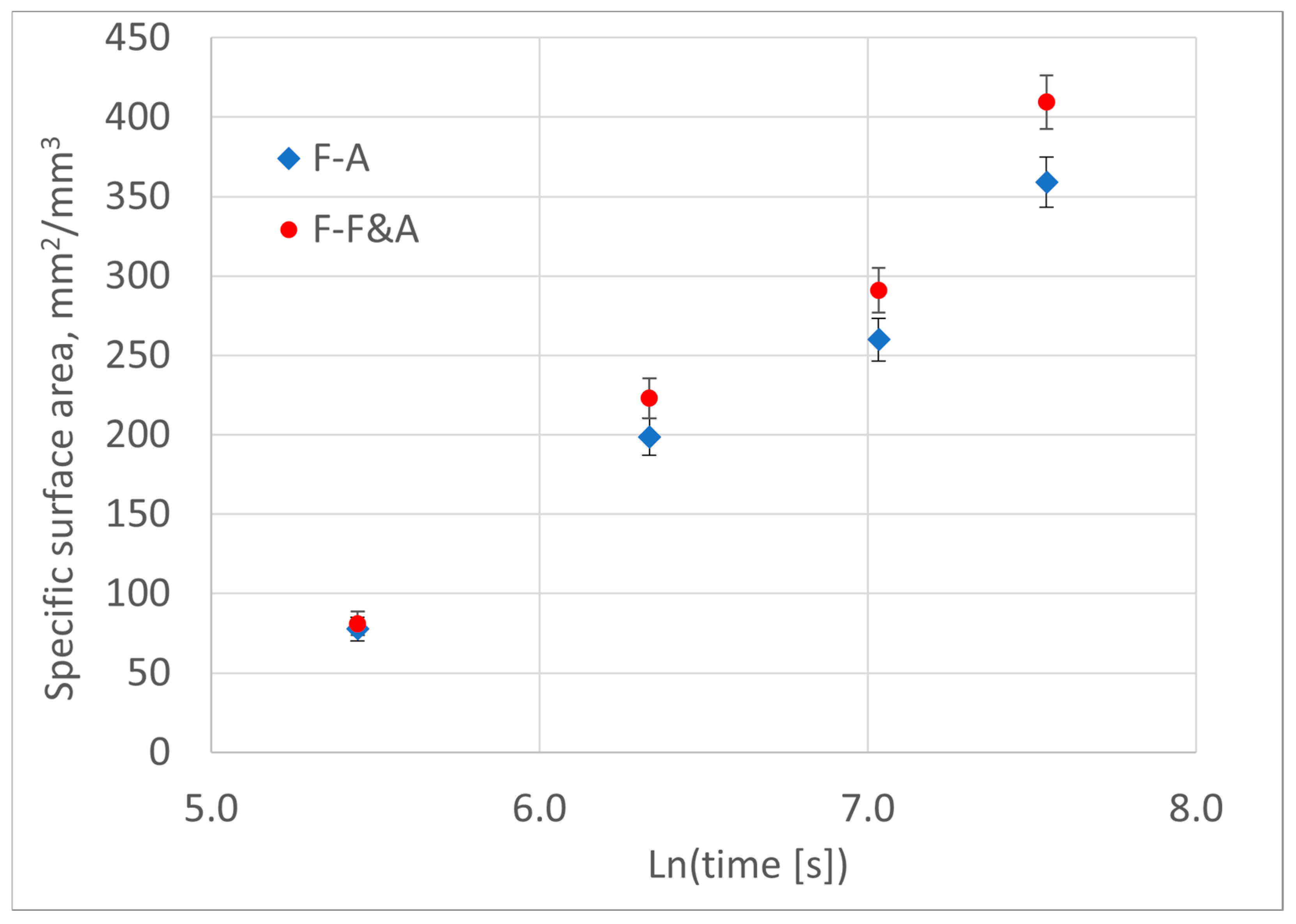
The deviation of the Avrami diagram, i.e., the graph of the left side of Equation (8) versus the natural logarithm of time, from a straight line may indicate a change in the transformation mechanism. The use of such a transformation provides the opportunity to detect moments of change in the kinetics of phase transformation processes based on an analysis of the graphical representation of the experimental results.
As can be seen in Equations (8) and (13), changes in the exponent n during the transformation can be associated with variations in the nucleation rate, the migration velocity of the boundary and/or the dimensionality of growth. During crystallization and solid-state phase transformations, the nucleation period can be shorter than the time required for complete transformation. If the speed and dimensionality of growth remain unchanged, the moment of nucleation termination can be seen in the Avrami diagrams. An indicator of the end of nucleation will be a decrease in the angular coefficient of the diagram.
These factors may not be accounted for in the general Avrami equation for transformation with very short incubation periods compared to the time required to complete the reaction. An illustrative example of this is provided in [
37].
A summary of example values of the exponent
n for different transformation mechanisms is provided in [
38]. If both the migration speed of the boundary and the nucleation rate do not increase, the exponent
n does not exceed four. The minimum value of
n is equal to ½ and applies to one-dimensional, diffusion-controlled growth (e.g., for large plates that no longer grow in the longitudinal directions due to full impingements of the sidewalls).
Another reason for reducing the slope of the Avrami diagram may be a reduction in the dimensionality of growth. Changes of this type are caused by the blocking effect (also referred to as “shielding” or “screening”) [
29,
39,
40]. A quantitative assessment of the correction to Equation (3) in the case of the anisotropic shape of the grains and their random spatial orientation is described in [
41].
For needle-shaped grains, three-dimensional growth will be reduced to two-dimensional after full impingements of the rapidly growing end walls. A similar situation after impingements of the sidewalls applies to the growth of thin plates. If the growth rate of plate grains varies depending on the crystallographic orientation of the growing surface, the growth dimension will decrease from the initial three to two and then to one, as the faster end walls will be blocked by the slower growing surfaces of adjacent grains.
One more factor that can change the linearity of the Avrami diagram is a change in the mechanism of the phase transformation. In the case of ADI heat treatment using the austempering method, such a change may occur because of the transition from the first to second stage of the process and possibly during the first stage.
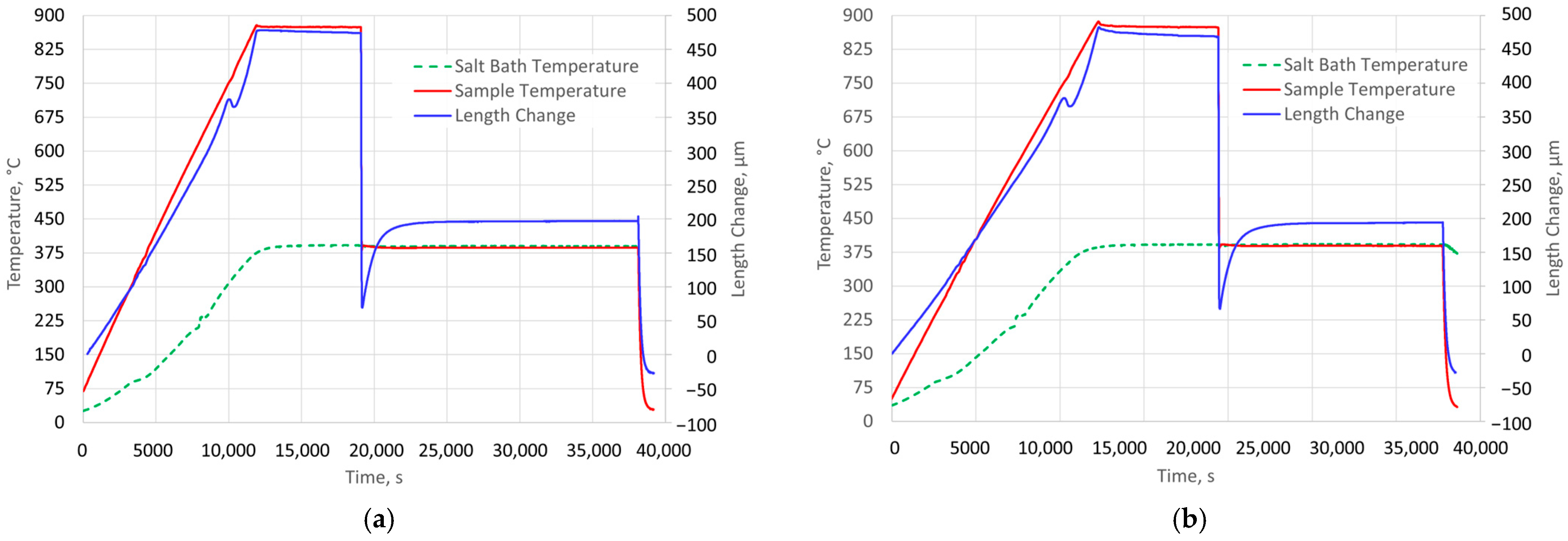
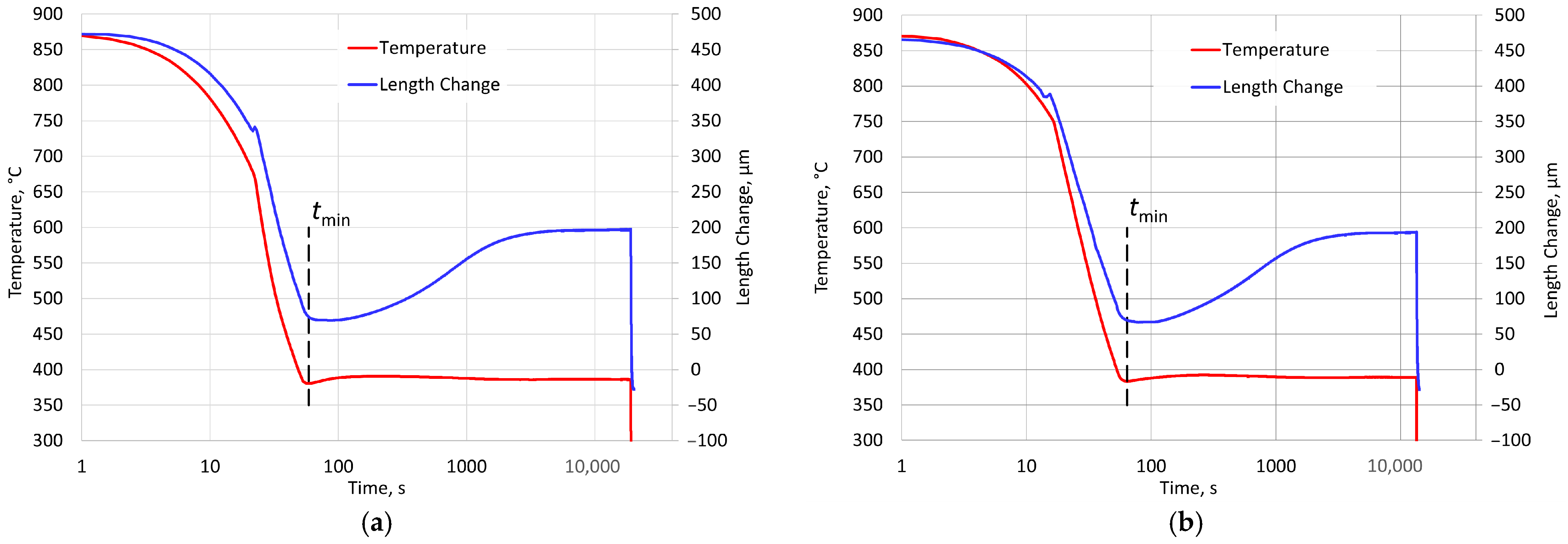
The aim of the research described in this article is to examine the possibility of analyzing changes in the kinetics of the first stage of austenite decomposition in the isothermal austempering process.
An excessive prolongation of low-temperature treatment is risky because it may lead to the onset of the second stage and deterioration of the properties of cast iron. This means that increasing the duration of this treatment is risky. On the other hand, it is not very effective due to the constantly decreasing rate of austenite decomposition. Compared to methods involving the preparation and testing of a series of samples, dilatometric analysis is continuous from a practical point of view. Therefore, the results of such an analysis can serve as a basis for a justified and more precise reduction in the time required for this procedure. It should also be noted that switching from a series of quenches to “online” dilatometry not only increases the accuracy of the time assessment but also speeds up the analysis and reduces the cost of testing.