Evaluation of Colonization by Candida albicans and Biofilm Formation on 3D-Printed Denture Base Resins
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of Specimens
2.2. Finishing and Polishing of Specimens
2.3. Analysis of the Surface Roughness
2.4. Analysis of Surface Wettability
2.5. Biofilm Formation
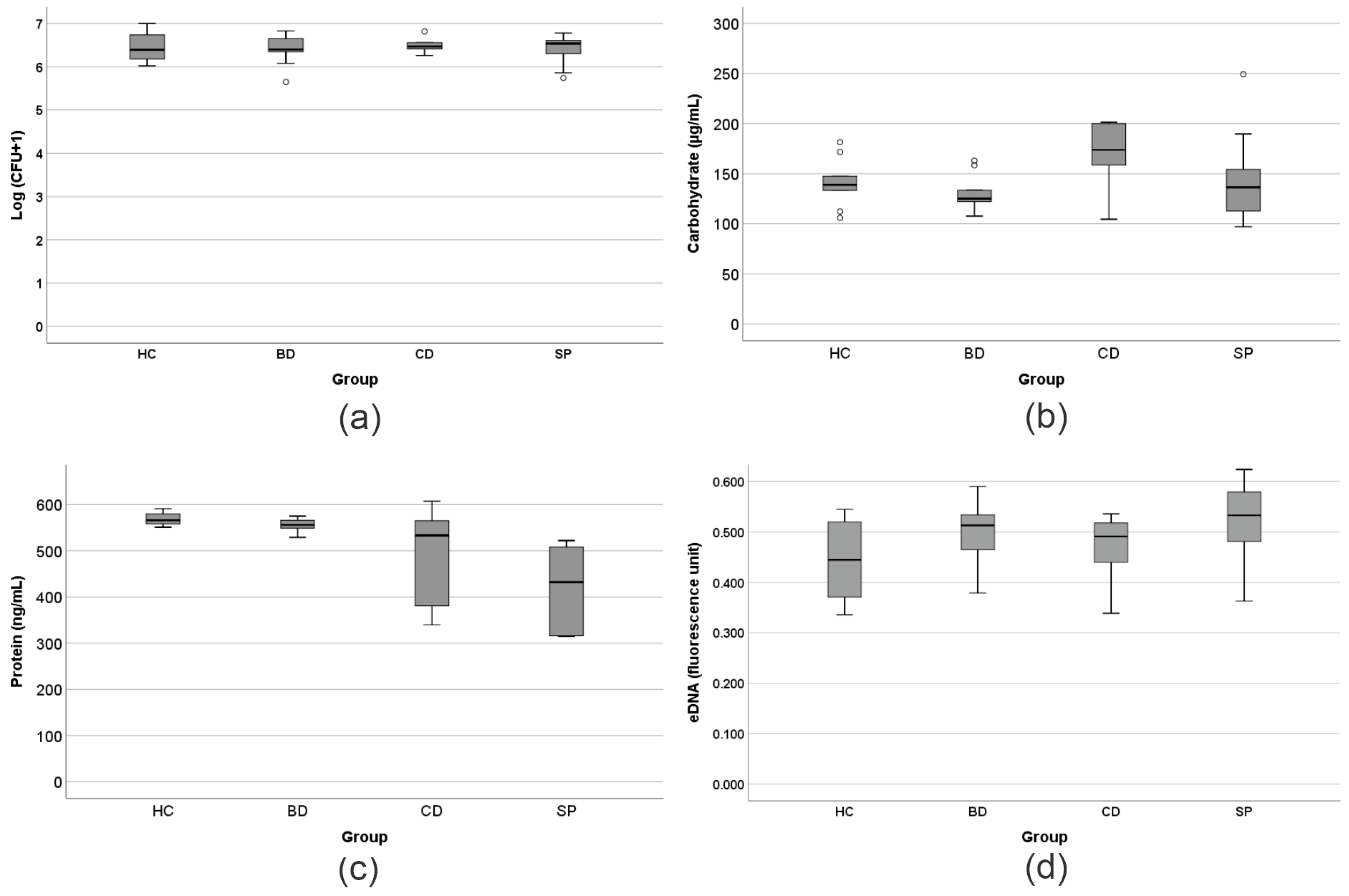
2.6. Determination of the Colony-Forming Units (CFU/mL)
2.7. Extraction of Biofilm’s Extracellular Polymeric Substances (EPS)
2.8. Quantification of Proteins in the EPS
2.9. Quantification of Carbohydrates in the EPS
2.10. Extraction and Quantification of the eDNA from the EPS
2.11. Qualitative Analysis of Biofilm Morphology
2.12. Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMMA | Polymethylmethacrylate |
| CAD/CAM | Computer-aided design and manufacturing |
| DLP | Digital light processing |
| CFU | Colony-forming units |
| EPS | Extracellular polymeric substance |
| eDNA | Extracellular DNA |
| SEM | Scanning electron microscopy |
| Sa | Surface roughness |
| BD | Bio Denture—Prizma® |
| CD | Denture Base Cosmos—Yller® |
| SP | Smart Print Bio Denture—Smart Dent® |
| HC | Heat-cured polymethylmethacrylate resin |
| STL | Standard triangle language |
| rpm | Revolutions per minute |
| BHIA | Brain heart infusion agar |
| BHI | Brain heart infusion |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
References
- Girundi, F.M.D.S.; Girundi, A.L.G.; Ribeiro, M.C.D.O.; Machado, R.M.M.; Gonçalves, T.M.S.V.; Del Bel Cury, A.A.; da Silva, W.J. Influence of Denture-Bearing Conditions on Masticatory Function and Patient-Reported Outcome Measures. J. Oral Rehabil. 2024, 51, 2316–2323. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Baik, A.; Almuzaini, S.A.; Farghal, A.E.; Alnazzawi, A.A.; Borzangy, S.; Aboalrejal, A.N.; AbdElaziz, M.H.; Mahmoud, I.I.; Zafar, M.S. Polymeric Denture Base Materials: A Review. Polymers 2023, 15, 3258. [Google Scholar] [CrossRef]
- Grachev, D.I.; Zolotnitsky, I.V.; Stepanov, D.Y.; Kozulin, A.A.; Mustafaev, M.S.; Deshev, A.V.; Arutyunov, D.S.; Tlupov, I.V.; Panin, S.V.; Arutyunov, S.D. Ranking Technologies of Additive Manufacturing of Removable Complete Dentures by the Results of Their Mechanical Testing. Dent. J. 2023, 11, 265. [Google Scholar] [CrossRef]
- Thu, K.M.; Molinero-Mourelle, P.; Yeung, A.W.K.; Abou-Ayash, S.; Lam, W.Y.H. Which Clinical and Laboratory Procedures Should Be Used to Fabricate Digital Complete Dentures? A Systematic Review. J. Prosthet. Dent. 2024, 132, 922–938. [Google Scholar] [CrossRef]
- Anadioti, E.; Musharbash, L.; Blatz, M.B.; Papavasiliou, G.; Kamposiora, P. 3D Printed Complete Removable Dental Prostheses: A Narrative Review. BMC Oral Health 2020, 20, 343. [Google Scholar] [CrossRef]
- Mubaraki, M.Q.; Moaleem, M.M.A.; Alzahrani, A.H.; Shariff, M.; Alqahtani, S.M.; Porwal, A.; Al-Sanabani, F.A.; Bhandi, S.; Tribst, J.P.M.; Heboyan, A.; et al. Assessment of Conventionally and Digitally Fabricated Complete Dentures: A Comprehensive Review. Materials 2022, 15, 3868. [Google Scholar] [CrossRef] [PubMed]
- Almuhayya, S.; Alshahrani, R.; Alsania, R.; Albassam, A.; Alnemari, H.; Babaier, R. Biofilm Formation on Three High-Performance Polymeric CAD/CAM Composites: An In Vitro Study. Polymers 2025, 17, 676. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A. Materials in Digital Dentistry—A Review. J. Esthet. Restor. Dent. 2020, 32, 171–181. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; de Carvalho, G.G.; dos Reis, A.C. Incorporation of Antimicrobial Agents into Dental Materials Obtained by Additive Manufacturing: A Literature Review. Saudi Dent. J. 2022, 34, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Temizci, T.; Bozoğulları, H.N. Effect of Thermal Cycling on the Flexural Strength of 3D-Printed, CAD/CAM-Milled and Heat-Polymerized Denture Base Materials. BMC Oral Health 2024, 24, 357. [Google Scholar] [CrossRef]
- Dimitrova, M.; Vlahova, A.; Kalachev, Y.; Zlatev, S.; Kazakova, R.; Capodiferro, S. Recent Advances in 3D Printing of Polymers for Application in Prosthodontics. Polymers 2023, 15, 4525. [Google Scholar] [CrossRef]
- Valencia, L.M.; Herrera, M.; de la Mata, M.; de León, A.S.; Delgado, F.J.; Molina, S.I. Synthesis of Silver Nanocomposites for Stereolithography: In Situ Formation of Nanoparticles. Polymers 2022, 14, 1168. [Google Scholar] [CrossRef]
- Poker, B.C.; Oliveira, V.C.; Macedo, A.P.; Gonçalves, M.; Ramos, A.P.; Silva-Lovato, C.H. Evaluation of Surface Roughness, Wettability and Adhesion of Multispecies Biofilm on 3D-Printed Resins for the Base and Teeth of Complete Dentures. J. Appl. Oral Sci. 2024, 32, e20230326. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Cuellar, E.; Guerrero-Barrera, A.L.; Avelar-Gonzalez, F.J.; Díaz, J.M.; de Santiago, A.S.; Chávez-Reyes, J.; Poblano-Sánchez, E. Characterization of Candida albicans and Staphylococcus aureus Polymicrobial Biofilm on Different Surfaces. Rev. Iberoam. Micol. 2022, 39, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Kurylec, J.; Ossa, A.; Orrego, S. Cyclic Strain of Poly(Methyl Methacrylate) Surfaces Triggered the Pathogenicity of Candida albicans. Acta Biomater. 2023, 170, 415–426. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, N.; Li, C.; Li, Z.; Zhang, N.; Li, B. Fungal Biofilm Formation and Its Regulatory Mechanism. Heliyon 2024, 10, e32766. [Google Scholar] [CrossRef]
- Ragupathi, H.; Pushparaj, M.M.; Gopi, S.M.; Govindarajan, D.K.; Kandaswamy, K. Biofilm Matrix: A Multifaceted Layer of Biomolecules and a Defensive Barrier against Antimicrobials. Arch. Microbiol. 2024, 206, 432. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-Forming Microorganisms Causing Hospital-Acquired Infections from Intravenous Catheter: A Systematic Review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef]
- Rahal, J.S.; Mesquita, M.F.; Henriques, G.E.P.; Nobilo, M.A.A. Surface Roughness of Acrylic Resins Submitted to Mechanical and Chemical Polishing. J. Oral Rehabil. 2004, 31, 1075–1079. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Carvalho-Silva, J.M.; Ferreira, I.; Schiavon, M.A.; Cândido Dos Reis, A. Silver vanadate nanomaterial incorporated into heat-cured resin and coating in printed resin: Antimicrobial activity in two multi-species biofilms and wettability. J. Dent. 2024, 145, 104984. [Google Scholar] [CrossRef] [PubMed]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Ludwig, T.G.; Goldberg, H.J. The Anthrone Method for the Determination of Carbohydrates in Foods and in Oral Rinsing. J. Dent. Res. 1956, 35, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeon, J.; Kim, J. Streptococcus mutans Extracellular DNA Levels Depend on the Number of Bacteria in a Biofilm. Sci. Rep. 2018, 8, 13313. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Ghannoum, M.A. Fungal Biofilms in the Clinical Laboratory. Curr. Fungal Infect. Rep. 2010, 4, 137–144. [Google Scholar] [CrossRef]
- An, R.; Rafiq, N.B. Candidiasis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560624/ (accessed on 23 September 2025).
- Vetsch, G.; Manoil, D.; Wulfman, C.; Parga, A.; Durual, S.; Srinivasan, M. Candida albicans Colonization on CAD-CAM Denture Resin Surface. J. Dent. 2025, 157, 105756. [Google Scholar] [CrossRef]
- Costa-Orlandi, C.B.; Sardi, J.C.O.; Pitangui, N.S.; De Oliveira, H.C.; Scorzoni, L.; Galeane, M.C.; Medina-Alarcón, K.P.; Melo, W.C.M.A.; Marcelino, M.Y.; Braz, J.D.; et al. Fungal Biofilms and Polymicrobial Diseases. J. Fungi 2017, 3, 22. [Google Scholar] [CrossRef]
- Freitas, R.F.C.P.; Duarte, S.; Feitosa, S.; Dutra, V.; Lin, W.S.; Panariello, B.H.D.; Carreiro, A.D.F.P. Physical, Mechanical, and Anti-Biofilm Formation Properties of CAD-CAM Milled or 3D Printed Denture Base Resins: In Vitro Analysis. J. Prosthodont. 2023, 32, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Al Haj Ebrahim, A.A.; Baba, N.Z. A Comparison of the Surface and Mechanical Properties of 3D Printable Denture-Base Resin Material and Conventional Polymethylmethacrylate (PMMA). J. Prosthodont. 2023, 32, 40–48. [Google Scholar] [CrossRef]
- Kurzendorfer-Brose, L.; Rosentritt, M. The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material. Materials 2025, 18, 1323. [Google Scholar] [CrossRef]
- Hahnel, S.; Rosentritt, M.; Handel, G.; Bürgers, R. In Vitro Evaluation of Artificial Ageing on Surface Properties and Early Candida albicans Adhesion to Prosthetic Resins. J. Mater. Sci. Mater. Med. 2009, 20, 249–255. [Google Scholar] [CrossRef]
- de Foggi, C.C.; Machado, A.L.; Zamperini, C.A.; Fernandes, D.; Wady, A.F.; Vergani, C.E. Effect of Surface Roughness on the Hydrophobicity of a Denture-Base Acrylic Resin and Candida albicans Colonization. J. Investig. Clin. Dent. 2016, 7, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Abualsaud, R.; Khan, S.Q. Hydrophobicity of Denture Base Resins: A Systematic Review and Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2022, 12, 139–159. [Google Scholar] [CrossRef]
- Bajunaid, S.O.; Baras, B.H.; Balhaddad, A.A.; Weir, M.D.; Xu, H.H.K. Antibiofilm and Protein-Repellent Polymethylmethacrylate Denture Base Acrylic Resin for Treatment of Denture Stomatitis. Materials 2021, 14, 1067. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Akutsu-Suyama, K.; Iwamiya, Y.; Terada-Ito, C.; Hiroi, Z.; Shibayama, M.; Satomura, K. Antimicrobial Surface Processing of Polymethyl Methacrylate Denture Base Resin Using a Novel Silica-Based Coating Technology. Clin. Oral Investig. 2023, 27, 1043–1053. [Google Scholar] [CrossRef]
- Hirasawa, M.; Tsutsumi-Arai, C.; Takakusaki, K.; Oya, T.; Fueki, K.; Wakabayashi, N. Superhydrophilic Co-Polymer Coatings on Denture Surfaces Reduce Candida albicans Adhesion—An In Vitro Study. Arch. Oral Biol. 2018, 87, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, J.H.; Kong, H.; Park, E.J. Biofilm Removal Effect of Diatom Complex on 3D Printed Denture Base Resin. Sci. Rep. 2024, 14, 4034. [Google Scholar] [CrossRef]
- Wei, X.; Gao, L.; Wu, K.; Pan, Y.; Jiang, L.; Lin, H.; Wang, Y.; Cheng, H. In Vitro Study of Surface Properties and Microbial Adhesion of Various Dental Polymers Fabricated by Different Manufacturing Techniques after Thermocycling. Clin. Oral Investig. 2022, 26, 7287–7297. [Google Scholar] [CrossRef]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A Review of the Role of Extracellular Polymeric Substances (EPS) in Wastewater Treatment Systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The Biofilm Matrix: Multitasking in a Shared Space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Hernández-Benítez, J.A.; Martines-Arano, H.; Rodríguez-Tovar, A.V.; Trejo-Valdez, M.; García-Pérez, B.E.; Torres-Torres, C. Multiphotonic Ablation and Electro-Capacitive Effects Exhibited by Candida albicans Biofilms. Bioengineering 2024, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.R.L.; Pianegonda, N.A.; Huynh, T.T.; Manefield, M.; MacLaughlin, S.A.; Rice, S.A.; Barker, P.J.; Blanksby, F.P. Evaluation of Hindered Amine Light Stabilisers and Their N-Chlorinated Derivatives as Antibacterial and Antifungal Additives for Thermoset Surface Coatings. Prog. Org. Coat. 2016, 99, 330–336. [Google Scholar] [CrossRef]
- Costa, T.; Sampaio-Marques, B.; Neves, N.M.; Aguilar, H.; Fraga, A.G. Antimicrobial Properties of Hindered Amine Light Stabilizers in Polymer Coating Materials and Their Mechanism of Action. Front. Bioeng. Biotechnol. 2024, 12, 1390513. [Google Scholar] [CrossRef] [PubMed]
- Abreu-Pereira, C.A.; Gorayb-Pereira, A.L.; Menezes Noveletto, J.V.; Jordão, C.C.; Pavarina, A.C. Zerumbone Disturbs the Extracellular Matrix of Fluconazole-Resistant Candida albicans Biofilms. J. Fungi 2023, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.; Piculell, M.; Modin, O.; Langenheder, S.; Persson, F.; Hermansson, M. Thickness Determines Microbial Community Structure and Function in Nitrifying Biofilms via Deterministic Assembly. Sci. Rep. 2019, 9, 5110. [Google Scholar] [CrossRef]
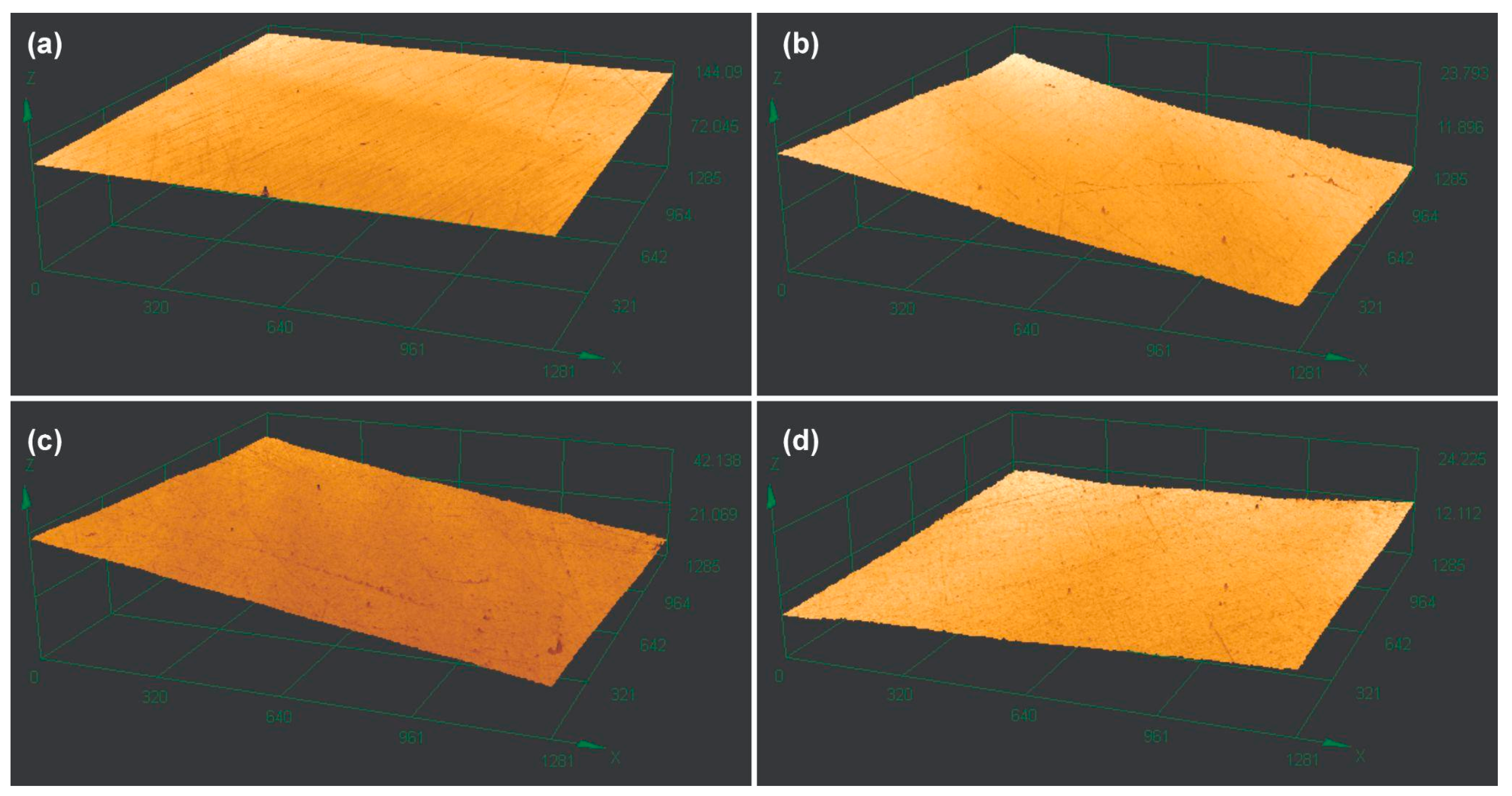
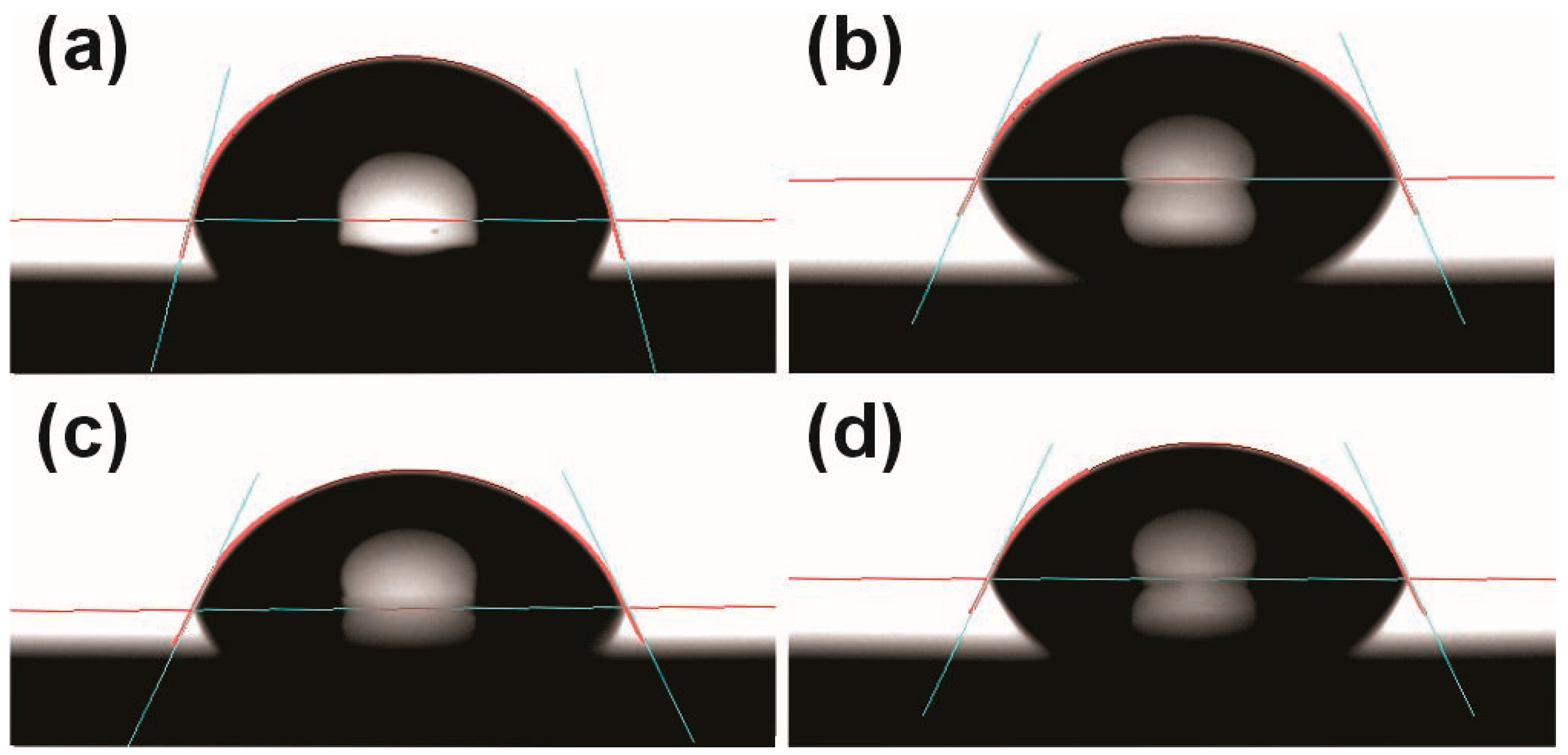
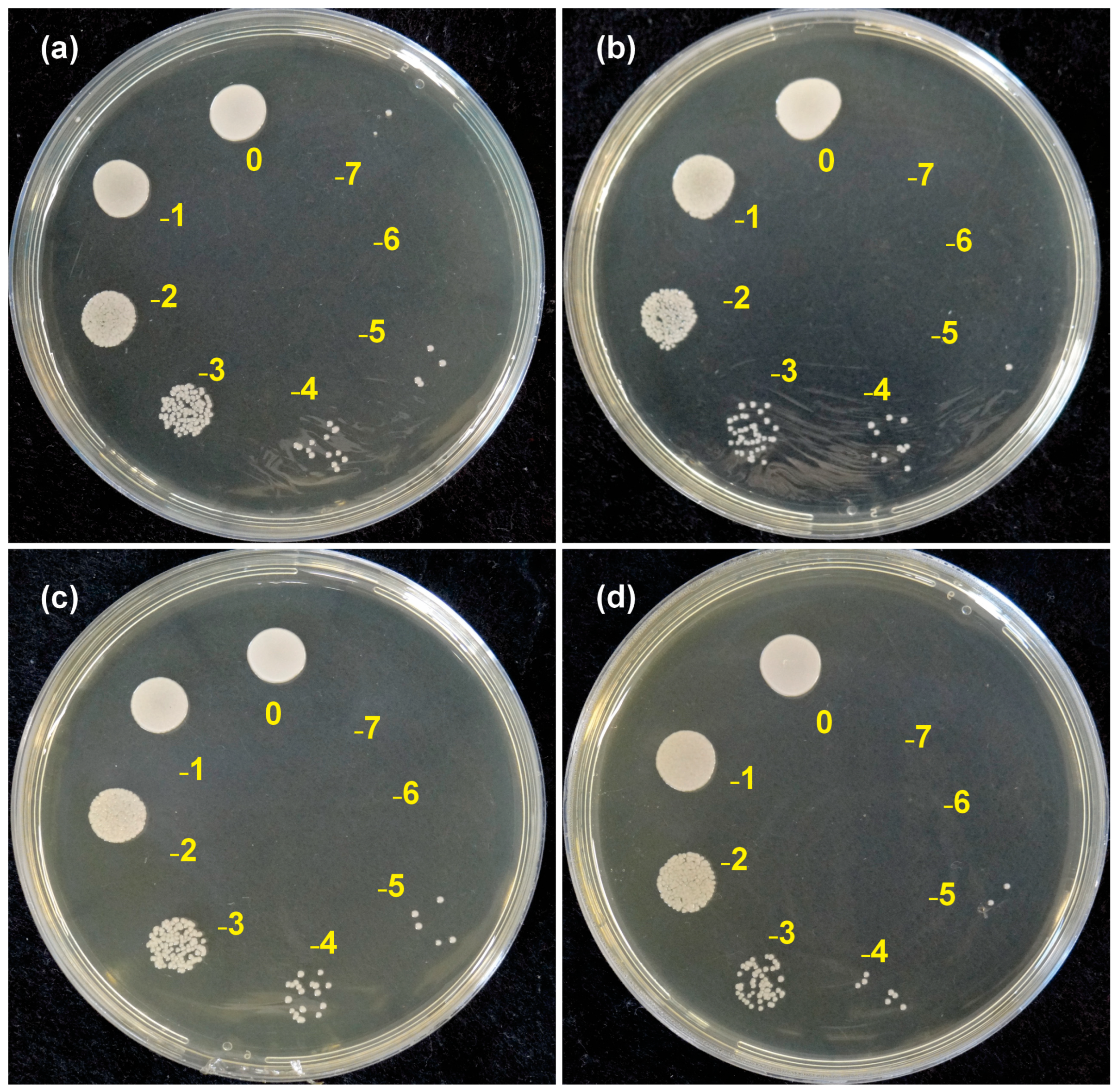


| Resin | Composition | Layer Height (mm) | Exposure Time (s) | Number of Base Layers | Base Exposure Time (s) | Lift/Retract Speed (mm/min) | Post-Curing (Cycles/min) |
|---|---|---|---|---|---|---|---|
| BD | Proprietary Acrylate Monomers; Pigmentation and Fillers; Acrylate Oligomers; Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (photoinitiator) | 0.05 | 3.80 | 8 | 35.00 | 60.0 | 3/1 |
| CD | Oligomers; Monomers; Photoinitiators; Stabilizer; Pigment | 0.05 | 3.50 | 10 | 40.00 | 65.00 | 1/10 |
| SP | Monomers, Oligomers, Photoinitiators, Pigments, Stabilizers | 0.05 | 4.30 | 10 | 25.00 | 60.00 | 1/9 |
| Group | Sa | p | Wettability | p |
|---|---|---|---|---|
| HC | 0.084 (0.018) * | 0.005 | 73.0 (3.5) | 0.004 |
| BD | 0.078 (0.015) * | 63.2 (5.2) ** | ||
| CD | 0.111 (0.013) | 68.7 (2.5) | ||
| SP | 0.096 (0.028) | 65.2 (3.1) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa, P.G.L.; Cruz-Araújo, S.R.; Freiria de Oliveira, C.A.; Silva, R.R.d.; Oliveira, V.d.C.; Pagnano, V.O.; Silva-Lovato, C.H.; Galo, R.; Stirke, A.; Melo, W.C.M.A.; et al. Evaluation of Colonization by Candida albicans and Biofilm Formation on 3D-Printed Denture Base Resins. Materials 2025, 18, 5018. https://doi.org/10.3390/ma18215018
Corrêa PGL, Cruz-Araújo SR, Freiria de Oliveira CA, Silva RRd, Oliveira VdC, Pagnano VO, Silva-Lovato CH, Galo R, Stirke A, Melo WCMA, et al. Evaluation of Colonization by Candida albicans and Biofilm Formation on 3D-Printed Denture Base Resins. Materials. 2025; 18(21):5018. https://doi.org/10.3390/ma18215018
Chicago/Turabian StyleCorrêa, Pedro Guilherme Lemos, Sarah Ribeiro Cruz-Araújo, Carolina Alves Freiria de Oliveira, Raiane Rodrigues da Silva, Viviane de Cássia Oliveira, Valéria Oliveira Pagnano, Claudia Helena Silva-Lovato, Rodrigo Galo, Arunas Stirke, Wanessa C. M. A. Melo, and et al. 2025. "Evaluation of Colonization by Candida albicans and Biofilm Formation on 3D-Printed Denture Base Resins" Materials 18, no. 21: 5018. https://doi.org/10.3390/ma18215018
APA StyleCorrêa, P. G. L., Cruz-Araújo, S. R., Freiria de Oliveira, C. A., Silva, R. R. d., Oliveira, V. d. C., Pagnano, V. O., Silva-Lovato, C. H., Galo, R., Stirke, A., Melo, W. C. M. A., & Macedo, A. P. (2025). Evaluation of Colonization by Candida albicans and Biofilm Formation on 3D-Printed Denture Base Resins. Materials, 18(21), 5018. https://doi.org/10.3390/ma18215018











